Abstract
The cortisol (C) and testosterone (T) responses to experimental stress have been linked to sport and health outcomes several days to years later. Here we examined the utility of these biomarkers, taken across a simulated Olympic weightlifting (OWL) competition, as predictors of future competitive performance in young athletes. Seventy junior athletes (46 males, 24 females) participated in a talent identification and development programme that replicated an OWL competition. Performance was indexed by the total load lifted, relative to body mass, with serum changes in C (∆C) and (∆T) concentrations profiled. We identified each athlete’s best performance in real competitions over two subsequent years via online resources. Hierarchical regression was used to predict changes in competitive performance at <12 (∆Total12) and 12-24 months (∆Total24). The simulated OWL event promoted a small positive ∆C (effect size [ES]=0.3) and ∆T (ES=0.5), but with large variation in ∆C (-58% to 200%) and ∆T (-21% to 71%). Performance improved after 12 (ES=1.5) and 24 months (ES=0.9). The ∆C was negatively related to the ∆Total12 and ∆Total24 when controlling for competitions entered (R2=13-24%). Sensitivity analyses confirmed the ∆C link to both outcomes (R2=9%). The serum C and T responses to a simulated OWL competition varied considerably between participants. Their competitive performance improved over the next two years and individual performance trajectories were related to the ∆C. Therefore, individual variation in the C responses to a competitive stressor may help forecast the training and/or competitive gain process in young developing athletes.
Keywords: Endocrine, Hypothalamic-pituitary-adrenal axis, Competition simulation, Genetics Stress
INTRODUCTION
The hypothalamic-pituitary-adrenal (HPA) axis and hypothalamic-pituitary-gonadal (HPG) axis are two major neuroendocrine systems that coordinate the release of cortisol (C) and testosterone (T) into blood circulation; both key signals involved in human development and expression of performance [1, 2, 3]. In sport, the stressors imposed by exercise, training and competition can promote acute and/or chronic changes in C (∆C) and T (∆T) concentrations [1, 4, 5, 6, 7, 8]. Such changes in the hormonal milieu help support the structural and/or functional development of different physiological systems (e.g., behavioural, cognitive, neuromuscular, energetic) that underpin athletic performance and training adaptation [1, 9], and on timescales reflecting both genomic and non-genomic actions [1].
One important feature of hormonal activation under stress is large individual variation. For example, the individual ∆C (e.g., -57% to 255%) and ∆T (e.g., -67% to 126%) differed considerably during Olympic weightlifting (OWL) competition [8, 10], despite exposure to similar physical and psychological loads. Personal factors can affect hormone release (e.g., age, life experiences, nutrition, training status) [1, 11]. Genetic factors are also potentially involved, as evidenced by twin similarities (intraclass correlation coefficients [ICC] >0.75) in the C response to exercise [12]. Others report some stability in the ∆C across physical and psychosocial stressors [13], and ∆T when performing different workouts [14]. These findings support the idea that C or T release under stress, if highly individual and stable over time, might reflect a phenotype that confers differential adaptations to identical stressors in sport [1].
Neuroendocrine studies endorse such a perspective, whereby the acute C responses to an experimental stressor predicted health-related trajectories in selected populations (e.g., police officers, soldiers) several months to years later [15, 16, 17]. Similarly, in team-sport competition, a relationship emerged between the ∆C and ∆T to a physical stressor and subsequent win-loss outcomes several days later [18, 19]. To our knowledge, no studies have assessed whether these biomarkers can predict individual performance in athletic competition over one or more years. The sport of OWL would provide an ideal experimental model, with competition producing heterogenous hormone responses and a quantifiable outcome (i.e., total load lifted). This research could add value in areas of talent identification, performance prognostics linked to biochemical changes, and individualisation of training programmes. Some control for time-of-day effects is however necessary, as the hormonal responses to strength-type exercise can vary across the day [4, 5].
This study investigated the serum C and T responses of young athletes to a simulated OWL competition, as predictive biomarkers of performance in real competition over two subsequent years. This timeframe was based on somewhat stable C responses to challenge (over 18 months) among children and youth [20], and similar results for athletes (ICC = 0.47) across real and simulated OWL competitions over a two-year period (unpublished data). Our first hypothesis was that C and T levels would rise across the simulated OWL competition, but with variable responses between athletes. The second hypothesis was that individual ∆C and/or ∆T would be related to changes in competitive performance at <12 and 12-24 months.
MATERIALS AND METHODS
Participants
Seventy-seven junior athletes were recruited for this study, but seven were removed from the final analysis due to a lack of follow-up data. The final cohort of males (n=46) and females (n=24) had a mean age (±SD) of 18.0±1.2 and 17.6±1.2 years, height of 173±8.4 and 163±6.8 cm, body mass (BM) of 77.6±17.0 and 65.9±17.3 kg, training experience of 4.4±1.1 and 4.4±0.8 years, and a personal best lift (combined total load) of 231±41.6 and 138±28.6 kg, respectively. Pre-screening revealed that the participants were healthy, injury-free, and not taking any doping agents. As registered athletes, they were routinely tested for illegal substances in and out of competition. One female did report using oral contraceptives, but her results were retained as they did not unduly bias any result. Given the testing format of this study (see below), we were unable to control for menstrual-phase differences between females, potentially affecting baseline T concentrations [21]. The expression of maximal strength does not appear to be influenced by menstrual phase [22, 23]. Written informed consent and parental consent were provided before study commencement. Ethical approval was provided by the Institute of Sport – National Research Institute, Poland.
Study design
A quasi-experimental design with prospective monitoring was used to address the study hypotheses. The athletes were invited to participate in a national talent identification and development programme for weightlifters in Poland, where testing replicated somewhat an OWL competition. This programme was scheduled over four consecutive days at the same indoor venue. Testing was conducted between 10 am and 1 pm to account for circadian variation in basal hormones, hormone responsiveness to exercise, and OWL performance itself [4, 5, 18, 24]. To ensure ecological validity, the athletes were instructed to maintain their normal dietary intake and to follow any established pre-competition routines (e.g., smelling salts, motivational feedback). They were also instructed to refrain from intense exercise (>2 days) before their assessment to reduce the confounding effects of muscle damage, oxidative and inflammatory responses [24, 25], and to get adequate sleep (>7 hours) to further eliminate any fatigue. As a national programme aimed at identifying and developing young talented weightlifters, we anticipated that some form of tapering schedule (e.g., a reduction in training volume) [8] would be adopted to ensure peak performance. The programme started within four days of the Polish age-group championships, so most athletes were still in the competitive phase of training.
Athlete testing was conducted in front of a small audience under International Weightlifting Federation rules (http://www.iwf.net/) with some modifications. Assessment began with a standard warm-up (15-20 minutes) using progressively heavier loads up to 90-93% of the first lift. Three single-lift trials were completed in the snatch and clean and jerk (CJ) exercises with the aim to lift the heaviest load possible. Between-trial rest periods were at least five minutes to reduce fatigue. For trained weightlifters, the snatch and CJ exercises are both highly reliable with coefficients of variation (CV) of <3% and ICC values >0.93. An independent judge was present to assess each lifting attempt as a success or failure. A failed attempt could be repeated by participants, but only if it occurred in the two initial trials. Weightlifting performance was indexed by the total combined load across the snatch and CJ exercises, as it determines the athlete’s placing in their weight class [9]. The total load lifted was also used to indicate current physical capacity, as it approached (96% on average) each participant’s personal best performance and both outcomes were strongly related (r=0.93).
Hormone testing
Two capillary blood samples were taken from each subject; before warming up and within five minutes of the last CJ attempt, equating to a sampling period of around 45 minutes. Logistical constraints (e.g., locality of room for blood collections) prevented us from sampling immediately after the last lift. Using a sterile lancet, a skin incision was made on the index finger of the non-dominant hand, after which a blood sample (~300 μL) was drawn and placed into a serum microvette (Greiner Vacuette, Germany) for clotting. After centrifugation, the supernatant was transferred to a labelled tube and stored at -80˚C for no longer than a month. The samples were assayed for T and C concentrations using enzyme-linked immunoassay kits (DRG, Germany). The lower detection limits for the T and C kits were 0.3 nmol·L-1 and 6 nmol·L-1, respectively. The CVs for duplicate samples were less than 4% and inter-assay kit CVs were less than 8%. For analysis, the pre- to post-competition ∆C and ∆T were calculated and then log transformed to approximate percentages.
Monitoring competitive performance
Over the next two years, we monitored athlete performance (to nearest 1 kg) and their BM (to nearest 0.1 kg) during registered OWL competitions using two internet resources; the International Weightlifting Results Project (http://www.iwrp.net) and a Polish OWL website (http://www.podnoszenieciezarow.pl/). All results were cross-referenced to check for correctness and missing data. To account for seasonal fluctuations in maximal strength, only the best OWL performance in each 12-month block was analysed. If achieved more than once, the load lifted at a lighter BM was selected, as it represents greater relative strength. Performance was divided by BM (i.e., kg⋅kg-1) to account for body size differences between weight classes and sex [8, 9]. Data were further expressed as a log-transformed change score at <12 (∆Total12) and 12-24 months (∆Total24) from initial testing, again to approximate percentages. Since athletes can vary in their competitive frequency, we also recorded the number of competitions entered over the 12-month (M=6.1, SD=2.9) and 24-month periods (M=11.4, SD=5.7), with the latter representing cumulative frequency.
Statistical analyses
All data were analysed using the R programming package. To assess hormone reactivity, we compared the ∆C and ∆T to a zero baseline and between sexes using a paired and unpaired T-test, respectively. Performance was examined with a two-way (Sex, Time) analysis of variance, followed by simple main effects and Tukey contrasts. Cohen’s effect sizes (ES) were also computed with 95% confidence intervals (CI), as follows; <0.2 = trivial, 0.2 to <0.5 = small, 0.5 to <0.8 = medium, 0.8+ = large [18]. To predict changes in future OWL performance, a three-step hierarchical regression was employed with the ∆Total12 and ∆Total24 entered as dependant variables. Control variables were included in step one, once identified through a stepwise selection process using the Bayesian information criterion. The control variables initially tested were participant age, sex (males=0, females=1), current physical capacity, training years (at best performance), and competitions entered. The ∆C and ∆T were added in step two, followed by the ∆C × ∆T interaction in step three. Diagnostic testing revealed that all statistical assumptions were met. Significance was set at p≤0.05 for all analyses.
RESULTS
The raw hormone (Table 1) and performance (Figure 1) results are presented to aid interpretation. We found no sex differences in the ∆C (p=0.494) or ∆T (p=0.937) across the simulated OWL competition. A small positive ∆C (ES=0.3 [95% CI 0.0, 0.6]) and ∆T (ES = 0.5 [95% CI 0.1, 0.8]) emerged when data were pooled, though only the T result was significant (Table 1). On an individual level, the C and T responses (as a % from baseline) ranged from -58% to 200% and -21% to 71%, respectively. Exploratory testing with partial correlations (controlling for sex) identified several correlates of the ∆C, but not ∆T, including training experience (r=-0.29), BM (r=0.25), pre-competition T (r=0.31) and C (r=-0.48).
TABLE 1.
Serum hormone concentrations before and after the simulated Olympic weightlifting competition in young male (n=46) and female (n=24) athletes. Data are presented as means±SD.
| Variable | Pre-competition | Post-competition | % change | p value | |
|---|---|---|---|---|---|
| Cortisol (nmol·L-1) | Combined | 435±170 | 493±198 | 11.2±60.5 | 0.064 |
| Males | 445±164 | 487±185 | |||
| Females | 414±182 | 505±226 | |||
| Testosterone (nmol·L-1) | Combined | 11.7±10.5 | 13.0±13.2 | 7.1±17.0 | 0.001 |
| Males | 16.8±9.50 | 18.8±12.9 | |||
| Females | 1.86±0.92 | 1.99±0.96 | |||
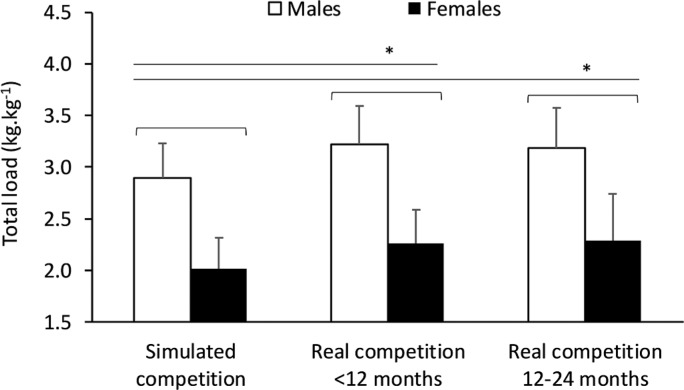
FIG. 1.
Olympic weightlifting performance in the simulated competition and best performance during real competitions over two years in young male (n=46) and female (n=24) athletes. Data are presented as means±SD. *Significant from the simulated competition p<0.01.
We observed a sex and time effect on OWL performance (both p<0.001), but no interaction (p=0.227). Males (3.10±0.39 kg·kg-1) had greater relative strength (p<0.001) than females (2.19±0.38 kg·kg-1) with a large ES difference of 2.4 (95% CI 2.0, 2.8). Simple testing for a time effect (p<0.001) with post-hoc contrasts revealed improvements (p<0.01) in the ∆Total12 (2.89±0.58 kg·kg-1) and ∆Total24 (2.88±0.0.59 kg·kg-1) from the simulated event (2.60±0.53 kg·kg-1). These differences represent large ES changes of 1.5 (95% CI 1.1, 1.8) and 0.93 (95% CI 0.60, 1.30), or gains of 11.4±7.7% and 10.8±11.6%, respectively. The change in performance did not differ significantly between the two follow-up periods (ES=-0.1 [95% CI -0.4, 0.3]). The individual performance changes in each 12-month block were strongly and positively correlated (r=0.71, p<0.001) when controlling for sex.
The regression models are depicted in Table 2. To aid interpretation, all coefficients have been back-transformed into percentages. In step one, only competitions entered was selected as a control variable, explaining 13% and 17% of variation in the ∆Total12 and ∆Total24, respectively. In step two, the ∆C contributed to the ∆Total12 (p=0.025), whereby a 1% increase in C (as a log value) predicted a 0.04% decline in performance from the predicted group mean (~8%) when controlling for competitions entered. Overall model fit tended to improve, but this was not statistically significant (p=0.069). The ∆C in step two also contributed to the ∆Total24 (p=0.006), such that a 1% increase in C predicted a 0.07% drop in performance from the mean response (~2%), whilst improving model fit by 6.8% (p=0.022). The ∆C × ∆T interactions (in step 3) were not significant predictors of performance when accounting for all other variables, nor did they enhance the fitted models (p>0.505).
TABLE 2.
Regression models predicting changes in Olympic weightlifting performance in real competitions.
| Models | Dependent variable: ΔTotal12 |
||||||
|---|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | ||
| Step 1 | |||||||
| Intercept | 0.07# | 0.04, 0.10 | 0.08# | 0.05, 0.12 | 0.08# | 0.05, 0.12 | |
| Competitions | 0.01** | 0.00, 0.02 | 0.01** | 0.00, 0.02 | 0.01** | 0.00, 0.02 | |
| Step 2 | |||||||
| ΔT | -0.04 | -0.13, 0.07 | -0.04 | -0.13, 0.07 | |||
| ΔC | -0.04* | -0.07, -0.01 | -0.03 | -0.07, 0.00 | |||
| Step 3 | |||||||
| ΔC × ΔT | -0.06 | -0.24, 0.16 | |||||
| Model fit | |||||||
| R2 | 0.127* | 0.172** | 0.163** | ||||
| ΔR2 | 0.044 | -0.008 | |||||
| Models | Dependent variable: ΔTotal24 |
||||||
|---|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | ||
| Step 1 | |||||||
| Intercept | 0.00 | -0.05, 0.06 | 0.02 | -0.04, 0.07 | 0.02 | -0.04, 0.07 | |
| Competitions | 0.01# | 0.01, 0.02 | 0.01# | 0.01, 0.02 | 0.01# | 0.01, 0.02 | |
| Step 2 | |||||||
| ΔT | -0.02 | -0.15, 0.14 | -0.01 | -0.15, 0.14 | |||
| ΔC | -0.07** | -0.11, -0.02 | -0.07** | -0.12, -0.02 | |||
| Step 3 | |||||||
| ΔC × ΔT | 0.11 | -0.18, 0.49 | |||||
| Model fit | |||||||
| R2 | 0.172# | 0.241# | 0.234# | ||||
| ΔR2 | 0.068* | -0.006 | |||||
Key: ∆Total12 = change in performance at 12 months, ∆Total24 = change in performance at 12-24 months, ∆C = change in cortisol from pre- to post-competition, ∆T = change in testosterone from pre- to post-competition. All coefficients have been back-transformed into percentages for interpretability. Significant at *p<0.05, **p<0.01, #p<0.001.
To test the robustness of our results, sensitivity analyses were conducted by removing the control variable and retesting the ∆T and ∆C in a stepwise manner. This procedure confirmed the ∆C as the only hormonal contributor to ∆Total12 (B=-0.05 [95% CI -0.08, -0.01], R2=8.6%, p=0.008) and ∆Total24 (B=-0.07 [95% CI -0.12, -0.02], R2=9.1%, p=0.006). We repeated these procedures with performance in the simulated OWL competition, as the dependant variable, and two control variables (i.e., gender, training experience). The ∆C and/or ∆T were not related to performance (p>0.136) at any stage of analysis. Further testing also revealed no association between pre-competition C or T concentrations and OWL performance (p>0.475).
DISCUSSION
This study examined whether the acute T and C responses of young athletes, measured in a simulated competitive environment, would prospectively predict future performance in real competitions. Consistent with our first hypothesis, serum C and T concentrations increased (small ES) during initial testing with evidence of large individual variability. Weightlifting performance also improved (large ES) within a year and this level of performance was maintained in year two. Aligning to our second hypothesis, the individual C under competitive stress contributed to some variance in future OWL performance.
The study participants experienced a small positive ∆C (11%) and ∆T (7%) in the simulated OWL event. Unpublished data from another OWL cohort (n=9) revealed a plasma volume shift of -14±3% in a comparable event; thus, our results could be partly attributed to a haemoconcentration effect. This cannot, however, explain the heterogeneity observed in this and other OWL studies [8, 10, 26]. Both hormones were unrelated (r<0.12) to the total load lifted and, given that participants were habituated to training and competition, we can rule out any familiarisation effect. Heritable and reliable differences in HPA-axis reactivity to stress offers one explanation [12, 13, 20], with corroborating reliability data (∆C ICC=0.47) on adults competing in OWL events over two years (unpublished). Since the ∆C was related to pre-competition C and T concentrations in this work, more complex feedback mechanisms involving the HPG- and HPA-axes appear to be involved. Further research is still needed on younger athletes to assess hormone reliability across different stressors and time periods. In addition, other factors can affect stress-induced hormone reactivity (e.g., early life experiences, personality traits, social support, nutrition, training status) [1, 11]; thus, their assessment would add value when attempting to explicate the drivers of individual stress responses.
Weightlifting performance improved considerably (~11%) within 12 months, especially when compared to gains (<3%) reported in other OWL studies [26, 27], before stabilising at 12-24 months. This difference can be attributed to athlete monitoring across a developmental period associated with normal gains in muscle size and strength [28, 29]. Notably, the ∆C was related (negatively) to the ∆Total12 and ∆Total24 after controlling for other variables and when assessed in isolation via sensitivity analyses. Cortisol often correlates with OWL performance [7] and maximal strength [6, 30], with recent work showing that mid-week ∆C can discriminate win-loss outcomes in professional rugby matches [18]. In other domains, the C response to an experimental stressor predicted health indices (e.g., resilience, depressive symptoms) on follow-up periods of six [17], 12 [16] and 48 months [15]. Whilst interpretation is complicated by other factors (e.g., stressor intensity, population tested), including both adaptive and maladaptive outcomes, this work highlights the potential of this dynamic C measure in predicting health-related trajectories. Given that performance did not improve further after 12 months in this study, and the positive correlation between individual trajectories in both follow-up periods, hormonal assessment (under competitive conditions) at a two-yearly interval would be a feasible approach for athlete testing.
Maximum growth rate amongst young athletes generally occurs at 12-15 years of age, before gradually declining into late adolescence [3]. Our BM data paralleled this trend, increasing within 12 months before stabilising at 12-24 months, as did OWL performance when normalised for BM. Hence, both neurological and morphological pathways are likely contributors to the observed strength changes [27, 31], potentially arising from training factors and/or natural maturation processes. Different maturity features (e.g., muscle size, sexual development) are thought to be controlled by the HPG- and HPA-axes [3]; thus, it’s conceivable that acute C responsivity to a stressor might operate as an intermediate signal that reflects adaptive potential during a key developmental period. Alternatively, the ∆C could reflect prior training exposure, as it correlated with training experience at study onset, and one that carries over to future gains (or losses) in competitive OWL performance. Others suggest that individual C responses might reflect differential adaptations to sport-related [1] or broader life stressors [17], or it could represent a preparative action (of glucocorticoids) for impending stress [2].
It was somewhat surprising that the ∆T was a poor predictor of the ∆Total12 and ∆Total24. This may well be a function of more complex release patterns. The release of T prior to, and after, a competitive encounter is thought to depend on social factors (e.g., mood, outcome anticipation) [32] driven by personal expectations, situational and environmental cues. As such, the utility of T could be realised over a shorter (3-4 days) time span in competitive sport [19]. Conversely, T might have some prognostic value of physical efforts over longer timeframes (i.e., a rugby game) when dominant behaviours are required [33], rather than brief maximal strength-based activities like OWL, where neural factors like intra- and inter-muscular coordination might predominate [31]. No performance linkages emerged when testing the ∆C × ∆T interaction. This interplay is perhaps a feature of acute settings where hormones are thought to contribute to flexible adjustments in OWL performance [10] and, to date, has only been demonstrated in male cohorts [10, 34].
Recent work on weightlifters highlighted the importance of muscle damage (e.g., creatine kinase), oxidative (e.g., malondialdehyde), and inflammatory (e.g., C-reactive protein) markers in describing exercise stress, as well as interactions between these biomarkers [24, 25]. The interrelationship between these outcomes and competition-induced activation of the HPG- and HPA-axes, including possible time-of-day effects on hormones and OWL performance [4, 5, 24], are worthwhile pursuits to help explain our results. The protocols used to simulate an OWL competition is another consideration when interpreting our results. Some modifications were necessary to accommodate the large number of participants within a short time span. For example, to ensure compliance and increase our sample size, participants were assessed as they became available. Hence, we tested small groups who may differ in weight category, which required different starting and incremental loads. For convenience athletes were also tested in a set order, irrespective of successes or failures, thereby contributing to a slightly longer (5-minute) rest period between the final two trials per exercise.
Other study limitations must be recognised, for example, the regression models only explained a small proportion of OWL performance. Moreover, we did not have access to athlete training loads and training structure over the experimental period, nor other information (e.g., dietary intake, illness or injuries) that could illuminate the unexplained variance. Still, profiling individual C dynamics might provide a useful adjunct to available training information, when up to 9% of performance variability could be explained by this biomarker. Sex-related differences in T production and muscle strength trajectories [28, 29] are other confounds in longitudinal studies on athletes, which was partly addressed by examining percentage changes in the study outcomes. Psychological factors (e.g., motivation, self-esteem) might also predict OWL performance [35], and we were unable to control for menstrual-related T fluctuations and its impact on female motivation [21]. Conversely, the basal and exercise activation of C is unaffected by the menstrual cycle [36, 37].
CONCLUSIONS
In summary, the serum C and T responses to a simulated OWL competition showed considerable variability between young athletes. Cohort monitoring over the next two years revealed improvements in competitive OWL performance and the individual ∆C predicted a small proportion of performance trajectories. This suggests that individual variation in C responsiveness to a competitive stressor may forecast, in some capacity, the training process and/or competitive outcomes of young developing athletes.
Acknowledgements
We acknowledge with gratitude the athletes and coaching staff who contributed to this project. This study was funded by the Institute of Sport – National Research Institute with in-kind support from the Polish Weightlifting Association.
Conflicts of interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Crewther BT, Cook C, Cardinale M, Weatherby RP, Lowe T. Two emerging concepts for elite athletes: The short-term effects of testosterone and cortisol on the neuromuscular system and the dose-response training role of these endogenous hormones. Sports Med. 2011;41(2):103–23. doi: 10.2165/11539170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM, Romero ML, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 3.Malina RM, Rogol AD, Cumming SP, Coelho e Silva MJ, Figueiredo AJ. Biological maturation of youth athletes: assessment and implications. Brit J Sport Med. 2015;49(13):852–9. doi: 10.1136/bjsports-2015-094623. [DOI] [PubMed] [Google Scholar]
- 4.Sedliak M, Finni T, Cheng S, Kraemer WJ, Häkkinen K. Effect of time-of-day-specific strength training on serum hormone concentrations and isometric strength in men. Chronobiol Int. 2007;24(6):1159–77. doi: 10.1080/07420520701800686. [DOI] [PubMed] [Google Scholar]
- 5.Chtourou H, Hammouda O, Aloui A, Chaabouni K, Makni-Ayedi F, Wahl M, et al. The effect of time of day on hormonal responses to resistance exercise. Biol Rhythm Res. 2014;45(2):247–56. [Google Scholar]
- 6.Argus CK, Gill ND, Keogh JWL, Hopkins WG, Beaven MC. Changes in strength, power, and steroid hormones during a professional rugby union competition. J Strength Cond Res. 2009;23(5):1583–92. doi: 10.1519/JSC.0b013e3181a392d9. [DOI] [PubMed] [Google Scholar]
- 7.Passelergue P, Robert A, Lac G. Salivary cortisol and testosterone variations during an official and a simulated weight-lifting competition. Int J Sports Med. 1995;16(5):298–303. doi: 10.1055/s-2007-973009. [DOI] [PubMed] [Google Scholar]
- 8.Crewther B, Obminski Z, Cook C. The effect of steroid hormones on the physical performance of boys and girls during an Olympic weightlifting competition. Pediatr Exerc Sci. 2016;28(4):570–87. doi: 10.1123/pes.2016-0070. [DOI] [PubMed] [Google Scholar]
- 9.Storey A, Smith HK. Unique aspects of competitive weightlifting: performance, training and physiology. Sports Med. 2012;42(9):769–90. doi: 10.1007/BF03262294. [DOI] [PubMed] [Google Scholar]
- 10.Crewther BT, Obmiński Z, Cook CJ. Serum cortisol as a moderator of the relationship between serum testosterone and Olympic weightlifting performance in real and simulated competitions. Biol Sport. 2018;35(3):215–21. doi: 10.5114/biolsport.2018.74632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Di Luigi L, Guidetti L, Baldari C, Romanelli F. Heredity and pituitary response to exercise-related stress in trained men. Int J Sports Med. 2003;24(8):551–8. doi: 10.1055/s-2003-43266. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Petrides JS, Gold PW, Chrousos GP, Deuster PA. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J Clin Endocrinol Metab. 1999;84(6):1944–8. doi: 10.1210/jcem.84.6.5746. [DOI] [PubMed] [Google Scholar]
- 14.Jensen J, Oftebro H, Breigan B, Johnsson A, Ohlin K, Meen HD, et al. Comparison of changes in testosterone concentrations after strength and endurance exercise in well trained men. Eur J Appl Physiol Occ Physiol. 1991;63(6):467–71. doi: 10.1007/BF00868080. [DOI] [PubMed] [Google Scholar]
- 15.Galatzer-Levy IR, Steenkamp MM, Brown AD, Qian M, Inslicht S, Henn-Haase C, et al. Cortisol response to an experimental stress paradigm prospectively predicts long-term distress and resilience trajectories in response to active police service. J Psychiatr Res. 2014;56:36–42. doi: 10.1016/j.jpsychires.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steudte-Schmiedgen S, Stalder T, Schwönfeld S, Wittchen HU, Trautmann S, Alexander N, et al. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology. 2015;59:123–33. doi: 10.1016/j.psyneuen.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: Social-evaluative threat and prior depressive episodes as moderators. J Affect Disord. 2012;143:223–30. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crewther BT, Potts N, Kilduff LP, Drawer S, Cook CJ. Can salivary testosterone and cortisol reactivity to a mid-week stress test discriminate a match outcome during international rugby union competition? J Sci Med Sport. 2018;21:312–6. doi: 10.1016/j.jsams.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Crewther BT, Sanctuary CE, Kilduff LP, Carruthers JS, Gaviglio CM, Cook CJ. The workout responses of salivary-free testosterone and cortisol concentrations and their association with the subsequent competition outcomes in professional rugby league. J Strength Cond Res. 2013;27(2):471–6. doi: 10.1519/JSC.0b013e3182577010. [DOI] [PubMed] [Google Scholar]
- 20.Hankin BL, Badanes LS, Smolen A, Young JF. Cortisol reactivity to stress among youth: stability over time and genetic variants for stress sensitivity. J Abnormal Psychol. 2015;124(1):54–67. doi: 10.1037/abn0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook CJ, Kilduff LP, Crewther BT. Basal and stress-induced salivary testosterone variation across the menstrual cycle and linkage to motivation and muscle power. Scand J Med Sci Sport. 2018;28(4):1345–53. doi: 10.1111/sms.13041. [DOI] [PubMed] [Google Scholar]
- 22.Lebrun CM, McKenzie DC, Prior JC, Taunton JE. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc. 1995;27(3):437–44. [PubMed] [Google Scholar]
- 23.Fridén C, Hirschberg AL, Saartok T. Muscle strength and endurance do not significantly vary across 3 phases of the menstrual cycle in moderately active premenopausal women. Clin J Sports Med. 2003;13(4):238–41. doi: 10.1097/00042752-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Ammar A, Chtourou H, Trabelsi K, Padulo J, Turki M, El Abed K, et al. Temporal specificity of training: intra-day effects on biochemical responses and Olympic-Weightlifting performances. J Sports Sci. 2015;33(4):358–68. doi: 10.1080/02640414.2014.944559. [DOI] [PubMed] [Google Scholar]
- 25.Ammar A, Chtourou H, Hammouda O, Turki M, Ayedi F, Kallel C, et al. Relationship between biomarkers of muscle damage and redox status in response to a weightlifting training session: effect of time-of-day. Physiol Int. 2016;103(2):243–61. doi: 10.1556/036.103.2016.2.11. [DOI] [PubMed] [Google Scholar]
- 26.Häkkinen K, Keskinen KL, Alén M, Komi PV, Kauhanen H. Serum hormone concentrations during prolonged training in elite endurance-trained and strength-trained athletes. Eur J Appl Physiol Occ Physiol. 1989;59(3):233–8. doi: 10.1007/BF02386193. [DOI] [PubMed] [Google Scholar]
- 27.Häkkinen K, Pakarinen A, Alen M, Kauhanen H, Komi PV. Neuromuscular and hormonal adaptations in athletes to strength training in two years. J Appl Physiol. 1988;65(6):2406–12. doi: 10.1152/jappl.1988.65.6.2406. [DOI] [PubMed] [Google Scholar]
- 28.Round JM, Jones DA, Honour JW, Nevill AM. Hormonal factors in the development of differences in strength between boys and girls during adolescence: a longitudinal study. Ann Hum Biol. 1999;26(1):49–62. doi: 10.1080/030144699282976. [DOI] [PubMed] [Google Scholar]
- 29.Ramos E, Frontera WR, Llopart A, Feliciano D. Muscle strength and hormonal levels in adolescents: Gender related differences. Int J Sports Med. 1998;19(8):526–31. doi: 10.1055/s-2007-971955. [DOI] [PubMed] [Google Scholar]
- 30.Crewther BT, Heke TOL, Keogh JWL. The effects of two equal-volume training protocols upon strength, body composition and salivary hormones in male rugby union players. Biol Sport. 2016;33:111–6. doi: 10.5604/20831862.1196511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 2007;37(2):145–68. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 32.Chichinadze K, Lazarashvili A, Chichinadze N, Gachechiladze L. Testosterone dynamics during encounter: role of emotional factors. J Comp Physiol A. 2012;198(7):485–94. doi: 10.1007/s00359-012-0726-1. [DOI] [PubMed] [Google Scholar]
- 33.Gaviglio CM, Crewther BT, Kilduff LP, Stokes KA, Cook CJ. Relationship between pregame concentrations of free testosterone and outcome in rugby union. Int J Sports Physiol Perform. 2014;9:324–31. doi: 10.1123/ijspp.2013-0106. [DOI] [PubMed] [Google Scholar]
- 34.Crewther BT, Thomas AG, Stewart-Williams S, Kilduff LP, Cook CJ. Is salivary cortisol moderating the relationship between salivary testosterone and hand-grip strength in healthy men? Eur J Sport Sci. 2017;17(2):188–94. doi: 10.1080/17461391.2016.1220628. [DOI] [PubMed] [Google Scholar]
- 35.Mahoney MJ. Psychological predictors of elite and non-elite performance in Olympic weightlifting. Int J Sport Psychol. 1989;20(1):1–12. [Google Scholar]
- 36.Boisseau N, Enea C, Diaz V, Dugué B, Corcuff JB, Duclos M. Oral contraception but not menstrual cycle phase is associated with increased free cortisol levels and low hypothalamo-pituitary-adrenal axis reactivity. J Endocrinol Invest. 2013;36(11):955–64. doi: 10.3275/8971. [DOI] [PubMed] [Google Scholar]
- 37.Kirschbaum C, Platte P, Pirke KM, Hellhammer DH. Adrenocortical activation following stressful exercise: Further evidence for attenuated free cortisol responses in women using oral contraceptives. Stress Med. 1996;112:137–43. [Google Scholar]