Abstract
The aim of this study was to evaluate the acute effect of aerobic (AER) and eccentric (ECC) exercise on glucose variability, correlating it with circulating markers of inflammation and oxidative stress in healthy subjects. Sixteen healthy subjects (32 ± 12 years old) wore a continuous glucose monitoring system for three days. Participants randomly performed single AER and ECC exercise sessions. Glucose variability was evaluated by glucose variance (VAR), glucose coefficient of variation (CV%) and glucose standard deviation (SD). Blood samples were collected to evaluate inflammatory and oxidative stress markers. When compared with the pre-exercise period of 0-6 h, all the indices of glucose variability presented comparable reductions 12-18 h after both exercises (∆AER: VAR= 151.5, ∆CV% = 0.55 and ∆SD = 3.1 and ECC: ∆VAR = 221.2 , ∆CV% = 3.7 and ∆SD = 6.5). Increased interleukin-6 (IL-6) levels after AER (68.5%) and ECC (30.8%) (P<0.001) were observed, with no differences between sessions (P = 0.459). Uric acid levels were increased after exercise sessions (3% in AER and 4% in ECC, P = 0.001). In conclusion, both AER and ECC exercise sessions reduced glucose variability in healthy individuals. Inflammatory cytokines, such as IL-6, and stress oxidative markers might play a role in underlying mechanisms modulating the glucose variability responses to exercise (clinicalTrials.gov NCT02262208).
Keywords: Glucose variability, Oxidative stress, Inflammation, Acute exercise aerobic, Acute exercise eccentric
INTRODUCTION
The treatment of hyperglycaemia in diabetes mellitus aiming at glycated haemoglobin (HbA1c) lower than 7% is recommended, reducing rates of acute and chronic complications of the disease [1]. While hyperglycaemia indicates sustained high levels of glucose, it is relevant to discuss glucose fluctuations, which may also be related to the pathogenesis of diabetic complications [2]. In addition, several consequences of excess glucose variability support this relationship ultimately due to excessive oxidative stress. Indeed, compared with the constant high glucose, the 24-h urinary excretion rates of free 8-iso prostaglandin F2a (8-iso PGF2), a stress oxidative marker, are increased when glucose oscillation is high, [3]. Furthermore, urinary excretion rates of 8-iso PGF2 are not correlated with insulin levels, HbA1c and plasma glucose, but are positively correlated with one of the glucose variability indices, MAGE [4]. However, not all studies have shown a relationship between glucose variability and 8-iso PGF2 [5-6]. Glucose variability, moreover, is a complex phenomenon that includes intraday and interday variability and also a combination of minor and major glucose fluctuations.
Although primarily studied in patients with diabetes, glucose variability has been explored in other settings, such as obesity, metabolic syndrome [7], cystic fibrosis [8] and also in healthy subjects [9]. Individuals with diabetes present higher indices of glucose variability when compared to controls without the disease, whereas in the population without diabetes age-related increases in glucose variability have been shown [10]. Therefore, the development of diabetes may be affected by a progressive loss of complexity in glucose profile, probably reflecting insulin resistance and a progressive failing of glucoregulation. Importantly, the loss of complexity may anticipate the hyperglycaemic states [7]. Insulin resistance and inflammation were suggested to be involved in the generation of these fluctuations, reflecting a possible relationship between specific physiological mechanisms and mathematical characteristics of glucose variation [11].
On the other hand, exercise is an efficacious non-pharmacological intervention for type 2 diabetes [12]. Even in non-diabetic subjects, physical activity interventions are associated with reduced HbA1c [13]. We have recently shown that acute bouts of aerobic or combined (aerobic and resistance) exercise can reduce glucose variability in patients with type 2 diabetes [14-15]. However, possible mechanisms involved in glucose variability changes induced by different types of exercises are still unknown.
In healthy subjects, increased glucose variability is observed during inactivity after a meal [16], reinforcing the role of exercise in changing glucose patterns irrespective of the absence of hyperglycaemia. Also, circulating markers of oxidative stress and inflammation, which can be influenced by different exercise protocols in healthy subjects [17], have been previously related to changes in glucose variability in type 2 diabetes [18,19]. We therefore hypothesized that different types of exercise modalities could have a distinct impact on glucose variability, and that changes in oxidative stress and inflammation could be involved in these responses.
In this way, we aimed to assess glucose variability in healthy subjects through glucose variance (VAR), the glucose coefficient of variation (CV%) and glucose standard deviation (SD), and correlated them with circulating markers of oxidative stress and inflammation at rest and after aerobic (AER) and eccentric (ECC) exercise sessions.
MATERIALS AND METHODS
Research Design and Participants
Sixteen healthy subjects participated in the experiments in a randomized crossover trial design. Exclusion criteria were regular practice of exercise (more than three exercise sessions per week), or having any chronic disease, mainly diabetes mellitus, hypertension, heart failure and cancer. At the entry of the study, clinical characteristics, self-reported usual physical activity (International Physical Activity Questionnaire – IPAQ; long-form) [20], anthropometric evaluation, and a 12-h fasting blood sample (glucose, HbA1c) were obtained for each subject. One week prior to the first experimental exercise session, subjects underwent maximal cardiopulmonary exercise testing and maximal strength testing.
Ethics
The protocol was approved by the Institutional Review Board at Hospital de Clínicas de Porto Alegre (Number: 120148) and all patients provided their written informed consent before participation.
Maximal Cardiopulmonary Exercise Testing
The maximal incremental exercise test was performed on an electrically braked cycle ergometer (ER-900, Jaeger, Würzburg, Germany) with increments of 20 W/minute, as previously described [21] During the test, gas exchange variables were measured by a previously validated system (Oxycon Delta, VIASYS, Healthcare GmbH, Jaeger, Germany). Heart rate was continuously monitored by a 12-lead electrocardiogram (Nihon Kohden Corporation, Japan) and blood pressure was measured with an automatic oscillometric device every 2 min.
Strength Testing
Strength was measured by one-repetition maximum test (1-RM), which was preceded by exercises at mild intensity for movement familiarization and warm-up. Proper technique was demonstrated and practised for the leg press exercise (Sculptor, Porto Alegre, Brazil). When new attempts were needed, a 5-min resting period was allowed between successive attempts [22].
Randomization
Subjects were randomly assigned to two experimental sessions according to a computer-generated sequence, which was handled by a researcher who was not involved in interventions. Such a process occurred once before subjects were recruited to the study, indicating the order of both 40-min sessions of AER (n = 16) and ECC (n = 16) exercise sessions, which were performed at least seven days apart.
Exercise Protocols
Two exercise sessions, consisting of either AER or ECC protocols, were executed by all participants. Exercise intensity was recorded for each individual by a heart rate monitor (Polar F1 TM, Polar Electro Oy, Helsinki, Finland), and a Borg 0-10 scale was used to register individuals’ perceived exertion every five minutes throughout the experimental sessions. In the AER session, subjects exercised on a cycle ergometer (Embreex 360, Brusque, Brazil). Each session included a 5-min warm-up at 20 W, followed by 40 min at 70% of the VO2peak (peak oxygen uptake per kilogram, ml · kg-1 · min- 1), as determined in the incremental exercise test, and 5 min of stretching exercise as cool down. In the ECC session, subjects initially performed a specific warm-up of 15 repetitions on the leg press. Thereafter, the main part of this session was conducted during 40 min, in which subjects completed six eccentric sets of 10 repetitions at 120% of 1-RM for each leg (the exercise was one-sided), with a completely unloaded concentric phase (lasting approximately two seconds), and 2-second eccentric phase. Resting between sets and exercises lasted 2 min.
Continuous Glucose Monitoring System (CGMS) Measurements
Subjects were admitted to the laboratory in the morning at approximately 9:00 a.m., 24 h before the exercise session, when a glucose sensor (Sof-Sensor, Guardian REAL-Time System/Medtronic, Northridge, USA) was inserted subcutaneously. This procedure is fully described elsewhere [15]. For each experimental session, glucose profiles were collected sequentially during three days: (i) previous to the allocated exercise session, (ii) on the exercise day, and (iii) the day following the exercise bout. Each sensor was not used longer than 72 h and subjects were oriented to avoid exercise workouts except for the protocol.
Glucose Variability Evaluation: Glucose variability was assessed from series of absolute values of glucose, obtained by CGMS, sampled every 5 minutes. The first set period for analysis was obtained 6 h before each exercise onset and was compared with a set periods in a 6-hour timeframe obtained until 18 h after each of the allocated exercise sessions. Glucose variability was evaluated using conventional analysis, constructed from the statistical properties of the series, obtaining the indices VAR, CV% and SD in each period [23-24].
Blood Collection: Venous blood was drawn 10 min before and immediately after each exercise bout. Approximately 9 mL of blood was collected from an antecubital vein using a disposable needle and vacutainer containing sodium heparin or ethylene diamine tetra-acetic acid (EDTA). The blood was centrifuged for 10 min at 1000g to separate plasma and evaluate total protein sulfhydryl and protein carbonyl; for interleukin-6 it was centrifuged for 15 min at 1500g and then stored at -80°C for further analysis.
Total Protein Sulfhydryl: Briefly, 45 μL of plasma was mixed with 120 μL of PBS and 35 μL of 30 mM Tris/3 mM EDTA (pH 8) in a microplate well. After reading baseline absorbances (412 nm), samples were reacted with 10 μL of 5,5′-dithiobis-(2-nitrobenzoic acid) (10 mM in ethanol) for one hour. Samples were read again (412 nm) and baseline absorbances were discounted. The obtained values were compared with those obtained with a cysteine standard curve and results were expressed as nmol –SH/mg protein [25].
Protein carbonyl: Briefly, protein content in samples was determined with the Bradford method, using a commercial kit (Bio-Rad, cod. 500-0001). A sample containing 1 mg of protein was reacted with 10 mM 2,4-dinitrophenylhydrazine for 30 min and subjected to protein precipitation with 10% TCA followed by centrifugation (11,000 xg, 3 min, 4°C). The pellet was washed with ethanol: ethyl acetate (1:1) and centrifuged (11,000 xg, 3 min, 4°C) three times. After, pellets were suspended in 6 M guanidine hydrochloride (in 20 mM KH2PO4, pH 2.4) and read in a spectrophotometer (380 nm). Blank samples reacted with 2 M HCl instead of 10 mM 2,4-dinitrophenylhydrazine and were run in parallel. Results were expressed as nmol carbonyl/mg protein [26].
Interleukin-6 (IL-6): The commercially available Human Ultrasensitive IL-6 Magnetic Bead Kit was used according to the manufacturer’s instructions (Life Technologies, catalogue number: LHC0063M, Carlsbad, United States), and data were collected using the Luminex (Carlsbad, United States).
Uric Acid: The enzymatic colorimetric method in plasma was used in analyses (Roche/Hitachi Cobas c 311, Cobas c 501/502, Mannheim, Germany).
FRAP (Ferric Reducing Ability of Plasma): The FRAP assay is able to detect non-enzymatic antioxidants present in plasma, based on the plasma mediated ferric reduction. Briefly, 7 μL of plasma was mixed with 18 μL of distilled water and reacted with 175 μL of FRAP reagent for 10 min. FRAP reagent was prepared as required by mixing 10 mL acetate buffer (300 mM, pH 3.6), 1 mL 2,4,6-Tris(2-pyridyl)-s-triazine (10 mM in 40 mM HCl), and 1 mL 20 mM FeCl3. A standard curve was built out of Fe(II) and run in parallel and absorbances were read at 593 nm. Results are expressed as mmol/L of plasma [27].
Statistical Analysis
Descriptive data are presented as mean and SD or SE, or median and interquartile range (P25-P75). Whenever we use delta values in results descriptions, such numbers were derived by the post values subtracted from pre values in each exercise session. Given that most variables presented skewed data distributions, responses to the interventions were compared by generalized estimating equations (GEE), and multiple comparisons were performed with the Bonferroni correction. In our exploratory analyses, we used Spearman correlation, testing the association between either inflammatory (IL-6) or oxidative (sulfhydryl; carbonyl; uric acid; FRAP) biomarkers with deltas of glucose variability indices. Statistical significance was accepted when P<0.05. Version 20.0 of SPSS software was used for statistical analysis.
RESULTS
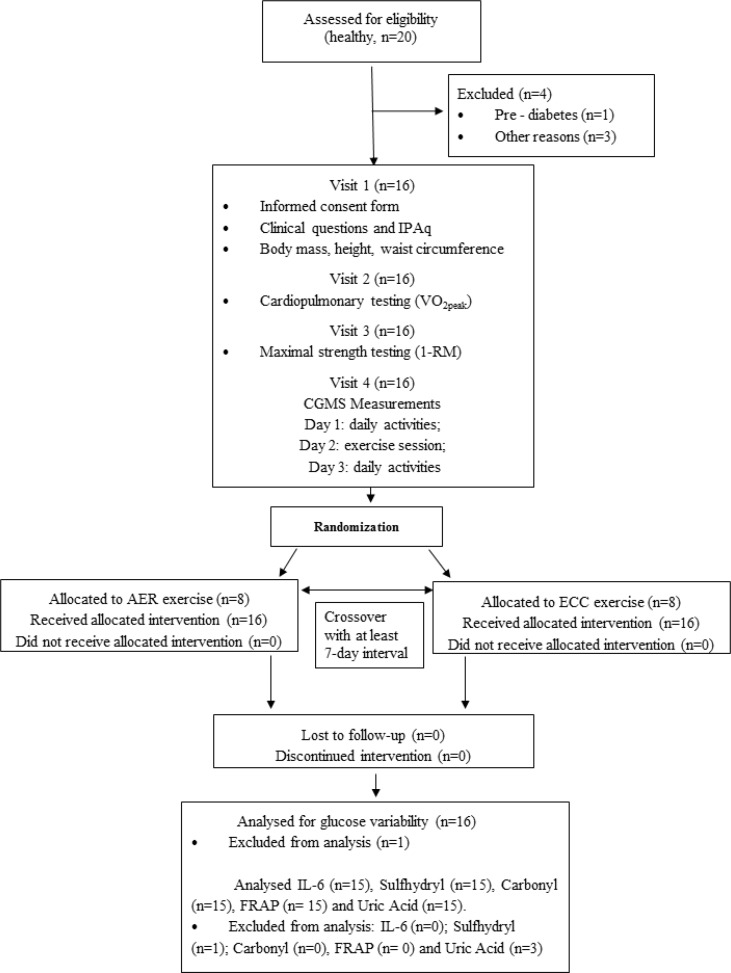
Twenty healthy subjects were screened for participation. One subject was excluded because pre-diabetes was diagnosed and three others withdrew for personal reasons including engagement in a physical activity programme and time constraints. Then, 16 subjects were included to participate in protocols and one was excluded from the analysis because of insufficient CGMS data. Demographic and clinical characteristics of participants are shown in Table 1. Patients were 32 ± 12 years old, predominantly women. Eight subjects were classified as insufficiently active, four were sufficiently active, while three were classified as very active. A flow diagram of the sample selection and study conduction is shown in Fig. 1.
TABLE 1.
Baseline characteristics of the healthy volunteers studied.
| No. of men/women | 6/9 |
| Age (yr) | 32 ± 12 |
| Anthropometrics | |
| Body weight (kg) | 69 ± 9 |
| Body mass index (kg /m2) | 23.8 ± 2.8 |
| Waist circumference (cm) | 85 ± 11 |
| Blood pressure (mmHg) | |
| Systolic | 113 ± 15 |
| Diastolic | 68 ± 10 |
| Heart rate (bpm) | 75 ±12 |
| HbAlc (%) | 5.0 ± 0.3 |
| Fasting blood glucose (mg/dl) | 84 ± 8 |
| Physical activity level (IPAQ), n (%) | |
| Insufficiently active | 8 (53) |
| Sufficiently active | 4 (27) |
| Very active | 3 (20) |
| VO2 peak (ml/kg/min) | 29.7 ± 5.6 |
| Heart rate peak (bpm) | 175 ± 13 |
| RERpeak | 1.2 ± 0.1 |
| Maximal strength testing (1-RM), kg | |
| Leg extension (right) | 60 ± 15 |
| Leg extension (left) | 59 ± 14 |
HbA1c: glycated hemoglobin; VO2peak: peak oxygen uptake per kilogram of body weight/fat-free mass; RERpeak: peak respiratory exchange ratio. **Data are expressed as mean ± SD. Categorical variables are presented as numbers (%).
FIG. 1.
Flow diagram.
Note: International Physical Activity Questionnaire (IPAQ); Continuous glucose monitoring system (CGMS); aerobic (AER) eccentric (ECC) exercise; ferric reducing ability of plasma (FRAP); interleukin-6 (IL-6).
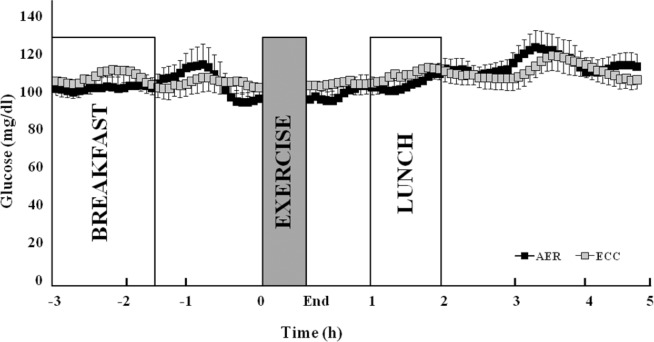
Both AER and ECC sessions elicited increases in heart rate and systolic blood pressure (P < 0.001), but no changes were observed in glucose levels (CGMS, average of 30 min before vs. 30 min after the exercise session, P= 0.859).
Fig. 2 presents absolute glucose levels evaluated by CGMS before and after both sessions. There were no changes in glucose levels in the first 5 h (Fig. 2) and also until the 18th hour after both exercise sessions (data not shown).
FIG. 2.
Absolute glucose levels (CGMS), detailed in the first 3 h and 5 h after aerobic (AER) and eccentric (ECC) exercise.
Note: data are reported as mean ± SEM. Generalized estimating equations (GEE); Bonferroni correction.
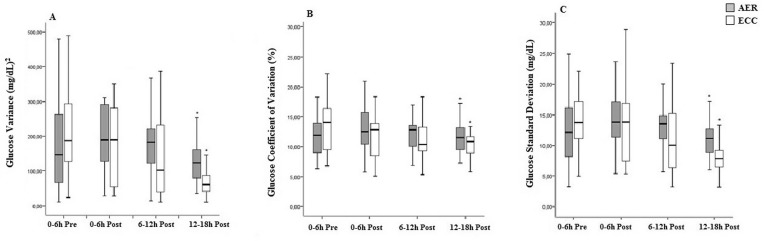
Fig. 3 shows the results of glucose variability, as evaluated by VAR, CV% and SD. When compared with the pre-exercise period of 0-6 h, all the indices of glucose variability presented comparable reductions 12-18 h after both exercise sessions (AER: ∆VAR= 151.52, ∆CV%= 0.55 and ∆SD= 3.12 and ECC: ∆VAR= 221.22 , ∆CV%= 3.67 and SD= 6.54 ). However, compared with the pre-exercise period of 0-6 h, none of the indices of glucose variability presented differences 0-6 and 6-12 h after both exercise sessions.
FIG. 3.
Glucose variability evaluated before and after aerobic (AER) or eccentric (ECC) exercise (n=15)
Note: glucose variance (panel A), glucose coefficient of variation (panel B), glucose standard deviation (panel C). Generalized estimating equations (GEE); Bonferroni correction. *P<0.05 vs. pre-exercise period (-6 to 0 h).
Table 2 shows the plasma biochemical analysis results obtained before and after exercise sessions. Interleukin-6 levels increased by 68.5% and 30.8% after the AER and ECC sessions, respectively. Among stress oxidative markers, only uric acid levels showed changes immediately after the experimental sessions (+3% and +4% for AER and ECC, respectively).
TABLE 2.
Biochemical analyses before and after aerobic (AER) or eccentric (ECC) exercise sessions.
| AER exercise | ECC exercise | Group | Time | Interaction | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | P | P | P | |
| IL-6 (pg/mL) | 0.89 ± 0.13 | 1.50 ± 0.21 | 1.30 ± 0.37 | 1.70 ± 0.70 | 0.459 | 0.016 | 0.551 |
| Sulfhydryl (nmol/mg protein) | 7.22 ± 0.38 | 6.98 ± 0.31 | 6.91± 0.38 | 6.98 ± 0.30 | 0.690 | 0.749 | 0.503 |
| Carbonyl (nmol/mg protein) | 0.56 ± 0.06 | 0.46 ± 0.07 | 0.42 ± 0.57 | 0.43 ± 0.06 | 0.077 | 0.413 | 0.335 |
| Uric Acid (mg/dL) | 4.50 ± 0.27 | 4.65 ± 0.28 | 4.13 ± 0.28 | 4.29 ± 0.30 | 0.001 | <0.001 | 0.639 |
| FRAP (mmol/L) | 864 ± 54 | 881 ± 46 | 832 ± 36 | 818± 49 | 0.056 | 0.951 | 0.357 |
Note: IL-6, Interleukin-6; FRAP, Ferric Reducing Ability of Plasma. Data are reported as estimated marginal mean ± SE. Repeated measures generalized estimating equations (GEE), *P < 0.001 vs. pre-exercise values (AER and ECC).
No correlations were found between changes in inflammatory or oxidative biomarkers with changes in glucose variability indices following AER sessions. However, in the ECC session there was an inverse correlation between delta of total protein sulfhydryl and delta of VAR (r = -0.59 P= 0.02) and between delta of total protein sulfhydryl and delta of SD (r = -0.56 P= 0.03). There were no significant correlations between other biomarkers and other indices of glucose variability.
DISCUSSION
In this trial, we showed that acute sessions of AER and ECC promote comparable reductions in glucose variability evaluated by VAR, CV% and SD in non-trained healthy individuals. Increased IL-6 levels and uric acid were observed after both exercise sessions and an oxidative stress marker (total protein sulfhydryl) inversely correlated with VAR and SD specifically in ECC exercise.
The reduction in glucose variability after AER or ECC exercise sessions is in accordance with our previous study, although performed in patients with type 2 diabetes [15]. This evidence indicates a specific effect of exercise in reducing glucose variability, not related to glucose homeostasis derangements. Changes in glucose variability irrespective of mean glucose were previously observed in subjects with or without metabolic syndrome and diabetes [11]. Interestingly, we did not find differences between the exercise modalities tested either in diabetic [15] or non-diabetic subjects (present data).
Comparable to our findings, previous studies showed that AER and ECC exercises are associated with increases in IL-6 [28-30], a cytokine that has both pro- and anti-inflammatory actions. In response to exercise, the increase in IL-6 without concomitant increases in tumour necrosis factor-α (TNF-α) and IL-1β determines production of interleukin 1 receptor antagonist (IL-1ra), IL-10 and soluble TNF-α receptors, all anti-inflammatory molecules that suppress inflammation [31]. Data from a systematic review [28] indicate that a single bout of AER exercise of moderate to high intensity (55% VO2max, 60% VO2max and 90% VO2max) is associated with an increase in IL-6 (145%). We used an intensity of AER exercise of 70% of the VO2peak (peak oxygen uptake per kilogram, ml · kg-1 · min- 1) with a single IL-6 evaluation after exercise, which can account for non-different IL-6 changes between protocols. We also point out that the correlation between IL-6 and glucose variability was tested based on single evaluations that limit us in a definitive conclusion.
The use of large muscle groups during the ECC exercise sessions could lead to increases in IL-6 levels, because the amount of muscle mass involved in exercise is important in the stimulation of a systemic response [30]. Although there is equivocal evidence whether eccentric protocols acutely induce an exacerbated increase in IL-6, varied intensities of resistance exercise (50%, 75%, 90%, and 110% 1-RM) with concentric-eccentric contractions seem to increase IL-6 in a healthy population [32]. Therefore, plasma IL-6 during recovery may present a delayed peak and slower decrease, as observed when ECC was performed with intensity of 150% of the isotonic 1-RM of the dominant knee extensors [33] or 300 eccentric repetitions on an isokinetic dynamometer [34].
Based on reports evaluating diabetic subjects [35-36], we hypothesized that inflammation would be related to glucose variability. Here we observed that the relationship between IL-6 responses after exercise, expressed by delta indices, did not correlate with glucose variability indices. Despite this observation, exercise elicited increases in IL-6 immediately after both sessions and decreases in glucose variability 12-18 h after the sessions. This variation may still provide insight for a linked response between inflammation and glucose variability, as IL-6 might initiate the secretion of anti-inflammatory cytokines, promoting a range of downstream benefits for glucose metabolism [28], However, these effects could be perceived later and would require analyses of downstream molecules in the inflammatory cascade and insulin signalling.
Oxidative stress markers were not substantially altered after the exercises. However, uric acid increased significantly after the exercise sessions, as observed previously in healthy adults [37]. This compound is generated through the catabolism of ATP and has antioxidant potential as a side effect [38]. At least in part, acid uric increase is likely induced by inhibition of the renal clearance of uric acid by lactate, which is accumulated in plasma during exercise [39]. Additionally, exercise stimulates purine catabolism, which accumulates hypoxanthine, xanthine and uric acid. The latter is released from the cells into the plasma and could act as a free radical scavenger and chelator of transitional metal ions in skeletal muscle. It would result in a sustained antioxidant effect after an oxidative challenge [40-41]. Besides that, plasma volume change is another possible cause of uric acid increase. Bloomer et al. found that plasma volume decreased significantly immediately after aerobic exercise performed for 60 minutes at 70% heart rate reserve. Unfortunately, we did not evaluate plasma volume in the present study [42].
Total protein sulfhydryl inversely correlated with glucose variability (VAR and SD) in ECC sessions. High levels of serum protein sulfhydryl groups reflect favourable redox status, indicating less oxidative stress [43]. The inverse correlation thus suggests that increasing protein sulfhydryl is related to less glucose variability. Inadequate antioxidant defence with oscillating glucose levels may induce more damage in protein structure than a constantly high glucose level [18, 44], which is a condition that could favour the development of diabetic complications [18, 45]. Some studies have reported a positive correlation of oxidative stress with glucose variability [36, 46]. In patients with type 2 diabetes, the serum levels of oxidative stress markers (evaluated by 8-iso PGF2) are positively correlated with mean amplitude of glycaemic excursion (MAGE) and standard deviation of HbA1c [36]. Likewise, the endogenous anti-oxidant molecule serum bilirubin is associated (r= 0.588, P < 0.001) with glucose variability (MAGE) in women with type 2 diabetes [46]. Because monitoring oxidative stress markers depends on the sampling timing, we cannot rule out potential changes in oxidative stress parameters with later measurements after exercise sessions.
Limitations
The small sample size may have influenced the analyses of glucose variability. In addition, we carried out measurements of blood only once before and immediately after both exercise sessions, which limits inferences about markers of inflammation and oxidative stress in the present study. Clinical insights offered by a study that uses only one bout of exercise are limited.
CONCLUSIONS
In conclusion, both AER and ECC exercise sessions were associated with reductions in glucose variability in healthy individuals. Inflammatory cytokines, such as IL-6, and oxidative stress might be part of the glucose variability controlling mechanism.
Acknowledgments
Funding/Support: This study was partially supported by Fundo de Apoio à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE), grant 12-0148 and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) PNPD 2546/2009. Role of funding source: the sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest
All other authors have no conflict of interest to declare.
REFERENCES
- 1.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 2.Smith-Palmer J, Brandle M, Trevisan R, Orsini FM, Liabat S, Valentine W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(3):273–284. doi: 10.1016/j.diabres.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 4.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 5.Altıncık A, Tuğlu B, Demir K, Çatlı G, Abacı A, Böber E. Relationship between oxidative stress and blood glucose fluctuations evaluated with daily glucose monitoring in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2016;29(4):435–439. doi: 10.1515/jpem-2015-0212. [DOI] [PubMed] [Google Scholar]
- 6.Siegelaar SE, Barwari T, Kulik W, Hoekstra JB, DeVries JH. No relevant relationship between glucose variability and oxidative stress in well-regulated type 2 diabetes patients. J Diabetes Sci Technol. 2011;5(1):86–92. doi: 10.1177/193229681100500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churruca J, Vigil L, Luna E, Ruiz-Galiana J, Varela M. The route to diabetes: Loss of complexity in the glycemic profile from health through the metabolic syndrome to type 2 diabetes. Diabetes Metab Syndr Obes. 2008;1:3–11. doi: 10.2147/dmso.s3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzese A, Valerio G, Buono P, Spagnuolo MI, Sepe A, Mozzillo E, De Simone I, Raia V. Continuous glucose monitoring system in the screening of early glucose derangements in children and adolescents with cystic fibrosis. J Pediatr Endocrinol Metab. 2008;21(2):109–116. doi: 10.1515/jpem.2008.21.2.109. [DOI] [PubMed] [Google Scholar]
- 9.Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, Nerup J, Borch-Johnsen K, Witte DR, ADAG Study Group Associations between features of glucose exposure and A1C: the A1C-derived average glucose (ADAG) study. Diabetes. 2010;59(7):1585–1590. doi: 10.2337/db09-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gude F, Díaz-Vidal P, Rúa-Pérez C, Alonso-Sampedro M, Fernández-Merino C, Rey-García J, Cadarso-Suárez C, Pazos-Couselo M, García-López JM, Gonzalez-Quintela A. Glycemic variability and its association with its association with demographics and lifestyles in a general adult population. J Diabetes Sci Technol. 2017;11(4):780–790. doi: 10.1177/1932296816682031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buscemi S, Verga S, Cottone S, Azzolina V, Buscemi B, Gioia D, Cerasola G. Glycaemic variability and inflammation in subjects with metabolic syndrome. Acta Diabetol. 2009;46(1):55–61. doi: 10.1007/s00592-008-0061-8. [DOI] [PubMed] [Google Scholar]
- 12.Umpierre D, Ribeiro PA, Kramer CK, Leitao CB, Zucatti AT, Azevedo MJ, Gross JL, Ribeiro JP, Schaan BD. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305(17):1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 13.Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Artero EG, Garrido-Miguel M, Martinez-Vizcaíno V. The effect of physical activity interventions on glycosylated hemoglobin (HbA1c) in non-diabetic populations: A systematic review and meta-analysis. Sports Med. 2018;48(5):1151–1164. doi: 10.1007/s40279-018-0861-0. [DOI] [PubMed] [Google Scholar]
- 14.Correa AP, Figueira FR, Umpierre D, Casali KR, Schaan BD. Inspiratory muscle loading: a new approach for lowering glucose levels and glucose variability in patients with Type 2 diabetes. Diabet Med. 2015;32(9):1255–1257. doi: 10.1111/dme.12798. [DOI] [PubMed] [Google Scholar]
- 15.Figueira FR, Umpierre D, Casali KR, Tetelbom PS, Henn NT, Ribeiro JP, Schaan BD. Aerobic and combined exercise sessions reduce glucose variability in type 2 diabetes: crossover randomized trial. PLoS One. 2013;8(3):e57733. doi: 10.1371/journal.pone.0057733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manohar C, Levine JA, Nandy DK, Saad A, Dalla Man C, McCrady-Spitzer SK, Basu R, Cobelli C, Carter RE, Basu A, Kudva YC. The effect of walking on postprandial glycemic excursion in patients with type 1 diabetes and healthy people. Diabetes Care. 2012;35(12):2493–2499. doi: 10.2337/dc11-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClean C, Harris R A, Brown M, Brown JC, Davison GW. Effects of exercise intensity on postexercise endothelial function and oxidative stress. Oxid Med Cell Longev. 2015;2015:723679. doi: 10.1155/2015/723679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacco F, Du X, Carratú A, Gerfen GJ, D’Apolito M, Giardino I, Rasola A, Marin O, Divakaruni AS, Murphy AN, Shah MS, Brownlee M. GLP-1 cleavage product reverses persistent ROS generation after transient hyperglycemia by disrupting an ROS-generating feedback joop. Diabetes. 2015;64(9):3273–3284. doi: 10.2337/db15-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasari PS, Gandomani BS, Teague AM, Pitale A, Otto M, Short KR. Glycemic variability is associated with markers of vascular stress in adolescents. The journal of pediatric. 2016;172:47–55. doi: 10.1016/j.jpeds.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 20.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 21.Umpierre D, Stein R, Vieira PJ, Ribeiro JP. Blunted vascular responses but preserved endothelial vasodilation after submaximal exercise in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2009;16(1):53–59. doi: 10.1097/HJR.0b013e32831c8489. [DOI] [PubMed] [Google Scholar]
- 22.Levinger I, Goodman C, Hare DL, Jerums G, Toia D, Selig S. The reliability of the 1RM strength test for untrained middle-aged individuals. J Sci Med Sport. 2009;12(2):310–316. doi: 10.1016/j.jsams.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron F J. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2015;7(2):253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 24.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riener CK, Kada G, Gruber HJ. Quick measurement of protein sulfhydryls with Ellman’s reagentand with 4,4′-dithiodipyridine. Anal BioanalChem. 2002;373:266–276. doi: 10.1007/s00216-002-1347-2. [DOI] [PubMed] [Google Scholar]
- 26.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 27.Benzie IF, Strain JJ. The ferric reducing ability of plasma (frap) as a measure of ‘‘antioxidant power’’: the frap assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 28.Brown WM, Davison GW, McClean CM, Murphy MH. A Systematic Review of the Acute Effects of Exercise on Immune and Inflammatory Indices in Untrained Adults. Sports Med Open. 2015;1(1):35. doi: 10.1186/s40798-015-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang CJ, Slusher AL, Whitehurst M, Wells M, Mock JT, Maharaj A, Shibata Y. Acute aerobic exercise mediates G protein-coupled receptor kinase 2 expression in human PBMCs. Life Sci. 2015;135:87–91. doi: 10.1016/j.lfs.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Fischer C.P. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 31.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 32.Uchida MC, Nosaka K, Ugrinowitsch C, Yamashita A, Martins E, Jr, Moriscot AS, Aoki MS. Effect of bench press exercise intensity on muscle soreness and inflammatory mediators. J Sports Sci. 2009;27(5):499–507. doi: 10.1080/02640410802632144. [DOI] [PubMed] [Google Scholar]
- 33.Willoughby DS, McFarlin B, Bois C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int J Sports Med. 2003;24(1):15–21. doi: 10.1055/s-2003-37197. [DOI] [PubMed] [Google Scholar]
- 34.MacIntyre DL, Sorichter S, Mair J, Berg A, McKenzie DC. Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur J Appl Physiol. 2001;84(3):180–186. doi: 10.1007/s004210170002. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman RP, Dye AS, Huang H, Bauer JA. Glycemic variability predicts inflammation in adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2016;29(10):1129–1133. doi: 10.1515/jpem-2016-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang CM, Hsieh CJ, Huang JC, Huang IC. Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus. Acta Diabetol. 2012;49(1):S171–177. doi: 10.1007/s00592-012-0398-x. [DOI] [PubMed] [Google Scholar]
- 37.Wiecek M, Maciejczyk M, Szymura J, Szygula Z, Kantorowicz M. Changes in non-enzymatic antioxidants in the blood following anaerobic exercise in men and women. PLoS One. 2015;10(11):e0143499. doi: 10.1371/journal.pone.0143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 2014;63(3):976–981. doi: 10.2337/db13-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kandar R, Stramova X, Drabkova P, Krenkova J. A monitoring of allantoin, uric acid, and malondialdehyde levels in plasma and erythrocytes after ten minutes of running activity. Physiol Res. 2014;63(6):753–762. doi: 10.33549/physiolres.932696. [DOI] [PubMed] [Google Scholar]
- 40.Hellsten Y, Tullson PC, Richter EA, Bangsbo J. Oxidation of urate in human skeletal muscle during exercise. Free Radic Biol Med. 1997;22(1-2):169–174. doi: 10.1016/s0891-5849(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 41.Hellsten Y, Svensson M, Sjodin B, Smith S, Christensen A, Richter EA, Bangsbo J. Allantoin formation and urate and glutathione exchange in human muscle during submaximal exercise. Free Radic Biol Med. 2001;31(11):1313–1322. doi: 10.1016/s0891-5849(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 42.Bloomer RJ, Farney TM. Acute plasma volume change with high intensity sprint exercise. J Strength Cond Res. 2013;27(10):2874–8. doi: 10.1519/JSC.0b013e318282d416. [DOI] [PubMed] [Google Scholar]
- 43.Jones DP, Sies H. The Redox Code. Antioxid Redox Signal. 2015;23(9):734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ihnat MA, Kamat CD, Piconi L, Kaltreider RC, Cariello A. Attenuated superoxide dismutase induction in retinal cells in response to intermittent high versus continuous high glucose. Am J Biochem Biotechnol. 2007;3:16–23. [Google Scholar]
- 45.Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care. 2009;32(10):1901–1903. doi: 10.2337/dc09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim LK, Roh E, Kim MJ, Kim MK, Park KS, Kwak SH, Cho YM1, Park KS, Jang HC, Jung HS. Serum bilirubin levels are positively associated with glycemic variability in women with type 2 diabetes. J Diabetes Investig. 2016;7(6):874–880. doi: 10.1111/jdi.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]