Significance
Vitamin A is a nutrient that is essential for the development of intestinal immunity. It is absorbed by gut epithelial cells, which convert it to retinol and retinoic acid. Here we show that the transcription factor retinoic acid receptor β (RARβ) allows epithelial cells to sense vitamin A in the diet and regulate vitamin A-dependent immunity in the intestine. We find that epithelial RARβ regulates several intestinal immune responses, including production of the immunomodulatory protein serum amyloid A, T-cell homing to the intestine, and B-cell production of immunoglobulin A. Our findings provide insight into how epithelial cells sense vitamin A to regulate intestinal immunity, and highlight why vitamin A is so important for immunity to infection.
Keywords: vitamin A, retinol, microbiota, intestinal epithelium, mucosal immunity
Abstract
Vitamin A is a dietary component that is essential for the development of intestinal immunity. Vitamin A is absorbed and converted to its bioactive derivatives retinol and retinoic acid by the intestinal epithelium, yet little is known about how epithelial cells regulate vitamin A-dependent intestinal immunity. Here we show that epithelial cell expression of the transcription factor retinoic acid receptor β (RARβ) is essential for vitamin A-dependent intestinal immunity. Epithelial RARβ activated vitamin A-dependent expression of serum amyloid A (SAA) proteins by binding directly to Saa promoters. In accordance with the known role of SAAs in regulating Th17 cell effector function, epithelial RARβ promoted IL-17 production by intestinal Th17 cells. More broadly, epithelial RARβ was required for the development of key vitamin A-dependent adaptive immune responses, including CD4+ T-cell homing to the intestine and the development of IgA-producing intestinal B cells. Our findings provide insight into how the intestinal epithelium senses dietary vitamin A status to regulate adaptive immunity, and highlight the role of epithelial cells in regulating intestinal immunity in response to diet.
The mammalian intestinal epithelium is a vital interface between the external environment and internal tissues. Epithelial cells interact with the environment of the gut lumen by absorbing dietary compounds and by associating with the resident bacterial communities that promote digestion. The intestinal epithelium also orchestrates development of the underlying immune system through the secretion of immunoregulatory proteins (1). Thus, epithelial cells are ideally positioned to capture information about the diet and the microbiota to regulate adaptive immunity. Although gut epithelial cells are known to detect intestinal microorganisms through various pathways involving pattern recognition receptors (1), little is known about how epithelial cells sense dietary components to regulate adaptive immunity.
Vitamin A is a fat-soluble nutrient that is essential for the development of adaptive immunity to intestinal microorganisms. It is required for IgA production by intestinal B cells (2), T-cell homing to the intestine (3), and the production of IL-17 by T helper 17 (Th17) cells (4). As a consequence, vitamin A-deficient diets result in severe immunodeficiency and increased infection rates (5).
Cells convert vitamin A into several related compounds, collectively known as retinoids. These include retinol and its derivative retinoic acid (RA). RA is a potent regulatory molecule that controls gene expression through RA receptors (RARα, RARβ, and RARγ), members of the nuclear receptor family that activate the transcription of specific target genes (6). The intestinal epithelium plays a central role in retinoid metabolism by absorbing dietary vitamin A and expressing RA-generating enzymes and RARs (7, 8). This suggests that the epithelium could help regulate vitamin A-dependent adaptive immunity. A recent study implicated epithelial RARα in the development of the epithelial barrier, as well as lymphoid follicles that support intestinal immune cell development (8). However, it is not clear whether epithelial cells regulate the development of specific vitamin A-dependent immunological pathways, including the development of gut-homing CD4+ T cells, IgA-producing B cells, and Th17 cell effector function.
Serum amyloid A proteins are a family of immunoregulatory proteins that highlight the integration of dietary and microbiota signals by the intestinal epithelium. SAAs are retinol-binding proteins that are expressed at the site of retinoid uptake (intestinal epithelium) and retinoid storage (liver), and that circulate retinol after systemic bacterial exposure (9). In the intestine, SAAs stimulate IL-17 expression by Th17 cells (10), thus shaping their effector functions. Expression of SAAs in the intestine and the liver requires both a microbial signal (microbiota colonization in the intestine and systemic bacterial challenge in the liver) (9, 10) and dietary vitamin A (9). The microbiota triggers intestinal epithelial SAA expression through a multicellular signaling circuit involving dendritic cells, innate lymphoid cells, and epithelial STAT3 (10). However, the mechanisms by which vitamin A regulates SAA expression are unknown.
Here we show that RARβ activates Saa expression through direct binding to retinoic acid response elements (RAREs) in Saa promoters. Consistent with the known role of SAAs in regulating Th17 cell effector function (10), we also find that epithelial RARβ regulates IL-17 production by Th17 cells. More generally, we show that epithelial RARβ regulates other known vitamin A-dependent adaptive immune responses, including the development of gut-homing CD4+ T cells and IgA-producing B cells. Our findings thus provide insight into how the intestinal epithelium senses dietary vitamin A status to control vitamin A-dependent adaptive immunity.
Results
RARβ Directs Retinoid-Dependent SAA Expression.
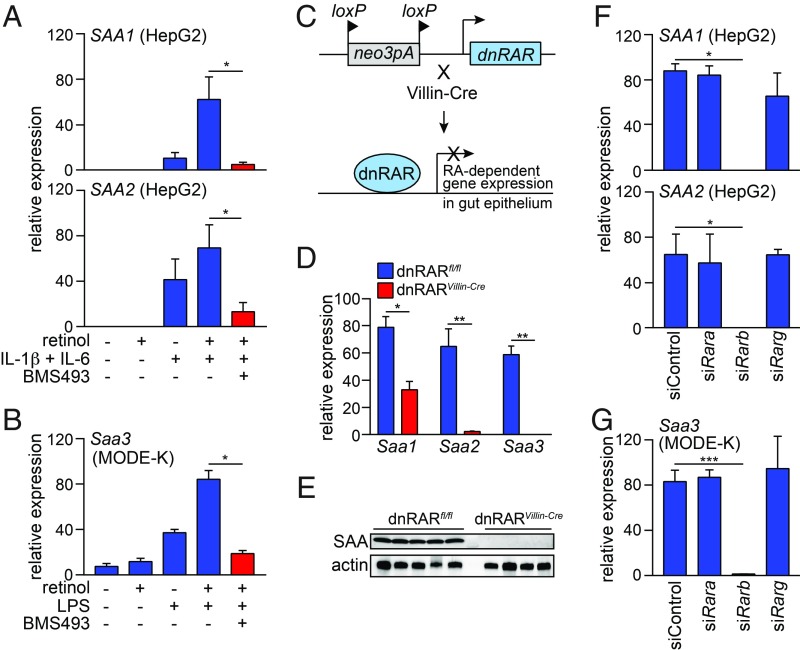
Dietary vitamin A is required for SAA expression in mouse intestine and liver, and the vitamin A derivatives retinol and RA stimulate SAA1 and SAA2 expression in the human liver cell line HepG2 (ref. 9; Fig. 1A). To determine whether retinoids also stimulate SAA expression in intestinal epithelial cells, we studied the mouse intestinal epithelial cell line MODE-K. MODE-K cells do not express Saa1 and Saa2; however, they do express Saa3, which is expressed in the intestine but not the liver. Saa3 expression increased with the addition of bacterial lipopolysaccharide (LPS) and retinol (Fig. 1B), showing that Saa3 expression in MODE-K cells is highest in the presence of both a bacterial signal and a retinoid.
Fig. 1.
RARβ directs retinoid-dependent SAA expression. (A and B) qPCR analysis of SAA (human) and Saa (mouse) expression. (A) SAA1 and SAA2 expression in HepG 2 cells treated for 24 h with retinol, IL-1β, IL-6, and the RAR inhibitor BMS493. (B) Saa3 expression in MODE-K cells treated for 24 h with retinol, LPS, and BMS493. n = 4 replicates/group; data represent two independent experiments. (C) Selective disruption of RAR activity in intestinal epithelial cells. Knock-in mice carry a neomycin resistance gene and 3 loxP-flanked polyadenylation sequences located upstream of an ORF encoding a dominant negative (dn)RAR. Breeding to Villin-Cre mice results in selective expression of the dnRAR in intestinal epithelial cells. (D) qPCR analysis of Saa transcripts in the small intestines of dnRARfl/fl mice and dnRARVillin-Cre mice. n = 4 mice/group; data represent two independent experiments. (E) Western blot of SAA in the small intestines of dnRARfl/fl mice and dnRARVillin-Cre mice. (F and G) qPCR analysis of SAA (human) and Saa (mouse) expression after siRNA knockdown of individual Rar isoforms. (F) SAA1 and SAA2 transcripts were analyzed in HepG2 cells treated with retinol, IL-1β and IL-6 as in A, and (G) Saa3 expression was analyzed in MODE-K cells treated with retinol and LPS as in B. n = 3 replicates/group; data represent two independent experiments. Means ± SEM are plotted. *P < 0.05; **P < 0.01; ***P < 0.001 as determined by Student’s t test.
To further our mechanistic understanding of how retinoids stimulate SAA expression, we tested whether RARs are required. We added the RAR inhibitor BMS493 to HepG2 cells in the presence of retinol and the cytokines IL-1β and IL-6, which are generated during systemic infection (11). We chose to use retinol instead of retinoic acid, as retinol is more stable than retinoic acid (12) and freely diffuses across membranes (13), and both HepG2 and MODE-K cells convert retinol to retinoic acid (14). BMS493 inhibited retinol-dependent SAA1 and SAA2 expression in HepG2 cells (Fig. 1A) and Saa3 expression in MODE-K cells (Fig. 1B), suggesting that RARs are required for retinoid-induced SAA expression in cells.
To determine whether RARs govern SAA expression in vivo, we studied mice with selective disruption of RAR activity in intestinal epithelial cells. The mice were derived from knock-in mice carrying three loxP-flanked polyadenylation sequences upstream of an ORF encoding a dominant negative form of human RARα (dnRAR) that disrupts RAR activity (15, 16). We crossed mice carrying the epithelial cell-restricted Villin-Cre transgene (17) with the loxP-flanked dnRAR knock-in mice (dnRARfl/fl) to selectively disrupt RAR activity in epithelial cells (Fig. 1C). Expression of Saa1-3 and SAA protein levels were lower in the dnRARVillin-Cre mice compared with dnRAR controls (Fig. 1 D and E), indicating that RAR activity regulates intestinal SAA expression in vivo.
The mouse genome encodes three RAR isoforms (RARα, RARβ, and RARγ), and we therefore sought to identify which isoform governs Saa expression. We used siRNAs to target individual Rar isoforms in HepG2 (SI Appendix, Fig. S1) and MODE-K cells (SI Appendix, Fig. S2). siRNA knockdown of Rarb suppressed SAA1 and SAA2 expression in HepG2 cells (Fig. 1F) and Saa3 expression in MODE-K cells (Fig. 1G), whereas knockdown of Rara and Rarg had little effect. Thus, RARβ is uniquely required for retinol-dependent Saa expression in cells.
RARβ Activates Saa3 Transcription by Binding Directly to Its Promoter.
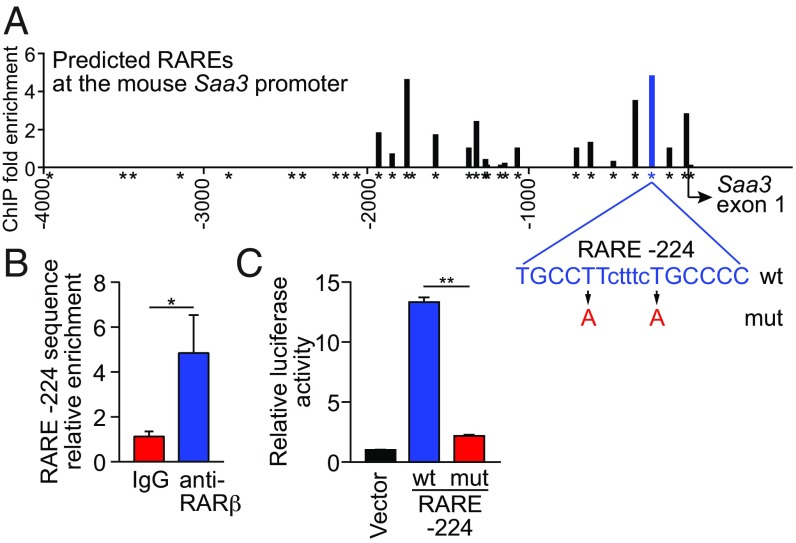
We next asked whether RARβ regulates SAA expression through direct binding to Saa promoters. RARs bind to canonical promoter sequences called RAREs, which consist of the direct repetition of two core motifs. Most RAREs are composed of two hexameric motifs, 5′-(A/G)G(G/T)TCA-3′, arranged as palindromes, direct repeats, or inverted repeats (18).
In silico analysis of the ∼4.1-kb mouse Saa3 promoter region, using NUBIScan (19), identified multiple potential RAREs. We selected the 30 RAREs identified by NUBIScan as having the highest statistical chance of being functional RAREs, and performed chromatin immunoprecipitation (ChIP) assays for RARβ binding at each (Fig. 2A and SI Appendix, Table S1). RARβ bound to the Saa3 promoter in MODE-K cells at multiple RAREs, including those located at -224, -327, and -1740 (Fig. 2A). We further verified binding of RARβ to RARE -224 (Fig. 2B) and showed promoter activity for the 4.1-kb region by a luciferase reporter assay (Fig. 2C). Introduction of point mutations into RARE -224 abolished reporter expression (Fig. 2 A and C), establishing that this RARE is essential for Saa3 promoter activity. Thus, RARβ activates Saa3 transcription by binding directly to its promoter. In silico analysis of mouse Saa1 and Saa2 also identified multiple putative RAREs, suggesting that RARβ also binds directly to these promoters (SI Appendix, Fig. S3 and Tables S2 and S3).
Fig. 2.
RARβ activates Saa3 transcription by binding directly to its promoter. (A) RARβ binding to the Saa3 promoter was measured by ChIP assay with an anti-RARβ antibody. Bound promoter sequences were detected by qPCR with primers flanking each predicted retinoic acid response element (RARE, indicated by *). (B) The RARE located 224 nt upstream of the Saa3 start site (RARE -224) was further validated by ChIP. n = 3 replicates per group; data represent three independent experiments. (C) Luciferase reporter assay for Saa3 promoter activity. A 4,103-bp fragment of the Saa3 promoter was fused to a firefly luciferase reporter and RARE -224 was mutated as shown in A. MODE-K cells were transfected with the wild-type (wt) or mutant (mut) reporter plasmids or empty vector and were treated with retinol and LPS for 24 h. n = 3 replicates/group; data represent two independent experiments. Means ± SEM are plotted. *P < 0.05; **P < 0.01, as determined by Student’s t test.
Epithelial RARβ Regulates Epithelial Immune Gene Expression and Promotes Host Resistance to Intestinal Bacterial Infection.
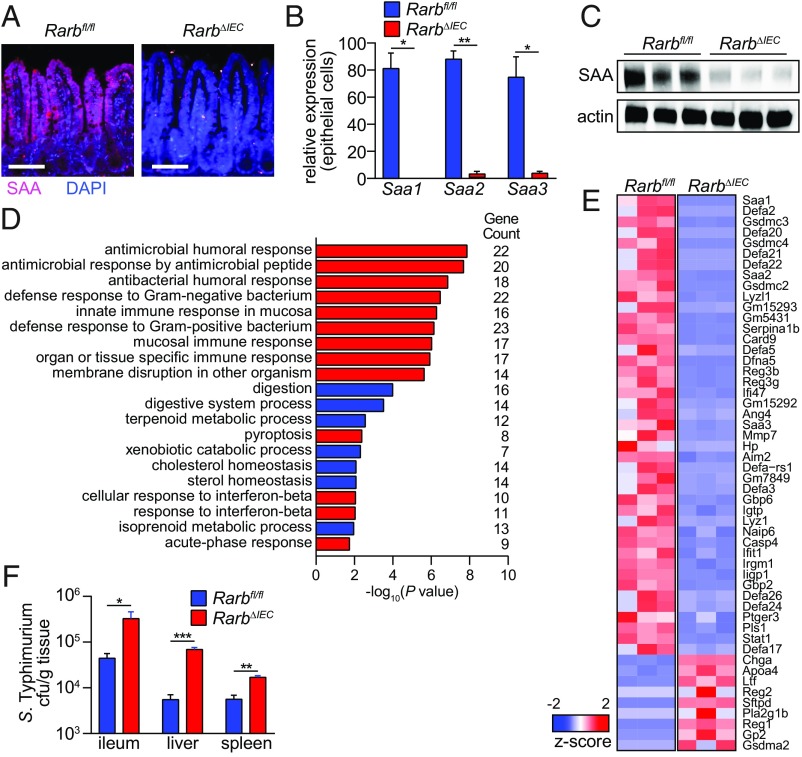
To determine whether RARβ controls intestinal SAA expression in vivo, we created mice with an intestinal epithelial cell-specific deletion of Rarb. We crossed mice with a loxP-flanked Rarb allele (Rarbfl/fl) (20) with Villin-Cre transgenic mice (17) to produce RarbΔIEC mice (SI Appendix, Fig. S4). SAAs were expressed throughout the small intestinal epithelium of Rarbfl/fl mice, but showed markedly reduced expression in RarbΔIEC mice (Fig. 3A). qPCR analysis of laser capture microdissected epithelial cells showed reduced abundance of transcripts encoding all three mouse Saa isoforms (Saa1, Saa2, and Saa3) (Fig. 3B), and SAA protein levels were reduced in the small intestines of RarbΔIEC mice (Fig. 3C).
Fig. 3.
RARβ controls an epithelial immune gene expression program and promotes host resistance to bacterial infection. (A–C) Epithelial RARβ controls SAA expression. (A) Immunofluorescence detection of SAA in the small intestines of Rarbfl/fl and RarbΔIEC mice. (Scale bars, 50 μm.) (B) qPCR analysis of Saa expression in small intestinal epithelial cells acquired by laser capture microdissection and (C) Western blot of small intestinal SAA from Rarbfl/fl and RarbΔIEC mice, with actin as a control. (D) Gene ontology biological process enrichment analysis of genes identified by RNA sequencing analysis as being differentially regulated in small intestines of Rarbfl/fl and RarbΔIEC mice. Immunological gene categories are highlighted in red. (E) Heat map displaying expression levels of the 52 genes that were identified as having immune functions by the gene ontology analysis shown in D, and which had a −log10(P value) > 5. (F) Bacterial burdens (colony-forming units) in the small intestine (ileum), spleen, and liver of Rarbfl/fl and RarbΔIEC littermates 48 h after oral infection with 1010 colony-forming units of Salmonella Typhimurium. n = 5 mice/group; data represent three independent experiments. Means ± SEM are plotted. *P < 0.05; **P < 0.01; ***P < 0.001 as determined by Student’s t test.
To broaden our insight into epithelial RARβ function, we identified other intestinal genes that were regulated by RARβ. We used RNAseq to compare the transcriptomes of Rarbfl/fl and RarbΔIEC mouse small intestines, finding 832 differentially abundant transcripts (21). Gene ontology term analysis identified gene categories that were highly represented among the differentially expressed genes, including multiple categories related to immunity and metabolism (Fig. 3 D and E and SI Appendix, Fig. S5). In addition to Saa1, Saa2, and Saa3 transcripts, the immunological gene category included transcripts encoding proteins involved in antimicrobial defense, including several members of the Defa (defensin) gene family, Reg3b, Reg3g, and Ang4 (Fig. 3E). Also represented were transcripts encoding proteins involved in inflammasome function and assembly, including Card9, Aim2, Naip6, and Casp4, and Gsdmc2, Gsdmc3, and Gsdmc4 (Fig. 3E). Thus, RARβ controls an immune gene transcriptional program in intestinal epithelial cells. This suggests that epithelial sensing of vitamin A status may regulate antimicrobial defense, as well as inflammasome function.
Our finding that RARβ regulates the expression of immune genes in the intestinal epithelium suggested that RARβ might promote resistance to bacterial infection of the intestine. We orally challenged RarbΔIEC and Rarbfl/fl mice with the gastrointestinal pathogen Salmonella enterica Serovar Typhimurium (Salmonella Typhimurium). Forty-eight hours later, RarbΔIEC mice had increased bacterial burdens in the ileum, liver, and spleen (Fig. 3F). The increased bacterial burdens did not arise from increased nonspecific barrier permeability (SI Appendix, Fig. S6) or altered microbiota taxonomic composition (SI Appendix, Fig. S7 A and B), as these were similar between RarbΔIEC and Rarbfl/fl mice. Thus, epithelial RARβ contributes to host resistance to intestinal bacterial infection and dissemination.
Epithelial RARβ Promotes Intestinal Th17 Cell Effector Function.
Th17 cells are a specialized subset of CD4+ T cells that require the transcription factor RORγt for lineage commitment (22). Th17 cells secrete a distinct set of cytokines, including IL-17A, IL-17F, and IL-22, and promote host defense against extracellular bacteria (23). SAA secretion by intestinal epithelial cells promotes Th17 cell effector functions (10). Although SAAs are not required for Th17 cell lineage commitment, they boost Th17 cell effector function by stimulating IL-17A production in differentiated Th17 cells (10).
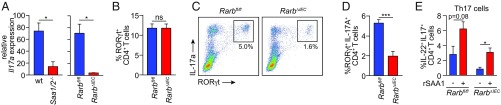
Our finding that epithelial RARβ governs SAA expression suggested that epithelial RARβ might also regulate IL-17 production by intestinal Th17 cells. In support of this idea, Il17a expression was lowered in the intestines of RarbΔIEC mice, paralleling the lowered Il17a expression in Saa1/2−/− mouse intestines (ref. 10; Fig. 4A). Overall frequencies of CD4+ RORγt+ Th17 cells were similar between Rarbfl/fl and RarbΔIEC mice (Fig. 4B), reflecting the fact that SAA is dispensable for Th17 cell lineage commitment (10). However, IL-17A production was reduced in RORγt+ Th17 cells from RarbΔIEC mice (Fig. 4 C and D), indicating that epithelial RARβ regulates Th17 cell effector function. IL-17A production was restored by the addition of recombinant SAA1 to cultured intestinal lamina propria cells from RarbΔIEC mice (Fig. 4E). This indicates that SAA1 is sufficient to rescue the defective Th17 effector function conferred by epithelial RARβ deficiency, and suggests that the defect in Th17 IL-17 production in RarbΔIEC mice is a result of the lowered SAA expression. Thus, epithelial RARβ promotes intestinal Th17 cell effector function, likely by activating Saa expression.
Fig. 4.
Epithelial RARβ regulates intestinal Th17 cell effector function. (A) qPCR analysis of Il17a transcripts in small intestines from wild-type (WT) and Saa1/2−/− mice, and Rarbfl/fl and RarbΔIEC mice. (B) RORγt+ CD4+ Th17 cells as a percentage of CD45+ CD3+ cells in the small intestinal lamina propria. n = 3 mice/group; data represent four independent experiments. (C and D) IL-17+ RORγt+ Th17 cells as a percentage of CD45+ CD4+ CD3+ cells in the small intestine. Representative flow cytometry plots (C) and data from multiple mice (D) are shown. n = 3 mice/group; data represent four independent experiments. (E) Recombinant SAA1 (rSAA1) rescues lowered IL-17+ IL-22+ CD4+ T cell numbers from RarbΔIEC mice. Small intestinal lamina propria cells were isolated and stimulated ex vivo with ionomycin, phorbol myristate acetate, brefeldin A, and rSAA1 for 4 h before flow cytometry analysis. n = 3 mice/group; data represent four independent experiments. Means± SEM are plotted. *P < 0.05; **P < 0.01, as determined by Student’s t test. ns, not significant.
Epithelial RARβ Promotes Development of Gut Homing T Cells and IgA-Producing B Cells.
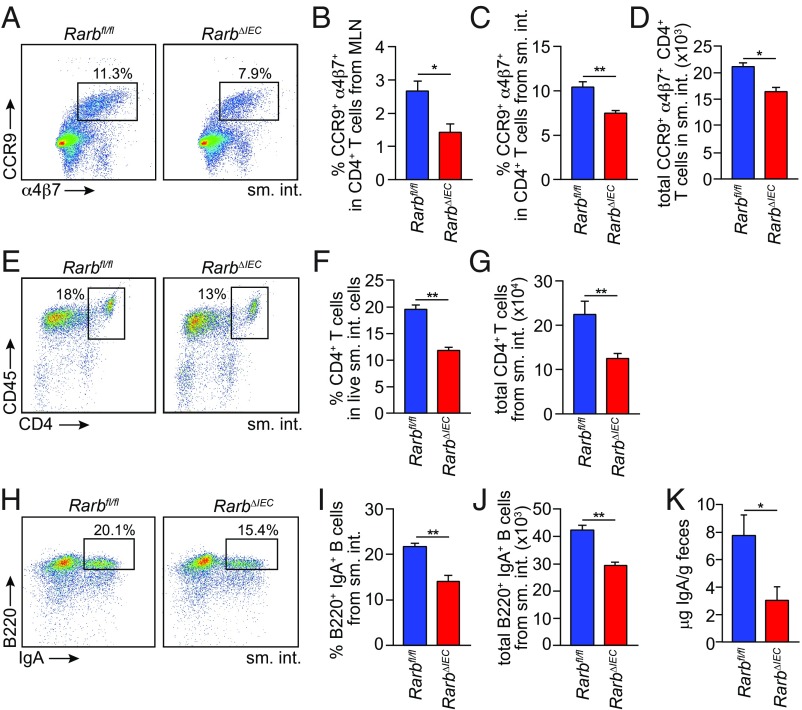
Vitamin A and its derivative RA are essential for the development of key intestinal adaptive immune cells, including gut homing CD4+ T cells (3) and IgA-producing B cells (2). RA also promotes the generation of Foxp3+ regulatory T cells (24, 25). We therefore sought to determine whether epithelial RARβ regulates the development of each of these cell populations. In the case of gut homing T cells, RA-producing dendritic cells (DCs) migrate to the mesenteric lymph nodes (MLN), where they imprint gut homing receptors on activated CD4+ T cells (3). RarbΔIEC mice had reduced frequencies of CD4+ T cells imprinted with the gut homing receptors CCR9 and α4β7 in the MLN and the small intestine (Fig. 5 A–C), reduced total numbers of small intestinal CCR9+ α4β7+ CD4+ T cells (Fig. 5D), and reduced overall numbers of small intestinal CD4+ T cells (Fig. 5 E–G). Thus, epithelial RARβ is essential for the development of gut homing CD4+ T cells.
Fig. 5.
Epithelial RARβ regulates the development of gut homing T cells and IgA-producing B cells. (A–D) Expression of the gut homing markers α4β7 and CCR9 on T cells (CD4+ CD45+ CD3+) from Rarbfl/fl and RarbΔIEC littermates. (A) Representative flow cytometry plots of small intestinal T cells. Frequencies of CCR9+ α4β7+ cells in CD4+ CD45+ CD3+ cells from MLN (B) and small intestine (C). Total gut homing CD4+ T-cell numbers (CCR9+ α4β7+ CD4+ CD45+ CD3+) are given in D. n = 3 mice/group; data represent four independent experiments. (E) Flow cytometry of CD4+ (CD45+ CD3+) T cells from the small intestines of Rarbfl/fl and RarbΔIEC littermates. CD4+ (CD45+ CD3+) T-cell frequencies are quantified in F and total small intestinal CD4+ T-cell numbers (CD4+ CD45+ CD3+) are given in G. n = 3 mice per group; data represent four independent experiments. (H) Flow cytometry analysis of IgA+ B220+ (CD45+ CD19+) B cells from Rarbfl/fl and RarbΔIEC littermates. IgA+ B220+ cell frequencies in CD45+ CD19+ B cells are quantified in I, and total numbers of small intestinal IgA+ B cells are shown in J. n = 3 mice per group; data represent four independent experiments. (K) Quantification of fecal IgA by ELISA. n = 4 mice/group; data represent two independent experiments. Means ± SEM are plotted. *P < 0.05; **P < 0.01 as determined by Student’s t test. sm. int., small intestine. MLN, mesenteric lymph nodes.
RA-producing DCs also induce IgA expression in gut homing B lymphocytes (2). RarbΔIEC mice had reduced frequencies and numbers of small intestinal IgA+ B cells (Fig. 5 H–J) and decreased fecal IgA concentrations (Fig. 5K), indicating that epithelial RARβ promotes the development of IgA-producing B cells. Although DC-produced RA also promotes the development of intestinal Foxp3+ regulatory T cells (24, 25), frequencies of Foxp3+ cells among small intestinal CD4+ T cells were similar between Rarbfl/fl and RarbΔIEC mice (SI Appendix, Fig. S8 A and B), indicating that epithelial RARβ is not required for the generation of intestinal regulatory T cells.
RARα is a closely related RAR isoform that affects several aspects of intestinal immunity, including Paneth and goblet cell development, numbers of RA-producing DCs, and overall B-cell numbers (8). We considered whether RARα and RARβ might have overlapping functions in the control of intestinal adaptive immunity. We found that RARbfl/fl and RarbΔIEC mice had similar numbers of small intestinal Paneth cells or goblet cells (SI Appendix, Fig. S9A), and similar frequencies of CD11c+ CD103+ cells (which include RA-producing DCs; SI Appendix, Fig. S9B) and B220+ B cells (SI Appendix, Fig. S9C). In contrast to RarbΔIEC mice, RaraΔIEC mice had elevated intestinal Saa expression (SI Appendix, Fig. S10 A and B). Numbers of intestinal gut homing T cells (SI Appendix, Fig. S11 A–D) and total CD4+ T cells (SI Appendix, Fig. S11 E–G) were also elevated relative to Rarafl/fl mice. Frequencies of intestinal IgA-producing B cells (SI Appendix, Fig. S11 H–J) and fecal IgA quantities (SI Appendix, Fig. S11K) were similar compared with Rarafl/fl mice. These data indicate that RARα is dispensable for CD4+ T-cell homing and B-cell expression of IgA, and show that epithelial RARα and RARβ regulate distinct aspects of intestinal immunity.
Discussion
The intestinal epithelium is a vital interface between the environment and the underlying immune system. Epithelial cells sense key environmental factors, including the microbiota and the host diet, and use these cues to orchestrate adaptive immunity in subepithelial tissues. The Saa genes highlight the environmental sensing function of the gut epithelium by requiring both microbiota and vitamin A for expression in the intestinal epithelium. We have now unraveled the molecular basis for how vitamin A directs SAA expression by showing that RARβ activates the expression of Saa genes through direct promoter binding. More generally, we show that epithelial RARβ regulates the function of Th17 cells, the development of gut homing T cells, and the development of IgA-producing B cells. Our findings thus provide important mechanistic insight into how the intestinal epithelium senses vitamin A to regulate intestinal adaptive immunity.
The three RAR isoforms (α, β, and γ) are conserved across species (26), suggesting a unique conserved function for each isoform. Supporting this idea, we found that RARβ was uniquely required for expression of Saa genes, and that RARα and RARβ have nonredundant functions in the regulation of intestinal immune function. Although RARα regulates the development of intestinal epithelial cell secretory lineages, RA-producing DCs, and overall B-cell populations (8), our findings show that RARβ regulates Th17 cell effector function, the development of gut homing T cells, and IgA-producing B cells. Thus, epithelial RARα and RARβ are both essential for intestinal immune homeostasis, but regulate distinct aspects of immunity.
Our finding that epithelial RARβ promotes IL-17 production by Th17 cells suggests that dietary vitamin A promotes Th17 cell effector function. This idea is supported by prior work showing that mice fed a vitamin A-deficient diet exhibit lowered intestinal IL-17 production (4). Furthermore, mice carrying a T-cell-specific Rara deletion show lowered IL-17 production by Th17 cells (4), indicating that T-cell-intrinsic RA signaling is required for Th17 cell effector function. Taken together, these findings indicate that Th17 cell effector function is a component of vitamin A-dependent intestinal immunity.
We propose that the function of SAAs in retinol transport could explain the requirement for both epithelial cell-intrinsic RARβ and T-cell-intrinsic RARα in Th17 cell effector function. Given that production of IL-17 by Th17 cells requires vitamin A (4), and that SAAs transport the vitamin A derivative retinol, it is possible that SAAs deliver retinol directly to Th17 cells for conversion to RA and activation of RARα. Alternatively, SAAs could deliver retinol to antigen-presenting cells (such as dendritic cells or macrophages) for conversion to RA and delivery to Th17 cells. Defective Th17 cell function could also help explain the increased susceptibility of RarbΔIEC mice to Salmonella Typhimurium infection, which require Th17 cell responses for effective clearance.
SAA could also in part account for the essential role of epithelial RARβ in the development of gut-homing T cells and IgA-producing B cells. Development of both groups of cells requires RA-producing DCs. These DCs convert retinol to RA, which imprints gut homing receptors on T cells and induces IgA expression in B cells (2, 3). Given the retinol transport function of SAAs, we propose that SAAs could deliver retinol from the epithelium to RA-producing DCs to serve as substrate for RA production. Future work will be directed at testing this idea.
Altogether, our findings provide insight into how the intestinal epithelium uses dietary cues to orchestrate adaptive immunity in the intestine (SI Appendix, Fig. S12). By showing that epithelial RARβ is a key regulator of vitamin A-dependent immunity, we highlight epithelial RARs as potential therapeutic targets for the modulation of intestinal immunity during infection or inflammation.
Methods
Additional methods are presented in SI Appendix, Extended Materials and Methods.
Mice.
RarbΔIEC mice were generated by crossing Rarbfl/fl mice (20) with Villin-Cre mice (Jackson Laboratories), which express Cre recombinase under the control of the intestinal epithelial cell-specific Villin promoter (17). dnRARVillin-Cre mice were generated by crossing dnRARfl/fl mice (16) with Villin-Cre mice. Saa1/2−/− mice were from F. de Beer (27); Rarafl/fl mice were from P. Chambon (28). Six- to 14-wk-old mice were used for all experiments. Because microbiota composition can affect intestinal immune cell frequencies, we used age- and sex-matched littermates that were cocaged to minimize microbiota differences. Experiments were performed according to protocols approved by the Institutional Animal Care and Use Committees of the University of Texas Southwestern Medical Center.
Cell Culture.
The MODE-K cell line was from Kaiserlian and coworkers (29). HepG2 cells were from the ATCC. Cells were cultured in DMEM with GlutaMAX, 10% FBS, 1× Penstrep, and 1× sodium pyruvate. Cells were maintained in 5% CO2 at 37 °C. Before adding retinol, LPS, cytokines, or siRNAs, cells were cultured in serum-free medium for 48 h. MODE-K cells were treated for 24 h with retinol (100 nM; Sigma) and LPS (100 ng/mL; Sigma). HepG2 cells were treated for 24 h with retinol (100 nM), IL-1β (50 pg/mL; R&D Systems), and IL-6 (100 pg/mL; R&D Systems).
Western Blots.
Total protein was isolated from cells or tissues as described (30). Blots were probed with antibodies against SAA (9), RARα (Affinity Bioreagents), RARβ (Invitrogen), RARγ (Thermo Fisher), and actin (Thermo Fisher).
ChIP Assays.
MODE-K cells were crosslinked in 1% formaldehyde in PBS for 3 min at room temperature, and quenched in 125 mM glycine at 4 °C for 10 min. Nuclei from fixed cells were pelleted and used for chromatin immunoprecipitation (Diagenode). Each reaction included chromatin from 5 × 106 cells, 5 μg goat anti-RARβ (Santa Cruz) or total goat IgG (Millipore), and 20 μL Magna protein A beads (Millipore). Bound Saa3 promoter sequences were quantified using SYBR Green-based real-time PCR (target sequence and primers listed in SI Appendix, Table S4). Relative enrichment of the Saa3 promoter was calculated as the ratio of specific antibody pull-down to input DNA.
Lymphocyte Isolation and Analysis.
Small intestinal lamina propria lymphocytes were isolated as previously described (30). Approximately 2 × 106 cells were treated with 50 ng/mL phorbol myristate acetate, 1 mM ionomycin, and 1 mg/mL brefeldin A for 4 h. To rescue IL-17 production, 5 μg/mL recombinant mSAA1 (R&D Systems) was added to lamina propria samples during the phorbol myristate acetate/ionomycin/brefeldin A stimulation. Cells were fixed and permeabilized for 30 min and stained with commercial antibodies from Biolegend, BD, and eBiosciences. Flow cytometry was performed on a LSRII, and data were analyzed with FlowJo software (TreeStar).
Salmonella Infection.
Salmonella enterica serovar Typhimurium (SL1344) was grown in Luria-Bertani broth with ampicillin (100 μg/mL) at 37 °C. Mice were infected intragastrically by gavage of 1010 colony-forming units per mouse. Colony-forming units in the small intestine, liver, and spleen were determined by dilution plating on Luria-Bertani plates containing ampicillin (100 μg/mL).
Statistical Analysis.
All statistical analyses were performed using two-tailed Student’s t test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ns, P > 0.05.
Supplementary Material
Acknowledgments
We thank Dr. P. Chambon for the Rarafl/fl and Rarbfl/fl mice. This work was supported by NIH Grant R01 DK070855 (to L.V.H.), Welch Foundation Grant I-1762 (to L.V.H.), the Walter M. and Helen D. Bader Center for Research on Arthritis and Autoimmune Diseases (L.V.H.), and the Howard Hughes Medical Institute (L.V.H.). S.G. was supported by NIH T32 AI007520, and T.A.H. was supported by the Burroughs Wellcome Foundation Minority Enrichment Program and the Dermatology Foundation.
Footnotes
The authors declare no conflict of interest.
Data deposition: RNAseq data have been deposited in the Gene Expression Omnibus repository with accession number GSE122471.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812069116/-/DCSupplemental.
References
- 1.Peterson LW, Artis D (2014) Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153. [DOI] [PubMed] [Google Scholar]
- 2.Mora JR, et al. (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314:1157–1160. [DOI] [PubMed] [Google Scholar]
- 3.Iwata M, et al. (2004) Retinoic acid imprints gut-homing specificity on T cells. Immunity 21:527–538. [DOI] [PubMed] [Google Scholar]
- 4.Hall JA, et al. (2011) Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 34:435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskaram P. (2002) Micronutrient malnutrition, infection, and immunity: An overview. Nutr Rev 60:S40–S45. [DOI] [PubMed] [Google Scholar]
- 6.Germain P, et al. (2006) International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev 58:712–725. [DOI] [PubMed] [Google Scholar]
- 7.Harrison EH. (2005) Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr 25:87–103. [DOI] [PubMed] [Google Scholar]
- 8.Jijon HB, et al. (2018) Intestinal epithelial cell-specific RARα depletion results in aberrant epithelial cell homeostasis and underdeveloped immune system. Mucosal Immunol 11:703–715, and erratum (2019) 12:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derebe MG, et al. (2014) Serum amyloid A is a retinol binding protein that transports retinol during bacterial infection. eLife 3:e03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sano T, et al. (2015) An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 163:381–393, erratum (2015) 163:273–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG (2016) IL-1β/IL-6/CRP and IL-18/ferritin: Distinct inflammatory programs in infections. PLoS Pathog 12:e1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolleson WH, et al. (2005) Photodecomposition and phototoxicity of natural retinoids. Int J Environ Res Public Health 2:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blomhoff R, Green MH, Berg T, Norum KR (1990) Transport and storage of vitamin A. Science 250:399–404. [DOI] [PubMed] [Google Scholar]
- 14.Rossi E, et al. (2007) Forced expression of RDH10 gene retards growth of HepG2 cells. Cancer Biol Ther 6:238–244. [DOI] [PubMed] [Google Scholar]
- 15.Damm K, Evans RM (1993) Identification of a domain required for oncogenic activity and transcriptional suppression by v-erbA and thyroid-hormone receptor α. Proc Natl Acad Sci USA 90:10668–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajaii F, Bitzer ZT, Xu Q, Sockanathan S (2008) Expression of the dominant negative retinoid receptor, RAR403, alters telencephalic progenitor proliferation, survival, and cell fate specification. Dev Biol 316:371–382. [DOI] [PubMed] [Google Scholar]
- 17.Madison BB, et al. (2002) Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277:33275–33283. [DOI] [PubMed] [Google Scholar]
- 18.Moutier E, et al. (2012) Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J Biol Chem 287:26328–26341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podvinec M, Kaufmann MR, Handschin C, Meyer UA (2002) NUBIScan, an in silico approach for prediction of nuclear receptor response elements. Mol Endocrinol 16:1269–1279. [DOI] [PubMed] [Google Scholar]
- 20.Chapellier B, et al. (2002) A conditional floxed (loxP-flanked) allele for the retinoic acid receptor β (RARbeta) gene. Genesis 32:91–94. [DOI] [PubMed] [Google Scholar]
- 21.Gattu S, et al. (2019) Epithelial retinoic acid receptor beta regulates serum amyloid A expression and vitamin A-dependent intestinal immunity. Gene Expression Omnibus. Available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE122471. Deposited November 13, 2018.
- 22.Ivanov II, Zhou L, Littman DR (2007) Transcriptional regulation of Th17 cell differentiation. Semin Immunol 19:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 cells. Annu Rev Immunol 27:485–517. [DOI] [PubMed] [Google Scholar]
- 24.Coombes JL, et al. (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med 204:1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun CM, et al. (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204:1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blomhoff R, Blomhoff HK (2006) Overview of retinoid metabolism and function. J Neurobiol 66:606–630. [DOI] [PubMed] [Google Scholar]
- 27.Eckhardt ER, et al. (2010) Intestinal epithelial serum amyloid A modulates bacterial growth in vitro and pro-inflammatory responses in mouse experimental colitis. BMC Gastroenterol 10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapellier B, et al. (2002) A conditional floxed (loxP-flanked) allele for the retinoic acid receptor α (RARalpha) gene. Genesis 32:87–90. [DOI] [PubMed] [Google Scholar]
- 29.Vidal K, Grosjean I, evillard JP, Gespach C, Kaiserlian D (1993) Immortalization of mouse intestinal epithelial cells by the SV40-large T gene. Phenotypic and immune characterization of the MODE-K cell line. J Immunol Methods 166:63–73. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, et al. (2017) The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 357:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanov II, et al. (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.