Significance
Leptin (LEP), produced and acting in the hippocampus, mediates enhancement by mild exercise (ME) of hippocampus-related memory and neurogenesis, which are further increased by an antioxidant carotenoid, astaxanthin (AX). Both are facilitated by the administration of ME or AX alone. The up-regulation of the LEP gene and LEP protein expression in the hippocampus by ME is further elevated when combined with AX. Consistently, the combined interventions increased hippocampal LEP protein. In LEP-deficient ob/ob mice, LEP replacement in the brain restored the ability of ME+AX to enhance hippocampal function. Thus, a combined lifestyle intervention based on ME, including yoga and tai chi, and specific dietary supplements that include antioxidants may together improve cognition and possibly retard cognitive decline in humans.
Keywords: hippocampal leptin, mild exercise, astaxanthin, antioxidant, spatial memory
Abstract
Regular exercise and dietary supplements with antioxidants each have the potential to improve cognitive function and attenuate cognitive decline, and, in some cases, they enhance each other. Our current results reveal that low-intensity exercise (mild exercise, ME) and the natural antioxidant carotenoid astaxanthin (AX) each have equivalent beneficial effects on hippocampal neurogenesis and memory function. We found that the enhancement by ME combined with AX in potentiating hippocampus-based plasticity and cognition is mediated by leptin (LEP) made and acting in the hippocampus. In assessing the combined effects upon wild-type (WT) mice undergoing ME with or without an AX diet for four weeks, we found that, when administrated alone, ME and AX separately enhanced neurogenesis and spatial memory, and when combined they were at least additive in their effects. DNA microarray and bioinformatics analyses revealed not only the up-regulation of an antioxidant gene, ABHD3, but also that the up-regulation of LEP gene expression in the hippocampus of WT mice with ME alone is further enhanced by AX. Together, they also increased hippocampal LEP (h-LEP) protein levels and enhanced spatial memory mediated through AKT/STAT3 signaling. AX treatment also has direct action on human neuroblastoma cell lines to increase cell viability associated with increased LEP expression. In LEP-deficient mice (ob/ob), chronic infusion of LEP into the lateral ventricles restored the synergy. Collectively, our findings suggest that not only h-LEP but also exogenous LEP mediates effects of ME on neural functions underlying memory, which is further enhanced by the antioxidant AX.
Positive lifestyle changes including physical activity and diet are believed to be beneficial for promoting brain health and slowing cognitive decline of Alzheimer’s disease (AD). Exercise is an important factor in improving hippocampus-related cognition by enhancing adult hippocampal neurogenesis (AHN) in rodents (1). Although dietary supplements such as docosahexaenoic acid (DHA) and epicatechin (EGCG) have been shown to potentiate the effects of voluntary exercise on memory function (2–4), voluntary exercise alone, not in combination with EGCG, improves AHN and memory functions (5, 6). However, previous animal studies were limited in their applicability to clinical trials because of the uncertainty concerning exercise intensity. To translate our mild exercise (ME) animal model to humans, we developed a quantitative evaluation system based on lactate threshold (LT) and showed that ME has beneficial effects on AHN and spatial memory in animals (7, 8) and on hippocampal memory function in young humans (9). This raises the question of whether, for translation to humans, a combined intervention of dietary supplements and ME could enhance memory function and neuronal plasticity.
While mechanisms of exercise-enhanced hippocampal function are not fully understood, several molecular factors including insulin-like growth factor 1 (IGF1) and brain-derived neurotrophic factor (BDNF) are well documented (10). Serum IGF1 enters the brain and aids the development of neuronal plasticity (11, 12). Circulating IGF1 promotes AHN through stimulation by exercise (13), but a peripheral deficiency of IGF1 through immunoneutralization diminishes the beneficial effects of exercise on AHN and spatial learning (14), suggesting that IGF1 is one mechanistic factor in exercise-induced improvement of hippocampal function. BDNF is essential for brain development and synaptic plasticity and is increased in the rodent hippocampus with running (15). Acute treadmill running at a low intensity below the LT enhances BDNF mRNA expression and neuronal activity in hippocampal regions (16). Furthermore, hippocampal androgens promote AHN mediated through chronic ME via a paracrine effect (17). These findings suggest multiple molecular mechanisms behind exercise-enhanced hippocampal function, including both peripheral growth factors and brain-derived factors.
Leptin (LEP), a hormone derived from adipose tissue, has a crucial role in regulating energy homeostasis and food intake mediated by the hypothalamus. LEP is also produced in the brain and is involved in various processes in the central nervous system (18–20). The expression of LEP mRNA and protein has been demonstrated in rodent (21, 22) and human brains (23), as well as in neuronal cells (24), pointing to LEP synthesis in the brain itself. Hippocampal LEP (h-LEP) enhances AHN and synaptic function (25, 26), which facilitates spatial learning and memory function (27), suggesting a neurotrophic role of LEP within the hippocampus.
LEP-based therapeutic treatment for neurodegenerative disease has been proposed because LEP gene delivery into the brain rescues impaired memory and AHN, and reduces amyloidosis in AD mice (28, 29). In a rat model of chronic unpredictable stress, hippocampal administration of LEP ameliorated depression-like behaviors (30). If endogenous LEP could be increased by lifestyle interventions including exercise and dietary supplements, it would be of significant interest for enhancement of cognitive function and neuronal plasticity.
Among potential dietary supplements, we focused on astaxanthin (AX), a red carotenoid with various health benefits, including enhancement of cognition. AX has highly antioxidant and antiinflammatory properties, with reportedly strong neuroprotective effects (31), and it enhances AHN and spatial memory (32), suggesting that it also has beneficial effects equivalent to those of ME on hippocampal functions. Hence, we hypothesized that ME-enhanced hippocampal neurogenesis and memory might be further improved with dietary AX via mediation by a neurotrophic factor such as h-LEP.
Here, we report that ME enhances spatial memory and AHN when combined with AX in wild-type (WT) mice but not in LEP-deficient ob/ob mice. Exploring the molecular mechanisms underlying the combination effects, DNA microarray and bioinformatics revealed that the LEP gene is a major player. We also demonstrate that LEP and its AKT/STAT3 signaling in the hippocampus are involved in the enhancement of memory function in the combined (ME+AX) intervention group. Thus, we propose that leptin produced or acting in the hippocampus (h-LEP) may be a key molecular factor in the synergistic benefits of ME combined with AX on hippocampus-based neurogenesis and memory function. ME+AX, which boosts, separately and together, the expression of h-LEP, may provide greater beneficial cognitive enhancement and neuroprotective effects in preventing neurodegenerative disorders, including AD, than does exercise alone.
Results
ME Combined with AX Induces Strong Enhancement of Hippocampus-Dependent Cognition and Neurogenesis.
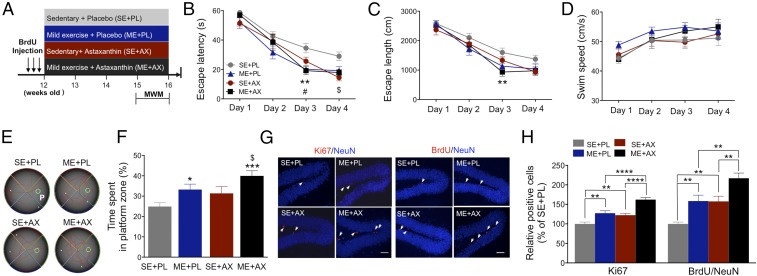
We first determined whether the combined intervention of ME+AX produces greater enhancement in spatial learning and memory in WT (C57BL/6J) mice (Fig. 1A). Escape latency and path length in all groups significantly decreased across the acquisition phase (SI Appendix, Table S1). There was a significant effect of intervention on escape latency (Fig. 1B) and path length (Fig. 1C). The ME with placebo (ML+PL) and ME+AX groups had significantly faster escape latencies on day 3. The sedentary with AX supplementation (SE+AX) group had a shortened escape latency on day 4. The path length taken by the ME+AX group was significantly shorter on day 3. There was no effect of any intervention on swimming speed (Fig. 1D). These results suggest that ME and AX each lead to independent enhancements in spatial learning ability. In the probe test, there were significant effects of ME or AX on spatial memory performance (Fig. 1F). The time spent in the platform (P) zone was increased in the ME+PL (132.9%) and ME+AX (160.6%) groups compared with the SE+PL group. The ME+AX group spent significantly more time in the P zone than did the SE+AX group (Fig. 1 E and F). These findings support enhanced effects of ME and AX on the retention of spatial memory.
Fig. 1.
ME combined with AX induces enhancing effects on spatial memory and neurogenesis. (A) Experimental design for hippocampal function and AHN. (B and C) Shorter escape latency and length following the acquisition phase (P < 0.0001). (B) Shorter escape latency following ME alone, AX alone, and combined intervention (#P < 0.05, ME+PL vs. SE+PL; **P < 0.01, ME+AX vs. SE+PL, $P < 0.05, SE+AX vs. SE+PL, n = 9–10/group). (C) ME combined with AX results in a shorter escape path length (**P < 0.01, ME+AX vs. SE+PL). (D) Swim speed does not differ between groups. (E) Representative swimming paths during the probe test. (F) ME-alone and AX-alone interventions increase time spent in the P zone during the probe test; combined intervention time is longer compared with SE+PL (*P < 0.05, ***P < 0.001, vs. SE+PL; $P < 0.05, vs. SE+AX). (G) Representative images of double-fluorescence staining for Ki67 (red) and BrdU (red), respectively, and NeuN (blue) in the dentate gyrus. (Scale bar: 50 μm.) (H) Ki67 and BrdU/NeuN cells are significantly increased with ME alone; the combination of ME and AX leads to a greater increase compared with ME or AX alone (**P < 0.01, ****P < 0.0001, n = 7–8/group). Data are presented as mean ± SEM.
We examined whether the greater effects of ME combined with AX on spatial memory would be mediated by at least an additive or possibly even a synergistic increase in the progression of AHN (Fig. 1A). There was a significant effect of ME and AX on the number of Ki67-positive cells (Fig. 1 G and H). Ki67-positive cells increased in the ME+PL (124.4%) and in the SE+AX (122.4%) groups compared with the SE+PL group. Ki67-positive cells were much higher in the ME+AX group (162.2%) compared with each treatment alone. Similarly, there was a significant effect of ME and AX on the number of BrdU/NeuN-positive cells (Fig. 1 G and H). BrdU/NeuN-positive cells increased in the ME+PL (158.5%) and SE+AX groups (157.7%) compared with the SE+PL group. These increases were further boosted in the ME+AX group (216.9%), implying that while ME and AX respectively enhance cell proliferation and maturation of newborn cells, their combination results in a strong additional effect.
LEP Is a Promising Molecular Mediator of Synergistic Effects.
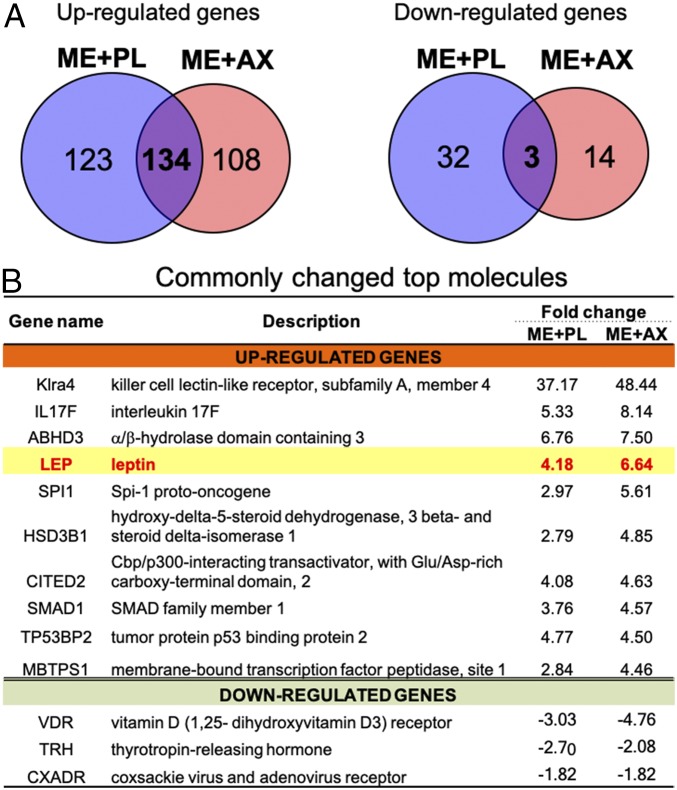
To determine the potential molecular factors underlying the synergistic interactions of ME combined with AX on hippocampal function, we examined the transcriptome in the hippocampus after ME alone and in combination with AX, and we compared the results with those of the SE+PL group using a whole-genome DNA microarray and ingenuity pathway analysis (IPA) with gene expression specific to hippocampal tissue. The DNA microarray using cutoff values (≧/≦ 1.5/0.75-fold) revealed 2,209 and 564 up- and down-regulated genes, respectively, in response to the ME alone. Interestingly, a much higher number of changes appeared in the combined intervention, where 3,891 and 411 up- and down-regulated genes were altered (SI Appendix, Fig. S1). IPA further revealed that annotated genes yielded 257 up-regulated and 35 down-regulated genes in the ME+PL group, and 242 up-regulated and 17 down-regulated genes in ME+AX group (SI Appendix, Table S2–S5). Among the annotated genes, 134 up-regulated genes and 3 down-regulated genes were altered in common and overlapped between ME alone and combined interventions (Fig. 2A). Of these, the top genes up- and down-regulated in common are shown in Fig. 2B. Of the top 3 up-regulated genes (Klra4, IL17F, and ABHD3), ABHD3 has an antioxidant function that is consistent with the action of AX (33). It is also noteworthy that the up-regulation of the LEP gene with ME alone was further elevated when combined with AX (Fig. 2B). These results suggest that antioxidant action is the key factor along with the LEP gene behind the synergy effect upon spatial memory and AHN by ME combined with AX.
Fig. 2.
The LEP gene is a potential molecule for mediating synergistic effects. (A) Venn diagrams show overlap of the up- and down-regulated genes between the ME+PL and the ME+AX groups. (B) The LEP gene is included in the list of common top up-regulated genes.
LEP in the Hippocampus, but Not in Plasma, Contributes to the Combined Effects of ME and AX.
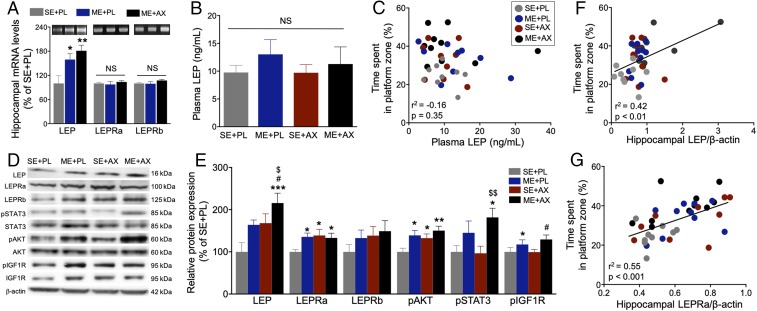
To validate the DNA microarray-based gene expressions, several specific genes (Igf1r, Stat3, Akt, and Mapk1) were validated using semiquantitative (sq) RT-PCR that employed individual samples from the pool (SI Appendix, Fig. S2). We also measured LEP and its receptor (LEPRa and LEPRb) mRNA in the hippocampus. While the LEP mRNA levels were increased in the ME+PL and ME+AX groups, there were no increments in the LEPRa or LEPRb mRNA levels after intervention (Fig. 3A). We further measured circulating plasma LEP levels to determine whether peripheral LEP contributes to the enhancement of spatial memory after the combination of ME and AX. Plasma LEP levels were not significantly different among groups (Fig. 3B), nor did they correlate with spatial memory (Fig. 3C). These findings demonstrate that h-LEP, not circulating LEP, is a potential target molecule underlying enhanced memory performance resulting from the combined intervention.
Fig. 3.
Combined ME and AX increase LEP in the hippocampus but not in plasma. (A) sqRT-PCR analysis showing that interventions of ME alone and ME combined with AX significantly increase LEP mRNA but not LEPRa or LEPRb mRNA levels (n = 7–8/group). (B and C) ELISA analysis shows no differences in plasma LEP between groups; plasma LEP is not correlated with spatial memory. (D and E) Western blotting analysis reveals LEP, LEPRa, LEPRb, pSTAT3, pAKT, and pIGF1R protein levels in the hippocampi of WT mice. Data are shown as protein levels relative to the SE+PL group and presented as mean ± SEM (n = 9–10/group). *P < 0.05, **P < 0.01, ***P < 0.001, vs. SE+PL; #P < 0.05, vs. ME+PL; $P < 0.05, $$P < 0.01, vs. SE+AX. (F and G) LEP and LEPRa levels are positively correlated with spatial memory. NS, not significant.
To validate the candidate molecular factors at the protein level, Western blotting was performed for LEP signaling in the hippocampus of WT mice (Fig. 3 D and E). There was a significant effect of ME and AX on LEP levels. LEP expression was much higher in the ME+AX group than in all other groups, which correlated with spatial memory (Fig. 3F). AX alone produced a slight tendency to increase the short form of LEPRa but not the long form of LEPRb (Fig. 3 D and E). The relative expression of LEPRa increased in the ME+PL, SE+AX, and ME+AX groups compared with the SE+PL group, and this was strongly correlated with spatial memory (Fig. 3G). In addition, phosphorylation of AKT (pAKT) showed a significant effect of ME and AX. The relative expression of pAKT increased in the ME+PL, SE+AX, and ME+AX groups compared with the SE+PL group. Phosphorylation of STAT3 (pSTAT3) was significantly increased in the ME+AX group (Fig. 3 D and E). These results suggest that h-LEP expression levels and the AKT/STAT3 signaling pathways are involved in the enhancing effects of the combined intervention.
AX Treatment Enhances Neuronal Cell Viability with LEP Expression.
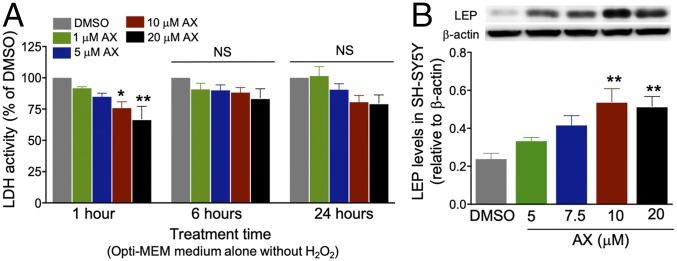
Human neuroblastoma SH-SY5Y cell lines synthesize endogenous LEP (24), and this provided an in vitro model to study whether AX directly induces beneficial effects on the viability of neuronal cells at least in part via LEP expression. AX at 10 and 20 µM significantly decreased LDH activity in Opti-MEM medium alone without H2O2 (CON) for one hour of incubation (Fig. 4A); AX treatment in CON medium increased LEP levels in a dose-dependent manner; in particular, 10 and 20 µM led to significant induction (Fig. 4B). In addition, DNA microarray analysis revealed that the AHBD3 and LEP genes were up-regulated by AX only (SI Appendix, Table S6). These results suggest that AX-induced effects upon neuronal cell viability may be, in part, mediated through increased expression of the antioxidant gene ABHD3 as well as endogenous LEP.
Fig. 4.
AX promotes neuronal cell viability with LEP induction. (A) AX treatments of 10 and 20 μM in Opti-MEM medium alone without H2O2 effectively and significantly decrease LDH release compared with the DMSO group (n = 3/group). (B) Western blots showing that 10 and 20 μM of AX significantly increases LEP expression compared with DMSO (n = 4–5/group). Protein levels normalized to the band intensity of β-actin. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, vs. DMSO. NS, not significant.
LEP Deficiency Restricts the Enhancement of Spatial Memory in Response to the Combination of ME and AX.
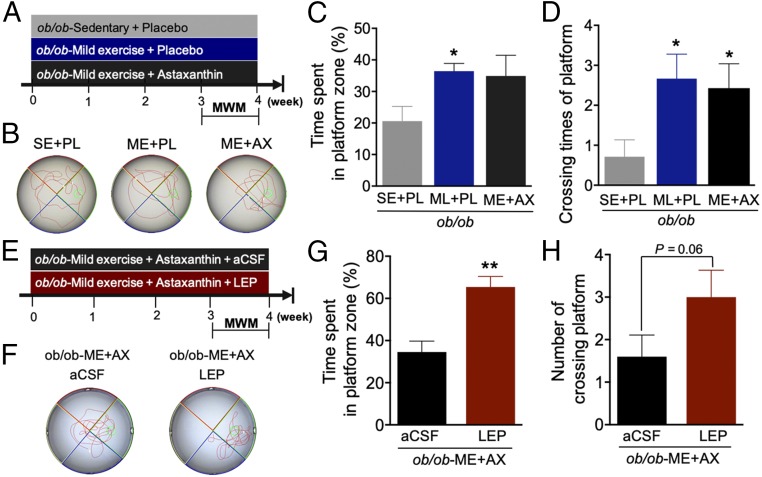
To determine whether LEP is required for regulating the synergistic effect on spatial memory with the combined intervention of ME and AX, we used LEP-deficient ob/ob mice. During the final week of a four-week intervention, we evaluated the effects of LEP deficiency on spatial learning and memory enhanced with ME alone and in combined interventions using the Morris water maze (MWM) test (Fig. 5A). There were no intervention effects on escape length and swimming speed (SI Appendix, Fig. S3 A and B). A tendency toward increased time spent in the P zone (Fig. 5 B and C) and significant effects on crossing times for the P zone (Fig. 5D) were seen. The WT mice with the combined intervention spent more time in the P zone compared with those in the ME-alone group (Fig. 1F). However, the ob/ob mice with the combined intervention did not exhibit a tendency to spend more time in the P zone compared with the ME-alone group (Fig. 5C). These results suggest that LEP deficiency restricts the enhancement of spatial memory as a response to the combining of ME and AX and thus LEP is the key to ME/AX synergy.
Fig. 5.
Central LEP involved in ME+AX-induced enhancement of spatial memory in LEP-deficient mice. (A) Experimental design for ME combined with AX in LEP-deficient (ob/ob) mice (n = 5–7/group). (B) Representative swimming paths during the probe test. (C) The ME-alone group showing increased time in the P zone (*P < 0.05 vs. SE+PL). (D) The ME-alone and the ME+AX interventions lead to increased crossing times for the P zone (*P < 0.05 vs. SE+PL). (E) The ob/ob mice continuously administered sCSF or LEP during the four-week combined intervention (n = 5/group). (F) Representative swimming paths during the probe test. (G) The percentage of time spent in the P zone with LEP administration is shown as significantly greater than that with aCSF infusion (**P < 0.01 vs. aCSF group). (H) There is a tendency for increased times in crossing the P zone in the LEP-administered group (P = 0.06). Data are presented as mean ± SEM.
In the WT mice, the ME-alone intervention increased pIGF1R in the hippocampus (Fig. 3E). Therefore, we also checked whether hippocampal IGF1R signaling is involved in ob/ob mice memory functions enhanced by ME alone. Both the ME+PL and ME+AX groups had significantly increased pIGF1R and pPI3K protein abundances compared with the SE+PL group (SI Appendix, Fig. S3 C and D). The level of pAKT protein was increased only in the ME+AX group (SI Appendix, Fig. S3E). These results reveal that hippocampal IGF1R signaling is also involved in the ME-alone enhancement of memory.
Increasing Hippocampal LEP Restores the Synergistic Effects of ME and AX on Spatial Memory in LEP-Deficient Mice.
Our data shows that ME combined with AX increased hippocampal LEP levels in WT mice (Fig. 3E), which positively correlated with spatial memory enhancement (Fig. 3F). This suggests that hippocampal LEP might mediate the combined effect of ME and AX on spatial memory. Therefore, we next examined whether increased central LEP levels in LEP-deficient mice were functionally involved in the synergistic enhancement of spatial memory performance.
For long-term increase of central LEP levels during four weeks, we administered intracerebroventricular (ICV) injections of LEP or artificial cerebrospinal fluid (aCSF, control group) to ob/ob mice using an osmotic minipump (Fig. 5E). No difference was noted in the escape latency achieved by the ob/ob mice administered LEP and those administered aCSF (SI Appendix, Fig. S4A). The LEP-administered group dramatically increased time spent in the P zone (Fig. 5 F and G), and there was a slight tendency toward increased crossing times for the P zone (Fig. 5H). In ob/ob mice, plasma LEP levels were virtually undetectable (0.62–0.85 ng/mL) compared with lean mice with SE+PL (17.5 ng/mL; SI Appendix, Fig. S4B). These findings demonstrate that the synergistic enhancement of spatial memory may be partly mediated through increased LEP acting in the brain.
Discussion
Our results reveal a key role for h-LEP in ME-stimulated dentate gyrus neurogenesis and demonstrate further enhancement of this and related spatial memory by an antioxidant that has neuroprotective and LEP-inducing effects in vitro. After verifying our hypothesis that ME combined with AX produces greater benefits for spatial memory and AHN than either ME or AX alone, our omics analysis on their combined effects on hippocampal gene expression revealed that up-regulation of the LEP gene by ME alone was further increased by the combination of ME and AX. Hippocampal, but not systemic, LEP expression increased with both ME and AX individually. A greater increase was observed with the combined intervention, which correlated with spatial memory performance. LEP deficiency in ob/ob mice restricted synergistic enhancement compared with ME alone, an effect that was reversed with the chronic infusion of LEP into the brain.
To further connect LEP and AX, in vitro AX treatment in a neuroblastoma cell line exerted a neurotrophic effect in neuronal cell viability (Fig. 4A) and directly increased LEP expression in a dose-dependent manner (Fig. 4B). Omics analysis also revealed that up-regulation by ME+AX of one gene (ABHD3) had an antioxidant function consistent with the action of AX (33). The function of the ABHD3 gene in the hippocampus is still unknown, although these results support the notion that AX diets and/or ME exert antioxidant effects associated with the role of ABHD3. Given that AX has a strong antioxidant capacity in the brain (34), it is possible that ME combined with AX may result in superior protective effects against oxidative stress associated with neurological disorders as well as promotion of healthy brain function.
It is also known that LEP has other extrahypothalamic roles in the brain based on its reported effects on dendritic morphogenesis, synaptogenesis, and AHN (25, 35, 36). LEP mRNA expression has been detected in the rodent (21) and the human (23) brain. We confirmed the expression of hippocampal LEP mRNA, which increased with ME alone and even more so with the combination of ME and AX (Fig. 3A). Importantly, plasma LEP in WT mice remained unchanged by the combined intervention (Fig. 3B). We also found that hippocampal, but not blood, LEP levels were positively correlated with intervention-enhanced spatial memory function (Fig. 3F), suggesting that brain-derived LEP, particularly in the hippocampus, mediates the enhancement of AHN and spatial memory when ME and AX are combined.
While brain-produced LEP is important, intraperitoneally administered LEP contributes to enhanced AHN (25). Exogenous LEP treatment also facilitates an improvement in long-term potential and spatial memory (27, 37). Furthermore, a previous study showed that administration of LEP into the hippocampus induces potential antidepressant effects against chronic stress (30).
LEP-deficient (ob/ob) mice, a model for diabetes with obesity, have been reported to show spatial learning and memory deficits in the MWM test (38). Thus, because of LEP’s crucial role in hippocampus-dependent cognitive function, we chose ob/ob mice to investigate whether it could be involved in increased spatial memory resulting from the combination of ME and AX. LEP-deficient mice did not exhibit any further increase in spatial memory performance with the combined intervention compared with ME alone (Fig. 5 C and D). When we applied a chronic ICV infusion of LEP over four weeks to leptin-deficient ob/ob mice, spatial memory induced by the combination of ME and AX was enhanced (Fig. 5 G and H). Therefore, a LEP increment in the brain may be partly required for inducing the synergistic effects of the combined intervention on spatial memory performance.
Like other mediators of additive/synergistic effects on cognition and neuronal plasticity, a combination of DHA and exercise has been reported to enhance learning, in part via increasing BDNF and synaptic protein expression (3, 4). As part of a complex signaling mechanism, in the absence of LEP, ME combined with AX increased pIGF1R and its downstream factors (pPI3K and pAKT) in the hippocampus of ob/ob mice (SI Appendix, Fig. S3). Considering prior evidence on the interaction between IGF1 and LEP signaling in the hippocampus (39), our results of increased LEP and IGF1R support the possibility that the enhancement of AHN and memory function by ME+AX may be due to the interplay of both LEP and IGF1R expression.
We also found that levels of hippocampal LEPRa, but not levels of LEPRb, and its downstream factors (pAKT and pSTAT3) were increased together with ME and AX (Fig. 3E). Interestingly, the LEPRa increase was correlated with memory increase (Fig. 3G). Hippocampal LEPRb has been principally recognized to play a physiological role in depression-like behaviors (40), but the role of LEPRa as a functional receptor in the hippocampus is uncertain. A recent study showed that LEPRa is predominantly expressed in the hippocampus and that the LEPRa mRNA is highly expressed in cultured astrocytes (41). Among many cellular signals in astrocytes, STAT3 appears to have an important role in response to LEP for the regulation of neuroendocrine function in obesity (42). Thus, our results raise the possibility that combined intervention induces synergistic enhancements of AHN and spatial memory, at least in part, through LEP action with astrocytic LEPRa/STAT3 signaling.
There are indications from animal models that coadministered exercise and dietary supplements provide superior benefits for hippocampus-based cognitive function (43). For example, the effect of voluntary running, regardless of exercise intensity, on spatial learning and memory performance was increased when combined with the dietary intervention of DHA (3, 4) and EGCG (2). However, a further study is needed to determine optimal exercise conditions for additional effects on hippocampal function, because two studies investigated the combined effects of voluntary exercise and specific dietary supplements (EGCG/β-alanine) on AHN in mice but failed to show any significant effect with either compound alone or in combination with exercise (5, 6). We previously reported that ME below the exercise intensity threshold enhances hippocampal memory function in both humans and rodents (7, 9). Here we have further demonstrated that ME is more effective for enhancing spatial memory and AHN when combined with AX, as evidenced by their combined contribution being greater than the sum of their respective neurogenic effects. Thus, our results suggest that ME defined by a physiological parameter of exercise intensity is highly feasible for translating to a human clinical trial to achieve better benefits of combining exercise with an antioxidant.
In conclusion, we have demonstrated that the beneficial effects of ME on hippocampus-based cognition and neuronal plasticity are strengthened by diets supplemented with an antioxidant, AX. Importantly, we have provided evidence that increased hippocampal h-LEP and its AKT/STAT3 signaling mediate the induction of at least the additive effect of ME combined with AX on neurogenesis and spatial memory (SI Appendix, Fig. S5); however, exogenous LEP also works in ob/ob mice. We have shown that AX has a neurotrophic effect that also increases LEP expression in an in vitro cell model. Recently, LEP administration and gene therapy were shown to have potential neuroprotective effects against AD in rodents (28, 44), suggesting that LEP has therapeutic potential in AD pathology. Thus, our findings advance the notion that ME combined with a dietary antioxidant such as AX, which induces endogenous h-LEP, may be an effective nonpharmacological strategy for preventing or improving cognitive function and brain health, and for slowing cognitive decline. This strategy may be particularly useful in vulnerable individuals, including the elderly.
Methods
Animal care and experiments were performed in accordance with procedures approved by the University of Tsukuba Animal Experiment Committee (approval 15–054). For fully detailed methods, please refer to the SI Appendix.
Based on the ventilatory threshold (VT), we determined the intensity of treadmill running for both WT and ob/ob mice (SI Appendix, Fig. S6). The mice from the exercise groups ran following the appropriate running protocol (four weeks) below their VT (SI Appendix, Table S7). The mice received a nonpurified basal diet with either AX or a placebo (AstaReal powder 20F; AstaReal Co. Ltd) at concentrations of 0.5% (SI Appendix, Table S8) (32).
Supplementary Material
Acknowledgments
We thank our colleagues in the H.S. laboratory (University of Tsukuba) for their kind technical assistance, and Drs. H. Ohmori and N. Omi (University of Tsukuba) for their helpful advice. This research was supported in part by special funds for Education and Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) granted to the Human High Performance (HHP) Research Project (1111501004); grants from the Japan Society for the Promotion of Science (JSPS) for the Global Initiative for Sports Neuroscience (GISN): For Development of Exercise Prescription Enhancing Cognitive Functions (HFH27016); KAKENHI Grants-in-Aid for Scientific Research (A) (18H04081); and KAKENHI Grants-in-Aid for Scientific Research on Innovative Areas: Next Generation Exercise Program for Developing Motivation, Body and Mind Performance (16H06405) (to H.S.).
Footnotes
Conflict of interest statement: J.S.Y. and H.S. are listed as inventors on patent application WO2017213197A1. H.S. has received a research grant and served as a scientific advisor for AstaReal Co. Ltd. All other authors declare that they have no competing interests.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1815197116/-/DCSupplemental.
References
- 1.van Praag H, Shubert T, Zhao C, Gage FH (2005) Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25:8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Praag H, et al. (2007) Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci 27:5869–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu A, Ying Z, Gomez-Pinilla F (2008) Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 155:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chytrova G, Ying Z, Gomez-Pinilla F (2010) Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res 1341:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibbons TE, et al. (2014) Voluntary wheel running, but not a diet containing (-)-epigallocatechin-3-gallate and β-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behav Brain Res 272:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya TK, et al. (2015) Exercise but not (-)-epigallocatechin-3-gallate or β-alanine enhances physical fitness, brain plasticity, and behavioral performance in mice. Physiol Behav 145:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue K, et al. (2015) Long-term mild exercise training enhances hippocampus-dependent memory in rats. Int J Sports Med 36:280–285. [DOI] [PubMed] [Google Scholar]
- 8.Inoue K, et al. (2015) Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS One 10:e0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suwabe K, et al. (2018) Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc Natl Acad Sci USA 115:10487–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci 30:464–472. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez AM, Torres-Alemán I (2012) The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci 13:225–239. [DOI] [PubMed] [Google Scholar]
- 12.Nishijima T, et al. (2010) Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 67:834–846. [DOI] [PubMed] [Google Scholar]
- 13.Aberg MA, Aberg ND, Hedbäcker H, Oscarsson J, Eriksson PS (2000) Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci 20:2896–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trejo JL, Llorens-Martín MV, Torres-Alemán I (2008) The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci 37:402–411. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Pinilla F, Vaynman S, Ying Z (2008) Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci 28:2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soya H, et al. (2007) BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun 358:961–967. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto M, et al. (2012) Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci USA 109:13100–13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esler M, et al. (1998) Leptin in human plasma is derived in part from the brain, and cleared by the kidneys. Lancet 351:879. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432. [DOI] [PubMed] [Google Scholar]
- 20.Li HY, Wang LL, Yeh RS (1999) Leptin immunoreactivity in the central nervous system in normal and diabetic rats. Neuroreport 10:437–442. [DOI] [PubMed] [Google Scholar]
- 21.Morash B, Li A, Murphy PR, Wilkinson M, Ur E (1999) Leptin gene expression in the brain and pituitary gland. Endocrinology 140:5995–5998. [DOI] [PubMed] [Google Scholar]
- 22.Ur E, Wilkinson DA, Morash BA, Wilkinson M (2002) Leptin immunoreactivity is localized to neurons in rat brain. Neuroendocrinology 75:264–272. [DOI] [PubMed] [Google Scholar]
- 23.Eikelis N, Wiesner G, Lambert G, Esler M (2007) Brain leptin resistance in human obesity revisited. Regul Pept 139:45–51. [DOI] [PubMed] [Google Scholar]
- 24.Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA (2004) Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology 145:4103–4112. [DOI] [PubMed] [Google Scholar]
- 25.Garza JC, Guo M, Zhang W, Lu XY (2008) Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem 283:18238–18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moult PR, Milojkovic B, Harvey J (2009) Leptin reverses long-term potentiation at hippocampal CA1 synapses. J Neurochem 108:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oomura Y, et al. (2006) Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides 27:2738–2749. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-González R, et al. (2014) Leptin gene therapy attenuates neuronal damages evoked by amyloid-β and rescues memory deficits in APP/PS1 mice. Gene Ther 21:298–308. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-González R, et al. (2011) Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J Alzheimers Dis 24:17–25. [DOI] [PubMed] [Google Scholar]
- 30.Lu XY, Kim CS, Frazer A, Zhang W (2006) Leptin: A potential novel antidepressant. Proc Natl Acad Sci USA 103:1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimmig B, Kim S-H, Nash K, Bickford PC, Douglas Shytle R (2017) Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience 39:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yook JS, et al. (2016) Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice. Mol Nutr Food Res 60:589–599. [DOI] [PubMed] [Google Scholar]
- 33.Long JZ, et al. (2011) Metabolomics annotates ABHD3 as a physiologic regulator of medium-chain phospholipids. Nat Chem Biol 7:763–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Amin MM, et al. (2015) The antioxidant effect of astaxanthin is higher in young mice than aged: A region specific study on brain. Metab Brain Dis 30:1237–1246. [DOI] [PubMed] [Google Scholar]
- 35.O’Malley D, et al. (2007) Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci 35:559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhar M, et al. (2014) Leptin induces hippocampal synaptogenesis via CREB-regulated microRNA-132 suppression of p250GAP. Mol Endocrinol 28:1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farr SA, Banks WA, Morley JE (2006) Effects of leptin on memory processing. Peptides 27:1420–1425. [DOI] [PubMed] [Google Scholar]
- 38.Jeon BT, et al. (2016) Effects of caloric restriction on O-GlcNAcylation, Ca2+ signaling, and learning impairment in the hippocampus of ob/ob mice. Neurobiol Aging 44:127–137. [DOI] [PubMed] [Google Scholar]
- 39.Marwarha G, Prasanthi JR, Schommer J, Dasari B, Ghribi O (2011) Molecular interplay between leptin, insulin-like growth factor-1, and β-amyloid in organotypic slices from rabbit hippocampus. Mol Neurodegener 6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo M, Huang TY, Garza JC, Chua SC, Lu XY (2013) Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int J Neuropsychopharmacol 16:857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koga S, et al. (2014) Effects of diet-induced obesity and voluntary exercise in a tauopathy mouse model: Implications of persistent hyperleptinemia and enhanced astrocytic leptin receptor expression. Neurobiol Dis 71:180–192. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Hsuchou H, He Y, Kastin AJ, Pan W (2015) Role of astrocytes in leptin signaling. J Mol Neurosci 56:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Pinilla F, Ying Z (2010) Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus. Neuroscience 168:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greco SJ, et al. (2010) Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis 19:1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.