Significance
Bruton’s tyrosine kinase (Btk) is a cytoplasmic tyrosine kinase critical for B cell signal transduction. While it has been known that its activation requires localization to the plasma membrane by lipid second messenger phosphatidylinositol (3–5)-triphosphate (PIP3), the structural basis for activation had been unclear. In this work, the interaction between the membrane-binding domain of Btk and PIP3 was studied in a reconstituted membrane system. Quantitative investigations through molecular diffusion and adsorption kinetics show that Btk undergoes dimerization-mediated activation in a PIP3 density-dependent manner, providing a ligand-counting mechanism with a sharp sensitivity to signal strength. This mechanism is not present in related kinases in T cells, Tec and Itk, highlighting the differences in B and T cell regulations.
Keywords: Bruton’s tyrosine kinase, PIP3, ultrasensitivity, signaling
Abstract
The transformation of molecular binding events into cellular decisions is the basis of most biological signal transduction. A fundamental challenge faced by these systems is that reliance on protein–ligand chemical affinities alone generally results in poor sensitivity to ligand concentration, endangering the system to error. Here, we examine the lipid-binding pleckstrin homology and Tec homology (PH-TH) module of Bruton’s tyrosine kinase (Btk). Using fluorescence correlation spectroscopy (FCS) and membrane-binding kinetic measurements, we identify a phosphatidylinositol (3–5)-trisphosphate (PIP3) sensing mechanism that achieves switch-like sensitivity to PIP3 levels, surpassing the intrinsic affinity discrimination of PIP3:PH binding. This mechanism employs multiple PIP3 binding as well as dimerization of Btk on the membrane surface. Studies in live cells confirm that mutations at the dimer interface and peripheral site produce effects comparable to that of the kinase-dead Btk in vivo. These results demonstrate how a single protein module can institute an allosteric counting mechanism to achieve high-precision discrimination of ligand concentration. Furthermore, this activation mechanism distinguishes Btk from other Tec family member kinases, Tec and Itk, which we show are not capable of dimerization through their PH-TH modules. This suggests that Btk plays a critical role in the stringency of the B cell response, whereas T cells rely on other mechanisms to achieve stringency.
Cellular decision making often relies on relatively small changes in fluctuating ligand concentrations. One prominent example is the response to the membrane-lipid second messenger phosphatidylinositol (3–5)-triphosphate (PIP3), which is involved in multiple signaling networks, including the PI3K/Akt/mTOR and T and B cell receptor pathways (1, 2). A wide variety of signaling proteins recognize PIP3 via pleckstrin homology (PH) domains, which leads to membrane recruitment and activation of the protein, typically by phosphorylation, and propagation of downstream signaling reactions (3–5). A simple reliance on the ligand concentration would result in a gradual, hyperbolic response to the PIP3 density (6, 7). Such a system would suffer a high probability of error, as well as limited sensitivity.
One general mechanism to overcome this physical limitation, referred to as coincidence detection, relies on the binding of multiple membrane targets via distinct binding domains (3, 4, 8), as exemplified by the protein kinase C family isozymes and the actin-nucleating WAVE (Wiskott–Aldrich syndrome protein family verprolin-homologous protein) complex (3, 9–13). In both cases, coincidence detection has the effect of producing spatial and temporal specificity, as well as a threshold-like response (4, 14). Many of these examples have experimental foundations in structural data (11, 15, 16) and solution-based binding and activity assays (7, 9, 17). However, quantitative information, such as molecular stoichiometry, kinetic rates, and binding affinities, has largely been inaccessible for these membrane-bound processes. This work addresses this shortcoming by quantitatively investigating Bruton’s tyrosine kinase (Btk) and associated membrane interactions in reconstituted systems via fluorescence spectroscopy and imaging.
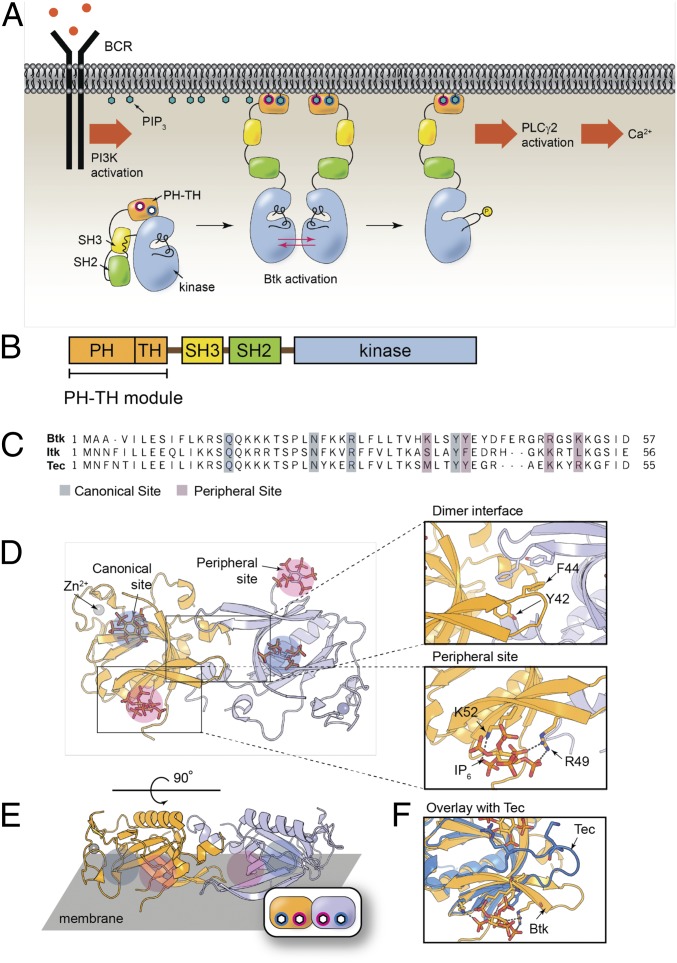
A member of the Tec family of tyrosine kinases, Btk plays a critical role in B cell signaling, as depicted in Fig. 1A. Upon signal initiation, the B cell receptor activates phosphoinositide 3-kinase (PI3K), which generates PIP3, resulting in recruitment of Btk to the membrane via the PH-TH module. Btk is then activated, allowing it to phosphorylate phospholipase C-γ2, leading to calcium flux and cellular activation (18, 19). Mutations in Btk have been shown to result in X-linked agammaglobulinemia, an autoimmune disease (20, 21). Btk is also a primary target in the treatment of some types of lymphoma and leukemia, such as with ibrutinib and its derivatives (19, 22, 23). Furthermore, the study of Btk could elucidate the mechanisms of closely related members of the Tec family, Tec and Itk, which have been shown to control T cell development and proliferation in a similar manner (24–26).
Fig. 1.
Btk in the B cell receptor (BCR) pathway and the PH-TH domain structure. (A) The B cell signaling pathway. (B) The domain architecture of Btk. (C) Sequence alignment of the PH-TH modules for Btk, Itk, and Tec. (D) Schematic depicting the Saraste dimer of the Btk PH-TH domain with two IP6 coordinated to the canonical (blue) and peripheral (magenta) phosphoinositide binding sites (PDB 4Y94). Zoom ins show the residues involved in the dimerization interface (Top) and the peripheral inositol phosphate binding site (Bottom). (E) Same structure rotated to show the orientation with respect to the membrane. (F) Comparison between Btk and Tec shows that Tec is missing key residues for the peripheral site and dimerization.
The structures of Tec kinases resembles that of Src and Abl families of cytoplamsmic tyrosine kinases in that they contain the catalytic domain and regulatory Src homology two and three domains (Fig. 1B) (27–32). A feature that distinguishes Btk and other Tec family members from other cytoplasmic tyrosine kinases is the PH-TH module, which consists of a PH domain fused to a zinc-bound Tec homology (TH) domain. In this paper, we focus on the lipid-interacting PH-TH module.
The activation of Btk involves the trans-autophosphorylation of the kinase domains. While localization of Btk to the membrane is expected to result in enhanced phosphorylation due to the local concentration and increased transphosphorylation, two key findings demonstrated that the PH-TH module may have a role in activation beyond simple membrane recruitment (33). First, Btk is strongly activated in solution by inositol hexaphosphate (IP6), a soluble inositol phosphate, and this activation is mediated by the PH-TH module. Second, crystal structures of the PH-TH module (Fig. 1D) are dimeric (referred to as the Saraste dimer, as it was first identified by Saraste and coworkers) (31, 34, 35). The crystal structure of the IP6:PH-TH complex (PDB: 4Y94) revealed two IP6 binding sites on each PH-TH module. One is the canonical binding site, common to other PH domains as a lipid binding site. The other, referred to here as the peripheral site, is proximal to the Saraste dimer interface (Fig. 1D). Mutations at the crystal dimer interface or at the peripheral binding site prevent the activation of Btk by IP6 (31). These observations led to the hypothesis that there is a transient dimerization of Btk that is promoted by IP6 binding, which activates Btk by trans-autophosphorylation.
In this paper, we demonstrate that PIP3 triggers the dimerization of the Btk PH-TH module on membranes in a switch-like manner that is dependent on the Saraste dimer interface. Critical for this behavior is the ability of the Btk PH-TH module to bind to two PIP3 lipids, creating a nonlinear response that is fourth order with respect to the PIP3 surface density. Further, we establish the physiological importance of the peripheral binding site and the Saraste dimer interface in the activation of live B cells through activity assays. These results illustrate how the PH-TH domain of Btk takes advantage of multiple interactions to attain a switch-like response in B cell signaling. The dimerization behavior and the resulting ultrasensitivity is found to be unique to Btk and is not displayed by Tec or Itk. This suggests that Btk provides a critical signaling juncture in B cells, while the corresponding regulation occurs elsewhere in T cells—which may explain why Btk has been an exceptionally effective target for treating B cell-related diseases.
Results and Discussions
The Btk PH-TH Module Dimerizes on Membranes.
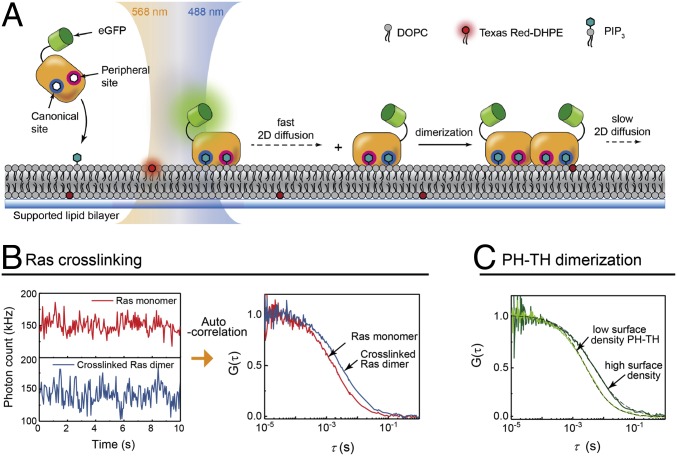
Every crystal structure of the Btk PH-TH module determined so far shows the Saraste dimer, even when there is no ligand bound (31, 34). The ability of IP6 to dimerize the PH-TH module was inferred indirectly by its effect on kinase activity. Nevertheless, dimerization has not been detected directly in solution, suggesting that the membrane plays a role in dimerization of the Btk PH-TH module. To address this, we monitored the density-dependent diffusion of Btk on supported lipid bilayers (SLBs) containing PIP3 (Fig. 2A). The enhanced green fluorescent protein (eGFP)-tagged Btk PH-TH module (henceforth referred to simply as PH-TH module) is introduced to SLBs containing PIP3 and a trace amount of Texas Red (TR) dye-labeled lipids. The dual-color excitation allowed the simultaneous measurement of the proteins and lipids by fluorescence correlation spectroscopy (FCS) or time-correlated single photon counting (TCSPC). TR lipid serves as a reference for each measurement, and the protein diffusion coefficient values reported for the PH-TH module here are shown relative to that of TR lipids measured simultaneously in the same spot.
Fig. 2.
Detection of 2D dimerization reaction on membrane surfaces by FCS. (A) In a dual-color FCS setup, TR-labeled lipid (TR-DHPE) and eGFP-labeled PH-TH domain adsorbed to the SLB by PIP3 are simultaneously measured. (B) For membrane-bound Ras, time-dependent fluorescence intensity fluctuation due to diffusion is recorded (Left), before (red) and after the addition of the RBD-LeuZ crosslinker (blue). The corresponding autocorrelation functions are shown on the Right. (C) In the case of the PH-TH domain dimerization reaction, the difference in diffusion can be clearly resolved between lower surface density (20 molecules/μm2) and a higher surface density (400 molecules/μm2), reflecting the dimer population increase.
In FCS, the time-dependent fluorescence intensity fluctuations due to fluorescent particles entering and exiting the focus area is recorded, as shown in Fig. 2B, Left. The autocorrelation function of the intensity fluctuations (Fig. 2B, Right) has a decay profile with a correlation time constant, τd, which is the average residence time of a diffusing particle in the focus area (36). FCS has been shown to be useful in measuring membrane-bound protein diffusion and, in particular, for the detection of low-affinity dimerization on membranes (37). Supported membranes are highly homogeneous, and proteins bound to them exhibit unencumbered Brownian motion, which enables lateral diffusion to be used as a robust indicator of dimerization. To illustrate the diffusion change on dimerization, the autocorrelation functions of Ras, a lipid-anchored protein, before and after crosslinking by the Ras-binding domain of c-Raf (RBD) fused to leucine zipper domains are shown and correspond to a reduction of the diffusion coefficient from 4.2 to 2.3 μm2/s (data for Ras are taken from ref. 37). A similar change, from 3.5 to 1.7 μm2/s, is observed for the diffusion constant of the wild-type Btk PH-TH module when the Btk surface density is increased, suggesting dimerization (Fig. 2C).
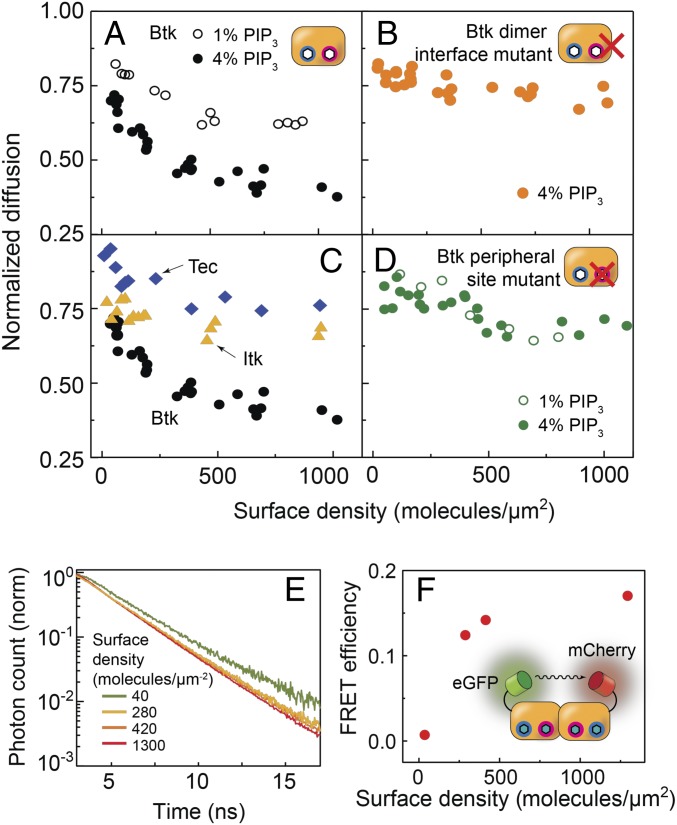
The equilibrium fraction of Btk dimers is a function of Btk monomer surface density. The ratio of the mobilities of monomeric wild-type PH-TH module bound to membrane via PIP3 with respect to the TR-labeled lipid is ∼0.85, as indicated by the diffusion coefficient at low surface densities. On a 4% PIP3 bilayer, this ratio approaches 0.4, indicating an appreciable population of slowly diffusing PH-TH dimers (Fig. 3A, filled black circles). The dimerization of the PH-TH module was further corroborated by density-dependent Förster resonance energy transfer (FRET) increase on 4% PIP3, in which eGFP and mCherry labels on separate Btk PH-TH modules were used as the donor and acceptor, respectively (Fig. 3 E and F). Notably, these SLB-based measurements are direct evidence for Btk dimerization.
Fig. 3.
Diffusion and FRET measurement. The density-dependent diffusion was measured for the wild-type Btk (A), Tec and Itk (C), Btk dimer interface mutant (B), and the peripheral site mutant (D) on SLBs containing 1% (empty circles) and 4% PIP3. (E) The fluorescence lifetime of eGFP tagged to wild-type PH-TH module in presence of mCherry-labeled wild-type PH-TH modules was measured as a function of PH-TH surface density on 4% PIP3 SLBs. (F) FRET efficiency increases as a function of the PH-TH surface density, consistent with dimerization.
Multiple PIP3 Binding Makes the PH-TH Module Ultrasensitive to the PIP3 Surface Density.
To examine how PIP3 modulates Btk PH-TH dimerization, protein diffusion was measured on SLBs containing either 1% or 4% PIP3. Previous measurements on vesicles containing PIP3 had shown an ultrasensitive activation of Btk occurring between 2% and 5% PIP3 (31). Fig. 3A, Left, shows diffusion measurements for 1% and 4% PIP3 bilayers. Compared with 4% PIP3 bilayers (filled black circles), the PH-TH module displays significantly diminished dimerization on 1% PIP3 bilayers (empty black circles), which leads to two conclusions. First, each PH-TH module interacts with multiple PIP3 lipids: if there were a 1:1 stoichiometric relationship between the PH-TH module and PIP3, then its behavior would be identical regardless of the PIP3 density, as long as the protein surface density is constant. Second, the additional PIP3 may be allosterically involved in a structural change or electrostatic interaction necessary for dimerization, in line with previous observations made by molecular dynamics (31).
A remarkable feature of the PIP3 density-dependent dimerization is an ultrasensitive response to the PIP3 concentration. For example, according to estimates based on the FCS data, the surface density of the PH-TH module required for 15% dimer fraction is 1,110 molecules/μm2 on membranes containing 1% PIP3 and 170 molecules/μm2 on membranes containing 4% PIP3 (see SI Appendix for details). Considering that the solution concentrations necessary to achieve these membrane surface densities of the PH-TH module are 34 and 0.9 nM for 1% and 4% PIP3 bilayers, respectively, this is a 38-fold difference.
Although the PIP3 window measured here is restricted by our experimental setup, these data suggest, in combination with the kinetic analysis (Fig. 4) and the calcium flux data (Fig. 5D), that the observed mechanisms here are relevant in the cellular context. The measured bulk PIP3 concentration has been shown to be two to three orders of magnitude less than this range depending on the cellular activation state (38, 39). However, this is assuming a homogeneous distribution of PIP3; it is likely that PIP3 densities are concentrated near the receptors upon activation (40). Unfortunately, this is difficult to measure quantitatively, and to our knowledge, there is no such measurement at this point (41). We also examined the specificity of the Btk PH-TH module toward PIP3 for recruitment and dimerization and found that both membrane recruitment of Btk and dimerization are highly specific to PIP3 (see SI Appendix for details).
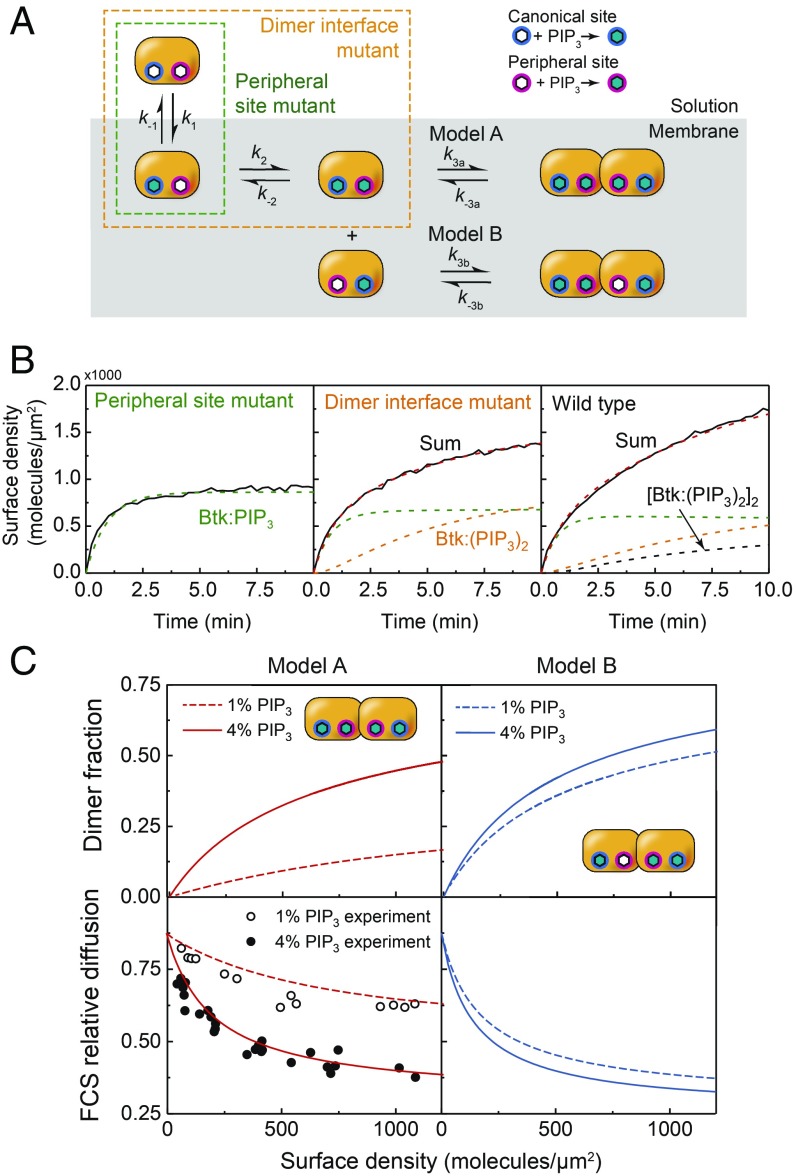
Fig. 4.
Btk PH-TH module adsorption kinetics onto PIP3 SLBs by TIRF microscopy. (A) A simple three-step sequential kinetic model for Btk PH-TH domain is tested using mutant constructs that can undergo a subset of reactions. (B) The membrane adsorption profile measured by TIRF on 4% PIP3 bilayers for each Btk construct. (C) Using the kinetic rate constants obtained from the sequential lipid binding and dimerization model, the equilibrium surface density of dimers was calculated for both model A and B cases at 1% and 4% PIP3 (Top). With the results from C, density-dependent FCS was simulated.
Fig. 5.
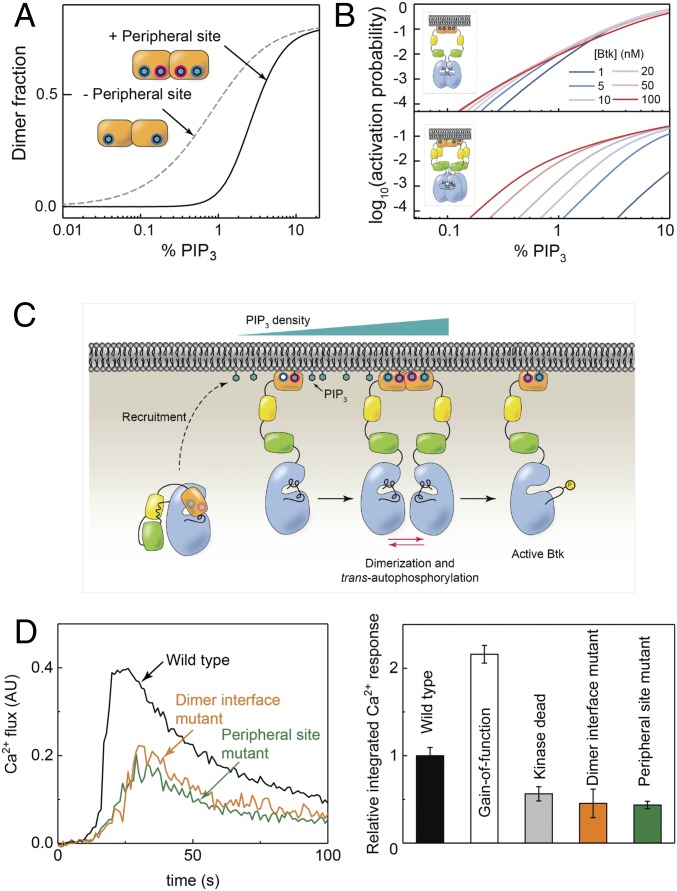
Fourth-order substrate detection by Btk. (A) Using the kinetic rate constants derived from the sequential kinetic model, the dimer fraction was calculated for the hypothetical case in which the PH-TH module may dimerize with only one PIP3 (second order) and for the actual case where two are required (fourth order). (B) An alternative method to achieve fourth-order PIP3 detection, single lipid binding followed by tetramerization to activation (Lower), was considered and compared with the observed mechanism (Upper). (C) The proposed activation mechanism for Btk. (D) Calcium flux for Btk-deficient chicken B cells transfected with variants of Btk and activated with BCR antibody (Left) and relative integrated calcium response for WT, gain-of-function (E41K), kinase dead (D521N), dimer interface mutant, and peripheral site mutant (Right).
Mutations Identify Critical Sites for Dimerization.
For this study, two double mutants of Btk PH-TH module were created based on the crystal structure: a Saraste interface mutant (Y42Q/F44Q) and a peripheral site mutant (K49S/R52S; Fig. 1D). The Saraste interface mutation is predicted to disrupt dimerization by replacing tyrosine and phenylalanine residues that provide intermolecular hydrophobic contacts in the crystal structures of the PH-TH module (35). The peripheral site mutation interferes with peripheral site binding by replacing lysine and arginine residues by serine (31). These mutations have been shown previously to impede Btk activation by IP6 in solution-based biochemical assays. However, a direct connection between peripheral site binding of lipids, dimerization, and phosphorylation had not been established.
Fig. 3B shows the density-dependent diffusion of the Saraste interface mutant. The diffusion remains relatively fast up to a high Btk surface density, indicating that this mutation does indeed impede dimerization. In light of the biochemical activity measurements in which the Saraste interface mutant is incapable of activation in the presence of IP6, the natural interpretation is that dimerization of Btk is required for activation via trans-autophosphorylation (31). The FCS diffusion measurements of the Saraste interface mutant establish the relevance of the crystallographically observed Saraste dimer for the membrane-bound dimerization we observe.
PIP3 binding at the peripheral site was also found to be necessary for dimerization, as mutation of this site resulted in diffusion behavior on 4% PIP3 bilayers nearly identical to that on 1% PIP3 bilayers—in other words, PIP3 concentrations cannot be differentiated without the peripheral site (Fig. 3D, Right). Unlike the canonical phosphatidylinositol binding site, which is common to many PH domains, the role of the peripheral site in PIP3 binding had been ambiguous, as there had been no direct evidence for PIP3 binding at this site. Our observations suggest that engagement of the peripheral site by PIP3 is important for the dimerization and activation of Btk on the membrane.
Tec and Itk PH-TH Modules Are Not Capable of Dimerization on PIP3-Containing Membranes.
We studied whether the PH-TH modules of Tec and Itk also exhibit dimerization and multiple lipid binding. A structure of the Tec PH-TH module is known (PDB: 2LUL); but the Itk PH-TH structure has not been determined, and neither protein has been studied extensively in the context of membrane association in vitro. Sequence alignments suggest that the peripheral site and the Saraste dimer interface seen in Btk are not maintained in these family members (Fig. 1B). The Itk and Tec PH-TH modules were examined by FCS on 4% PIP3 bilayers. Both showed very little protein density-dependent change in diffusion (Fig. 3C), indicating that if there is dimerization, the affinity is very weak (37). This suggests that these proteins do not undergo a PH-TH dimerization-dependent activation, which aligns with the idea that other kinases, such as Lck, are largely responsible for activating Itk and Tec once they are recruited to the membrane (26, 27). It has been shown that Tec and Itk may function as a tunable signal for gene expression rather than an immediate output upon T cell activation, contrary to the case for Btk and its response to B cell activation (24–26, 42).
Adsorption Kinetics Are Consistent with Btk Dimerization Driven by Multiple PIP3 Binding.
The diffusion measurements of Btk PH-TH module on membranes indicated that Btk binds at least two PIP3 and dimerizes, suggesting that the adsorption kinetics would be complex due to these multiple processes. Based on the result that a mutation to the canonical site eliminates binding entirely (SI Appendix, Fig. S2) and the initial rate of adsorption of all PH-TH constructs are essentially equivalent (SI Appendix, Fig. S3), the first binding step is through the canonical site. Given that the peripheral site eliminates dimerization (Fig. 3D), a PIP3 must bind the peripheral site in at least one PH-TH monomer before dimerization can occur; this is step two. However, based on our FCS data alone, it was not possible to distinguish whether the dimerization requires binding of PIP3 to both binding sites on both PH-TH monomers, or if there are dimer species that are only bound to three PIP3. To resolve this ambiguity, the kinetic adsorption data were considered (Fig. 4B). A sequential multistep interaction scheme was constructed, illustrated in Fig. 4A. Each PH-TH mutant was assumed to undergo a different subset of these reactions, and this strategy allowed a sequential estimation of the rate constants for each reaction (SI Appendix, Fig. S4).
Although it is possible to construct more complicated models, these three steps represent the simplest possible mechanisms, and we find that model A successfully predicts, quantitatively, all of the FCS dimerization measurements and total internal reflection flourescence (TIRF) adsorption data (see SI Appendix for details). This comparison provides a rigorous validation for the kinetic model, as FCS is an orthogonal experiment measuring an entirely independent quantity (diffusion rate) from the TIRF measurements. Fig. 4C shows the equilibrium dimer fraction and simulated FCS data for each model, calculated using the respective kinetic rate constants. It is clear that for model B, there is an insufficient difference between the dimer fractions for 1% and 4% PIP3 to account for the experimental FCS results. Model A, however, captures both the PIP3 dependence and the overall shape of the PH-TH surface density dependence, suggesting that a PH-TH dimer is bound to at least four PIP3 lipids. Note that although the possibility of higher-order oligomers cannot be ruled out, but this model of dimerization is sufficient to describe these data.
Because each PH-TH dimer requires four PIP3 molecules, the dimer concentration scales with the fourth power of the PIP3 surface density. This fourth-order nonlinearity creates a sharp PIP3 concentration threshold, which can be contrasted to a hypothetical second-order lipid sensing scenario where only one PIP3 binding at the canonical site is required for dimerization (Fig. 5A). This ligand-counting mechanism may be classified as a type of coincidence detection based on binding multiple ligand molecules.
The Threshold for Activation by PIP3 Is Relatively Robust to Variation in Protein Concentration.
According to the model based on the FCS and adsorption data, Btk employs a system that is dependent on both the ligand and protein concentrations, with an overall fourth-order detection of PIP3 surface density. One advantage of such a system is that it is much less sensitive to changes in protein concentration than a system that uses only 1:1 binding. To illustrate this, we considered a different scenario in which a protein forms a 1:1 complex with its ligand, in which the fourth-order ultrasensitivity to ligand concentration arises solely from tetramerization of the 1:1 PIP3:protein complex. Fig. 5B shows the activation probability of Btk (defined as the fraction of phosphorylated Btk on the membrane at equilibrium) as a function of PIP3 surface density for a range of Btk concentrations, which was calculated based on the kinetic rate constants obtained from TIRF measurements and the catalytic rate constant of Src kinase (see SI Appendix for details). In the case where tetramerization is required, the variation of protein concentration significantly perturbs the threshold PIP3 density (Fig. 5B, Lower). However, in the case where the PIP3 detection is achieved by dimerization, the PIP3 threshold remains relatively stable over the same protein concentration range (Fig. 5B, Upper). This illustrates how this particular mechanism has the effect of decoupling PIP3 sensitivity from Btk concentration, buffering the system with respect to variations in protein expression.
Reconstitution of Btk Variants in Knockout B Cells Corroborate in Vitro Findings.
To test whether these findings have a direct impact on B cell activity in a live cell context, calcium flux in a Btk-knockout chicken B cell line, DT40, was measured with various Btk mutants transiently transfected (Fig. 5D). Wild-type Btk shows expression level-dependent calcium flux, demonstrating that the B cell activation is dependent on the Btk concentration (SI Appendix, Fig. S8). The negative control, the kinase dead mutant D521N, shows a clear reduction of calcium flux, while the positive control, the gain-of-function mutant E41K (whose PH-TH module has been modified to bind PIP2) shows saturating activation, even at a lower Btk concentration than the wild type (43–46). For both the dimer interface and peripheral site mutants, there is a comparable decrease of calcium flux to the kinase dead mutant. This suggests that Btk membrane recruitment through both the canonical and peripheral sites, as well as its dimerization, contributes to the overall activation of Btk in B cells.
Conclusions
In this work, we have established that dimerization of the Btk PH-TH module can be observed on reconstituted membrane surfaces. By measuring diffusion and membrane binding kinetics, we show that PIP3 binding at the peripheral site of the PH-TH module, in addition to the canonical site, is necessary for dimerization, and this requirement gives rise to a sharp threshold in PIP3 concentration for activation. These findings could further our understanding of how to specifically target Btk in the context of diseases such as chronic lymphocytic leukemia (21, 23). For instance, finding a way to block dimerization of the PH-TH module might be a new avenue for inhibiting Btk.
A key consequence of PIP3 binding at the peripheral site is that it makes Btk extremely sensitive to the PIP3 density, a tightly regulated but dynamic signal that influences numerous signaling pathways. Activation by trans-autophosphorylation is a positive feedback mechanism for a switch-like response to signal (47–49). By requiring the peripheral site to bind a PIP3 for activation by dimerization, the Btk PH-TH module creates an even sharper PIP3 concentration threshold for activation. The mutational studies in B cells also supported the prediction that these protein–membrane interactions are critical in B cell activation. Together, they provide detailed insights to how B cells may be instituting a minimalist coincidence detection, and more broadly, how lipid-binding domains have evolved to enhance the integrity of cellular signal transduction.
The dependence of Btk activation on PIP3-triggered dimerization on the membrane had been puzzling, because membrane localization is sufficient to activate most kinases. We have shown that the PH-TH modules of the closely related kinases Itk and Tec do not dimerize on the membrane, suggesting that, for Itk and Tec, the enhanced local concentration that results from membrane recruitment might suffice for activation. The molecular differences in kinase domains that are responsible for their differing requirements for PH-TH dimerization requires further study (26). In addition, these differences may provide insights to how T cells and B cells have adapted to different roles in the course of the development of the immune system.
Methods
Sample Preparation.
The Btk PH-TH module constructs were purified from bacterial recombinant expression, as described previously (31). They were then introduced to supported lipid bilayers composed primarily of 1,2-dioleoyl-sn-glycero-3-phosphocholine and PIP3, prepared on glass coverslips as described elsewhere (50). See SI Appendix for more details.
Microscopic Measurements.
Dual-color FCS measurements were performed on a home-built confocal system integrated into an inverted microscope. The experimental methods have been published previously (51). For FRET, fluorescence lifetime of the donor fluorophore (eGFP) in the presence of an acceptor fluorophore (mCherry) was measured by TCSPC. The adsorption and desorption kinetics of eGFP-labeled Btk PH-TH domain constructs were obtained by bulk TIRF microscopy. The data were fit based on the change in surface density as determined by the fluorescence intensity and TIRF calibration. See SI Appendix for details.
Btk Knockout B Cell Calcium Activity Assay.
Btk-deficient DT40 cells were transiently transfected with pEGFP-N1-nBtk (wild type or mutant). After washing and resting before analysis, calcium flux was monitored by flow cytometry before and after stimulation with 1:1,000 dilution of anti-chicken BCR monoclonal antibody M4 or 1 μM Ionomycin. (See SI Appendix for details.)
Supplementary Material
Acknowledgments
We acknowledge Angelica Gonzalez-Sanchez and Han Li for pilot experiments. We also thank Dr. Neel H. Shah, Dr. Scott D. Hansen, and Joseph B. DeGrandchamp for critical reading of the manuscript. The primary support for this work was provided by the National Health Institute (NIH), under Grant P01AI091580. Additional support was provided by the National Cancer Institute, NIH, under Grant U01CA202241.
Footnotes
The authors declare no competing interests.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819309116/-/DCSupplemental.
References
- 1.Czech MP. (2000) PIP2 and PIP3: Complex roles at the cell surface. Cell 100:603–606. [DOI] [PubMed] [Google Scholar]
- 2.Kane LP, Weiss A (2003) The PI-3 kinase/Akt pathway and T cell activation: Pleiotropic pathways downstream of PIP3. Immunol Rev 192:7–20. [DOI] [PubMed] [Google Scholar]
- 3.Newton AC. (2009) Lipid activation of protein kinases. J Lipid Res 50:S266–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemmon MA. (2008) Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 9:99–111. [DOI] [PubMed] [Google Scholar]
- 5.Lemmon MA, Ferguson KM (2000) Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J 350:1–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrell JE Jr, Machleder EM (1998) The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science 280:895–898. [DOI] [PubMed] [Google Scholar]
- 7.Papayannopoulos V, et al. (2005) A polybasic motif allows N-WASP to act as a sensor of PIP(2) density. Mol Cell 17:181–191. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin S, Wang J, Gambhir A, Murray D (2002) PIP(2) and proteins: Interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 31:151–175. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JE, Giorgione J, Newton AC (2000) The C1 and C2 domains of protein kinase C are independent membrane targeting modules, with specificity for phosphatidylserine conferred by the C1 domain. Biochemistry 39:11360–11369. [DOI] [PubMed] [Google Scholar]
- 10.Newton AC. (1995) Protein kinase C: Structure, function, and regulation. J Biol Chem 270:28495–28498. [DOI] [PubMed] [Google Scholar]
- 11.Hurley JH, Meyer T (2001) Subcellular targeting by membrane lipids. Curr Opin Cell Biol 13:146–152. [DOI] [PubMed] [Google Scholar]
- 12.Ziemba BP, et al. (2014) Single-molecule studies reveal a hidden key step in the activation mechanism of membrane-bound protein kinase C-α. Biochemistry 53:1697–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groves JT, Kuriyan J (2010) Molecular mechanisms in signal transduction at the membrane. Nat Struct Mol Biol 17:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlton JG, Cullen PJ (2005) Coincidence detection in phosphoinositide signaling. Trends Cell Biol 15:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, et al. (2010) Structure and control of the actin regulatory WAVE complex. Nature 468:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK (2000) Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404:151–158. [DOI] [PubMed] [Google Scholar]
- 17.Prehoda KE, Scott JA, Mullins RD, Lim WA (2000) Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science 290:801–806. [DOI] [PubMed] [Google Scholar]
- 18.Scharenberg AM, Humphries LA, Rawlings DJ (2007) Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol 7:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendriks RW, Yuvaraj S, Kil LP (2014) Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer 14:219–232. [DOI] [PubMed] [Google Scholar]
- 20.Tsukada S, et al. (1993) Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72:279–290. [DOI] [PubMed] [Google Scholar]
- 21.Rip J, Van Der Ploeg EK, Hendriks RW, Corneth OBJ (2018) The role of Bruton’s tyrosine kinase in immune cell signaling and systemic autoimmunity. Crit Rev Immunol 38:17–62. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JC, et al. (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal Singh S, Dammeijer F, Hendriks RW (2018) Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer 17:57, and erratum (2019) 18:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnadieu E, et al. (2001) Differential roles of Lck and Itk in T cell response to antigen recognition revealed by calcium imaging and electron microscopy. J Immunol 166:5540–5549. [DOI] [PubMed] [Google Scholar]
- 25.Nayar R, et al. (2012) TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T cell differentiation. Proc Natl Acad Sci USA 109:E2794–E2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreotti AH, Joseph RE, Conley JM, Iwasa J, Berg LJ (2018) Multidomain control over TEC kinase activation state tunes the T cell response. Annu Rev Immunol 36:549–578. [DOI] [PubMed] [Google Scholar]
- 27.Heyeck SD, Wilcox HM, Bunnell SC, Berg LJ (1997) Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J Biol Chem 272:25401–25408. [DOI] [PubMed] [Google Scholar]
- 28.Sicheri F, Moarefi I, Kuriyan J (1997) Crystal structure of the Src family tyrosine kinase Hck. Nature 385:602–609. [DOI] [PubMed] [Google Scholar]
- 29.Hantschel O, et al. (2003) A myristoyl/phosphotyrosine switch regulates c-Abl. Cell 112:845–857. [DOI] [PubMed] [Google Scholar]
- 30.Filippakopoulos P, et al. (2008) Structural coupling of SH2-kinase domains links Fes and Abl substrate recognition and kinase activation. Cell 134:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q, et al. (2015) Autoinhibition of Bruton’s tyrosine kinase (Btk) and activation by soluble inositol hexakisphosphate. eLife 4:e06074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah NH, Amacher JF, Nocka LM, Kuriyan J (2018) The Src module: An ancient scaffold in the evolution of cytoplasmic tyrosine kinases. Crit Rev Biochem Mol Biol 53:535–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grasberger B, Minton AP, DeLisi C, Metzger H (1986) Interaction between proteins localized in membranes. Proc Natl Acad Sci USA 83:6258–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyvönen M, Saraste M (1997) Structure of the PH domain and Btk motif from Bruton’s tyrosine kinase: Molecular explanations for X-linked agammaglobulinaemia. EMBO J 16:3396–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baraldi E, et al. (1999) Structure of the PH domain from Bruton’s tyrosine kinase in complex with inositol 1,3,4,5-tetrakisphosphate. Structure 7:449–460. [DOI] [PubMed] [Google Scholar]
- 36.Lakowicz JR. (2006) Principles of Fluorescence Spectroscopy (Springer, New York: ), 3rd Ed, p xxvi, p 954. [Google Scholar]
- 37.Chung JK, et al. (2018) K-Ras4B remains monomeric on membranes over a wide range of surface densities and lipid compositions. Biophys J 114:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbin JA, Dirkx RA, Falke JJ (2004) GRP1 pleckstrin homology domain: Activation parameters and novel search mechanism for rare target lipid. Biochemistry 43:16161–16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa C, et al. (2015) Measurement of PIP3 levels reveals an unexpected role for p110β in early adaptive responses to p110α-specific inhibitors in luminal breast cancer. Cancer Cell 27:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czech MP. (2003) Dynamics of phosphoinositides in membrane retrieval and insertion. Annu Rev Physiol 65:791–815. [DOI] [PubMed] [Google Scholar]
- 41.Guillou H, Stephens LR, Hawkins PT (2007) Quantitative measurement of phosphatidylinositol 3,4,5-trisphosphate. Methods Enzymol 434:117–130. [DOI] [PubMed] [Google Scholar]
- 42.Tomlinson MG, et al. (2004) Expression and function of Tec, Itk, and Btk in lymphocytes: Evidence for a unique role for Tec. Mol Cell Biol 24:2455–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavran JM, et al. (1998) Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem 273:30497–30508. [DOI] [PubMed] [Google Scholar]
- 44.Rameh LE, et al. (1997) A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J Biol Chem 272:22059–22066. [DOI] [PubMed] [Google Scholar]
- 45.Pilling C, Landgraf KE, Falke JJ (2011) The GRP1 PH domain, like the AKT1 PH domain, possesses a sentry glutamate residue essential for specific targeting to plasma membrane PI(3,4,5)P(3). Biochemistry 50:9845–9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dingjan GM, et al. (1998) Severe B cell deficiency and disrupted splenic architecture in transgenic mice expressing the E41K mutated form of Bruton’s tyrosine kinase. EMBO J 17:5309–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Q, et al. (2015) Identifying three-dimensional structures of autophosphorylation complexes in crystals of protein kinases. Sci Signal 8:rs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamontanara AJ, Georgeon S, Tria G, Svergun DI, Hantschel O (2014) The SH2 domain of Abl kinases regulates kinase autophosphorylation by controlling activation loop accessibility. Nat Commun 5:5470. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara H, et al. (2014) Positive feedback within a kinase signaling complex functions as a switch mechanism for NF-κB activation. Science 344:760–764. [DOI] [PubMed] [Google Scholar]
- 50.Lin WC, Yu CH, Triffo S, Groves JT (2010) Supported membrane formation, characterization, functionalization, and patterning for application in biological science and technology. Curr Protoc Chem Biol 2:235–269. [DOI] [PubMed] [Google Scholar]
- 51.Chung JK, Lee YK, Lam HYM, Groves JT (2016) Covalent ras dimerization on membrane surfaces through photosensitized oxidation. J Am Chem Soc 138:1800–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.