Abstract
Background
Chondrocyte apoptosis and catabolism are 2 major factors that contribute to the progression of osteoarthritis (OA). Loganin, an iridoid glycoside present in several herbs, including Flos lonicerae, Cornus mas L, and Strychnos nux vomica, is a valuable medication with anti-inflammatory and anti-apoptotic effects. Our study examines these effects and explores the potential benefits of loganin in the OA treatment.
Material/Methods
To clarify the roles of loganin in OA and its specific signaling pathway, chondrocytes were administrated with IL-1β and supplemented with or without LY294002 (a classic PI3K/Akt inhibitor). The apoptotic level, catabolic factors (MMP-3 and MMP-13 and ADAMTS-4 and ADAMTS-5), extracellular matrix (ECM) degradation, and activation of the PI3K/Akt pathway were evaluated using western blotting, PCR, and an immunofluorescent assay. The degenerative condition of the cartilage was evaluated using the Safranin O assay in vivo. The expression of cleaved-caspase-3 (C-caspase-3) was measured using immunochemistry.
Results
The data suggested that loganin suppressed the apoptotic level, reduced the release of catabolic enzymes, and decreased the ECM degradation of IL-1β-induced chondrocytes. However, suppressing PI3K/Akt signaling using LY294002 alleviated the therapeutic effects of loganin in chondrocytes. Our in vivo experiment showed that loganin partially attenuated cartilage degradation while inhibiting the apoptotic level.
Conclusions
This work revealed that loganin treatment attenuated IL-1β-treated apoptosis and ECM catabolism in rat chondrocytes via regulation of the PI3K/Akt signaling, revealing that loganin is a potentially useful treatment for OA.
MeSH Keywords: Apoptosis, Osteoarthritis, Phosphatidylinositol 3-Kinases
Background
Osteoarthritis (OA) is one of the chronic joint diseases that affect and disable 100 million people globally, especially the elderly population, and causes large societal and economic burden [1]. OA patients suffer joint pain, stiffness, and mobility difficulties, which leads to a low quality of life. Modern treatment of this disease focuses on attenuating its symptoms through the usage of non-steroidal anti-inflammatory drugs (NSAIDs), and often ends with joint replacement surgery [2]. It was important that our study elucidate the pathogenesis of OA and search for a potential treatment strategy that avoids surgery. However, the specific pathological mechanism of OA has not yet been clarified.
The pathological process of OA is evidenced by the aggravated degradation of articular cartilage and other joint components such as subchondral bone, synovium, ligaments, and muscles. The molecular events of chondrocytes (the only cells found in healthy cartilage), such as apoptosis, autophagic cell death, and necrosis, are closely involved in the pathological process of OA [3,4]. The death process of chondrocytes can be triggered by mechanical stress, the degradation of extracellular matrix (ECM), inflammation, or oxidative stress [5,6]. Several inflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor (TNF)-α bound with their specific ligands, initiate the formation of a death-related signaling complex, activating the caspase signaling pathway and ultimately inducing the death of target cells [7].
The decrease of chondrocytes leads directly to a decrease in cartilage function. Chondrocytes attach, move, and populate affected areas with the help of the ECM [8]. Furthermore, the ECM forms a hydrodynamic system that stabilizes the internal pressure of cartilage and maintains cartilage function [9]. Loss of the ECM is considered irreversible and plays an essential role in the cartilage degradation process [10]. Excessive ECM catabolic metabolism has been detected in the pathogenesis of OA [11]. Previous works have noted the upregulation of collagenolytic MMPs (matrix metalloproteinases) and aggrecanase ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) in the chondrocytes of OA patients [10,11].
There are currently no effective treatments to alleviate the progress of OA. Therefore, our study is timely and important in its attempt to facilitate solutions that prevent chondrocyte cell death and attenuate ECM degradation. Loganin is an iridoid glycoside extracted from several herbs including Flos lonicerae, Cornus mas L. and Strychnos nux vomica. Loganin has been demonstrated to regulate immune function and has anti-inflammatory effect that is induced by inhibition of nuclear factor (NF)-κB signaling and decreasing the release of TNF-α, IL-6, and other inflammatory cytokines [12,13]. Loganin protects against oxidative stress-related apoptosis via inhibition of JNK (natural killer), p38, and MAPK (mitogen-activated protein kinases) phosphorylation in neuronal cells [12]. Loganin attenuates apoptotic levels in TNF-α-treated SW10 cells via regulation of Smad2 cell signaling [13]. In consideration of the anti-inflammatory and anti-apoptotic effects of loganin, we attempted to determine whether it has potential effects on the treatment of OA. This study explored the specific mechanism underlying the potential protective effects of loganin against OA.
Material and Methods
Reagents
Loganin was acquired from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). IL-1β was acquired from R&D Systems (St. Paul, MN, USA). Antibodies were acquired from Santa Cruz (Santa Cruz, CA, USA) and Cell Signaling Technology (Beverly, MA, USA). Other reagents not mentioned here were acquired from Sigma-Aldrich.
Chondrocyte culture and treatment
Primary chondrocytes were extracted from rats as previously described [14]. Sprague Dawley male rats (180 g to 220 g) were euthanized using CO2. The cartilages of knee joints were collected and cut using Microscissors, digested by 0.25% Trypsin-EDTA for 30 minutes and type II collagenase for 5 hours. After filtration using 150-μM mesh, the tissue fragments were incubated with DMEM containing 10% fetal bovine serum and 1% antibiotics in a cell incubator of 5% CO2 at 37°C. The culture medium was changed every 2 days, and chondrocytes at P0 were passaged once reaching 70% to 80% confluence. Chondrocytes at P2 were treated for 2 hours with loganin or IL-1β (10 ng/mL) with or without the specific PI3K inhibitor LY294002 (50 μM) [15]. The cells were then collected for further tests.
Viability assay
The chondrocyte viability was tested using the Cell Counting Kit 8 (CCK-8) method. Chondrocytes were plated in 96-well plates, and their viability upon pretreatment with different concentrations of loganin or IL-1β, as described, was evaluated at 24 hours. And 10 μL CCK-8 solution was then added to each well and incubated for 1 hour at 37°C. Absorbance values at 450 nm were recorded with a microplate reader 2 hours after addition of the CCK-8 solution (10 μL) into each well.
Quantitative RT-PCR
The levels of ADAMTS-4 and ADAMTS-5, and MMP-3 and MMP-13, as well as collagen and aggrecan mRNA were measured. mRNA was isolated from the chondrocytes in each group using TRIzol reagent, and cDNA synthesis was performed according manufacturer’s protocol for the Thermo Scientific RevertAid First Strand cDNA Synthesis Kit. PCR was processed by SYBR® Premix Ex Taq™. The mRNA levels for ADAMTS-4, ADAMTS-5, MMP-3, MMP-13, collagen, and aggrecan levels were compared to that of β-actin to obtain the Ct (threshold cycle).
Western blot assay
Chondrocyte proteins were extracted using lysis buffer and quantified using a Bio-Rad protein assay kit. The equivalent of 60 μg of proteins were separated on a 10–12% gradient gel and transferred on PVDF membrane. The membranes were treated with a 5% skim milk-TBST solution for 1.5 hours, treated with specific primary and secondary antibodies, and then the signal visualized and quantified using a ChemiDoc XRS+ Imaging System (Bio-Rad).
TUNEL method
The TUNEL method is a technique used to measure DNA fragmentation in apoptotic cells. After fixation in 4% paraformaldehyde (PFA) for 15 minutes, chondrocytes were incubated with 3% hydrogen peroxide and 0.1% Triton X-100 for 15 minutes. In accordance with the kit manufacturer protocols, chondrocytes were incubated with in situ cell death detection reagents and then treated with DAPI. Data were visualized using a Nikon ECLIPSE Ti microscope (Tokyo, Japan).
Immunofluorometric assay
Collagen II was measured using immunofluorescence analysis. Chondrocytes seeding on coverslips were treated with 4% PFA for 15 minutes and then administrated using 0.1% Triton X-100 for 10 minutes. The chondrocytes were then administrated with 1% BSA for 30 minutes, treated with primary antibody overnight at 4°C and a specific secondary antibody for an additional hour, and stained using DAPI for 10 minutes [16]. Specimens were viewed under a Nikon ECLIPSE Ti microscope (Tokyo, Japan).
Model and treatment of OA
Male Sprague-Dawley Rats (3 months old, 180–220 g) were used in this study. Rats were assigned to following groups: sham, OA, and OA+loganin. The OA group rats were revived from surgery following the transection of their right anterior cruciate ligament (ACL) and resection of the right medial meniscus. The sham group received the same surgical preparation but without ACL transection or meniscus resection. After surgery, animals in the OA+loganin group were treated with loganin (20 mg/kg/day; subcutaneous injection) [17].
Histological assay
Cartilage degeneration was measured by Safranin O-Fast Green staining of 4% paraformaldehyde-fixed paraffin section (5-μm). The deparaffinized and rehydrated specimens were then viewed under a light microscope. The degenerative condition of the cartilage was quantified by the Osteoarthritis Research Society International (OARSI) system [18].
Immunochemistry
Cleaved-caspase-3 (C-caspase-3) levels in cartilage were measured via immunochemistry staining on 4% paraformaldehyde-fixed paraffin section (5-μm). The deparaffinized and rehydrated specimens were treated by 3% hydrogen peroxide, and antigen retrieval was finished using microwave heating for 10 minutes. The samples were treated with 5% BSA for 30 minutes, and primary antibody overnight. Finally, the sections were administrated with a specific secondary antibody for 1 hour. All images were captured using light microscopy.
Statistical analysis
Data are presented as mean ± standard deviation. Statistical analysis was performed using SPSS software (version 20.0). Statistical comparisons of the data between each group were analyzed by one-way ANOVA and Tukey’s post-hoc test. P<0.05 was considered as significant difference.
Results
Effect of loganin on chondrocyte viability
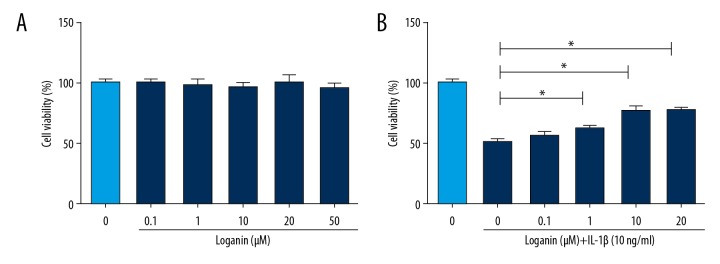
To explore the appropriate treatment concentration of loganin in chondrocytes, we tested the cytotoxicity of loganin. As seen in the CCK-8 analysis results in Figure 1A, loganin in the concentration range of 0.1–20 μM did not exhibit cytotoxic effect on cells. When loganin was administered to chondrocytes combined with IL-1β, it exerted a protective effect (Figure 1B).
Figure 1.
Effect of loganin and IL-1β on the cell viability of chondrocyte. (A) Cells were administered by various concentrations of loganin alone for 1 day. (B) Cells were administered with various concentrations of loganin supplemented with IL-1β (10 ng/mL) for 1 day. Results are expressed as the mean ± standard deviation. * P<0.05, ** P<0.01, *** P<0.001 in comparison to the control cells.
Loganin treatment decreased apoptosis in chondrocytes
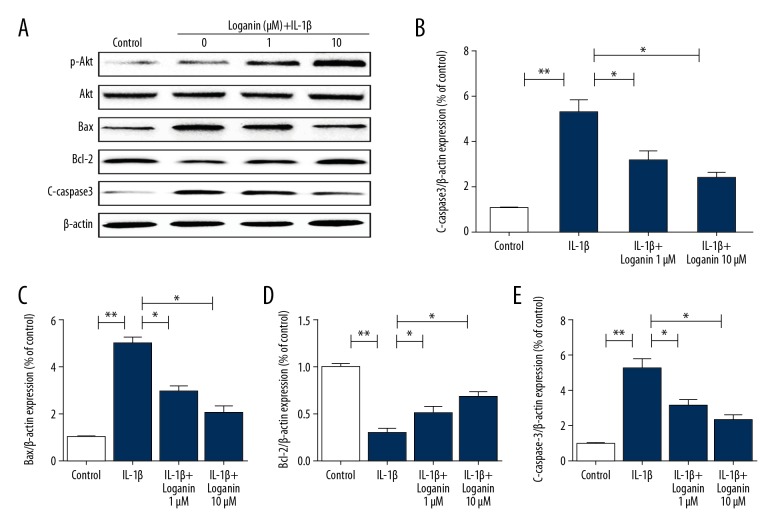
The PI3K/Akt pathway is closely involved in various cell biological processes. As shown in Figure 2, loganin treatment increased the ratio of p-Akt/t-Akt, suggesting that loganin activated the PI3K/Akt signaling. Furthermore, western blotting results showed increased expression of C-caspase-3 and Bax in the IL-1β-treated group, which was markedly reversed by loganin. Loganin also enhanced the expression of Bcl-2 when compared to the IL-1β group.
Figure 2.
Loganin treatment decrease apoptosis in chondrocytes. (A–E) Western blotting analysis of p-Akt, t-Akt, Bax, Bcl-2, and C-caspase-3 in each group. Results are expressed as the mean ± standard deviation. * P<0.05, ** P<0.01, *** P<0.001 in comparison to the control cells.
Inhibiting PI3K/Akt signaling suppressed the anti-apoptotic effects of loganin
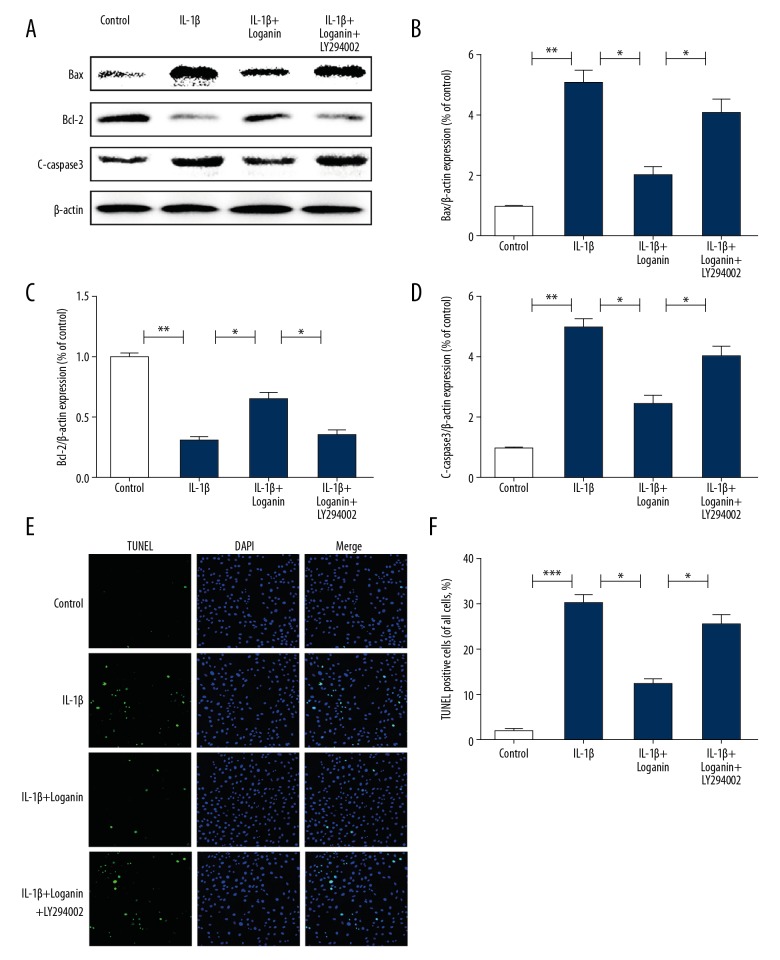
In order to explore whether PI3K/Akt signaling was involved in the anti-apoptotic effects of loganin, we used the classic PI3K/Akt signaling inhibitor LY294002 to treat cells with loganin. Our results showed that LY294002 abolished the anti-apoptotic effects of loganin, as noted by upregulated levels of Bax and C-caspase-3, and downregulated Bcl-2, which was consistent with the TUNEL results (Figure 3A–3F).
Figure 3.
Suppression of PI3K/Akt signaling suppressed anti-apoptotic effects of loganin. (A–D) Western blotting analysis of p-Akt, t-Akt, Bax, Bcl-2 and C-caspase-3 in each group. (E–F) TUNEL assay of each group. Results are expressed as the mean ± standard deviation. * P<0.05, ** P<0.01, *** P<0.001 in comparison to the control cells.
Loganin decreased catabolic enzyme levels via regulation of PI3K/Akt signaling
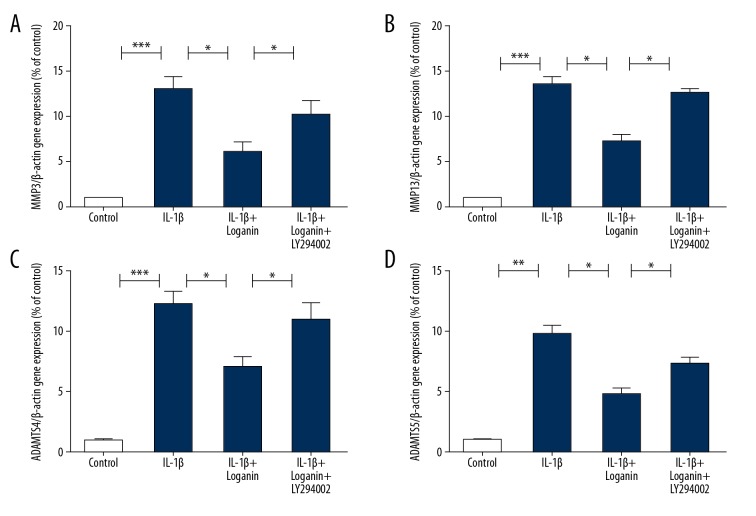
Since catabolic enzymes, including MMPs and ADAMTSs, play a critical role in OA development, we explored the ability of loganin to influence the production of the catabolic factors MMP-3 and MMP-13, and ADAMTS-4 and ADAMTS-5, which play essential roles in cartilage degeneration. These results revealed that loganin decreased the release of MMP-3 and MMP-13, and ADAMTS-4 and ADAMTS-5 in the IL-1β-stimulated group. However, the downregulated productions of these catabolic factors due to loganin were suppressed by LY294002 treatment, showing that loganin decreased catabolic activities via regulation of PI3K/Akt (Figure 4).
Figure 4.
Loganin decreased catabolic enzymes via regulation of PI3K/Akt signaling. (A–D) The mRNA expressions of (A) MMP-3, (B) MMP-13, (C) ADAMTS-4, (D) ADAMTS-5 were detected by PCR. Results are expressed as the mean ± standard deviation. * P<0.05, ** P<0.01, *** P<0.001 in comparison to the control cells.
Loganin decreased the degradation of ECM via activation of PI3K/Akt signaling in IL-1β-treated chondrocytes
As noted in Figure 5A and 5B, IL-1β markedly attenuated the mRNA expressions of collagen II and aggrecan. However, loganin inhibited ECM-degrading activity, and its effects were reversed by LY294002. The immunofluorescence results for collagen-II were consistent with the mRNA results, suggesting that PI3K/Akt signaling might regulate the ECM metabolic activity by loganin in chondrocytes (Figure 5C).
Figure 5.
Loganin decreases the degradation of ECM through regulation of PI3K/Akt signaling in IL-1β-treated group. (A) Immunofluorescence assay of collagen II protein in chondrocytes of each group. (B, C) The mRNA expressions of (B) collagen II, (C) aggreacan were measured by PCR. Results are expressed as the mean ± standard deviation. * P<0.05, ** P<0.01, *** P<0.001 in comparison to the control cells.
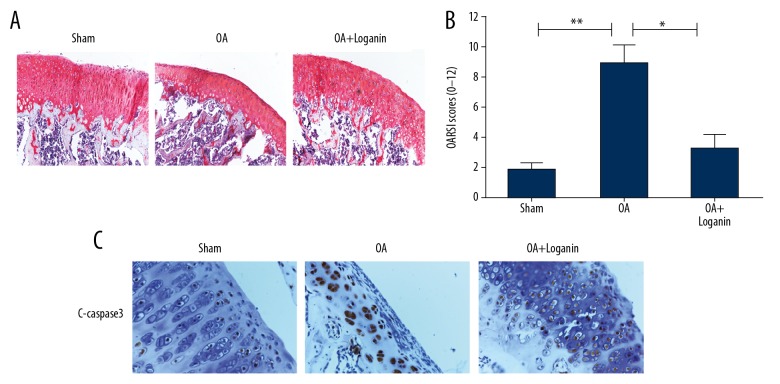
Loganin decreased the apoptotic level and ECM degradation in cartilage of rats with OA
OA severity was measured using Safranin O staining in histological analysis. When compared with the sham group, in the OA group, the cartilage layer exhibited thinning with a reduced number of chondrocytes. However, this cartilage degeneration was attenuated by loganin treatment (Figure 6A, 6B). Immunohistochemical results revealed that loganin also decreased the level of C-caspase-3 in the cartilage of the OA rats (Figure 6C).
Figure 6.
Loganin decreased apoptotic level and ECM degradation in rat cartilage of OA. (A, B) Safranin O staining and quantification analysis were used to measure the cartilage degeneration in each group at 8 weeks after surgery. (C) immunohistochemical assay of C-caspase-3 in each group. Results are expressed as the mean ± standard deviation. * P<0.05, ** P<0.01, *** P<0.001 in comparison to the control rats.
Discussion
Previous studies have demonstrated the close relationship between chondrocyte apoptosis and the severity of cartilage degeneration [5]. Decreasing chondrocyte numbers have been noted to occur with aging in OA cartilage [19]. Hence, clarifying the mechanism of chondrocyte apoptosis is critical in developing potential therapeutic strategies for OA patients. In contrast to passive necrosis, apoptosis is a programmed cell death that initiates due to a harmful situation such as infection, trauma, or oxidative stress [20,21]. Apoptosis is a normal, spatio-temporal controlled cell activity [22]. However, excessive apoptosis contributes to many pathological conditions, including autoimmunity.
The apoptotic process is regulated via 2 signaling pathways, the death receptor-related pathway as well as the mitochondrial-related pathway. Both pathways activate executioner caspases [23]. Caspase-3 is the most typical executioner caspase; it acts as a scavenger for cell substrates such as cytoskeletal and nuclear proteins, and contributes to the typical apoptotic decomposition of the cell via DNA fragmentation [24]. Inhibiting caspase signaling has been shown to suppress chondrocyte death and cartilage degradation in vivo [25].
Loganin protects against oxidative stress-related apoptosis via inhibition of JNK, p38, and MAPK phosphorylation in neuronal cells [12]. Loganin attenuates apoptotic levels in TNF-α treated SW10 cells via the regulation of Smad2 signaling [13]. Our study demonstrated that loganin could attenuate the expressions of the apoptotic proteins Bax and C-caspase-3, and enhance the expression of the anti-apoptotic protein Bcl-2 in IL-1β-treated group. The OA+loganin group showed lower apoptosis than rats of the OA group, demonstrating the potential anti-apoptotic effects of loganin.
Chondrocyte apoptosis is a process that has not yet been clarified. Several signaling pathways contribute to apoptosis, including p38, p53, NF-κB, MAPK, and JNK. MAPK signaling has been demonstrated to be related to the cellular senescence process [26,27]. Fas induction causes apoptosis by activating caspase-8 [28]. The NF-κB signaling pathway is a potential inducer for chondrocyte apoptosis [29,30]. The PI3K/Akt pathway is an important component in the growth and survival of cells during various biological processes, including the development of OA [31]. Akt can phosphorylate and inactivate a proapoptotic factor (Bad), leading to downregulation of the apoptotic level [32]. Akt is known to inactivate pro-caspase-9 and phosphorylate caspase-9, thus interfering with signaling of apoptosis [33]. Loganin treatment has been reported to improve insulin resistance through regulation of PI3K/Akt signaling in the liver of T2DM models [34]. Moreover, loganin exerted neuroprotective effects on NSC34 cells via activating PI3K/Akt in mice with spinal muscular atrophy [17]. In this present work, our results demonstrated that loganin can activate PI3K/Akt signaling and attenuate the apoptotic level in IL-1β-treated chondrocytes. However, inhibiting PI3K/Akt signaling using the specific inhibitor LY294002 partially reversed the anti-apoptotic effects of loganin, further revealing that it exerted a protective effect via regulation of PI3K/Akt signaling.
Various studies have shown that ECM metabolism is controlled by catabolic enzymes, including MMPs and ADAMTSs, the levels of which are increased in IL-1β-induced chondrocytes [35]. In addition, mechanical stress triggers danger-associated molecular patterns which release and degrade cartilage ECM [36]. Chondrocyte apoptosis and autophagic death initiate danger-associated molecular patterns, because both lead to secondary necrosis [37]. Therefore, treatments that suppress the release of these catabolic factors are potential therapeutic strategies to treat OA. MMP-3 and MMP-13 degrade a wide array of ECM components, including collagen II and other proteoglycans, and enhance the expression of other MMPs [38,39]. ADAMTS-5 siRNA attenuated ECM degradation in a previous in vivo study [40]. ECM catabolism is regulated by various signaling pathways. IL-6/Stat3 signaling mediated the release of catabolic factors in OA patients [41]. Oleocanthal-inhibited, LPS-induced expression of MMP-13 and ADAMTS-5 induction was observed in human primary OA chondrocyte through regulation of the MAPKs/NF-κB pathways [42]. Inhibition of PI3K/Akt/NF-κB pathway attenuated the inflammatory response and subsequent expression of MMP-3, MMP-13, ADAMTS-4, ADAMTS-5 in human OA chondrocyte [43]. IL-1β can potentially activate Akt signaling by increasing the release of MMP-13 via activation of the MAPK, JNK, p38, and JAK-STAT pathways [44].
In this work, we attempted to show the roles of loganin in ECM metabolism and its potential mechanism. Our results revealed that loganin markedly attenuated the expressions of the MMP-3 and MMP-13 and ADAMTS-4 and ADAMTS-5 genes, subsequently decreasing the degradation of collagen II and aggrecan in IL-1β-induced group. During in vivo experiments, the OA+loganin group showed higher deposition of proteoglycans in their cartilage, as well as a lower OARSI score, than the rats of the OA group. However, suppressing the PI3K/Akt signaling by LY294002 attenuated the therapeutic effects of loganin in chondrocytes. Together, the results revealed that loganin treatment restored the function of chondrocytes via PI3K/Akt signaling. However, there are still some limitations in this study. In order to better elucidate the apoptotic pathway regulated by Akt signaling, the specific mechanism between Akt and apoptosis need to be studied in this work, and more animal experiments need to prove this. We will provide further evidence in future studies.
Conclusions
In conclusion, this work revealed that loganin treatment suppressed IL-1β-treated apoptosis and ECM catabolism in rat chondrocytes. Suppression of the PI3K/Akt signaling attenuated the therapeutic effects of loganin. Our in vivo experiments also demonstrated that loganin attenuated cartilage degeneration. Our results revealed that loganin may be a potentially useful agent for OA.
Footnotes
Source of support: The present study was supported by Research Foundation Project of Hainan Medical University (Grant No. HYPY201925)
Conflict of interest
None.
References
- 1.Wight L, Owen D, Goldbloom D, et al. Pure ankle dislocation: A systematic review of the literature and estimation of incidence. Injury. 2017;48:2027–34. doi: 10.1016/j.injury.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Litwic A, Edwards MH, Dennison EM, et al. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn K, D’Lima DD, Hashimoto S, et al. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Zhai X, Meng R, Li H, et al. MiR-181a modulates chondrocyte apoptosis by targeting glycerol-3-phosphate dehydrogenase 1-like protein (GPD1L) in osteoarthritis. Med Sci Monit. 2017;23:1224–31. doi: 10.12659/MSM.899228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Carlo M, Jr, Loeser RF. Cell death in osteoarthritis. Curr Rheumatol Rep. 2008;10:37–42. doi: 10.1007/s11926-008-0007-8. [DOI] [PubMed] [Google Scholar]
- 6.Gao G, Ding H, Zhuang C, et al. Effects of hesperidin on H(2)O(2)-treated chondrocytes and cartilage in a rat osteoarthritis model. Med Sci Monit. 2018;24:9177–86. doi: 10.12659/MSM.913726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HA, Blanco FJ. Cell death and apoptosis in ostearthritic cartilage. Current Drug Targets. 2007;8:333–45. doi: 10.2174/138945007779940025. [DOI] [PubMed] [Google Scholar]
- 8.Petursson F, Husa M, June R, et al. Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res Ther. 2013;15:R77. doi: 10.1186/ar4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matyas JR, Adams ME, Huang D, et al. Discoordinate gene expression of aggrecan and type II collagen in experimental osteoarthritis. Arthritis Rheum. 1995;38:420–25. doi: 10.1002/art.1780380320. [DOI] [PubMed] [Google Scholar]
- 10.Catterall JB, Stabler TV, Flannery CR, et al. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Res Ther. 2010;12:R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Pan Q, Mao Z, et al. Kaempferol inhibits interleukin-1beta stimulated matrix metalloproteinases by suppressing the MAPK-associated ERK and P38 signaling pathways. Mol Med Rep. 2018;18:2697–704. doi: 10.3892/mmr.2018.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon SH, Kim JA, Hong SI, et al. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 2011;58:533–41. doi: 10.1016/j.neuint.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Chao G, Tian X, Zhang W, et al. Blocking Smad2 signalling with loganin attenuates SW10 cell cycle arrest induced by TNF-alpha. PLoS One. 2017;12:e0176965. doi: 10.1371/journal.pone.0176965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Y, Wang Y, Wang Y, et al. Transient receptor potential vanilloid 5 mediates Ca2+ influx and inhibits chondrocyte autophagy in a rat osteoarthritis model. Cell Physiol Biochem. 2017;42:319–32. doi: 10.1159/000477387. [DOI] [PubMed] [Google Scholar]
- 15.Xu D, Jin H, Wen J, et al. Hydrogen sulfide protects against endoplasmic reticulum stress and mitochondrial injury in nucleus pulposus cells and ameliorates intervertebral disc degeneration. Pharmacol Res. 2017;117:357–69. doi: 10.1016/j.phrs.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Zhang H, Zheng B, et al. Retinoic acid induced-autophagic flux inhibits ER-stress dependent apoptosis and prevents disruption of blood-spinal cord barrier after spinal cord injury. Int J Biol Sci. 2016;12:87–99. doi: 10.7150/ijbs.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng YT, Chen CS, Jong YJ, et al. Loganin possesses neuroprotective properties, restores SMN protein and activates protein synthesis positive regulator Akt/mTOR in experimental models of spinal muscular atrophy. Pharmacol Res. 2016;111:58–75. doi: 10.1016/j.phrs.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Kraus VB, Huebner JL, DeGroot J, et al. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2010;18(Suppl 3):S35–52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto S, Ochs RL, Komiya S, et al. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–38. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- 21.Ou Y, Tan C, An H, et al. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med Sci Monit. 2012;18:BR247–52. doi: 10.12659/MSM.882901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson G. Active role of chondrocyte apoptosis in endochondral ossification. Microsc Res Tech. 1998;43:191–204. doi: 10.1002/(SICI)1097-0029(19981015)43:2<191::AID-JEMT10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Lamkanfi M, Declercq W, Kalai M, et al. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–61. doi: 10.1038/sj.cdd.4400989. [DOI] [PubMed] [Google Scholar]
- 24.Enari M, Sakahira H, Yokoyama H, et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 25.D’Lima D, Hermida J, Hashimoto S, et al. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–21. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Liu WZ, Liu T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–4. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 27.Fan DX, Yang XH, Li YN, et al. 17beta-estradiol on the expression of g-protein coupled estrogen receptor (GPER/GPR30) mitophagy, and the PI3K/Akt signaling pathway in ATDC5 chondrocytes in vitro. Med Sci Monit. 2018;24:1936–47. doi: 10.12659/MSM.909365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Zhou L, Nan Z, et al. Knockdown of cMyc activates Fas-mediated apoptosis and sensitizes A549 cells to radiation. Oncol Rep. 2017;38:2471–79. doi: 10.3892/or.2017.5897. [DOI] [PubMed] [Google Scholar]
- 29.Cai L, Chen WN, Li R, et al. Acetazolamide protects rat articular chondrocytes from IL-1beta-induced apoptosis by inhibiting the activation of NF-kappaB signal pathway. Can J Physiol Pharmacol. 2018;96:1104–11. doi: 10.1139/cjpp-2018-0334. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang Z, Ye G, Huang B. Kaempferol alleviates the interleukin-1beta-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-kappaB. Med Sci Monit. 2017;23:3925–31. doi: 10.12659/MSM.902491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Ni S, Miao KS, et al. PI3K/Akt and caspase pathways mediate oxidative stress-induced chondrocyte apoptosis. Cell Stress Chaperones. 2019;24(1):195–202. doi: 10.1007/s12192-018-0956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamada H, Nito C, Endo H, et al. Bad as a converging signaling molecule between survival PI3-K/Akt and death JNK in neurons after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:521–33. doi: 10.1038/sj.jcbfm.9600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 34.Dai B, Wu Q, Zeng C, et al. The effect of Liuwei Dihuang decoction on PI3K/Akt signaling pathway in liver of type 2 diabetes mellitus (T2DM) rats with insulin resistance. J Ethnopharmacol. 2016;192:382–89. doi: 10.1016/j.jep.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Blasioli DJ, Kaplan DL. The roles of catabolic factors in the development of osteoarthritis. Tissue Eng Part B Rev. 2014;20:355–63. doi: 10.1089/ten.teb.2013.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komori T. Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res. 2013;352:191–98. doi: 10.1007/s00441-012-1546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eguchi T, Kubota S, Kawata K, et al. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol Cell Biol. 2008;28:2391–413. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Ma J, Gu JH, et al. Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1beta is mediated by curcumin via inhibition of NF-kappaB signaling in rat chondrocytes. Mol Med Rep. 2017;16:1837–45. doi: 10.3892/mmr.2017.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu X, You H, Yuan X, et al. Protective effect of lentivirus-mediated siRNA targeting ADAMTS-5 on cartilage degradation in a rat model of osteoarthritis. Int J Mol Med. 2013;31:1222–28. doi: 10.3892/ijmm.2013.1318. [DOI] [PubMed] [Google Scholar]
- 41.Latourte A, Cherifi C, Maillet J, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76:748–55. doi: 10.1136/annrheumdis-2016-209757. [DOI] [PubMed] [Google Scholar]
- 42.Scotece M, Conde J, Abella V, et al. Oleocanthal inhibits catabolic and inflammatory mediators in lps-activated human primary osteoarthritis (OA) chondrocytes through MAPKs/NF-kappaB pathways. Cell Physiol Biochem. 2018;49:2414–26. doi: 10.1159/000493840. [DOI] [PubMed] [Google Scholar]
- 43.Lu C, Li Y, Hu S, et al. Scoparone prevents IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes through the PI3K/Akt/NF-kappaB pathway. Biomed Pharmacother. 2018;106:1169–74. doi: 10.1016/j.biopha.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 44.Greene MA, Loeser RF. Function of the chondrocyte PI-3 kinase-Akt signaling pathway is stimulus dependent. Osteoarthritis Cartilage. 2015;23:949–56. doi: 10.1016/j.joca.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]