Significance
Humans have a long history of using natural resources, especially plants, to induce nonordinary states of consciousness. Imbibing substances derived from plants have been linked to ancient and elaborate knowledge systems and rituals. While archaeological evidence of the consumption of psychotropics, such as alcohol or caffeine, dates back thousands of years, evidence of the use of other psychoactive substances has been more difficult to document. This article presents the results of chemical analyses of organic residues found in a 1,000-year-old ritual bundle recovered from the highland Andes. The analyses provide evidence of the use of multiple psychoactive plants associated with a sophisticated botanical knowledge system among ritual specialists (shamans) during pre-Columbian times.
Keywords: archaeometry, liquid chromatography mass spectrometry, hallucinogen, exchange, shamanism
Abstract
Over several millennia, various native plant species in South America have been used for their healing and psychoactive properties. Chemical analysis of archaeological artifacts provides an opportunity to study the use of psychoactive plants in the past and to better understand ancient botanical knowledge systems. Liquid chromatography tandem mass spectrometry (LC-MS/MS) was used to analyze organic residues from a ritual bundle, radiocarbon dated to approximately 1,000 C.E., recovered from archaeological excavations in a rock shelter located in the Lípez Altiplano of southwestern Bolivia. The site is located at an elevation of ∼3,900 m above sea level and contains evidence of intermittent human occupations during the last 4,000 years. Chemical traces of bufotenine, dimethyltryptamine, harmine, and cocaine, including its degradation product benzoylecgonine, were identified, suggesting that at least three plants containing these compounds were part of the shamanic paraphernalia dating back 1,000 years ago, the largest number of compounds recovered from a single artifact from this area of the world, to date. This is also a documented case of a ritual bundle containing both harmine and dimethyltryptamine, the two primary ingredients of ayahuasca. The presence of multiple plants that come from disparate and distant ecological areas in South America suggests that hallucinogenic plants moved across significant distances and that an intricate botanical knowledge was intrinsic to pre-Columbian ritual practices.
Throughout history and across diverse cultures, humans have sought out and utilized various substances to alter perception and ordinary conscious experiences (1–3). Entheogens are a group of substances with known psychoactive effects that are used within spiritual and religious ritual contexts, often with a goal of awakening, transcendence, and/or personal development (4). Many Native American cultures, both past and present, have drawn on their botanical knowledge and incorporated particular species into ritual, social, and medicinal practices (5–14). There are numerous plant species native to South America that contain psychoactive compounds, and their use by ancient specialists provides significant clues concerning past knowledge systems and the importance of certain species for cultural practices (5, 11, 15). Hallucinogenic plants, in particular, have been used in numerous American contexts to establish a bridge between society and supernatural forces (6, 7, 9, 16, 17). In South America, the ethnographic documentation of the consumption of some critical plants has been linked with a deeper history of their use by means of archaeological research (18). Archaeometric studies have demonstrated the consumption of a few plant species such as coca (Erythroxylum coca) (19–22), vilca or cebil (Anadenanthera spp.) (17, 23, 24), tobacco (Nicotiana spp.) (12, 25), and yage/ayahuasca (Banisteriopsis caapi) (15). This paper adds to those previous studies by demonstrating the consumption of many of these resources together. Using liquid chromatography tandem mass spectrometry (LC-MS/MS), we tested for the presence of psychoactive compounds in the materials that composed a 1,000-year-old ritual bundle excavated in a dry rock shelter from southwestern Bolivia.
The Ritual Bundle from the Cueva del Chileno
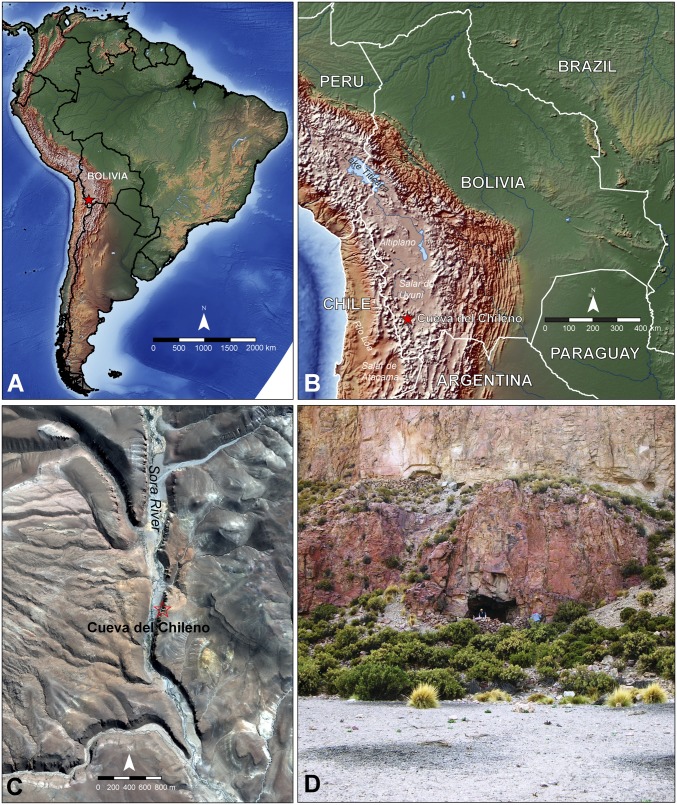
The Sora River valley, located in the Lípez highlands of southwestern Bolivia, is a narrow basin outlined by two parallel ignimbrite outcrops running from north to south. Several rock shelters found along these geological formations preserve evidence of human occupation that extends into the late Pleistocene (26, 27). One of these rock shelters, locally known as Cueva del Chileno (situated at 3,890 m above sea level), was excavated in 2008 and 2010 (Fig. 1). The earliest evidence of human occupation at the site consists of a hearth associated with obsidian and chert stone tools, radiocarbon dated by accelerator mass spectrometry (AMS) to 2136–1778 B.C.E. (AA91560, 3648 ± 48 BP, δ13C −22.2) (28). Above this occupation level were two strata of plastered floors, which were associated with a boulder-lined, circular structure that likely served as a funerary enclosure (SI Appendix, Fig. S1). The structure was likely remodeled and destroyed during pre-Hispanic times. Within the rubble above the plastered upper floor we found remains of “ritual trash” including a large spherical ground stone, turquoise beads, colored strings, a bundle of cut braids of human hair, and a remarkably well-preserved ritual bundle containing paraphernalia for consuming psychotropic substances (Fig. 2). We presume that human mummies were intentionally removed from the structure during episodes of desecration (28). The rubble fill was eventually capped by llama and sheep dung as the rock shelter was used as shelter for domesticated herds by indigenous pastoralists in the more recent past (SI Appendix, Fig. S2).
Fig. 1.
The study area is located in the south-central Andes (A), in the Lípez highlands of southwestern Bolivia (B). An aerial view of the Sora River Valley (C) shows Cueva del Chileno on its eastern side (c/o GeoEye Foundation), and a photograph (D) of the exterior of the rock shelter during excavation.
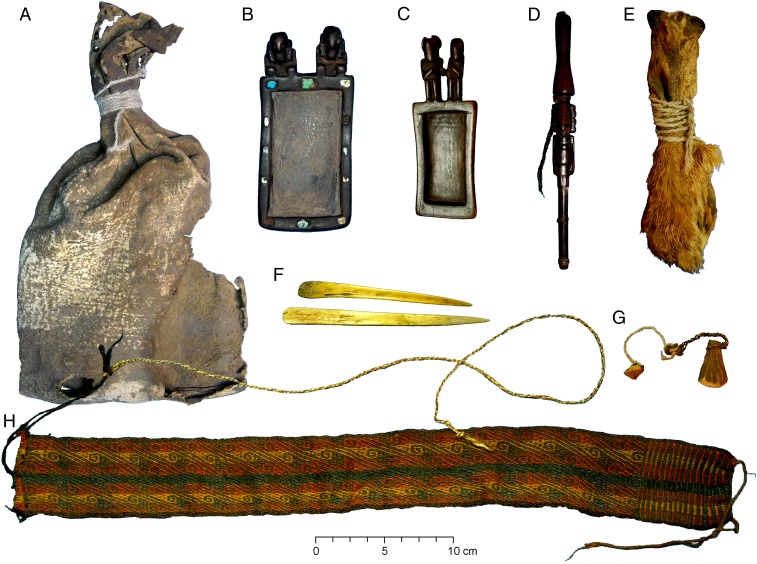
Fig. 2.
The Cueva del Chileno ritual bundle consisting of: outer leather bag (A), expertly carved and decorated wooden snuffing tablets with anthropomorphic figurines (B and C), intricate anthropomorphic snuffing tube with two human hair braids attached to it (D), animal-skin pouch constructed of three fox snouts (L. culpaeus) stitched together (E), two camelid (L. glama) bone spatulas (F), two small pieces of dried plant material attached to wool and fiber strings (G), and a polychrome woven textile headband (H). Artifacts (E and G) were tested using LC-MS/MS analysis.
The ritual bundle consisted of a large leather bag (280 × 165 mm, Fig. 2A), which contained two expertly carved and decorated wooden snuffing tablets with anthropomorphic figurines (Fig. 2 B and C), an intricate anthropomorphic snuffing tube with two real human hair braids attached to it (Fig. 2D), two camelid (Lama glama) bone spatulas (Fig. 2F), a colorful woven textile thought to be a headband (Fig. 2H), two small pieces of dried plant material attached to wool and fiber strings (Fig. 2G), and an unusual animal-skin pouch constructed of three fox snouts (Lycalopex culpaeus) stitched together (Fig. 2E) (28). A small sample of the outer leather bag of the ritual bundle was AMS radiocarbon dated (AA84156, 1042 ± 52 BP, δ13C −22.7) to cal. 905–1170 CE (28). This period is associated with the disintegration of the Tiwanaku state and the emergence of regional polities (29, 30). During the previous five centuries, Tiwanaku’s sphere of influence had spread across the south-central Andes extending from modern-day western Bolivia toward southern Peru and northern Chile. Ritual/ceremonial items associated with Tiwanaku, such as snuff paraphernalia, have been described as one of the means by which Tiwanaku ideology spread into other regions (28–32). However, snuffing paraphernalia in northern Chile and Argentina (and presumably in the eastern tropical lowlands) was part of a long tradition that preceded the arrival of Tiwanaku and that continued well after Tiwanaku’s disintegration, around 900 CE (33–35).
The use of psychoactive substances by ancient Tiwanaku may have been connected to a complex religious tradition with deep roots in earlier Andean and Amazonian cultures. Ritual specialists, or shamans, acted as intermediaries between realms, entering into altered mental/physical states to connect living people with venerated ancestors thought to exist in other realms (28, 31, 36–43). Presumably, the consumption of psychoactive substances facilitated the communication between ritual specialists, ancestors, and deities. The recovery of the ritual bundle at Cueva del Chileno provides an opportunity to investigate the kinds of psychoactive substances that were consumed in the region: Which substances did the owner(s) of this kit use, and what does this tell us about shamanic knowledge during this time period?
Results
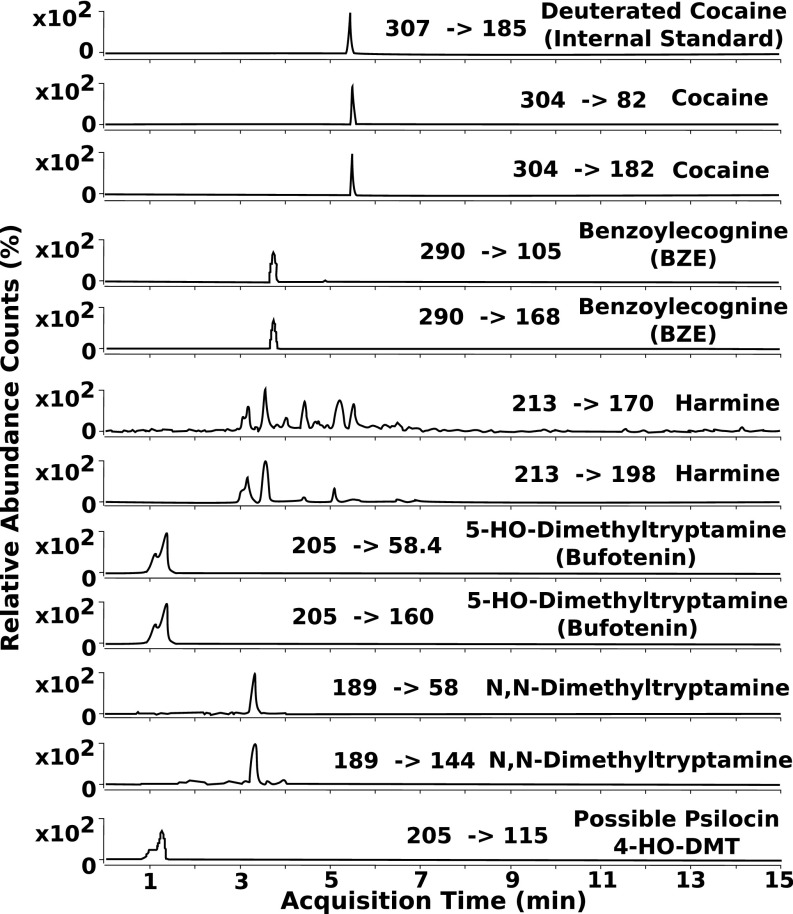
We sampled two artifacts from the ritual bundle (i): the fox-snout pouch interior was gently scraped to produce a small sample from adhering debris, and (ii) a superficial section from the larger fragment of archaeological plant stem tissue was collected. The scraping from the fox-snout pouch revealed multiple psychotropic compounds, suggesting that it contained multiple plants that were ingested for their psychoactive properties. Specifically, the pouch chromatograms indicated the presence of at least five psychotropic compounds: cocaine, benzoylecgonine (BZE), harmine, bufotenine, dimethyltryptamine (DMT), and a peak possibly related to psilocin (Fig. 3).
Fig. 3.
LC-MS/MS results from the fox-snout pouch indicating the presence of cocaine, BZE, harmine, bufotenine, DMT, and peak potentially corresponding to psilocin.
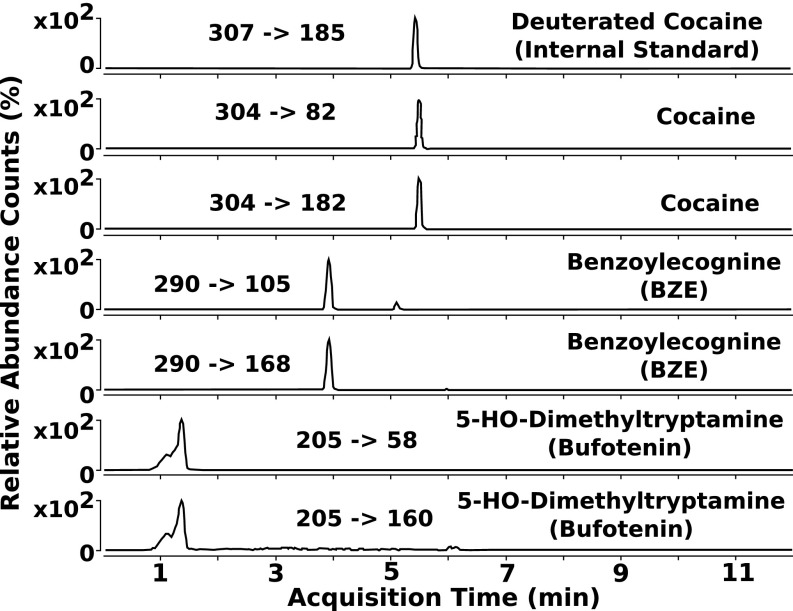
Regarding the archaeological plant stem, the chromatogram showed peaks that correspond to the presence of cocaine, BZE, and bufotenine (Fig. 4). None of the modern plants considered here contain these three substances together, nor do we know of any species that contains these three compounds in concert. Therefore, some, and possibly all, of these chemical compounds may be exogenous. Due to the destructive nature of chemical analysis, we only collected a very small surface sample of the archaeological plant. Therefore, the sample tested may reflect the chemistry of the items that plant surface was in contact with, rather than the biogenic signal of the plant itself. Two interpretations could explain this anomaly (i): that some of these compounds are biogenic to the archaeological plant on the string or (ii) that all of the compounds are exogenous.
Fig. 4.
LC-MS/MS results from the larger piece of plant tissue indicating the presence of cocaine, BZE, and bufotenine.
Discussion
The analyses indicated the presence of five psychoactive compounds derived from at least three different plant sources recovered from the bundle, the largest number of psychoactives present in a single archaeological assemblage reported from South America. Cocaine and BZE were identified in both the fox-snout pouch and archaeological plant samples, suggesting the presence and use of E. coca leaves by the owner of this kit. E. coca has a very long history of use in South America and is still widely used today (18–20). While not exclusively used as an entheogen, the leaves contain active ingredients including cocaine and BZE, and are consumed for social, ritual, and medicinal purposes (44). Coca leaves are chewed (or brewed into a tea), and the active ingredient, cocaine, acts as a mild stimulant and as an anesthetic. Coca leaves are also used to help with symptoms of altitude sickness and gastrointestinal disorders (45, 46). Chemical tests that were run on a number of pre-Columbian mummies from South America (especially Chile and Peru) show the presence of cocaine and/or its metabolite BZE, including in young infants, who likely received the chemical through their mother’s breastmilk (24, 47). The fox-snout pouch likely carried coca leaves, and we hypothesize that the presence of these compounds on the archaeological plant come from coca leaves rubbing against the surface of the plant on the string. Therefore, we do not think that the archaeological plant on the string is a piece of stem from an E. coca plant, but instead these two distinct items (coca leaves and this plant stem on the textile string) were in close contact inside the ritual bundle.
The presence of bufotenine suggests that seeds from Anadenanthera were carried in the fox-snout pouch and may have been related to the archaeological plant. Within a South American context, bufotenine has been most widely documented in the seeds of Anadenanthera, while the stem/bark of the plant has not yielded evidence of bufotenine content (instead having trace amounts of DMT-oxide) (48). It is possible that the bufotenine is endogenously present in the unknown archaeological plant sample and that further morphological work will be needed to identify this plant stem. For example, a study of Brosimum acutifolium, a plant found in northern South America, contains bufotenine in its latex (produced in the stem/bark), and is used as a shamanic potion by people of the Guiana plateau (49). Further analysis of this preserved ancient plant will be needed to confirm a species identification.
Anadenanthera colubrina (often called vilca or cebil) and Anadenanthera peregrina (called yopo) were widely used by South American horticultural tribes and are known to contain psychoactive tryptamines. The primary component in Anadenanthera is bufotenine (5-OH-DMT), and the genus also contains trace amounts of the tryptamines N,N-dimethyltryptamine (DMT) and 5-methoxy-N-N-dimethyltryptamine (23, 50). These tryptamines have a range of documented psychoactive effects depending on ingestion route and are taken as a stimulant and/or for their hallucinogenic properties (51–53). The seeds (which contain the psychoactive compounds) are ground as snuff and inhaled, mixed into a beverage such as chicha (an alcoholic drink brewed widely in South America), or given in an enema (23, 51, 53). Previous Tiwanaku-period archaeological finds of snuff trays and tubes have been thought to be related to inhalation of Anadenanthera, and while only a few have been analyzed in depth, those that were tested indicated Anadenanthera presence (17, 53). A recent study of mummies from the Azapa Valley, Chile, dated to between 500 and 1100 CE, showed that two individuals, male and female, had consumed Anadenanthera, as evidenced by bufotenine present in their hair (24). The identification of bufotenine in the scraping from the fox-snout pouch indicates Anadenanthera seeds were carried and used, likely ground into a powder on the snuff trays and inhaled using the snuff tube found within the ritual bundle.
The pouch also contained harmine, DMT, and a peak was observed that may be from a fungus with psilocin. In South American botanicals, harmine is found in highest quantities in the B. caapi plant, most commonly prepared as the main ingredient in “ayahuasca” (54). The presence of DMT is potentially confounding, as this tryptamine is found in low concentrations in A. colubrina and A. peregrina and in higher concentrations in Psychotria viridis. Therefore, the DMT presence could be derived from the same Anadenathera material contributing bufotenine to the pouch scraping, or it could be from an independent source, such as Psychotria. While psilocin was not in our initial compound screenings, the presence of a peak at the retention times and transitions, corresponding to psilocin, suggests it was in the original sample. Psilocin is also a tryptamine, 4-hydroxy-dimethyltryptamine, and it may be possible that its presence in the sample is derived from a psilocin containing fungus.
Recently scholars have sought out evidence of ayahuasca use during pre-Columbian times. In contemporary contexts, ayahuasca is a hypernym for a range of psychotropic concoctions and is traditionally associated with Amazonian cultures (55). The primary ingredient in ayahuasca is B. caapi, which may be boiled and brewed as a tea (alone), although it is often mixed with other plants, most commonly P. viridis (known as chacruna). B. caapi is grown in tropical areas of northern South America while P. viridis is thought to have been limited to Amazonian lowland areas in the past (56, 57). B. caapi contains the β-carboline alkaloids harmine, harmaline, and tetrahydraharmine while P. viridis contains DMT (58). When these two plants are combined in ayahuasca preparations they have dynamic interacting effects: The β-carboline alkaloids prevent the breakdown of the DMT in the digestive tract and then act as monoamine oxidase inhibitors, thus allowing the DMT to activate the central nervous system, causing vivid hallucinogenic experiences for consumers (57).
Scholars have debated the historical use of ayahuasca, with some suggesting it has relatively recent origins, while others argue that it may have been used for centuries, or even millennia (24). Archaeological evidence of ayahuasca consumption is still lacking. However, Ogalde et al. (15, 59) analyzed hair from 32 Tiwanaku period mummies dated between 400 and 900 CE from the Azapa Valley of northern Chile and found chemical traces of harmine in the hair of an infant and of an adult male, indicating Banisteriopsis consumption. They noted that the presence of harmine alone suggests that the consumption of Banisteriopsis was not for hallucinogenic purposes (since harmine is a monoaminoxidase inhibitor with psychoactive effects but not hallucinogenic ones), but rather for medicinal or therapeutic reasons (15, 59). The presence of Banisteropsis in archaeological contexts, therefore, does not necessarily indicate that it was used as a hallucinogen/entheogen. The combination of Banisteropsis with other plants to induce hallucinations, it has been argued, may have developed in more recent times (24, 60).
Of particular interest is the possibility that ayahuasca (a blend of various plants) was used in this ancient context. Given the presence of harmine (Banisteriopsis) and DMT (via multiple potential sources), ayahuasca must be considered. It is likely that shamans ingested plants containing harmine and DMT simultaneously to achieve a hallucinogenic state, either through a beverage, such as ayahuasca, or through a composite snuff that contained these plants in a single mixture (61, 62). The Piaroa of southern Venezuela use both A. peregrina and Banisteriopsis in their snuff preparations (and consume additional doses of Banisteriopsis before snuff inhalation) (63). It is possible that a similar preparation was used by shamans in our study area. However, the plants containing these psychotropic compounds may have also been used separately, potentially even as medications. For example, recent work on the bark of A. colubrina has indicated chemical compounds that aid in pain treatment (antinociceptive effects) (62). Anadenanthera, Banisteriopsis, and Erythroxylum could have also been used for medicinal/therapeutic uses, such as during pregnancy or in early childhood, as evidenced by the presence of Banisteriopsis and Erythroxylum in young individuals (59). Although our results do not provide conclusive evidence for the early use of ayahuasca in the form most commonly observed in modern South American indigenous communities (as an entheogen using Banisteriopsis and Psychotria together), it is evidence that this combination was possibly used as early as 1,000 y ago.
One significant compound we did not identify in the ritual bundle is mescaline, a phenethylamine alkaloid (3,4,5-trimethoxyphenethylamine), which is known for its hallucinogenic effects and has a history of being used in the Americas as an entheogen (6, 7, 9). Echinopsis pachanoi (previously listed as Trichocereus pachanoi), also known as San Pedro cactus, is native to South America and contains mescaline. Much of the evidence of E. pachanoi in South America is found in iconographic representations on pottery, lithic sculptures, and textiles (7, 64–66). However, direct evidence of the use of E. pachanoi for mescaline, such as via archaeobotanical or chemical residue data, is still lacking, as confirmed by our results.
While evidence of direct consumption of any of these plants is absent (no human remains were associated with this archaeological context), the substantial evidence of the presence of hallucinogenic plants is compelling. Few studies have recovered direct evidence of psychoactives in South American archaeological contexts, and this case systematically demonstrates evidence for multiple psychoactive plants found together in a single artifact. Importantly, as others have noted, these plants come from distant and diverse ecological zones (23, 60). Because these plants are foreign to the Lípez highlands, it remains to be established whether they were acquired through trading networks or directly by the shamans themselves. In either case, their role as articulators of ideology must have been strongly reinforced by these interregional contacts. These erudite and well-trained ritual specialists must have also been very influential individuals in ancient Andean society. With the disintegration of Tiwanaku, at around 1000 CE, rituals involving snuff paraphernalia vanished in the core area, nonetheless, shamanic cults endured in other regions (28, 31). The snuffing tablets and the inhalation tube recovered at Cueva del Chileno show greater affinity with a regional tradition that continued to evolve after Tiwanaku’s collapse. The “circumpuneño” tradition comprised the Atacama region in northern Chile, the Jujuy puna in northwestern Argentina, and the Lípez highlands of southwestern Bolivia, and involved the consumption of hallucinogens and mortuary ritual that included burial desecration and human decapitation (35, 41, 42).
Conclusions
Chemical analysis of organic residues from this ancient bundle indicates that the ritual specialists in this region had extensive knowledge of and access to various plants with psychoactive properties. Our results indicate that this is the largest number of psychoactive compounds found in association with a single archaeological artifact from South America. The chemical residues of at least five compounds that are known to have psychotropic effects on humans, present in the fox-snout pouch, imply that multiple plants were used to induce extraordinary states of consciousness, potentially within a range of ritual and healing contexts. The plants that were used in the rituals included Erythroxylum, Anadenanthera, Banisteriopsis, and potentially other sources contributing DMT and psilocin (such as Psychotria leaves and hallucinogenic fungi). This is conclusive evidence for the presence of at least three psychoactive plants, the highest number of such specimens recovered from a single South American artifact, and suggests intriguing evidence that ritual specialists might have manipulated and used multiple plants together in their psychoactive preparations.
Materials and Methods
The ritual bundle was carefully opened in La Paz under environmentally controlled conditions, and the entire sampling process was documented with photographs and notes. Due to the irreplaceable nature of these precious artifacts, only very small samples were removed for analyses. A small, surface piece of the archaeological plant on the string was removed with a sterilized scalpel, and a scraping from the interior of the fox-snout pouch was collected and analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS). This method is highly sensitive and very effective for analyzing small samples and is capable of detecting the presence of minute amounts of specific compounds.
The analysis of the artifacts was performed using LC-MS/MS in a modification of a published procedure (67). The expansion of the assay allowed for the detection of cocaine and its products, as well as monitoring of several compounds of potential interest (SI Appendix, Table S1). Deuterated internal standards (benzoylecgonine-d3, cocaine-d3), as well as nondeuterated drug standards for each of the drugs were obtained from Cerilliant. Solid phase extraction columns (Clin II, 691–0353T) were obtained from SPEWare. All solvents were HPLC grade or better, and all chemicals were American Chemical Society (ACS) grade.
Several milligrams of each artifact were pulverized and then sonicated in methanol (30 min at room temperature). Deuterated cocaine and deuterated BZE were added as internal standards to the artifact samples. Phosphate buffer (pH 2.7; 1.5 mL) was added, and the sample was sonicated at 75 °C for 3 h. The buffer was decanted into clean glass tubes, and sodium phosphate buffer (pH 6.0; 1 mL) was added. Solid-phase mixed mode extraction columns (Clin II, 691–0353T) were conditioned with methylene chloride:methanol:ammonium hydroxide (78:20:2 vol/vol 2 mL), ethyl acetate (2 mL), methanol (2 mL), and 0.1 M hydrochloric acid (1 mL). The samples were allowed to flow through the columns, and then the columns were washed with deionized water (2 mL), 0.1 M hydrochloric acid (2 mL), methanol (2 mL), and ethyl acetate (2 mL). The columns were allowed to dry between washes under nitrogen pressure (30 psi; 2 min). The drugs were finally eluted, using freshly prepared methylene chloride: methanol:ammonium hydroxide (78:20:2 vol/vol 3 mL). The extracts were evaporated to dryness under nitrogen at 40 °C and reconstituted in methanol (50 μL) for injection into the LC-MS/MS instrument.
An Agilent Technologies 1200 Series liquid chromatograph pump coupled to a 6430 triple quadrupole mass spectrometer (MS), operating in positive electrospray chemical ionization mode (ESI), was used for analysis. The liquid chromatographic column was a Zorbax Eclipse XDB C18 (4.6 × 50 mm × 1.8 μm), the column temperature was held at 40 °C, and the injection volume was 5 μL. The mobile phase consisted of Solvent A: 20 mM ammonium acetate (pH 6.4) and Solvent B: methanol. At time 0, the solvent composition was 85% A; 15% B, which changed to 50% of each after 4 min then returned to the original settings, at 6 min, where it was held a further 4 min. The gas temperature was 350 °C, the gas flow was 8 L/min, and the nebulizer pressure was 40 psi. Nitrogen was used as the collision gas and the capillary voltage was 4,000 V.
Accurate identification of chemical compounds in unknown samples requires the use of reference data from known compounds. We selected a number of compounds of interest (based on previous studies of South American psychoactives). In some cases, those compounds are well documented for LC-MS/MS analysis, while others were confirmed through LC-MS/MS analysis of modern plant samples. E. coca is commonly used by modern indigenous peoples living in the highlands of Peru and Bolivia. Coca leaves contain cocaine and its primary degradation product, BZE. The leaves of P. viridis contain N,N-DMT. The vines and bark of B. caapi contain the compounds of harmine and harmaline and also contain small amounts of 5-methoxy-DMT. A. colubrina seeds were analyzed and contain 5-OH-DMT, along with trace amounts of DMT and 5-methoxy-DMT. E. pachanoi (San Pedro cactus) contains the psychedelic compound mescaline. Two transitions were selected for each compound. SI Appendix, Table S2 shows the optimized fragment voltages for the parent ion (M +1) as well as the collision energy for fragmentation of the product ions. Each subsequent analysis required the ratio between the quantitative ion and the qualifier ion to be within ±20% to meet the criterion for a positive result.
Supplementary Material
Acknowledgments
We thank Cindy Coulter and Margaux Garnier of Immunalysis Corporation, the National Geographic Foundation, and the Stahl Foundation (UC Berkeley Archaeological Research Facility); the Ministry of Cultures and Tourism of Bolivia and the Mancomunidad de Lípez, including the regional authorities of San Agustin and Alota, for their endorsement of our research, and all the men and women of the local communities that assisted in the excavations; and Darwin Palomino, José Moller, Carlos Revilla, Carlos Capriles, Alejandra Domic, Blaine Maley, BrieAnna Langlie, Maria Bruno, and Eduardo Machicado for their contributions in the excavations. Funds for archaeological fieldwork in the Sora valley (Lípez) were provided by National Geographic Society Grant 8742-10, MONOPOL Ltda., and the Bartolome de Las Casas Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11087.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902174116/-/DCSupplemental.
References
- 1.Wadley G. (2016) How psychoactive drugs shape human culture: A multi-disciplinary perspective. Brain Res Bull 126:138–151. [DOI] [PubMed] [Google Scholar]
- 2.VanPool CS. (2009) The signs of the sacred: Identifying shamans using archaeological evidence. J Anthropol Archaeol 28:177–190. [Google Scholar]
- 3.Goodman J, Lovejoy PE, Sherratt A, eds (1995) Consuming Habits: Drugs in History and Anthropology (Routledge, London: ). [Google Scholar]
- 4.Ruck CAP, Bigwood J, Staples D, Ott J, Wasson RG (1979) Entheogens. J Psychedelic Drugs 11:145–146. [DOI] [PubMed] [Google Scholar]
- 5.Angelo ZD, Capriles JM (2004) La importancia de las plantas psicotrópicas para la economía de intercambio y relaciones de interacción en el altiplano sur andino. Chungara Revista de Antropología Chilena 36:1023–1035. [Google Scholar]
- 6.Carod-Artal FJ. (2015) Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurologia 30:42–49. [DOI] [PubMed] [Google Scholar]
- 7.Carod-Artal FJ, Vázquez-Cabrera CB (2006) [Mescaline and the San Pedro cactus ritual: Archaeological and ethnographic evidence in northern Peru]. Rev Neurol 42:489–498. [PubMed] [Google Scholar]
- 8.Duke GS. (2011) Continuity, cultural dynamics, and alcohol: The reinterpretation of identity through chicha in the andes. Identity Crisis: Archaeological Perspectives on Social Identity, Chacmool Archaeology Conference Proceedings, eds Amundsen-Meyer L, Engel N, Pickering S (University of Calgary, Calgary, Canada), pp 263–272.
- 9.El-Seedi HR, De Smet PA, Beck O, Possnert G, Bruhn JG (2005) Prehistoric peyote use: Alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas. J Ethnopharmacol 101:238–242. [DOI] [PubMed] [Google Scholar]
- 10.Valdez LM, Taboada J (2017) Coca Leaves in the Context of the Central Andean Wari State. Trading Spaces: The Archaeology of Interaction, Migration, and Exchange, Chacmool Archaeology Conference Proceedings, eds Patton M, Manion J (Chacmool Archaeol Assoc, University of Calgary, Calgary, Canada), pp 136–151.
- 11.Torres CM. (1995) Archaeological evidence for the antiquity of psychoactive plant use in the Central Andes. Annuli dei Musei Civici Roverero 11:291–326. [Google Scholar]
- 12.Echeverría J, Planella MT, Niemeyer HM (2014) Nicotine in residues of smoking pipes and other artifacts of the smoking complex from an Early Ceramic period archaeological site in central Chile. J Archaeol Sci 44:55–60. [Google Scholar]
- 13.Tushingham S, Snyder CM, Brownstein KJ, Damitio WJ, Gang DR (2018) Biomolecular archaeology reveals ancient origins of indigenous tobacco smoking in North American Plateau. Proc Natl Acad Sci USA 115:11742–11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eerkens JW, et al. (2018) Dental calculus as a source of ancient alkaloids: Detection of nicotine by LC-MS in calculus samples from the Americas. J Archaeol Sci Rep 18:509–515. [Google Scholar]
- 15.Ogalde JP, Arriaza BT, Soto EC (2009) Identification of psychoactive alkaloids in ancient andean human hair by gas chromatography/mass spectrometry. J Archaeol Sci 36:467–472. [Google Scholar]
- 16.de Smet PAGM. (1985) A multidisciplinary overview of intoxicating snuff rituals in the western hemisphere. J Ethnopharmacol 13:3–49. [DOI] [PubMed] [Google Scholar]
- 17.Torres CM, et al. (1991) Snuff powders from pre-Hispanic San Pedro de Atacama: Chemical and contextual analysis. Curr Anthropol 32:640–649. [Google Scholar]
- 18.Allen CJ. (2002) The Hold Life Has: Coca and Cultural Identity in an Andean Community (Smithsonian Inst Press, Washington, DC: ), 2nd Ed. [Google Scholar]
- 19.Dillehay TD, et al. (2010) Early Holocene coca chewing in northern Peru. Antiquity 84:939–953. [Google Scholar]
- 20.Indriati E, Buikstra JE (2001) Coca chewing in prehistoric coastal Peru: Dental evidence. Am J Phys Anthropol 114:242–257. [DOI] [PubMed] [Google Scholar]
- 21.Naranjo P. (1981) Social function of coca in pre-Columbian American. J Ethnopharmacol 3:161–172. [DOI] [PubMed] [Google Scholar]
- 22.Springfield AC, Cartmell LW, Aufderheide AC, Buikstra J, Ho J (1993) Cocaine and metabolites in the hair of ancient Peruvian coca leaf chewers. Forensic Sci Int 63:269–275. [DOI] [PubMed] [Google Scholar]
- 23.Torres CM, Repke DB (2012) Anadenanthera: Visionary Plant of Ancient South America (Routledge, New York: ), 2nd Ed. [Google Scholar]
- 24.Brown EL. (2012) Investigating the use of coca and other psychoactive plants in Pre-Columbian mummies from Chile and Peru. An analytical investigation into the feasibility of testing ancient hair for drug compounds. Doctoral thesis (University of Bradford, Bradford, UK).
- 25.Echeverría J, Niemeyer HM (2013) Nicotine in the hair of mummies from San Pedro de Atacama (Northern Chile). J Archaeol Sci 40:3561–3568. [Google Scholar]
- 26.Albarracin-Jordan J, Capriles JM (2011) The Paleoamerican occupation of Cueva Bautista: Late Pleistocene human evidence from the Bolivian highlands. Curr Res Pleistocene 28:95–98. [Google Scholar]
- 27.Capriles JM, et al. (2016) High-altitude adaptation and late Pleistocene foraging in the Bolivian Andes. J Archaeol Sci Rep 6:463–474. [Google Scholar]
- 28.Albarracin-Jordan J, Capriles JM, Miller MJ (2014) Transformations in ritual practice and social interaction on the Tiwanaku periphery. Antiquity 88:851–862. [Google Scholar]
- 29.Janusek JW. (2008) Ancient Tiwanaku (Cambridge Univ Press, Cambridge, UK: ). [Google Scholar]
- 30.Delaere C, Capriles JM, Stanish C (2019) Underwater ritual offerings in the Island of the Sun and the formation of the Tiwanaku state. Proc Natl Acad Sci USA 116:8233–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albarracin-Jordan J. (2007) La formación del estado prehispánico en los Andes: origen y desarrollo de la sociedad segmentaria indígena (Fundación Bartolomé de las Casas, La Paz, Bolivia: ). [Google Scholar]
- 32.Stanish C, et al. (2010) Tiwanaku trade patterns in southern Peru. J Anthropol Archaeol 29:524–532. [Google Scholar]
- 33.Llagostera A. (2015) Albores del psicotropismo en San Pedro de Atacama: Pipas vs. tabletas. Chungara (Arica) 47:469–488. [Google Scholar]
- 34.Llagostera A. (2006) Contextualización e iconografía de las tabletas psicotrópicas Tiwanaku de San Pedro de Atacama. Chungara (Arica) 38:83–111. [Google Scholar]
- 35.Horta Tricallotis H. (2012) El estilo circumpuneño en el arte de la parafernalia alucinógena prehispánica (Atacama y Noroeste Argentino). Estud Atacameños:5–34. [Google Scholar]
- 36.Harner MJ. (1973) Hallucinogens and Shamanism (Oxford Univ Press, Oxford: ). [Google Scholar]
- 37.Glowacki M. (2005) Food of the gods or mere mortals? Hallucinogenic Spondylus and its interpretive implications for early andean society. Antiquity 79:257–268. [Google Scholar]
- 38.Narby J, Huxley F, eds (2001) Shamans Through Time: 500 Years to the Path of Knowledge (Tarcher & Penguin, New York: ). [Google Scholar]
- 39.Richardin P, Lavier C, Horta H, Figueroa V, Lira N (2015) Radiocarbon dating of Atacama (Chile) snuff trays: An update on Stylistic and chronological correlations. Radiocarbon 57:775–784. [Google Scholar]
- 40.Salazar D, Niemeyer HM, Horta H, Figueroa V, Manríquez G (2014) Interaction, social identity, agency and change during Middle Horizon San Pedro de Atacama (northern Chile): A multidimensional and interdisciplinary perspective. J Anthropol Archaeol 35:135–152. [Google Scholar]
- 41.Nielsen AE. (2018) La parafernalia para consumo de alucinógenos de “calilegua” (Jujuy, Argentina): Procedencia, cronología y relaciones circumpuneñas. Bol Mus Chil Arte Precolomb 23:71–100. [Google Scholar]
- 42.Berenguer J, Acevedo N (2015) Tubos de hueso de ave como implementos chamánicos en el desierto de atacama, siglos XI-XV. Bol Mus Chil Arte Precolomb 20:51–72. [Google Scholar]
- 43.Bélisle V. (March 1, 2019) Hallucinogens and altered states of consciousness in Cusco, Peru: A path to local power during Wari State expansion. Camb Archaeol J, 10.1017/S0959774319000015.
- 44.Novák M, Salemink CA, Khan I (1984) Biological activity of the alkaloids of Erythroxylum coca and Erythroxylum novogranatense. J Ethnopharmacol 10:261–274. [DOI] [PubMed] [Google Scholar]
- 45.Gay GR, Inaba DS, Sheppard CW, Newmeyer JA, Rappolt RT (1975) Cocaine: History, epidemiology, human pharmacology, and treatment. A perspective on a new debut for an old girl. Clin Toxicol 8:149–178. [DOI] [PubMed] [Google Scholar]
- 46.Plowman T. (1986) Coca chewing and the botanical origins of coca (Erythroxylum spp.) in South America. Coca and Cocaine: Effects on People and Policy in Latin America, eds Pacini D, Franquemont C (Cultural Survival Inc., Cornell University Latin American Studies Program and the Transcript Printing Co, Peterborough, NH), Cultural Survival Report No. 23 pp 5–33.
- 47.Cartmell LW, Aufderheide AC, Springfield A, Weems C, Arriaza B (1991) The frequency and antiquity of prehistoric coca-leaf-chewing practices in Northern Chile: Radioimmunoassay of a cocaine metabolite in human-mummy hair. Lat Am Antiq 2:260–268. [Google Scholar]
- 48.Damascena NP, et al. (2014) Antioxidant and orofacial anti-nociceptive activities of the stem bark aqueous extract of Anadenanthera colubrina (Velloso) Brenan (Fabaceae). Nat Prod Res 28:753–756. [DOI] [PubMed] [Google Scholar]
- 49.Moretti C, Gaillard Y, Grenand P, Bévalot F, Prévosto J-M (2006) Identification of 5-hydroxy-tryptamine (bufotenine) in takini (Brosimumacutifolium Huber subsp. acutifolium C.C. Berg, Moraceae), a shamanic potion used in the Guiana Plateau. J Ethnopharmacol 106:198–202. [DOI] [PubMed] [Google Scholar]
- 50.Schultes RE, Holmstedt B, Lindgren J-E, Rivier L (1977) De plantis toxicariis e mundo novo tropicale commentationes xviii: Phytochemical examination of Spruce’s ethnobotanical collection of Anadenathera peregrina. Bot Mus Lealf Harv Univ 25:273–287. [Google Scholar]
- 51.Altschul SvonR. (1972) The Genus Anadenanthera in Amerindian Cultures (Harvard University, Cambridge, MA: ). [Google Scholar]
- 52.Ott J. (2001) Pharmañopo-psychonautics: Human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine. J Psychoactive Drugs 33:273–281. [DOI] [PubMed] [Google Scholar]
- 53.Pochettino ML, Cortella AR, Ruiz M (1999) Hallucinogenic snuff from Northwestern Argentina: Microscopical identification of Anadenanthera colubrina var. cebil (Fabaceae) in powdered archaeological material. Econ Bot 53:127–132. [Google Scholar]
- 54.Morales-García JA, et al. (2017) The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci Rep 7:5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caiuby Labate B, Cavnar C, Gearin AK (2017) Introduction: The shifting journey of ayahuasca in diaspora. The World Ayahuasca Diaspora: Reinventions and Controversies, eds Caiuby Labate B, Cavnar C, Gearin AK (Routledge, New York), pp 1–18.
- 56.Rätsch C. (2005) The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Applications (Park Street, Rochester, VT). US edition (translated).
- 57.Schultes RE, Hofmann A, Rätsch C (2001) Plants of the Gods: Their Sacred, Healing, and Hallucinogenic Powers (Healing Arts, Rochester, VT: ), 2nd Ed. [Google Scholar]
- 58.Callaway JC, Brito GS, Neves ES (2005) Phytochemical analyses of Banisteriopsis caapi and Psychotria viridis. J Psychoactive Drugs 37:145–150. [DOI] [PubMed] [Google Scholar]
- 59.Ogalde JP, Arriaza BT, Soto EC (2010) Uso de plantas psicoactivas en el norte de Chile: Evidencia química del consumo de ayahuasca durante el Periodo Medio (500–1000 d.C.). Lat Am Antiq 21:441–450. [Google Scholar]
- 60.Ogalde JP, et al. (2017) Consumo prehispánico de sustancias psicoactivas en el norte de Chile sugiere redes tempranas de intercambio con el altiplano central y la amazonía. Interciencia 42:459–463. [Google Scholar]
- 61.Holmstedt B, Lindgren JE (1967) Chemical constituents and pharmacology of South American snuffs. Ethnopharmacologic Search for Psychoactive Drugs, ed Efron DH. (Pub Health Serv Publication 1645, Washington, DC: ), pp 339–373. [PubMed] [Google Scholar]
- 62.De Smet PAGMD, Rivier L (1985) Intoxicating snuffs of the Venezuelan Piaroa Indians. J Psychoactive Drugs 17:93–103. [DOI] [PubMed] [Google Scholar]
- 63.Rodd R. (2002) Snuff synergy: Preparation, use and pharmacology of yopo and Banisteriopsis caapi among the Piaroa of southern Venezuela. J Psychoactive Drugs 34:273–279. [DOI] [PubMed] [Google Scholar]
- 64.Glass‐Coffin B. (2010) Shamanism and San Pedro through time: Some notes on the archaeology, history, and continued use of an entheogen in Northern Peru. Anthropol Conscious 21:58–82. [Google Scholar]
- 65.Burger R. (2011) What kind of hallucinogenic snuff was used at Chavín de Huántar? An iconographic identification. Ñawpa Pacha 31:123–140. [Google Scholar]
- 66.Mulvany EN. (1994) Posibles fuentes de alucinógenos en Wari y Tiwanaku: Cactus, flores, y frutos. Chungara (Arica) 26:185–209. [Google Scholar]
- 67.Moore C, Coulter C, Crompton K (2007) Determination of cocaine, benzoylecgonine, cocaethylene and norcocaine in human hair using solid-phase extraction and liquid chromatography with tandem mass spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci 859:208–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.