Significance
Viral infections after organ transplantation remain a significant cause of morbidity, reducing both allograft and patient survival. At this time, pretransplant screening of potential donors and recipients is based on serological status and is limited to certain viruses. Recently, a multiplex unbiased array, called VirScan, was developed. This tool allows the detection of antibodies against viruses, using a synthetic human virome. Here, we tested the value of VirScan in the follow-up of a cohort of kidney transplant recipients. We found that VirScan is easy to use, cost-effective, safe, and reproducible, and gives an unbiased approach to monitor patients after transplantation, but in addition, the anti-viral antibody responses were largely conserved in patients, regardless of immunosuppressive treatment.
Keywords: virus, screening, transplant, immunosuppression
Abstract
At this time, pretransplant viral screening of donors and recipients is based on serological status and limited to certain viruses. After transplantation, patient follow-up is based on a monitoring strategy using ELISA or PCR. Such approaches exclude other emerging viruses that can affect the transplant outcome. Recently, a multiplex unbiased array, VirScan, was developed. This tool allows the detection of antibodies against viruses, using a synthetic human virome, with minimal serum and cost. We decided to test the value of VirScan in the follow-up of a cohort of transplant recipients. We enrolled 45 kidney transplant recipients and performed virus serological profiling at day 0 and day +365, using VirScan. We compared the results obtained with ELISA/PCR assays. We detected antibody responses to 39 of the 206 species of virus present in the VirScan library, with an average of 12 species of virus per sample. VirScan gave similar results to PCR/ELISA screening tests. Using VirScan, we found that anti-viral antibody responses were largely conserved in patients during the first year after transplantation, regardless of immunosuppressive treatment. Our study suggests VirScan offers an unprecedented opportunity to screen and monitor posttransplant virus infection in a cost-effective, easy, and unbiased manner.
Kidney transplantation is recognized as the best therapeutic option for end-stage renal failure (1). However, the use of immunosuppressive drugs to prevent allograft rejection is associated with an increasing rate of opportunistic infections (2). Among them, viral infections remain a significant cause of morbidity, reducing both allograft and patient survival through the occurrence of virus-associated malignancies and kidney inflammation, and/or a playing a potent role in allograft rejection (3). Transplant recipients are exposed to virus transmission from the allograft but also, because of the immunosuppression therapy, to virus reactivation.
At this time, pretransplant serological screening of a potential donor and recipients is limited to antibodies targeting only certain virus species, including HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), human herpes virus 5 (HHV5 or CMV), HHV4 (or EBV), and human T-lymphotropic virus I/II (4). Therefore, current screening approaches risk missing important emerging viruses, such as West Nile virus (5) or lymphocytic choriomeningitis virus (6), that can adversely affect transplant outcomes. A limitation of the current screening methods is that clinical immunoassays aimed at detecting recent or past virus exposures remain largely singleplex assays, targeting one virus exposure at a time. Therefore, cost and sample requirements generally prohibit screening against a wide range of virus exposures, especially those that are of low prevalence. What is needed is an unbiased method of screening against a much larger number of potential virus exposures.
Recently, a technology named VirScan was developed that has been demonstrated to be a robust platform capable of very high complexity serological screening for virus exposure across the entire human virome, that is, all viruses known to infect humans, using a synthetic peptide array (7). VirScan is based on immunoprecipitation combined with next-generation sequencing of a bacteriophage library containing peptides representing viruses known to infect humans. The VirScan library displays viral peptides, each 56 amino acids in length, from 206 species of viruses, corresponding to 1,000 different strains known to infect humans. Serum antibodies are allowed to bind to phages displaying their cognate epitopes, and after immunoprecipitation of those phages with bound antibodies, next-generation sequencing is used to identify the recognized epitopes. Because VirScan is based on the presence of IgG, the assay provides information on both semirecent and past history of viral infections over the individual’s lifetime. Importantly, only minimal volume of serum is needed for VirScan (1 μL), and the cost is $25 per sample (excluding labor or capital depreciation) (7). Here, we describe the potential value of VirScan in the context of post–kidney transplant follow-up.
Methods
Study Design and Patients.
From 2014 to 2015, we prospectively enrolled 45 consecutive kidney transplant recipients in our transplant department (Hôpital Necker-Enfants Malades, Paris, France). At the time of transplantation (day 0), all donors and recipients were screened for HHV4, HHV5, HHV8, HIV 1 and 2, HCV, and HBV, using ELISA-based assays. After transplantation, based on clinical or biological assumption of viral infection, appropriate PCR tests were performed. All patients received CMV prophylaxis based on day 0 serological status of the donor/recipient pairs (i.e., donor−/recipient− received acyclovir for 4 mo, donor+/recipient− received ganciclovir for 6 mo, and donor+/recipient+ received ganciclovir for 4 mo).
For each of the 45 patients, serum was collected on the day of transplantation (D0) and at 1 y posttransplant (D+365). All serum samples (n = 90) were analyzed with VirScan (SI Appendix, Fig. S1A).
Our institutional review board Commité de Protection des Personnes Ile de France 2 (CPP IdF2) approved the study, and written informed consent was obtained from each patient.
VirScan Construction and Procedure.
VirScan is a high-throughput method of serological profiling requiring only 2 µg Ig (1 µL serum) to detect antiviral antibodies from all known human viruses (7). Briefly, the virome peptide library consists of 93,904 distinct 56–amino acid peptides tiling across the proteomes of 206 species of virus. DNA sequences encoding the peptides were cloned into a T7 bacteriophage display vector for screening. Amplification and sequencing of the insert DNA from bound phage reveals peptides targeted by potential antiviral antibodies from the sample (SI Appendix, Fig. S1B). For determining whether a patient has been infected with a certain virus, a minimum threshold of 3 virus-specific enriched peptides per virus was required for all viruses except for HHV5, where a minimum threshold of 5 virus-specific enriched peptides was used to account for the larger size of the viral proteome (7). To validate this threshold, a threshold performance analysis is provided in Datasets S1 and S2. In Table S1, we have performed an analysis of the specificity and sensitivity (comparing VirScan with ELISA) for all thresholds from 1 to 10 (threshold performance) of the following viruses: CMV, HCV, HIV, and EBV. In Table S2 (fraction positive), we added the fraction of patients who are positive at each threshold for each of the 15 most commonly detected viruses. The tables demonstrate that raising the threshold begins to impair the ability to detect common infections such as rhinovirus A, respiratory syncytial virus, and adenovirus C.
Statistical Analysis.
VirScan analysis for specific antibody–virus hits was performed as previously described (7). The means represented on the various graphics are associated with SEM. Fisher’s exact test was used to calculate P value for the significance of association of virus exposures between two populations. To identify peptide number and mean seroconversion differences between populations, we used Student t test to calculate a P value for the significance of association of virus peptide number and virus exposure with one population versus another. Statistical analyses were performed using xlstat software.
Results
Recipient Characteristics.
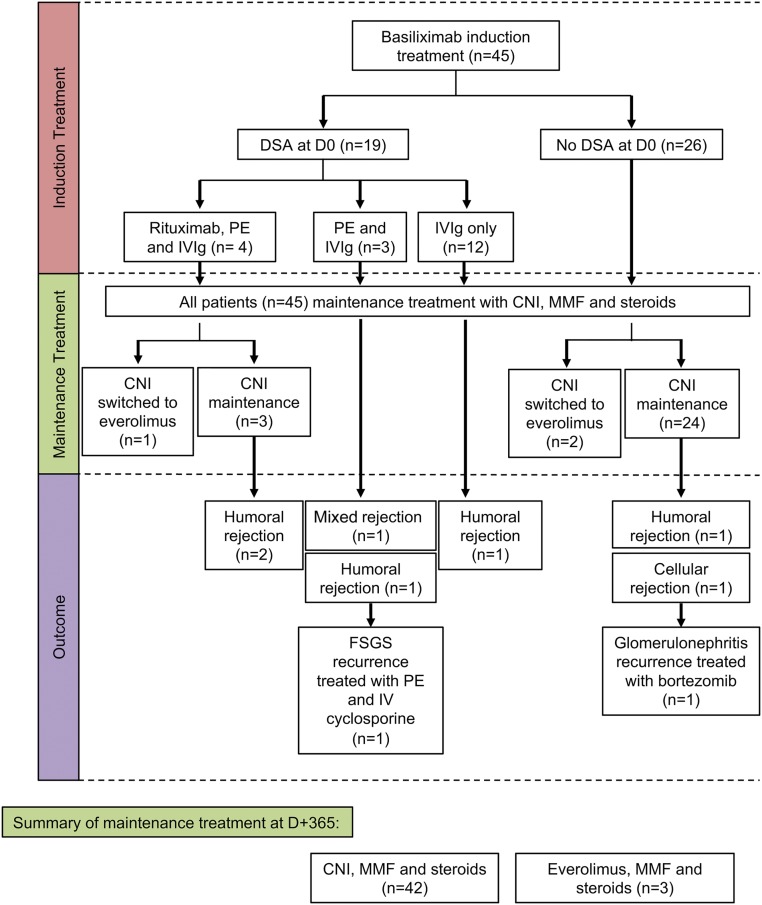
Demographic characteristics of the 45 patients are listed in Table 1 and SI Appendix, Table S1. Briefly, there was a discreet male predominance (sex ratio male/female, 3.5), and the mean age at transplantation was 49 y (range, 22–77 y). Deceased donors made up the vast majority (83%) of transplanted kidneys, with a mean donor age of 55 y (range, 18–64 y). Fourteen patients had already had a previous kidney transplant, and four additional patients received immunosuppressive therapy before kidney transplantation for their native kidney disease. Among the 45 patients, 19 (42%) had donor-specific antibodies before transplantation (D0) and were classified as highly sensitized patients. Induction therapy was based on basiliximab in all patients (Fig. 1). In addition, patients with preformed donor-specific antibodies also received rituximab (375 mg/m2), plasmapheresis exchanges and i.v. Ig (IVIg, 2 g/kg over the course of 2 d, every 3 wk ×4 doses; n = 4), plasmapheresis exchanges (five sessions) and IVIg (n = 3), or IVIg only (n = 12). Maintenance regimens included steroids, mycophenolate mofetil, and calcineurin inhibitors in all but three patients who were switched from calcineurin inhibitor to everolimus because of presumed drug-related kidney toxicity. During the follow-up, seven patients (five in the highly sensitized group) developed acute kidney rejection (humoral, n = 5; cellular, n = 1; and mixed, n = 1). All rejection episodes were treated with pulses of steroids, but patients with humoral rejection also received rituximab, plasma exchanges, and IVIg. Two patients with humoral rejection were given thymoglobulin, and an additional two patients had recurrent diseases on the allografted kidney, including focal and segmental glomerulosclerosis and proliferative glomerulonephritis. The patient with focal and segmental glomerulosclerosis recurrence received additional plasmapheresis exchange and i.v. cyclosporine, and the patient with proliferative glomerulonephritis was treated with bortezomib, a proteasome inhibitor.
Table 1.
Demographic characteristics
| Demographic and clinic characteristics | Patients (n = 45) |
| Recipients demographic data | |
| Sex ratio, male/female | 3.5 |
| Age, y (range) | 49 (22-77) |
| Origin, n (%) | |
| European | 26 (58) |
| North African | 10 (22) |
| Black African | 5 (11) |
| Asian | 4 (9) |
| Causes of end-stage renal disease, n (%) | |
| Diabetic nephropathy | 3 (7) |
| Vascular nephropathy | 2 (4) |
| Glomerular diseases | 14 (31) |
| CAKUT | 11 (24) |
| ADPKD | 5 (11) |
| Other | 10 (22) |
CAKUT: Congenital anomalies of the kidney and urinary tract; ADPKD: Autosomal polycystic kidney disease.
Fig. 1.
Flowchart description of the 45 patients. DSA, donor-specific antibody; CNI, calcineurin inhibitors; MMF, mycophenolate mofetil; PE, plasmapheresis exchange.
Unbiased Approach Using VirScan versus Conventional Virus Monitoring.
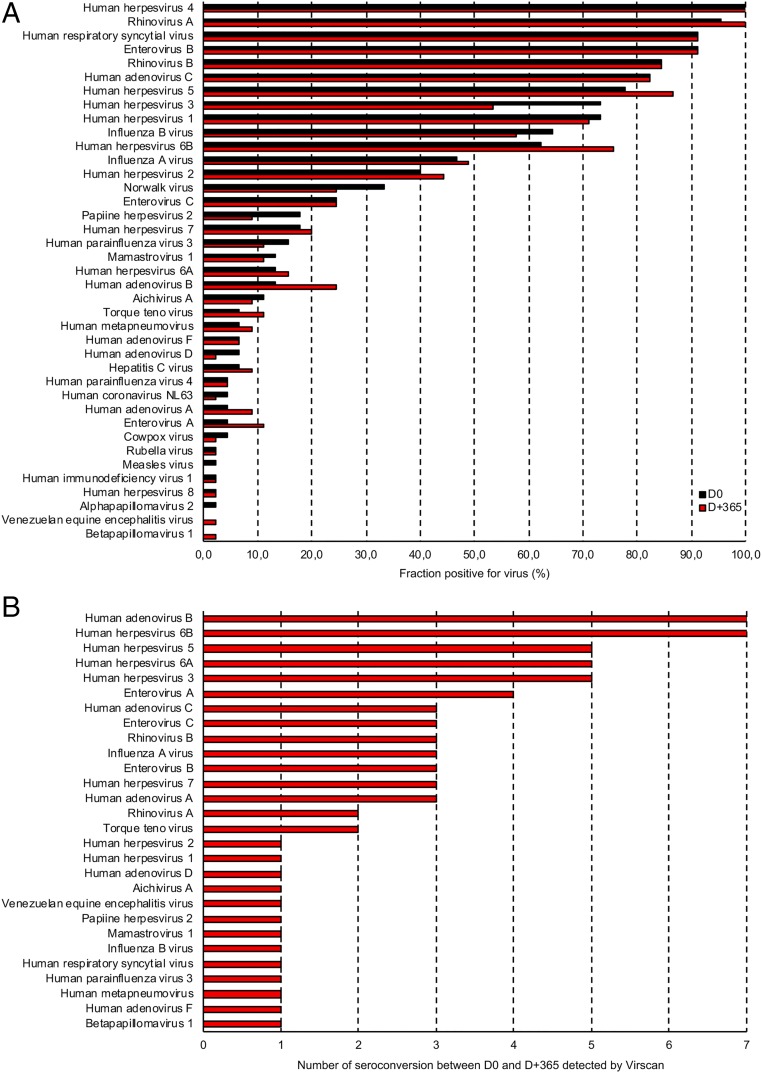
We first explored the value of VirScan versus candidate approaches. Screening each of the 90 serum specimens (D0 and D+365) detected antibody responses to 39 of the 206 species of virus in the VirScan library, with antibodies against an average of 12 species of virus per sample. Viral exposure at D0 and D+365 are summarized in Fig. 2A. The most frequently detected viruses at D0 included prevalent upper respiratory tract viruses such as rhinovirus, adenovirus, enterovirus, and human respiratory syncytial virus. We also detected antibody responses to influenza A and B and viruses responsible for gastroenteritis (i.e., Norwalk virus, Mamastovirus, Aichivirus A). As expected, we also found a high prevalence of antibodies against herpes viruses at D0, including HHV4 (100%), HHV5 (78%), and HHV3 (73%). Of note, all the raw data are available on Dataset S3.
Fig. 2.
Virus seroprevalence at D0 and D+365. (A) The bar graphs depict the percentage of samples that were positive for all viruses detected using VirScan. (B) The bar graphs depict the number of seroconversions between D0 and D+365, using VirScan.
Then, we compared the serum from patients with positive conventional ELISA at D0 with VirScan and found 100% concordance for HIV1, HIV 2, HHV8, HCV, HHV4, and 98% for HHV5 (Table 2). Patient 29 was ELISA (IgG and IgM) and PCR negative at D0 for HHV5 but was found “positive” using VirScan with 12 specific peptides enriched. By D+90, the ELISA-based HHV5 test result was positive for IgM and then positive for both IgG and IgM at D+365, but the HHV5 PCR remained negative at D+90 and D+345. The D+365 VirScan results revealed a boost in HHV5-enriched peptides from 12 to 37 specific hits between D0 and D+365 (SI Appendix, Fig. S2 and Table S2). This intriguing case is complex to interpret. Either a false-negative or false-positive VirScan result cannot be excluded at D0 in a patient receiving a CMV prophylaxis (ganciclovir).
Table 2.
VirScan and ELISA test concordance at D0
| Virus | Number of VirScan tests positive at D0 | Number of ELISA tests positive at D0 | Concordance ratio |
| HHV 5 | 34 | 33 | 98% (44/45) |
| HHV 4 | 45 | 45 | 100% |
| HIV 1 | 1 | 1 | 100% |
| HIV 2 | 0 | 0 | 100% |
| HCV | 4 | 4 | 100% |
| HHV 8 | 1 | 1 | 100% |
To further validate VirScan specificity and sensibility, we compared all negative ELISA and PCR assays at D0 with VirScan. Interestingly, we observed that all negative ELISA and PCR were also found negative with VirScan (SI Appendix, Tables S3 and S4). We concluded that VirScan was similar to conventional tests for viral pretransplant screening.
Next, we examined the sensitivity of VirScan at detecting viral seroconversion. Among the 45 patients, four of them (patients 8, 18, 21, and 32) developed HHV5 infection confirmed by PCR during the first year of transplantation. Interestingly, we observed a burst of antibody response to peptides in these four patients at D+365, confirming the sensitivity of VirScan (SI Appendix, Table S5). Interestingly, in addition to HHV5 infection, patient 21 was also diagnosed with rhinopharyngitis resulting from metapneumovirus assessed by PCR on a nasal swab. In this specific case, VirScan detected a seroconversion at D+365, with the occurrence of antibodies directed against one enriched peptide corresponding to a public epitope of metapneumovirus. However, the antibody response did not reach the high-stringency cutoff of three peptides. Nevertheless, this result is strongly suggestive of an infection, particularly as metapneumovirus has a very small genome with a limited total number of peptides (144 peptides).
We then investigated the case of patients who had VirScan seroconversion during the first year of transplantation. In addition to the four patients discussed here, we found that 32 among the 45 patients had at least one virus seroconversion, with a mean of 2 [seroconversion ranging from 1 to 8 maximum] seroconversions per patient (Fig. 2B and SI Appendix, Fig. S3). Viruses most frequently counted as positive seroconversions during the course of the year posttransplantation were generally those known to commonly infect humans, especially viruses responsible for upper respiratory tract infection (Fig. 2B). Importantly, viral infection was suspected by physicians in all cases during the follow-up, but was never proved molecularly. However, a number of patients had fluctuations of the number of antibodies responding to peptides around the threshold for seropositivity. This gray zone, difficult to interpret, may represent a low level of virus reactivation in the setting of immune suppression.
We concluded that VirScan was similar to conventional ELISA-based assays to retrospectively diagnose viral infection, and may help in the retrospective diagnosis of unscreened viruses.
Effect of Immunosuppressive Drugs on the Antibody Response to Virus Peptides.
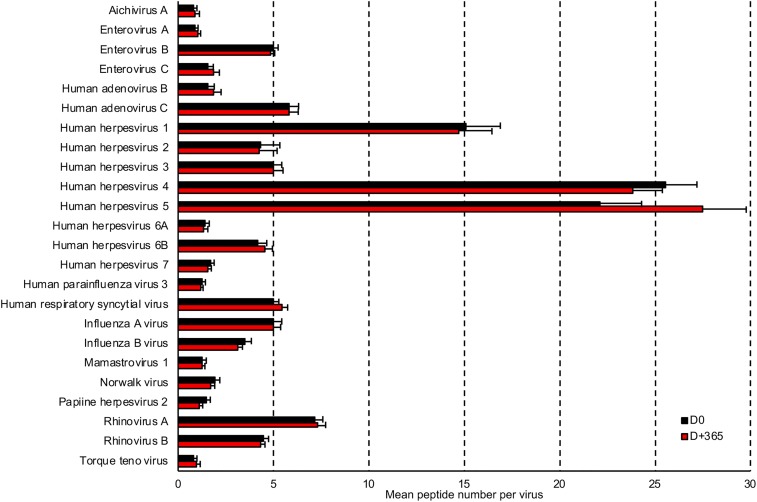
VirScan provides epitope-level resolution of antibody targets, so we examined the scope and precise targets of the immune response at D0 and D+365. For those viruses that were considered positive at D0, we found a striking conservation of antibody responses despite the use of immunosuppressive drugs. Indeed, across all individuals, there was no change in the mean number of peptides recognized per virus between D0 and D+365 (Fig. 3 and SI Appendix, Fig. S4 A and B). Strikingly, both the number of recognized epitopes and the exact protein fragment targets were almost completely conserved for each patient, even in patients who had undergone immunosuppressive treatments using plasmapheresis exchange, IVIg, rituximab, or bortezomib (SI Appendix, Fig. S4 A and C). These data suggest that the antiviral humoral immunity is not significantly altered by the examined immunosuppressive regimens, including rituximab treatment.
Fig. 3.
Antibody response to peptides is conserved during the first year of transplantation. The bar graphs depict the differences in mean enriched peptide number for the most prevalent viruses (>10%) between D0 and D+365.
Discussion
Here, we report the use of VirScan technology to follow a cohort of transplant recipients. We demonstrate that VirScan is an easy, cost-effective, safe, reproducible, and unbiased approach to monitoring patients after transplantation. Importantly, we also provide evidence that immunosuppressive regimens, including plasmapheresis exchange, rituximab, or IVIg, do not modify the anti-viral antibody response. This also appears to be the case for bortezomib, but the data are limited to a single patient and will have to be confirmed in larger cohort. This study allows a global examination of the stability of the immune response and its epitope-level response to immunosuppression. Importantly, our goal here was not to replace PCR for the diagnosis of early viral infection, but to demonstrate the utility of an unbiased approach to follow immunocompromised patients.
VirScan allows high-throughput virus antibody detection and requires minimal sample and cost (i.e., $25 per sample, excluding labor or capital depreciation) (7). With 1 µL serum, VirScan is able to detect the immune response to 206 species of viruses annotated to have human tropism (7). Moreover, contrary to current biological tests that are mainly limited to one virus at a time to address specific clinical hypotheses, VirScan is not restricted by limiting diagnostic assumptions, and favors the discovery of unexpected viral infection profiles. The diagnosis of an active viral disease usually relies on serological test or nucleic acid–based methods (8). Serological detection depends on antibody formation, whereas nucleic acid–based methods consist of direct amplification of specific regions of viral genomic sequences. The nucleic acid technique is a reliable diagnostic tool for active viral infection, as it detects viruses without delay. However, this method can fail when viruses have already been cleared or when viruses are not present in the sampled fluid. In contrast, humoral response to infection can persist over years, and serological tests can therefore identify both ongoing and cleared infections (9).
Using this multiplex approach (7), we could detect antibody response to 39 of the 206 species of virus in VirScan library, and an average of 12 species of virus per sample. ELISA tests performed at D0 for six species of virus showed concordance with VirScan that was close to 100%, and viral infection documented with PCR was found in four of five patients with VirScan. The most frequently detected viruses were generally those known to commonly infect humans, and our seroprevalence results were consistent with the one previously reported (7). Virus seroprevalence and seroconversion detected by VirScan in our transplant cohort are also consistent with the known epidemiology of viral infection after kidney transplantation (2, 10). We found that the antibody response to virus peptides was largely conserved between D0 and D+365, as assessed by the highly conserved number of enriched peptide between these two points. Higher immunosuppressive regimen at induction (plasmapheresis exchange, IVIg, and rituximab), as well as rejection treatment, did not significantly change virus seroprevalence between D0 and D+365. Antibodies involved in antiviral immunity are produced by long-lived antibody-secreting cells that are able to persist for years in survival niches and are likely to be more resistant to classical immunotherapies (11, 12). It is also possible that the design of the study with a seroprevalence explored at D0 and D+365 misses an early decrease of antibodies after treatment, followed by a rebound of synthesis.
Although these data point to VirScan as a promising tool, we must acknowledge two concerns. The first is related to the empirical peptide threshold that determines seropositivity, as demonstrated in the case of metapneumovirus infection. In addition, several patients in our study had variations (either positive or negative) around the threshold between D0 and D+365, in what we have called the gray zone. The second concern is that VirScan does not effectively allow for the detection of viruses with extensive posttranslational modifications and primarily conformational epitopes, including the BK virus, a major concern in the posttransplant setting (13).
In conclusion, our study suggests that VirScan may offer an unprecedented opportunity for easy and unbiased viral profiling of posttransplant recipients. Moreover, we discovered that anti-viral antibodies are largely conserved during the first year after transplantation, regardless of the immunosuppression regimen. This multiplex analysis could improve the epidemiology of viral infections in allograft recipients and help us to better understand virus and host immune system interactions in the setting of allograft transplantation.
Supplementary Material
Acknowledgments
This project received funding from the Emmanuel Boussard Foundation (London, UK), the Day Solvay Foundation (Paris, France), INSERM, Assistance Publique-Hôpitaux de Paris, and the University of Paris, Descartes. S.J.E. was supported by NIH Grant 5U24AI118633-03.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821166116/-/DCSupplemental.
References
- 1.Tonelli M, et al. (2011) Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11:2093–2109 [DOI] [PubMed] [Google Scholar]
- 2.Fishman JA. (2007) Infection in solid-organ transplant recipients. N Engl J Med 357:2601–2614 [DOI] [PubMed] [Google Scholar]
- 3.Kotton CN, Fishman JA (2005) Viral infection in the renal transplant recipient. J Am Soc Nephrol 16:1758–1774 [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9(Suppl 2):S1–S155 [DOI] [PubMed] [Google Scholar]
- 5.Rabe IB, et al. ; WNV Transplant Investigation Team (2013) Fatal transplant-associated west nile virus encephalitis and public health investigation-California, 2010. Transplantation 96:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer SA, et al. ; LCMV in Transplant Recipients Investigation Team (2006) Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med 354:2235–2249 [DOI] [PubMed] [Google Scholar]
- 7.Xu GJ, et al. (2015) Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science 348:aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vlaminck I, et al. (2013) Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 155:1178–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammarlund E, et al. (2003) Duration of antiviral immunity after smallpox vaccination. Nat Med 9:1131–1137 [DOI] [PubMed] [Google Scholar]
- 10.Weikert BC, Blumberg EA (2008) Viral infection after renal transplantation: Surveillance and management. Clin J Am Soc Nephrol 3:S76–S86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manz RA, Hauser AE, Hiepe F, Radbruch A (2005) Maintenance of serum antibody levels. Annu Rev Immunol 23:367–386 [DOI] [PubMed] [Google Scholar]
- 12.Radbruch A, et al. (2006) Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol 6:741–750 [DOI] [PubMed] [Google Scholar]
- 13.Wang M, et al. (1999) Human anti-JC virus serum reacts with native but not denatured JC virus major capsid protein VP1. J Virol Methods 78:171–176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.