Significance
Posttraumatic stress disorder (PTSD) is an important risk factor for suicidal ideation, attempts, and death by suicide. Understanding of the biology underlying suicidality in PTSD is limited. In this study, we used positron emission tomography to evaluate the metabotropic glutamate receptor type 5 (mGluR5) as a potential treatment target and biomarker of suicidal ideation in individuals with PTSD and major depressive disorder (MDD). We found higher availability of mGluR5 in individuals with PTSD relative to healthy control and MDD groups. Furthermore, higher mGluR5 availability was associated with scan-day suicidal ideation among individuals with PTSD, but not MDD. Findings identify mGluR5 as a biomarker for intervention and potentially suicide risk management in PTSD.
Keywords: PTSD, mGluR5, suicidal ideation, PET
Abstract
Recent evidence implicates dysregulation of metabotropic glutamatergic receptor 5 (mGluR5) in pathophysiology of PTSD and suicidality. Using positron emission tomography and [18F]FPEB, we quantified mGluR5 availability in vivo in individuals with PTSD (n = 29) and MDD (n = 29) as a function of suicidal ideation (SI) to compare with that of healthy comparison controls (HC; n = 29). Volume of distribution was computed using a venous input function in the five key frontal and limbic brain regions. We observed significantly higher mGluR5 availability in PTSD compared with HC individuals in all regions of interest (P’s = 0.001–0.01) and compared with MDD individuals in three regions (P’s = 0.007). mGluR5 availability was not significantly different between MDD and HC individuals (P = 0.17). Importantly, we observed an up-regulation in mGluR5 availability in the PTSD-SI group (P’s = 0.001–0.007) compared with PTSD individuals without SI. Findings point to the potential role for mGluR5 as a target for intervention and, potentially, suicide risk management in PTSD.
Posttraumatic stress disorder (PTSD) is an important risk factor for suicidal ideation, suicidal attempts, and death by suicide (1, 2). Despite awareness of this risk, there is limited understanding of the biology underlying suicidality in PTSD. As a result, there are limited pharmacologic options to treat individuals with PTSD at high risk for suicide. Thus, there is an urgent need to study the neurobiology of suicidality in PTSD.
The metabotropic glutamate receptor type 5 (mGluR5) has emerged as a target of interest for PTSD and suicide research. mGluR5 is implicated in mood and anxiety symptoms in both human (3, 4) and animal studies (5, 6). We recently reported significantly higher mGluR5 availability in individuals with PTSD relative to matched controls across many brain regions (4). Also, a postmortem work found up-regulation of mGluR5 gene expression in the locus coeruleus associated with suicide in tissue from depressed individuals (7). mGluR5 activation moderates the function of N-methyl-d-aspartate glutamate receptors (NMDA-R) (8–10), an ionotropic glutamate receptor critical to synaptic plasticity (11, 12) and emotional learning (13, 14). NMDA-R functioning has also been implicated in the pathophysiology of suicidal behavior (15–17). The NMDA-R antagonist, ketamine, has demonstrated efficacy in reducing suicidal ideation. Also, a polymorphism in the GRIN2B gene was associated with both impulsivity (18) and suicide attempt (19). Thus, dysregulation in mGluR5 may affect the development of suicidal behavior both directly and through downstream effects. However, the relationship between mGluR5 and suicidal behavior including suicidal ideation, in individuals with PTSD or other psychiatric diagnosis, has not been explored in vivo.
The present study used positron emission tomography (PET) to examine the relationship between mGluR5 availability and suicidal ideation in vivo in individuals with PTSD compared to those with major depressive disorder (MDD). Using [18F]FPEB—a radioligand with high selectivity and specificity (20) for the negative allosteric modulator site on mGluR5—we sought to quantify mGluR5 availability in three groups of individuals: those with (i) Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) PTSD with or without MDD, (ii) DSM-5 MDD without PTSD, and (iii) healthy comparison controls (HC). Based on our previous work (4, 3), we hypothesized that mGluR5 availability would be higher in individuals with PTSD relative to MDD and HC. Furthermore, we hypothesized that greater dysregulation in mGluR5 availability would be observed in individuals with suicidal ideation (SI) in the PTSD group. We also sought to examine whether dysregulation in mGluR5 availability would be associated with symptoms of PTSD and suicidality, such as anxiety and avoidance.
Results
Primary Analyses.
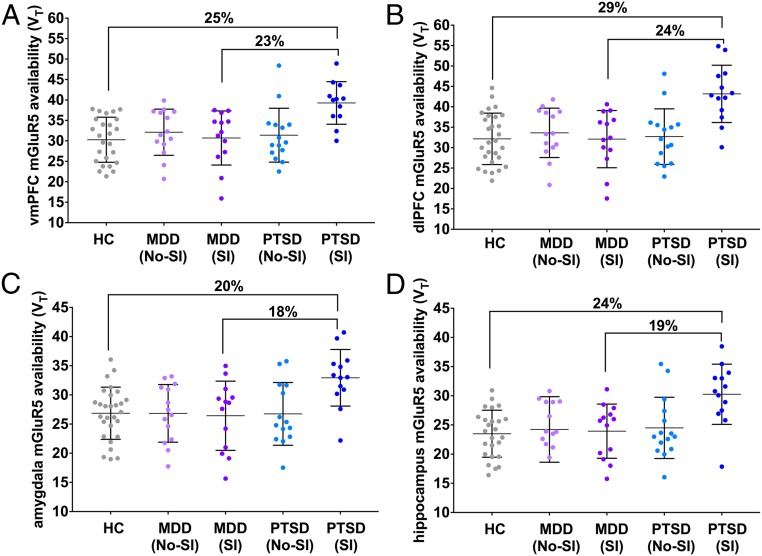
Groups were matched and did not differ significantly in age, race, gender, or depressive symptom severity. Similarly, diagnostic subgroups (i.e., PTSD with scan-day SI compared with PTSD without SI) did not differ significantly on the noted variables. First, a multivariate ANOVA was conducted to evaluate group differences in mGluR5 availability. A significant main effect of diagnosis was observed (F4,74 = 3.35, P = 0.009). Post hoc Tukey’s HSD tests suggested that mGluR5 availability was significantly higher in the PTSD group compared with HC in each of the five regions of interest; P values ranged from P < 0.001 [dorsolateral prefrontal cortex (dlPFC)] to P = 0.01 [ventromedial PFC (vmPFC)], an average of 19% higher (see Table 1 for mean regional VT values, % differences, and Cohen’s d values). Similarly, mGluR5 availability was higher in the PTSD group compared with MDD in the orbitofrontal cortex (OFC) (15%, P = 0.007), dlPFC (17%, P = 0.007), and hippocampus (15%, P = 0.007; SI Appendix, Table S1). There were no differences in mGluR5 availability between the MDD and HC groups (1–8% difference, P’s = 0.25–0.60), as previously shown by our group and others (3, 21).
Table 1.
Regional [18F]FPEB VT and significance values across reported suicidal ideation in both PTSD and MDD groups
| Region | MDD-No SI, n = 14 | MDD-SI, n = 15 | PTSD-No SI, n = 15 | PTSD-SI, n = 14 | Cohen’s d (PTSD) | P value (PTSD) |
| dlPFC | 33.63 (6.04) | 33.84 (9.39) | 32.71 (6.80) | 43.18 (7.01) | 1.51 | 0.001 |
| vmPFC | 32.11 (5.64) | 32.34 (8.54) | 31.39 (6.59) | 40.81 (7.45) | 1.34 | 0.001 |
| OFC | 30.64 (5.32) | 30.60 (8.78) | 30.39 (6.21) | 38.46 (3.94) | 1.55 | 0.003 |
| Amygdala | 26.83 (4.93) | 27.23 (7.22) | 26.75 (5.38) | 32.93 (4.84) | 1.21 | 0.007 |
| Hippocampus | 24.62 (5.61) | 25.01 (6.01) | 24.49 (5.25) | 30.26 (5.15) | 1.11 | 0.004 |
Mean (SD) reported. dlPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex.
To evaluate the relationship between mGluR5 availability and SI, ANOVAs with brain regions as dependent variables and SI as the independent variable were conducted within each clinical group. The main effect of SI was significant in the PTSD group (F7,16 = 4.03, P = 0.01), but not the MDD group (F8,16 = 0.27, P = 0.96; Table 1 and SI Appendix, Fig. S1). Post hoc tests in the PTSD group revealed significantly higher mGluR5 availability in individuals reporting scan-day SI in each of the five regions of interest (P values ranging from 0.001 to 0.007, average 24% difference; Fig. 1, Table 1, and SI Appendix, Fig. S2). In keeping with results from our prior work, we found a significant positive correlation between mGluR5 availability and scores on the avoidance subscale of the PTSD checklist for DSM-5 (PCL-5) in the vmPFC (r = 0.57, P = 0.007). Finally, peripheral cortisol samples were collected from plasma in the PTSD group at the beginning of the scan day and again immediately before scan. Cortisol levels at scan time were inversely correlated with mGluR5 availability in dlPFC (r = −0.69, P = 0.02) and OFC (r = −0.72, P = 0.02).
Fig. 1.
mGluR5 availability among PTSD individuals (with and without suicidal ideation), MDD individuals (with and without SI), and matched HCs in representative regions: (A) hippocampus; (B) amygdala; (C) dorsolateral prefrontal cortex; (D) ventromedial prefrontal cortex. Percentage differences reflect PTSD: SI vs. HC and MDD-SI vs. PTSD-SI. Error bars represent SD.
Secondary Analyses.
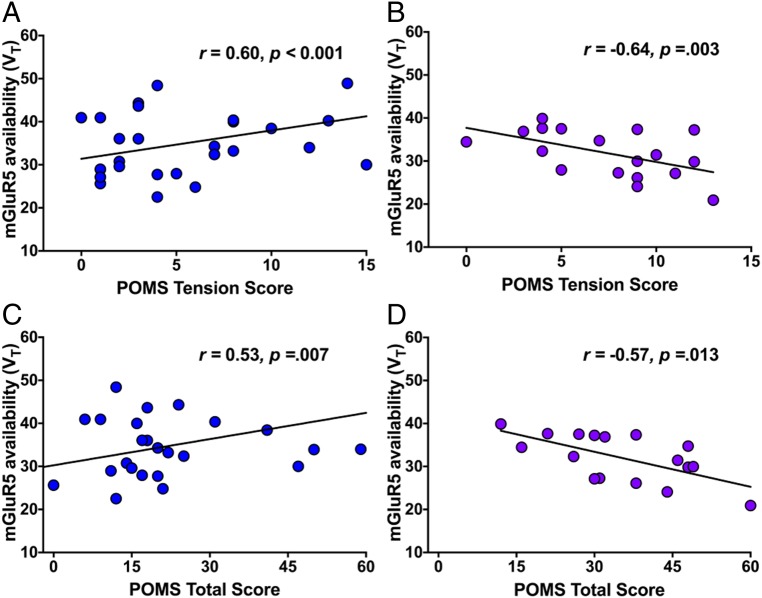
We conducted further analyses to investigate relationships between mGluR5 availability and suicide-related endophenotypic variables. Interestingly, we observed a divergent pattern of associations between mGluR5 availability and both total and subscales on the Profile of Mood States (POMS) in PTSD compared with MDD groups. POMS total score (POMS-total) was positively correlated with mGluR5 availability in the PTSD group (vmPFC, r = 0.60, P < 0.001; Fig. 2) and inversely correlated with mGluR5 availability in the MDD group (vmPFC, r = −0.64, P = 0.003; dlPFC, r = −0.67, P = 0.002; hippocampus, r = −0.55, P = 0.01). Similarly, PTSD group mGluR5 availability was positively correlated with subscores on the POMS tension (POMS-T) (vmPFC, r = 0.53, P = 0.007) and anxiety (POMS-A) (vmPFC, r = 0.47, P = 0.008) subscales in various regions, while inverse correlations were observed in the MDD group for both subscales (POMS-T: dlPFC, r = −0.52, P = 0.01; vmPFC, r = −0.57, P = 0.01; POMS-A: dlPFC, r = −0.55, P = 0.01; OFC, r = −0.38, P = 0.01).
Fig. 2.
(A) Positive association between mGluR5 availability in the vmPFC and score on the tension subscale of the POMS (POMS-T) among individuals with PTSD. (B) Negative association between mGluR5 availability in the vmPFC and POMS-T score among individuals with MDD. (C) Positive association between mGluR5 availability in the vmPFC POMS-total score among individuals with PTSD. (D) Negative association between mGluR5 availability in the vmPFC and POMS-total score among individuals with MDD.
Upon further examination, we detected that the association between mGluR5 availability and POMS-total in the PTSD group was driven by the PTSD-SI group (vmPFC, r = −0.72, P = 0.005); no significant association between POMS-total and mGluR5 availability was observed in individuals without SI in the PTSD group. To further examine the relationship between SI, mGluR5 availability, and POMS-total (mood variability), we repeated the primary analysis examining mGluR5 availability in PTSD-SI and PTSD without SI with POMS-total score included in the model. Results were similar to the original analysis in the PTSD group; the main effect of SI was significant (F5,19 = 3.58, P = 0.02), while the main effect of POMS-total (F5,19 = 2.37, P = 0.09) and the interaction of POMS-total ×SI (F5,19 = 1.38, P = 0.27) were not.
Discussion
In this investigation, we observed significantly higher mGluR5 availability in PTSD relative to both HC and MDD individuals in frontolimbic brain regions. These findings both confirm and extend results of our previous in vivo study, which showed mGluR5 up-regulation in PTSD relative to HC. Significantly, here we show that higher mGluR5 availability was also associated with scan-day suicidal ideation in the PTSD group only: PTSD-SI individuals exhibited significantly up-regulated frontolimbic mGluR5 availability compared with PTSD individuals without SI. No difference in mGluR5 availability was observed as a function of SI in the MDD group. Furthermore, dysregulation in mGluR5 in PTSD was associated with suicide-related endophenotypes including mood disturbance and anxiety. Thus, mGluR5 may represent a promising treatment target for the reduction of suicidal ideation in PTSD specifically.
At present, the mechanisms by which mGluR5 may be up-regulated in PTSD and in suicidal ideation in those with PTSD are not well understood. However, we hypothesize a combination of depression in hypothalamic-pituitary-adrenal (HPA) axis function (22–24) and up-regulation in scaffolding proteins that traffic and lock mGluR5 at the membrane (25, 26) might contribute to dysregulation in mGluR5 availability in PTSD suicidality. There is support for prominent differences between PTSD and MDD in HPA axis regulation. Specifically, studies suggest reduced glucocorticoid signaling in PTSD. For example, both epigenetic and postmortem studies suggest that down-regulation of FKBP5, a gene that controls the sensitivity of glucocorticoid receptors to cortisol, is associated with PTSD (27). Furthermore, FKBP5 has been shown to interact with childhood trauma exposure to increase risk for suicide attempt (28). By contrast, there is significant evidence of HPA axis hyperactivity in MDD, including heightened basal cortisol relative to comparison controls (29). These differences in cortisol level may help explain observed differences in mGluR5 availability across diagnostic groups; both the HPA axis and adrenal glucocorticoid system functioning may regulate glutamatergic neurotransmission, and therefore mGluR5 availability. Preclinical literature suggests that administration or stimulated release (30) of corticosterone reduces expression of mGluR5. Consistent with preclinical work, we observed an inverse correlation between mGluR5 availability and cortisol in PTSD individuals. Our preliminary data suggest that the reduced levels of cortisol in PTSD may contribute to mGluR5 up-regulation via trafficking and stabilization of mGluR5 at the cell surface. Specifically, we found up-regulation of the SHANK-1 (a glutamatergic scaffolding protein) gene in PTSD individuals postmortem (4). Notably, HPA axis hypoactivity, as observed in PTSD, has also been associated with suicidal behavior; studies suggest reduced glucocorticoid signaling in PTSD, and down-regulation of FKBP5 has been associated with risk for suicide attempt (28, 31, 32) and death by suicide (33, 34). Other insight into the relationship between suicidal behavior and mGluR5 might come from two candidate gene studies that have suggested that genetic alterations in the scaffolding protein Homer1 may be associated with suicide attempts (35, 36). Similarly, PSD-95, another glutamatergic scaffolding protein, has been associated with death by suicide (15). Together, while preliminary, these findings suggest that the location of mGluR5 (at the synapse versus internalized) may play a significant role in suicidality in PTSD. Taken together, these findings support the possibility that mGluR5 up-regulation in PTSD and suicidality occurs as a result of a combination of mGluR5 location at the cell and dampened glucocorticoid signaling.
With respect to suicidal ideation, the contrast in our findings across MDD and PTSD groups raises a crucial question: Is the neurobiology of suicide consistent across psychiatric diagnoses? A recent review of gene wide association studies that focused on suicide in both MDD and bipolar depression revealed inconsistent genetic findings and infrequent replication of results across studies (37). One possible explanation for such discrepancies concerns the failure to account for common comorbid diagnoses such as PTSD and other anxiety disorders. If the neurobiology of suicide varies as a function of psychiatric diagnosis, the failure to account for comorbidity could bias results. Our findings arguably provide some support for the presence of distinct neurobiological pathways to suicidal behavior: mGluR5 was associated with suicidal ideation in PTSD, but not MDD. Importantly, it is also possible that distinct pathways to suicidal behavior exist but are not directly related to diagnostic status. For example, Fudalej et al. (33) found that the association between a specific gene and death by suicide was stronger in individuals who were intoxicated at time of death. They argued that the differences in strength of association pointed to different neurobiological signatures for impulsive (more commonly associated with intoxication) versus planned suicide. Further work is needed before a definitive conclusion concerning sources of variability in the neurobiological mechanisms underlying suicide can be reached.
Careful characterization of the essential elements of constructs like SI and attempt (e.g., ideation reflecting hopelessness, ideation reflecting feeling overwhelmed, lethality of attempt method) should also be a priority of future work. In our sample, we observed a relationship between SI and POMS-total (mood variability) in the PTSD group. However, no significant interaction between POMS-total and SI was observed in ANOVA analyses. Thus, while SI in PTSD was related to mood variability in our sample, mood variability did not account for or significantly impact the relationship between SI and mGluR5 availability. We also examined bivariate relationships between SI and other relevant clinical variables in an effort to understand the nature of the construct (e.g., whether ideation in PTSD individuals in this sample reflected hopelessness, occurred in those with more severe symptom presentation, etc.). SI was not associated with PTSD or depressive symptom severity [Clinician Administered PTSD Scale for DSM-5 (CAPS) or Montgomery–Asberg Depression Scale (MADRS) total scores], hopelessness, or working memory or executive dysfunction. Future work should continue to explore the nature of SI in PTSD and in relation to mGluR5 availability.
Our exploratory findings also hold potentially significant implications for both research and treatment. We observed differential associations between mGluR5 availability and symptoms of depression and anxiety in PTSD and MDD groups. The observed pattern of findings suggests a potentially distinct role for mGluR5 in the pathology of PTSD relative to MDD. Higher frontolimbic mGluR5 was associated with more severe symptom experience across a number of domains in the PTSD group. The association between mGluR5 availability and symptoms of avoidance, also observed in our previous study, is particularly notable: relative to other symptom clusters, symptoms of avoidance have a stronger relationship with functional impairment (38, 39). Observed associations in the PTSD group are consistent with preclinical literature, which implicates mGluR5 in fear conditioning (5, 40, 41) and suggests that antagonism of mGluR5 can produce anxiolytic effects (42–44). Together, these findings support the potential importance of exploring down-regulation of mGluR5 as a treatment for symptom reduction in PTSD. By contrast, in MDD, lower frontolimbic mGluR5 availability was associated with more severe symptom presentation in a number of domains. In vivo findings of mGluR5’s role in MDD have been mixed (3, 45, 46), likely due to the difference in sample demographics. The role and significance of mGluR5 in the pathophysiology of MDD thus warrants additional exploration to clarify discrepant findings.
A number of study limitations should be noted. First, the participants were matched for age, sex, and smoking status, but not for history of trauma exposure. Future research should consider the potential impact of trauma exposure on mGluR5 availability in individuals who do not meet diagnostic criteria for PTSD. Second, diurnal variation has been shown to affect mGluR5 availability. We attempted to scan participants at roughly the same time of day (13:36 ± 2.3 h) to control for this potential confound. Third, analyses focused on select frontal and limbic brain regions, selected a priori based on strength of empirical evidence supporting their role in the pathophysiology of both PTSD and SI. Other regions, including anterior cingulate cortex and insula, have been shown to play significant roles in the neurobiology of PTSD and SI. Further work is needed to determine whether the observed alterations in mGluR5 availability are also apparent in these regions. Fourth, there is no region devoid of mGluR5 in the human brain (47, 48). As such, it is not possible to measure specific binding; rather, our outcome measure was VT, which includes both specific and nonspecific binding. Fifth, study sample size, specifically within diagnostic groups (SI compared with and without SI) may affect generalizability of results. Sixth, variability in SI severity in the present sample was limited due to study exclusion criteria. As such, SI was examined dichotomously. This strategy limits conclusions concerning a potential incremental relationship between SI severity and mGluR5 availability. Finally, SI was measured at a single time point. As such, variability in SI over time and the relationship between change in SI and mGluR5 variability was not assessed or accounted for in analyses.
This study investigates the role of mGluR5 in suicidality in vivo in both PTSD and MDD. We found a strong suggestion for mGluR5 dysregulation in suicidality in PTSD, but not in MDD. Greater dysregulation in mGluR5 was also associated with suicide-related endophenotypes including symptoms of mood dysregulation and anxiety. Direct and indirect treatments that might regulate mGluR5 have been investigated for other disorders (49–51), and thus pharmaceutics that might regulate mGluR5 are readily available for human clinical trials. The next step in this work might be to examine whether down-regulating mGluR5 via one of these mechanisms might aide with decreases in PTSD symptoms. Given the paucity of Food and Drug Administration-approved treatments for PTSD and the high risk of suicidality, continued investigation of the role of the glutamatergic system, and mGluR5 specifically, as targets for treatment and suicide risk management in PTSD should be prioritized.
Methods
Participants.
Twenty-nine individuals with PTSD (mean ± SD age = 35.55 ± 9.72 y; 16 females), 29 individuals with MDD (mean ± SD age = 36.69 ± 13.98 y; 14 females), and 29 healthy comparison control participants (mean ± SD age = 37.39 ± 12.01 y; 14 females) completed the study. Groups were matched by age, race, sex, and smoking status (Table 2). Sixteen of the 29 individuals with PTSD met the criteria for MDD. Of note, some participants [MDD, n = 18 (22); PTSD, n = 16 (21); HC, n = 18 (21, 22)] were also included in samples reported on previously. Eight individuals within the MDD group met criteria for comorbid DSM-5 anxiety disorders. Eleven PTSD participants and 6 MDD participants were medicated (using selective serotonin reuptake inhibitors or serotonin and norepinephrine reuptake inhibitors) at the time of the study. Participants ranged in age from 18 to 55 (see Table 2 for additional demographic information). All participants completed physical, psychiatric, and neurological examination at an initial screening visit to establish diagnosis and rule out any major medical or neurological illnesses. Screening included electrocardiography, complete blood counts, serum chemistries, thyroid function test, liver function test, urinalysis and urine toxicology screening, and plasma pregnancy tests (for women). Diagnosis was confirmed using the Structured Clinical Interview for DSM-5 (52). Symptoms were further assessed using the CAPS-5 (53), the PCL-5 (54), MADRS (55), and POMS (56), a measure of mood disturbance. The presence of SI was established based on participants’ scan-day report on both the MADRS and self-report Beck Depression Inventory (BDI) II (57). Scan-day SI was defined as endorsement of a “1” or higher on BDI item 9, a “2” or “3” on MADRS item 10 with overt endorsement of suicidal thoughts, or both. All but five participants included in the SI groups endorsed SI on both measures. Of note, individuals reporting active suicidal ideation were excluded from participation, which affected variability in reported SI. Across diagnoses four participants endorsed a “2” or higher on the BDI, and three participants endorsed a “3” on the MADRS. Reported criterion A traumatic events in the PTSD group included sexual assault (n = 4), witnessing of shooting (n = 3), sexual abuse (n = 6), physical abuse (n = 4), human trafficking (n = 1), car accident (n = 5), military combat (n = 3), and robbery at gunpoint (n = 1). Ten individuals in the MDD group reported a history of trauma exposure, including physical abuse (n = 3), car accident (n = 5), and natural disaster (n = 2). Information concerning trauma exposure was not collected from HC participants. Additional exclusion criteria for both MDD and PTSD groups were mild-to-severe substance use disorder (past 6 mo), or moderate-to-severe substance use disorder (past 12 mo) except for nicotine; positive urine toxicology or pregnancy tests at screening or before any scan; history of loss of consciousness for more than 5 min; and contraindications to magnetic resonance imaging (MRI) scans. Individuals in the MDD group could not meet criteria for current or historical diagnosis of PTSD. HC individuals could not meet current or lifetime criteria for any DSM-5 psychiatric diagnosis except for nicotine use disorders or have a first degree relative who met such criteria. The study was approved by the Yale University Human Investigation Committee and the Radioactive Drug Research Committee. All participants provided written informed consent.
Table 2.
PET study participant characteristics
| Variable | PTSD, n = 29 | PTSD-SI, n = 14 | PTSD-No SI | MDD, n = 29 | MDD-SI, n = 15 | MDD-No SI | Healthy Controls, n = 29 | P value* |
| Sex (M:F) | 16:13 | 6:8 | 10:5 | 14:15 | 6:9 | 8:6 | 14:15 | 0.47 |
| Age, y | 35.55 (9.72) | 32.38 (6.37) | 38.35 (10.60) | 36.69 (13.98) | 40.31 (14.24) | 35.63 (13.72) | 37.39 (12.01) | 0.84 |
| No. of smokers | 7 | 4 | 3 | 7 | 4 | 3 | 6 | 0.42 |
| Medicated | 11 | 4 | 7 | 6 | 3 | 3 | — | 0.82 |
| BMI (scan day) | 30.55 (5.57) | 30.02 (7.03) | 31.00 (4.59) | 27.83 (5.40) | 29.25 (4.56) | 27.20 (5.65) | 27.72 (3.28) | 0.16 |
| CAPS | 68.23 (23.45) | 70.05 (13.50) | 62.37 (20.05) | — | — | — | — | — |
| PCL-S | 54.1 (12.7) | 57.30 (8.50) | 39.10 (13.50) | — | — | — | — | — |
| MADRS | 19.41 (7.46) | 20.08 (8.20) | 19.07 (7.44) | 20.63 (8.65) | 23.56 (6.67) | 19.90 (7.34) | — | 0.61 |
| HAM-D | 15.53 (6.63) | 16.22 (6.12) | 14.90 (7.34) | 18.83 (4.62) | 17.75 (4.99) | 21.00 (4.24) | — | 0.27 |
Mean (SD) reported. *P values obtained from independent-sample t tests comparing PTSD, MDD, and HC (where applicable). Subsequent analyses confirmed no significant differences on the noted variables between PTSD individuals and either HC or MDD individuals. BMI, Body Mass Index; CAPS, Clinician Administered PTSD Scale; HAM-D, Hamilton depression rating scale; PCL, PTSD Checklist; MADRS, Montgomery–Asberg Depression Rating Scale; M:F, male:female.
MRI and PET Procedures.
T1-weighted MRI scans were acquired for all participants on a 3T scanner (Trio, Siemens Medical Systems). This was done both to evaluate potential structural abnormalities and to facilitate coregistration with PET data. The radiotracer [18F]FPEB was synthesized onsite at the Yale PET Center (as previously) (20). The [18F]FPEB was injected i.v. using a bolus plus infusion (B/I) paradigm over 120 min (20, 58). Equilibrium was reached at 60 min, and emission data were acquired 90–120 min after the start of injection on the high-resolution research tomograph (HRRT; Siemens/CTI). The HRRT has an intrinsic spatial resolution of ∼2.5 mm full width at half the maximum. Venous sampling was conducted at 15, 20, 25, 30, 40, 50, 60, 75, 90, 100, and 120 min post injection to permit calculation of a metabolite-corrected venous input function. This procedure has been validated by our research team in previous studies (3, 20). Venous sampling at baseline and 2 h later (immediately before scan) was also conducted for quantification of peripheral cortisol levels in PTSD participants only. A 6-min transmission scan was obtained for attenuation correction. Head motion was tracked using the Polaris Vicra optical tracking system (Vicra, NDI System Waterloo). There were no significant differences in the injected dose or mass between HC, MDD, or PTSD groups (overall injected M = 168 MBq, SD = 0.85). Dynamic scan data were reconstructed with corrections for attenuation, normalization, randoms, scatter, dead time, and motion using the ordered-subset expectation maximization-based MOLAR algorithm (59).
PET Image Analysis.
All PET images were first coregistered to participant’s T1-weighted MRI images using a six-parameter mutual information algorithm (FLIRT, FSL 3.2, Analysis Group, FMRIB). Images were then coregistered to the MR template via nonlinear transformation using the Bioimagesuite software (version 2.5; www.bioimagesuite.com). Regions of interest were identified using the Anatomical Automatic Labeling for SPM2 template. As detailed below, primary regions of interest included three subdivisions of the prefrontal cortex: vmPFC, OFC, and dlPFC, as well as the hippocampus and amygdala. Gray matter segmentation was conducted using the computational anatomy toolbox for SPM2 (CAT). No appropriate reference region completely devoid of mGluR5 is available in the human brain (60). As such, our outcome measure was calculated as volume of distribution (VT: ratio of metabolite-corrected radioligand concentration in region of interest to radioligand concentration in plasma, calculated at equilibrium). VT was estimated using the equilibrium analysis method with venous input function (3, 4, 20). No differences were observed in free fraction in plasma. Therefore, as previously, [18F]FPEB VT represents mGluR5 availability.
Statistical Analysis.
Statistical analyses were completed using SPSS Statistics v22 (IBM). For the PET portion of the study, independent-sample t tests and one-way ANOVAs were used to assess differences between demographic and clinical characteristics across groups. Primary analyses focused on five brain regions, selected on the basis of the strength of empirical evidence supporting their role in the pathophysiology of both PTSD and suicidal behavior, including ideation (61–63). This included three frontal/cortical regions (dlPFC, vmPFC, and OFC) and two subcortical regions (amygdala and hippocampus). Group differences in mGluR5 availability were assessed using a multivariate ANOVA with group as the independent variable and Tukey’s HSD post hoc comparison tests. Within-group differences in SI were similarly evaluated using multivariate ANOVAs performed separately in MDD and PTSD groups with brain regions as dependent variables and SI as the independent variable. In both cases, Tukey’s HSD tests were again performed to evaluate region-specific differences. Finally, to explore potentially meaningful associations between mGluR5 availability and clinical and demographic variables, Pearson correlations were used after we confirmed (using Kolmogorov–Smirnov tests) that primary variables of interest were normally distributed. A Bonferroni correction was used for all post hoc tests to control for potential family-wise error. A priori power analyses confirmed a minimum of 80% power to conduct all planned analyses.
Supplementary Material
Acknowledgments
We thank the staffs at the Yale PET Center, the National Center for PTSD (West Haven Campus), and the Yale Center for Clinical Investigation (YCCI), and the individuals who took part in the PET study. Funding support was provided by the Veterans Affairs National Center for PTSD (R.H.P., R.S.D., J.H.K., and I.E.), National Institute of Mental Health (NIMH) Grants K01MH092681 and R01MH104459 (to I.E.) and K08 MH117351-01 (to M.T.D.), National Institute on Drug Abuse Grant T32 DA022975-9 (to M.T.D.), a Dana Foundation Grant (to I.E.), and the YCCI.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818871116/-/DCSupplemental.
References
- 1.Bernal M, et al. ; ESEMED/MHEDEA Investigators (2007) Risk factors for suicidality in Europe: Results from the ESEMED study. J Affect Disord 101:27–34. [DOI] [PubMed] [Google Scholar]
- 2.Cougle JR, Keough ME, Riccardi CJ, Sachs-Ericsson N (2009) Anxiety disorders and suicidality in the National Comorbidity Survey-Replication. J Psychiatr Res 43:825–829. [DOI] [PubMed] [Google Scholar]
- 3.Abdallah CG, et al. (2017) Metabotropic glutamate receptor 5 and glutamate involvement in major depressive disorder: A multimodal imaging study. Biol Psychiatry Cogn Neurosci Neuroimaging 2:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes SE, et al. ; Traumatic Stress Brain Study Group (2017) Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proc Natl Acad Sci USA 114:8390–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tronson NC, et al. (2010) Metabotropic glutamate receptor 5/Homer interactions underlie stress effects on fear. Biol Psychiatry 68:1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethna F, Wang H (2016) Acute inhibition of mGluR5 disrupts behavioral flexibility. Neurobiol Learn Mem 130:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandley MJ, et al. (2014) Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int J Neuropsychopharmacol 17:1569–1578. [DOI] [PubMed] [Google Scholar]
- 8.Uslaner JM, et al. (2009) Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 57:531–538. [DOI] [PubMed] [Google Scholar]
- 9.Clifton NE, Morisot N, Girardon S, Millan MJ, Loiseau F (2013) Enhancement of social novelty discrimination by positive allosteric modulators at metabotropic glutamate 5 receptors: Adolescent administration prevents adult-onset deficits induced by neonatal treatment with phencyclidine. Psychopharmacology (Berl) 225:579–594. [DOI] [PubMed] [Google Scholar]
- 10.Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B (2004) Functional interaction between NMDA and mGlu5 receptors: Effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology 29:1259–1269. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie CF, Ressler KJ (2005) Emotional learning and glutamate: Translational perspectives. CNS Spectr 10:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosjean B, Tsai GE (2007) NMDA neurotransmission as a critical mediator of borderline personality disorder. J Psychiatry Neurosci 32:103–115. [PMC free article] [PubMed] [Google Scholar]
- 13.Ressler KJ, et al. (2004) Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61:1136–1144. [DOI] [PubMed] [Google Scholar]
- 14.Barret O, et al. (2010) Quantitation of glutamate mGluR5 receptor with 18F-FPEB PET in humans. J Nucl Med 51:215. [Google Scholar]
- 15.Dean B, et al. (2016) Changes in cortical N-methyl-D-aspartate receptors and post-synaptic density protein 95 in schizophrenia, mood disorders and suicide. Aust N Z J Psychiatry 50:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fudalej S, et al. (2017) An association between genetic variation in the glutamatergic system and suicide attempts in alcohol-dependent individuals. Am J Addict 26:595–601. [DOI] [PubMed] [Google Scholar]
- 17.Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS (2015) Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 20:1057–1068. [DOI] [PubMed] [Google Scholar]
- 18.Dorval KM, et al. (2007) Association of the glutamate receptor subunit gene GRIN2B with attention-deficit/hyperactivity disorder. Genes Brain Behav 6:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokolowski M, Ben-Efraim YJ, Wasserman J, Wasserman D (2013) Glutamatergic GRIN2B and polyaminergic ODC1 genes in suicide attempts: Associations and gene-environment interactions with childhood/adolescent physical assault. Mol Psychiatry 18:985–992. [DOI] [PubMed] [Google Scholar]
- 20.Park E, et al. (2015) Test-retest reproducibility of the metabotropic glutamate receptor 5 ligand [18F]FPEB with bolus plus constant infusion in humans. Eur J Nucl Med Mol Imaging 42:1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLorenzo C, et al. (2015) Characterization of brain mGluR5 binding in a pilot study of late-life major depressive disorder using positron emission tomography and [11C]ABP688. Transl Psychiatry 5:e693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yehuda R, et al. (1995) Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry 152:982–986. [DOI] [PubMed] [Google Scholar]
- 23.Boscarino JA. (1996) Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam veterans: Findings and clinical implications. J Consult Clin Psychol 64:191–201. [DOI] [PubMed] [Google Scholar]
- 24.Goenjian AK, et al. (1996) Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry 153:929–934. [DOI] [PubMed] [Google Scholar]
- 25.Wagner KV, et al. (2015) Homer1/mGluR5 activity moderates vulnerability to chronic social stress. Neuropsychopharmacology 40:1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu JC, et al. (1999) Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23:583–592. [DOI] [PubMed] [Google Scholar]
- 27.Mehta D, Binder EB (2012) Gene × environment vulnerability factors for PTSD: The HPA-axis. Neuropharmacology 62:654–662. [DOI] [PubMed] [Google Scholar]
- 28.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch M-A (2010) Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology 35:1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stetler C, Miller GE (2011) Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom Med 73:114–126. [DOI] [PubMed] [Google Scholar]
- 30.Wagner KV, et al. (2013) Homer1 mediates acute stress-induced cognitive deficits in the dorsal hippocampus. J Neurosci 33:3857–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brent D, et al. (2010) Association of FKBP5 polymorphisms with suicidal events in the treatment of resistant depression in adolescents (TORDIA) study. Am J Psychiatry 167:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch M-A (2012) Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res 46:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fudalej S, et al. (2015) Association between FKBP5 functional polymorphisms and completed suicide. Neuropsychobiology 72:126–131. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Ortiz JM, García-Gutiérrez MS, Navarrete F, Giner S, Manzanares J (2013) Gene and protein alterations of FKBP5 and glucocorticoid receptor in the amygdala of suicide victims. Psychoneuroendocrinology 38:1251–1258. [DOI] [PubMed] [Google Scholar]
- 35.Rao S, et al. (2017) Resequencing three candidate genes discovers seven potentially deleterious variants susceptibility to major depressive disorder and suicide attempts in Chinese. Gene 603:34–41. [DOI] [PubMed] [Google Scholar]
- 36.Strauss J, et al. (2012) Association study of early-immediate genes in childhood-onset mood disorders and suicide attempt. Psychiatry Res 197:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokolowski M, Wasserman J, Wasserman D (2014) Genome-wide association studies of suicidal behaviors: A review. Eur Neuropsychopharmacol 24:1567–1577. [DOI] [PubMed] [Google Scholar]
- 38.Lunney CA, Schnurr PP (2007) Domains of quality of life and symptoms in male veterans treated for posttraumatic stress disorder. J Trauma Stress 20:955–964. [DOI] [PubMed] [Google Scholar]
- 39.Schnurr PP, Lunney CA (2008) Exploration of gender differences in how quality of life relates to posttraumatic stress disorder in male and female veterans. J Rehabil Res Dev 45:383–393. [DOI] [PubMed] [Google Scholar]
- 40.Schulz B, et al. (2001) The metabotropic glutamate receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) blocks fear conditioning in rats. Neuropharmacology 41:1–7. [DOI] [PubMed] [Google Scholar]
- 41.Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE (2002) The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci 22:5219–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballard TM, et al. (2005) The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: A comparison. Psychopharmacology (Berl) 179:218–229. [DOI] [PubMed] [Google Scholar]
- 43.Mikulecká A, Mareš P (2009) Effects of mGluR5 and mGluR1 antagonists on anxiety-like behavior and learning in developing rats. Behav Brain Res 204:133–139. [DOI] [PubMed] [Google Scholar]
- 44.Krystal JH, et al. (2010) Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs 24:669–693. [DOI] [PubMed] [Google Scholar]
- 45.Esterlis I, et al. (2017) Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: An [11C] ABP688 and PET imaging study in depression. Mol Psychiatry 23:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deschwanden A, et al. (2011) Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry 168:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeLorenzo C, et al. (2011) In vivo positron emission tomography imaging with [11C]ABP688: Binding variability and specificity for the metabotropic glutamate receptor subtype 5 in baboons. Eur J Nucl Med Mol Imaging 38:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kågedal M, et al. (2013) A positron emission tomography study in healthy volunteers to estimate mGluR5 receptor occupancy of AZD2066: Estimating occupancy in the absence of a reference region. Neuroimage 82:160–169. [DOI] [PubMed] [Google Scholar]
- 49.Blackshaw LA, Page AJ, Young RL (2011) Metabotropic glutamate receptors as novel therapeutic targets on visceral sensory pathways. Front Neurosci 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brodkin J, Busse C, Sukoff SJ, Varney MA (2002) Anxiolytic-like activity of the mGluR5 antagonist MPEP: A comparison with diazepam and buspirone. Pharmacol Biochem Behav 73:359–366. [DOI] [PubMed] [Google Scholar]
- 51.Matosin N, et al. (2014) Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: Implications for novel mGluR-based therapeutics. J Psychiatry Neurosci 39:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.First MB, Spitzer RL, Gibbon M, Williams JB (1997) User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version (American Psychiatric Pub, Washington, DC: ). [Google Scholar]
- 53.Blake D, et al. (1990) A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behav Ther 18:187–188. [Google Scholar]
- 54.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL (2015) The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. J Trauma Stress 28:489–498. [DOI] [PubMed] [Google Scholar]
- 55.Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- 56.McNair DM, Lorr M, Droppleman LF (1971) Manual: Profile of Mood States (Educational and Industrial Testing Service, San Diego: ). [Google Scholar]
- 57.Beck AT, Steer RA, Brown GK (1996) Manual for the Beck Depression Inventory-II (Psychological Corporation, San Antonio, TX: ), Vol 1, p 82. [Google Scholar]
- 58.Sullivan JM, et al. (2013) Kinetic analysis of the metabotropic glutamate subtype 5 tracer [(18)F]FPEB in bolus and bolus-plus-constant-infusion studies in humans. J Cereb Blood Flow Metab 33:532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carson RE, Barker WC, Liow J-S, Johnson CA (2003) Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. 2003 IEEE Nuclear Science Symposium Conference Record (IEEE, Piscataway, NJ).
- 60.Patel S, et al. (2007) Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl Med Biol 34:1009–1017. [DOI] [PubMed] [Google Scholar]
- 61.Cox Lippard ET, Johnston JA, Blumberg HP (2014) Neurobiological risk factors for suicide: Insights from brain imaging. Am J Prev Med 47(Suppl 2):S152–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lutz PE, Mechawar N, Turecki G (2017) Neuropathology of suicide: Recent findings and future directions. Mol Psychiatry 22:1395–1412. [DOI] [PubMed] [Google Scholar]
- 63.Mann JJ. (1998) The neurobiology of suicide. Nat Med 4:25–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.