Abstract
STUDY QUESTION
In women undergoing IVF or ICSI cycles, do recombinant gonadotrophins differ from urinary-derived highly purified human menopausal gonadotropin (HP-hMG) or highly purified follicle-stimulating hormone (HP-FSH) in the total amount of gonadotrophins required to reach a live birth?
SUMMARY ANSWER
The difference between recombinant and urinary-derived HP-hMG or HP-FSH in the required amount to reach a live birth in IVF/ICSI cycles appears small.
WHAT IS KNOWN ALREADY
At present, gynecologists can choose between recombinant FSH (rFSH), urinary-derived HP-hMG and HP-FSH. These products are equally effective and safe, but it is unknown how these gonadotrophins compare in terms of IU required to reach a live birth.
STUDY DESIGN, SIZE AND DURATION
We conducted a search in Medline, Embase and CINAHL up to July 2018. We included randomized controlled trials (RCTs) that compared rFSH with HP-hMG or HP-FSH for ovarian stimulation in couples scheduled for IVF or ICSI treatment. From each randomized trial, we extracted the outcome data and information on participants, methods, interventions and funding.
PARTICIPANTS/MATERIALS, SETTING AND METHODS
Women undergoing ovarian stimulation with rFSH, HP-hMG or HP-FSH were included. We extracted data for the mean amount of gonadotrophins with SD, clinical pregnancy rate, live birth rate and cumulative live birth rate per woman from the included RCTs. We summarized these outcomes by calculating the individual and pooled mean difference (MD) or relative risk (RR) with 95% CI. We used the Review Manager software to perform the meta-analyses. We applied a random effect model to pool the data. We estimated the total amount of gonadotrophins used per extra live birth by STATA 14.2 and R software.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 28 studies with 7553 women were included in this review, of which 24 studies provided information on the total amount of gonadotrophins per woman who started an IVF/ICSI cycle. The total amount of gonadotrophins varied significantly between studies. The MDs in total amount were −37 IU (seven studies; N = 3220; 95% CI, −115 to 41; I2 = 68%) for rFSH versus HP-hMG and −31 IU (17 studies; N = 3629; 95% CI, −290 to 228; I2 = 97%) for rFSH versus HP-FSH. For rFSH versus HP-hMG, the RR for clinical pregnancy, live birth and cumulative live birth were 0.90 (95% CI, 0.81–1.00), 0.88 (95% CI, 0.78–0.99) and 0.91 (95% CI, 0.80–1.04), respectively. For rFSH versus HP-FSH, the RR for clinical pregnancy and live birth were 1.03 (95% CI, 0.94–1.13) and 1.03 (95% CI, 0.90–1.18), respectively; the data on cumulative live birth rate were lacking. The estimated difference in mean gonadotrophin amount per extra live birth was 789 IU (95% CI, −9.5 to 1570) for rFSH versus HP-hMG and −365 IU (95% CI, −2675 to 1945) for rFSH versus HP-FSH.
LIMITATIONS, REASONS FOR CAUTION
There was severe heterogeneity in the total amount of gonadotrophins between studies. A small fraction of women did not start gonadotrophin treatment; this was usually not accounted for in the provided mean amount of gonadotrophins per study and might have affected the averaged total amount of gonadotrophins but is unlikely to have affected the differences in the amount between rFSH and HP-hMG or HP-FSH.
WIDER IMPLICATIONS OF THE FINDINGS
The differences in the required amount to reach a live birth between rFSH, HP-hMG and HP-FSH appear to be small. Decision-making should be based on convenience, availability, actual costs and patient preferences.
STUDY FUNDING/COMPETING INTERESTS
The authors declare no conflict of interest. No external funding was either sought or obtained for this study.
REGISTRATION NUMBER
Prospero CRD42016038238
Keywords: pregnancy, gonadotrophins dosage, IVF, FSH, gonadotrophins, ovulation stimulation, ICSI
WHAT DOES THIS MEAN FOR PATIENTS?
Gonadotrophins are injectable drugs that are used to stimulate oocyte growth as part of IVF treatment. Three types of gonadotrophins are used most frequently: urinary-derived FSH, human menopausal gonadotrophin and recombinant FSH. These gonadotrophins are known to be equally effective and safe but it is unknown whether they differ in required amount. Hence, a literature review was performed to evaluate whether the three gonadotrophins differ in amount required to achieve a pregnancy leading to the birth of a child. The authors found no substantial differences in required amount between the gonadotrophins. It is concluded that the gynecologists should choose the hormone based on convenience, availability, actual costs and patient preferences.
Introduction
Ovarian stimulation with gonadotrophins to induce the development of multiple follicles is the first phase of most ART (Macklon et al., 2006). At present, gynecologists can choose between three commercially available gonadotrophins for ovarian stimulation in IVF/ICSI: rFSH, highly purified (HP)-hMG and HP-FSH.
Recombinant FSH (rFSH) is manufactured by recombinant DNA technology using a Chinese hamster ovary cell line transfected with the genes encoding human FSH (follitropin a and follitropin b) or derived from a cell line of human fetal retinal origin (FE 999049). It has 99% purity, does not contain any LH activity and is very similar to natural FSH (Out et al., 1995; Olsson et al., 2014). HP urinary gonadotrophins (HP-hMG and HP-FSH) are derived from urine of postmenopausal women. HP-hMG contains <5% of co-purified proteins and a 1:1 ratio of FSH and LH bioactivity and due to purification steps HP-FSH contains <0.1 IU LH and <5% of co-purified proteins (Lunenfeld and Lunenfeld, 1997; Wolfenson et al., 2005).
The various gonadotrophin products are heterogeneous in isoform composition. The urinary preparations have more acidic isoforms compared to the recombinant products (Lispi et al., 2006). Basic FSH isoforms have a high receptor affinity in vitro and a short half-life in vivo compared to acidic FSH isoforms (Practice Committee of American Society for Reproductive Medicine, Birmingham, Alabama, 2008). It has been suggested that due to the lower acidity rFSH might be more biopotent in vivo and that, as a consequence, a lower total dose and less stimulation days compared to highly purified urinary-derived gonadotrophins would be required (Andersen and Ezcurra, 2011).
It has been widely discussed in the literature that the differences in live birth between gonadotrophins are limited. What we were interested in is their economic value. The actual cost in euros or dollars differs widely between countries and therefore our focus was on the resource use that determines eventual cost differences. This resource use was expressed as amount of IU used. Therefore, we systematically examined the evidence on the total amount of recombinant and highly purified urinary-derived gonadotropins required to reach a live birth after ovarian stimulation in couples undergoing IVF or ICSI treatment.
Materials and Methods
The review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The protocol for this systematic review is registered in the Prospective Register of Systematic Reviews (PROSPERO) in April 2016 (Registration: Prospero CRD42016038238, available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016038238).
Literature search
We conducted a literature search in the databases Medline, Embase and CINAHL up to July 2018. We used the same search terms as the Cochrane review on the effectiveness of recombinant FSH versus urinary-derived gonadotrophins (van Wely et al., 2011) (Supplementary data).
Inclusion criteria and study selection
We screened all records for eligibility by analyzing the title and abstract. We included randomized controlled trials comparing rFSH with HP-hMG or HP-FSH for ovarian stimulation in couples with an indication for IVF or ICSI treatment. We excluded studies that compared rFSH with hMG or pituitary extract-FSH, because these products are no longer available for ovarian stimulation. There was no language restriction. After the eligibility screening we analyzed the studies by reading the full text. The studies were selected by two reviewers (E.B. and M.W.).
Data extraction and outcomes
We extracted data on female age, previous ART cycles, the number of women in each study arm, intervention details such as agonist or antagonist use, total amount of gonadotrophins in IU per woman who started an IVF/ICSI cycle, clinical pregnancy, live birth, cumulative live birth following fresh and cryocycles and funding per included study. An overview of study characteristics is shown in Table I. Some trials reported the total number of ampoules used. We transformed this data into the amount of gonadotrophins in IU. Our primary outcome was the total amount of gonadotrophins in IU per woman who started an IVF/ICSI cycle required per live birth. Secondary outcomes were the total amount of gonadotrophins in IU per woman who started IVF/ICSI cycle, clinical pregnancy, cumulative live birth following fresh and cryocycles and oocytes retrieved.
Table I.
Characteristics of studies in a systematic review and meta-analysis of gonadotrophin required for a live birth.
| Study | Couples (n) | Age women (yrs) | Previous cycles (n) | Intervention | Control | Starting dose (IU) | Downregulation protocol | IVF/ICSI | Embryo transfers (N) | Funding |
|---|---|---|---|---|---|---|---|---|---|---|
| Abate et al. (2009) | 401 | 26–38 | <3 Oocyte retrievals | rFSH | HP-FSH | 225 | Long GnRH agonist | IVF/ICSI | 1–3 | No |
| Andersen et al. (2006) | 731 | 21–37 | <4 | rFSH | HP-hMG | 225 | Long GnRH agonist | IVF | 1–2 | Ferring |
| Antoine et al. (2007) | 150 | 18–39 | <3 | rFSH | HP-FSH | 225 | Long GnRH agonist | IVF/ICSI | 1– | IBSA |
| Baker et al. (2009) | 152 | 18–39 | <3 | rFSH | HP-FSH | 300 | Long GnRH agonist | IVF/ICSI | 1–3 | IBSA |
| Bergh et al. (1997) | 235 | 18–38 | <4 ART cycles | rFSH | HP-FSH | 150 | Long GnRH agonist | IVF/ICSI | 2–3 | Serono |
| Bosch et al. (2008) | 280 | 18–37 | 0 | rFSH | HP-hMG | 225 | Antagonist | IVF/ICSI | 1–3 | No |
| Devroey et al. (2012) | 749 | 21–34 | <2 Cos cycles | rFSH | HP-hMG | 150 | Antagonist | ICSI | Max 1 | Ferring |
| Dickey et al. (2002) | 177 | 18–39 | No inclusion criteria | rFSH | HP-FSH | 250 | Long GnRH agonist | IVF | Max 4 | Ferring |
| Dickey et al. (2003) | 120 | 18–39 | No inclusion criteria | rFSH | HP-FSH | 225 | Long GnRH agonist | IVF | Max 4 | Ferring |
| EISG (2002) | 781 | 23–26 | <4 ART cycles | rFSH | HP-hMG | 225 | Long GnRH agonist | IVF/ICSI | 1–3 | Ferring |
| Ferraretti et al. (1999) | 141 | 29–45 | No ovarian response in previous cycle | rFSH | HP-FSH | 300 | No | IVF/ICSI | Max 3 | No |
| Franco et al. (2000) | 120 | <37 | Not reported | rFSH | HP-FSH | 150 | Long GnRH agonist | ICSI | Max 4 | Serono |
| Frydman et al. (2000) | 278 | 18–38 | <4 ART cycles | rFSH | HP-FSH | 150 | Long GnRH agonist | IVF | 2–5 | Serono |
| Gallego et al. (2003) | 100 | <40 | 0 | rFSH | HP-FSH | 225 | Long GnRH agonist | IVF | Max 3 | Not reported |
| Germond et al. (2000) | 79 | >35 | Not reported | rFSH | HP-FSH | 225 | No | IVF/ICSI | 2–3 | Not reported |
| Ghosh et al. (1999) | 47 | <37 | <4 IVF cycles | rFSH | FSH-HP | 150 rFSH and 225 FSH-HP | Long GnRH agonist | IVF | Max 3 | Not reported |
| Hompes et al. (2008) | 506 | 18–39 | 0 | rFSH | HP-hMG | 225 | Long GnRH agonist | IVF | 1–2 | Ferring |
| Hoomans et al. (1999) | 169 | 18–39 | <3 | rFSH | HP-FSH | 150 rFSH and 225 FSH-HP | Long GnRH agonist | IVF | Max 3 | Organon |
| Hugues et al. (2001) | 88 | 18–38 | Not reported | rFSH | HP-FSH | 100 rFSH and 150 FSH-HP | Short GnRH agonist | IVF | Max 3 | No |
| Kilani et al. (2003) | 100 | 18–37 | <3 | rFSH | HP-hMG | 150 | Long GnRH agonist | IVF/ICSI | 1–2 | No |
| Lenton et al. (2000) | 168 | 18–38 | 0 ART cycles | rFSH | HP-FSH | 150 | Long GnRH agonist | IVF/ICSI | 1–2 | Serono |
| Liu et al. (2015) | 508 | >37 | <3 | rFSH | HP-FSH | 300 | Long GnRH agonist | IVF/ICSI | Max 3 | No |
| Mohamed et al. (2006) | 257 | >39 | No inclusion criteria | rFSH | HP-FSH | 300 | Long GnRH agonist | IVF | Max 3 | No |
| Nardo et al. (2000) | 110 | Not reported | Not reported | rFSH | HP-FSH | Not reported | Long GnRH agonist | IVF | Max 3 | Not reported |
| O’Dea et al. (1993) | 114 | Not reported | Not reported | rFSH | HP-FSH | 150 rFSH and 225 FSH-HP | Long GnRH agonist | IVF | Not reported | Serono |
| Schats et al. (2000) | 496 | 18–38 | <3 ART cycles | rFSH | HP-FSH | 150 | Long GnRH agonist | IVF/ICSI | Max 3 | Serono |
| Selman et al. (2002) | 267 | 18–38 | 0 | rFSH | HP-FSH | 225 | Long GnRH agonist | IVF/ICSI | Max 3 | No |
| Ye et al. (2012) | 127 | >34 | 0 | rFSH | HP-hMG | 225 | Short GnRH agonist | IVF/ICSI | 2–3 | No |
rFSH: recombinant FSH, HP: highly purified.
In our protocol (Prospero CRD42016038238) we intended to report duration of stimulation. Although we did evaluate this outcome we decided not to present this analysis, since some days more or less stimulation time does not represent resource use, while total amount of gonadotropins used does.
Assessment of study quality
To check the quality of the article we used the risk of bias tool created by the Cochrane Collaboration (Cochrane Handbook). We looked at the following six domains: randomization sequence generation, allocation concealment, performance and detection bias, completeness of outcome data, selective outcome reporting and other potential sources. Risk of bias per study was expressed in the methodological quality graph as colored dots, with red dots meaning high risk of bias, yellow meaning unclear risk of bias and green meaning low risk of bias. Pooled evidence was scored using Grade Profiler 3.6.1 (GRADEpro Guideline Development Tool [Software]; McMaster University, 2015 [developed by Evidence Prime, Inc.]; available from gradepro.org.) as very low, low, moderate or high.
Statistical analysis
We retrieved for each individual study means and SD for the total amount of gonadotrophins per woman who started an IVF/ICSI cycle. We tried to retrieve these data and data on clinical pregnancy, live birth and cumulative live birth following fresh and cryocycles per woman treated with gonadotrophins. The outcomes were summarized by calculating the individual and pooled mean difference (MD) or relative risk (RR) with 95% CI for rFSH versus HP-hMG and rFSH versus HP-FSH. We used the Review Manager software (Review Manager [RevMan] Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) to perform these meta-analyses. Presence of heterogeneity was assessed by the I2 statistic. An I2 >50% was taken to indicate substantial heterogeneity. We calculated the mean amount of gonadotrophin used per clinical pregnancy per woman in STATA 14.2 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP). We estimated the MD and 95% CI in amount of gonadotrophins per extra clinical pregnancy, per extra live birth and per extra cumulative live birth achieved for the two gonadotrophin comparisons by bootstrapping techniques using Metafor (www.metaforproject.org) in R version 3.3.1. An extra, not pre-planned, analysis was done for oocytes retrieved and amount of gonadotrophin per extra oocyte retrieved.
Results
Search
We identified 362 records and hand searching resulted in another 11 records. After we removed the duplications, there were 348 records to screen for title and abstract. We excluded 302 records based on the inclusion and exclusion criteria. We assessed a total of 46 articles for eligibility by reading the full text after which we excluded another 18 studies expressing 19 comparisons. A summary of the search is presented in a flow diagram (Fig. 1).
Figure 1.
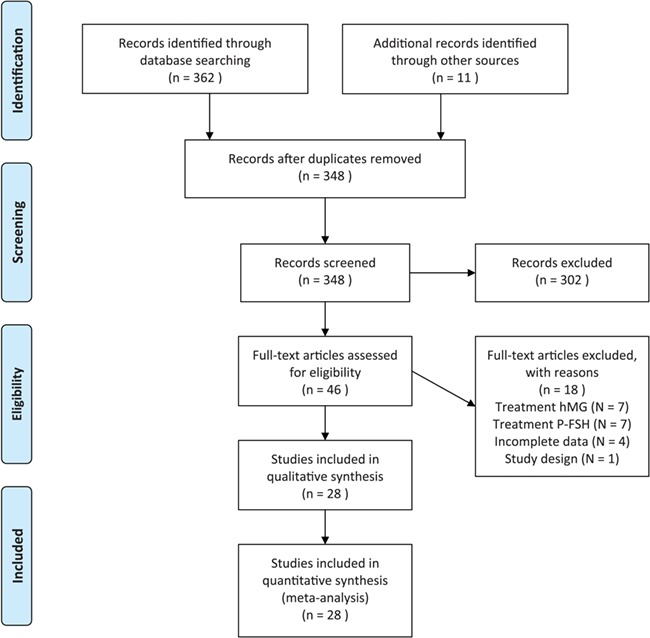
Flow diagram showing the search for articles for inclusion in the systematic review and meta-analysis. hMG = human menopausal gonadotropin. P-FSH = purified follicle-stimulating hormone.
Included studies
We included the 28 studies that met all selection criteria in the review. The total number of women was 7553. Seven studies compared rFSH with HP-hMG (n = 3397) and all provided data on the amount of gonadotrophin used. In total, 3220 women (95%) actually had started gonadotrophin treatment. Twenty-one studies compared rFSH with HP-FSH (n = 4156), but only 17 (n = 3775) provided data on amount of gonadotrophin used. In total, 3629 women (96%) actually had started gonadotrophin treatment. The studies varied in terms of female age, previous ART cycles, starting dose, downregulation protocol, fertilization method, number of embryo transfers and funding. The characteristics of each study can be found in Table I.
Assessment of study quality
The results of the assessment of the risks of bias using the Cochrane tool are summarized in Figs 2 and 3. The quality of the studies varied between low and high. Older studies performed before 2000 were more likely to be of lower quality. Five studies were only available as an abstract, all comparing rFSH versus HP-FSH (Ferraretti et al., 1999; Gallego et al., 2003; Germond et al., 2000; Ghosh et al., 1999; O’Dea et al., 1993). We considered these five studies to be of low quality, mainly due to incomplete outcomes, reporting bias and uncertainty about selection bias and other forms of bias.
Figure 2.
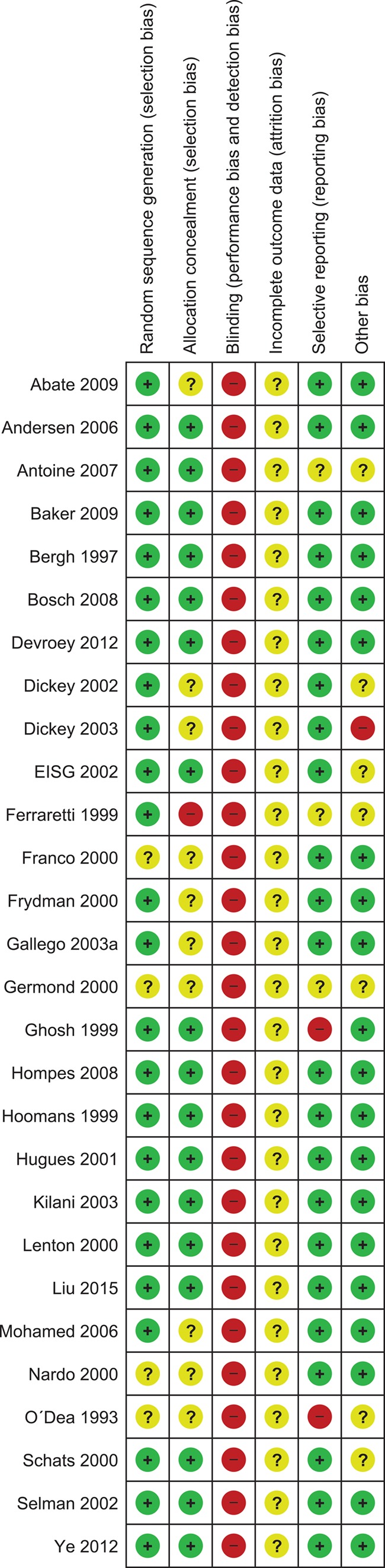
Methodological quality summary: authors’ judgments about each methodological quality item for each study included. Red dots: high risk of bias, yellow dots: unclear risk of bias, green dots: low risk of bias.
Figure 3.
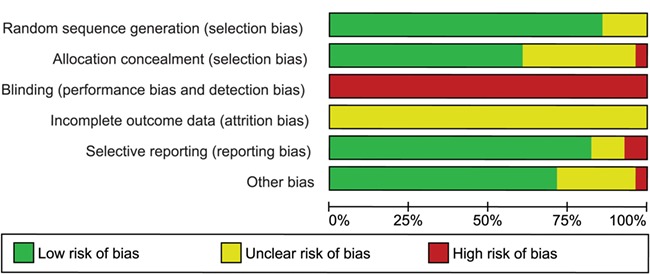
Methodological quality graph: authors’ judgments about each methodological quality item presented as percentages across all studies included.
In two studies we could not determine whether the data were intention-to-treat or not (Gallego et al., 2003; Liu et al., 2015). None of the trials used double blinding. Although double blinding is unlikely to have impact on pregnancy outcomes, it might have biased the outcome gonadotrophin amount.
Outcomes
Amount of gonadotrophins per woman that started IVF/ICSI cycle
Of the seven trials comparing rFSH with HP-hMG, three trials found no evidence of a difference in the amount of gonadotrophins, one found that more rFSH was required and three trials found that less rFSH was required. Pooling the data, we found no evidence of a difference in the amount of gonadotrophins (seven studies; N = 3220; MD, −37 IU; 95% CI, −115 to 41; I2 = 68%; low quality of evidence) (Fig. 4a).
Figure 4.
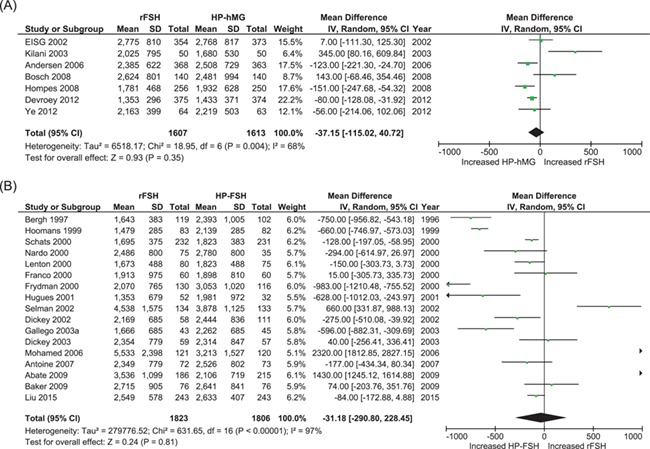
Meta-analyses comparing total amount of gonadotrophins used (IU) per woman that started an IVF/ICSI cycle. (A) Comparing recombinant FSH (rFSH) with urinary-derived highly purified hMG (HP-hMG). (B) Comparing rFSH with highly purified FSH (HP-FSH).
Of the 17 trials comparing rFSH with HP-FSH, seven trials found no evidence of a difference in the amount of gonadotrophins, three found that more rFSH was required and seven found that less rFSH was required. Pooling the data, we found no evidence of a difference in the amount of gonadotrophins (17 studies; N = 3629; MD −31 IU; 95% CI, −290 to 228; I2 = 97%; low quality of evidence) (Fig. 4b). For both comparisons there was severe statistical heterogeneity.
Pregnancy outcomes
Of the seven trials comparing rFSH and HP-hMG, all trials had data on clinical pregnancy and live birth rate and three trials (N = 2109) had data on cumulative live births following fresh and cryo-embryo transfers (Fig. 5).
Figure 5.
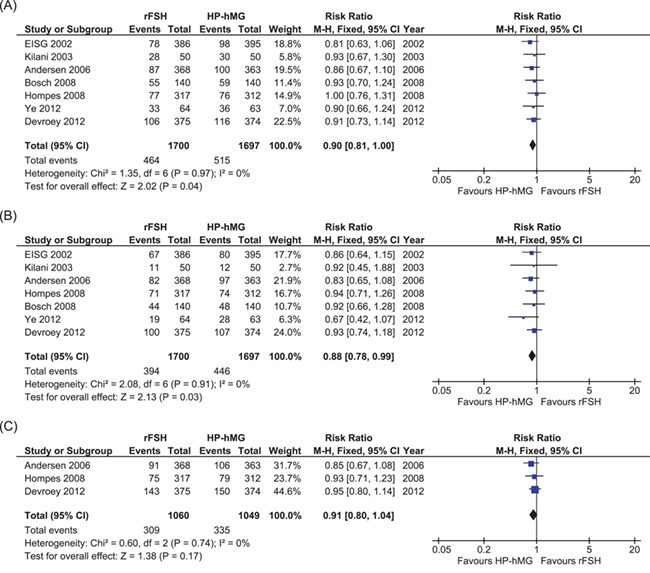
Meta-analyses comparing rFSH with HP-hMG. (A) Comparing clinical pregnancy. (B) Comparing live birth. (C) Comparing cumulative live birth.
We found a slightly lower clinical pregnancy rate and live birth rate in the rFSH-treated women than in the HP-hMG-treated women (seven studies, N= 3397. Clinical pregnancy: RR, 0.90; 95% CI, 0.81–1.00. Live birth: RR, 0.88; 95% CI, 0.78–0.99; high quality of evidence). We found insufficient evidence of a difference in cumulative live birth rate (three studies, N = 2109. Cumulative live birth: RR, 0.91; 95% CI, 0.80–1.04). There was no indication of heterogeneity for these outcomes across studies (I2 = 0%, high quality of evidence).
Of the 21 trials comparing rFSH and HP-FSH, all trials had data on clinical pregnancy rate and 12 trials had data on live birth rate (Fig. 6). One trial had data on cumulative ongoing pregnancies following frozen-thawed embryo transfers (Out et al., 1995), but no trials had data on cumulative live birth rate.
Figure 6.
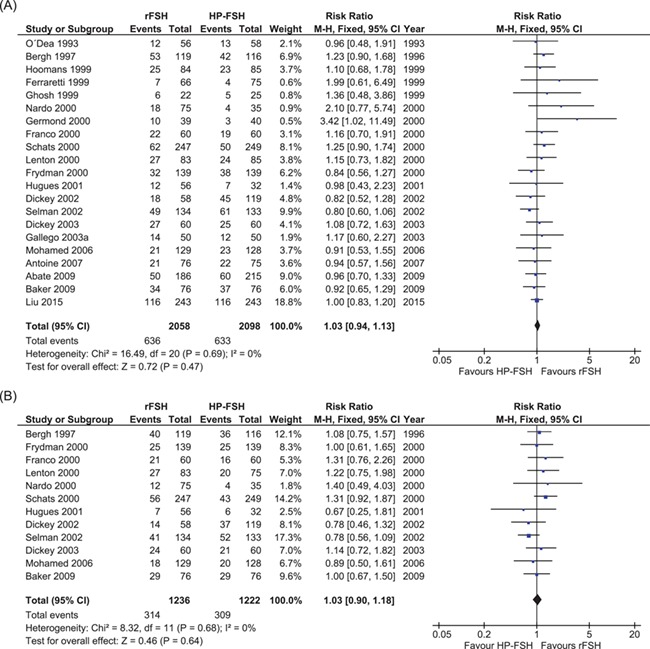
Meta-analyses comparing rFSH with HP-FSH. (A) Comparing clinical pregnancy. (B) Comparing live birth.
We found little or no difference in clinical pregnancy rate nor in live birth rate in the rFSH-treated women compared to HP-FSH-treated women (21 studies, N = 4165. Clinical pregnancy: RR, 1.03; 95% CI, 0.94–1.13. Twelve studies, N = 2458. Live birth: RR, 1.03; 95% CI, 0.90–1.18). There was no indication of heterogeneity across studies (I2 = 0%, high quality of evidence).
Required amount to reach a clinical pregnancy
Comparingv rFSH with HP-hMG, we found that the required amount to achieve a clinical pregnancy was 7168 IU (95% CI, 5007 to 9330) for rFSH and 5774 IU (95% CI, 4061 to 7488) for HP-hMG. The difference in mean gonadotrophin amount per extra clinical pregnancy was 720 IU (95% CI, −29 to 1470) for rFSH versus HP-hMG (Fig. 7).
Figure 7.
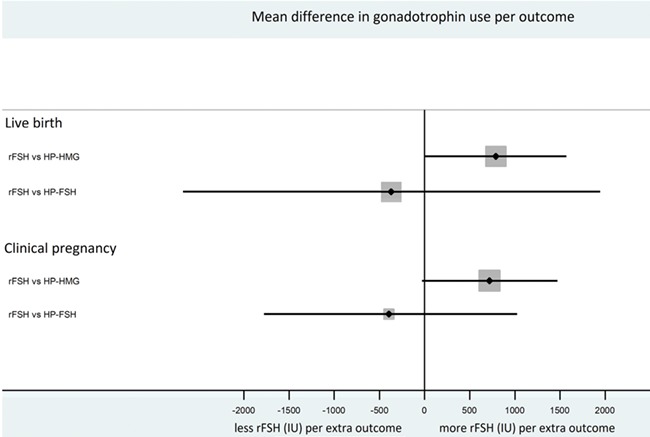
Forest plot showing the difference in gonadotrophin use per extra live birth and clinical pregnancy.
Comparing rFSH with HP-FSH, we found that the required amount to achieve a clinical pregnancy was 8017 IU (95% CI, 6714 to 9320) for rFSH and 9079 IU (95% CI, 7297 to 10861) for HP-FSH. The difference in mean gonadotrophin amount per extra clinical pregnancy was −380 IU (95% CI, −1778 to 1017) for rFSH versus HP-FSH (Fig. 7).
Required amount to reach a live birth
Comparing rFSH with HP-hMG, we found that the required amount to achieve a live birth was 8222 IU (95% CI, 5743 to 10 703) for rFSH and 6548 IU (95% CI, 4605 to 8491) for HP-hMG. The difference in mean gonadotrophin amount per extra live birth was 789 IU (95% CI, −9.5 to 1570) for rFSH versus HP-hMG (Fig. 7). In the three trials with data on cumulative live births the difference in mean gonadotrophin amount per extra live birth was 310 IU (95% CI, −158 to 778) for rFSH versus HP-hMG.
Comparing rFSH with HP-FSH, we found that the required amount to achieve a live birth was 9880 IU (95% CI, 8080 to 11489) for rFSH and 11 076 IU (95% CI, 8583 to 13924) for HP-FSH. The difference in mean gonadotrophin amount per extra live birth was −365 IU (95% CI, −2675 to 1945) for rFSH versus HP-FSH (Fig. 7).
Required amount for one more oocyte
Of the seven trials comparing rFSH and HP-hMG, all trials had data on the number of oocytes retrieved. Of the 21 trials comparing rFSH and HP-FSH, all trials had data on the number of oocytes retrieved. MD in number of oocytes retrieved was 1.83 (95% CI, 1.20 to 2.64; N = 3397; I2 = 70%; low quality evidence) for rFSH versus HP-HMG and was −0.57 (95% CI, −0.20 to 1.33; N = 2458; I2 = 80%; low quality evidence) for rFSH versus HP-FSH.
Comparing rFSH with HP-HMG, we found that the required amount per oocyte retrieved was 198 IU (95% CI, –38 to 433) for rFSH and 230 IU (95% CI, 107 to 352) for HP-HMG. The estimated difference in mean gonadotrophin amount per extra oocyte was –50 IU (95% CI, –276 to 226) for rFSH versus HP-FSH.
Comparing rFSH with HP-FSH, we found that the required amount per oocyte retrieved was 260 IU (95% CI, 17 to 503) for rFSH and 286 IU (95% CI, 153 to 418) for HP-FSH. The estimated difference in mean gonadotrophin amount per oocyte was −18 IU (95% CI, −322 to 304) for rFSH versus HP-FSH.
In all comparisons both oocyte number and amount of gonadotrophin differed across studies, creating severe statistical heterogeneity. This limits the external validity of the estimated required amount of gonadotrophin for one more oocyte presented here.
Discussion
In this systematic review and meta-analysis we evaluated the evidence on the total amount of gonadotrophins required to reach a live birth after ovarian stimulation with rFSH in comparison to HP-hMG or HP-FSH in couples undergoing IVF or ICSI treatment. We found no evidence of a difference in amount of gonadotrophins per woman who started an IVF/ICSI cycle and per live birth between rFSH and HP-hMG or HP-FSH.
The main strength of our study is that we were able to collect data for 7553 women from studies with a strong design. Also, the results are applicable to a general IVF/ICSI population and are important from a social-economic perspective.
A limitation lies in the severe clinical heterogeneity in mean total amount of gonadotrophins used across individual studies. Study year and country could partly explain this heterogeneity. Older studies often used higher starting doses.
Not all studies reported early drop outs, and for those studies we can thus not be certain that all women started gonadotrophin treatment. This might have affected the averaged total amount of gonadotrophins.
A further limitation of our review is that we did not evaluate potential differences in patient characteristics such as female age and indication for IVF. These factors could influence resource use. To take these differences into account an individual participant data meta-analysis would be necessary.
In our meta-analysis we focused on the total amount of gonadotrophins required to reach a clinical pregnancy. Previous meta-analyses focused on the effectiveness and safety of the different types of gonadotrophin (Coomarasamy et al., 2008; Al-Inany et al., 2009; van Wely et al., 2011; Gerli et al., 2013). These meta-analyses suggested that HP-hMG results in slightly more clinical pregnancies and live births than rFSH, possibly due to the LH activity in HP-hMG, but the relation to the required gonadotrophin amount per live birth was not studied.
We consider the required gonadotrophin amount per clinical pregnancy or per live birth to be representative of the biopotency of a gonadotrophin. A more biopotent product would require a lower total dose and less stimulation days in comparison to a less biopotent product. In general, basic FSH isoforms, such as rFSH, have a higher receptor affinity in vitro compared to more acid isoforms, such as HP-hMG and HP-FSH, but more acid isoforms have a longer plasma half-life compared to basic FSH isoforms. There has been much discussion on the presumed differences in biopotency of the various gonadotrophins related to isoform composition and acidity. Some studies describe that it is unknown whether these differences influence biopotency (Practice Committee of American Society for Reproductive Medicine, Birmingham, Alabama, 2008; Smitz et al., 2016), while others suggest that in view of the higher biopotency in vitro, rFSH might also be more biopotent in vivo (Barrios-De-Tomasi et al., 2002; Andersen et al., 2004; Andersen and Ezcurra, 2011), and one study suggests that, because of the longer plasma half-lives, more acid isoforms have an higher in vivo bioactivity (Wolfenson et al., 2005).
It can be argued that gonadotrophin amount per oocyte would better represent biopotency. Although not pre-defined, we added this outcome to our analysis and found insufficient evidence of a difference between rFSH and HP-HMG, and rFSH and HP-FSH. This analysis was hampered by the heterogeneity in the data.
Our results suggest that, although rFSH, HP-hMG and HP-FSH differ in isoform composition and acidity, as well as in LH activity, these gonadotrophins have a comparable biopotency.
We conclude that the differences in required amount of gonadotrophin to reach a live birth between rFSH, HP-hMG and HP-FSH are small. Decision-making should thus be based on convenience, availability, actual costs and patient preferences.
Supplementary Material
Acknowledgements
The authors thank the authors of the original papers for providing extra data.
Authors’ roles
E.B., M.W. and F.M. wrote the protocol. E.B. and M.W. managed the literature search and performed the meta-analysis. M.W. performed further calculations and estimations in STATA and R. All authors critically revised the manuscript and approved the final version.
Funding
No external funding was either sought or obtained for this study.
Conflict of interest
None declared.
References
- Abate A, Nazzaro A, Salerno A, Marzano F, Pavone Cossut MR, Perino M.. Efficacy of recombinant versus human derived follicle stimulating hormone on the oocyte and embryo quality in IVF-ICSI cycles: randomised, controlled, multi-centre trial. Gynecol Endocrinol 2009;25:479–484. [DOI] [PubMed] [Google Scholar]
- Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI.. Highly purified hMG achieves better pregnancy rates in IVF cycles but not ICSI cycles compared with recombinant FSH: a meta-analysis. Gynecol Endocrinol 2009;25:372–378. [DOI] [PubMed] [Google Scholar]
- Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod 2006;21:3217–3227. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Ezcurra D. What is the clinical relevance of follicle-stimulating hormone isoforms in fertility treatment? Reprod Biol Insights 2011;4:1–10. [Google Scholar]
- Andersen CY, Westergaard LG, van Wely M. FSH isoform composition of commercial gonadotrophin preparations: a neglected aspect? Reprod Biomed Online 2004;9:231–236. [DOI] [PubMed] [Google Scholar]
- Antoine JM, De Mouzon J, Nicollet B, Salle B, Urbancsek J, Grudzinskas JG.. Effectiveness and tolerability of hFSH compared to rFSH in ICSI: the European study. IBSA Satelite Symposium abstract, ESHRE, Lyon. 2007.
- Baker VL, Fujimoto VY, Kettel LM, Adamson GD, Hoehler F, Jones CE, Soules MR.. Clinical efficacy of highly purified urinary FSH versus recombinant FSH in volunteers undergoing controlled ovarian stimulation for in vitro fertilization: a randomized, multicenter, investigator-blind trial. Fertil Steril 2009;91:1005–1011. [DOI] [PubMed] [Google Scholar]
- Barrios-De-Tomasi J, Timossi C, Merchant H, Quintanar H, Avalos JM, Andersen CY, Ulloa-Aguirre A.. Assessment of the in vitro and in vivo biological activities of the human follicle-stimulating isohormones. Mol Cell Endocrinol 2002;186:189–198. [DOI] [PubMed] [Google Scholar]
- Bergh C, Howles CM, Borg K, Hamberger L, Josefsson B, Nilsson L, Wikland M.. Recombinant human follicle stimulating hormone (r-hFSH; Gonal-F) versus highly purified urinary FSH (Metrodin HP): results of a randomized comparative study in women undergoing assisted reproductive techniques. Hum Reprod 1997;12:2133–2139. [DOI] [PubMed] [Google Scholar]
- Bosch E, Vidal C, Labarta E, Simon C, Remohi J, Pellicer A.. Highly purified hMG versus recombinant FSH in ovarian hyperstimulation with GnRH antagonists—a randomized study. Hum Reprod 2008;23:2346–2351. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Afnan M, Cheema D, van der Veen F, Bossuyt PM, van Wely M.. Urinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IVF or ICSI treatment: a systematic review and meta-analysis. Hum Reprod 2008;23:310–315. [DOI] [PubMed] [Google Scholar]
- Devroey P, Pellicer A, Nyboe Andersen A, Arce JC.. A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril 2012;97:561–571. [DOI] [PubMed] [Google Scholar]
- Dickey RP, Nichols JE, Steinkampf MP, Gocial B, Thornton M, Webster BW, Bello SM, Crain J, Marshall DC.. Highly purified human-derived follicle-stimulating hormone (Bravelle) has equivalent efficacy to follitropin-beta (Follistim) in infertile women undergoing in vitro fertilization. Reprod Biol Endocrinol 2003;1:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey RP, Thornton M, Nichols J, Marshall DC, Fein SH, Nardi RV.. Comparison of the efficacy and safety of a highly purified human follicle-stimulating hormone (Bravelle) and recombinant follitropin-beta for in vitro fertilization: a prospective, randomized study. Fertil Steril 2002;77:1202–1208. [DOI] [PubMed] [Google Scholar]
- European and Israeli Study Group on Highly Purified Menotropin versus Recombinant Follicle-Stimulating Hormone (EISG) Efficacy and safety of highly purified menotropin versus recombinant follicle-stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: a randomized, comparative trial. Fertil Steril 2002;78:520–528. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Gianaroli L, Magli MC, Feliciani E, Gergolet M, Fortini D. Recombinant FSH versus urinary FSH in non-down regulated poorly responding patients. Abstract book, 11th World Congress of In vitro Fertilization and Human Reproductive Genetics, Vol. 263, 1999, abstract P196. [Google Scholar]
- Franco JG Jr, Baruffi RL, Coelho J, Mauri AL, Petersen CG, Garbellini E.. A prospective and randomized study of ovarian stimulation for ICSI with recombinant FSH versus highly purified urinary FSH. Gynecol Endocrinol 2000;14:5–10. [DOI] [PubMed] [Google Scholar]
- Frydman R, Howles CM, Truong F. A double-blind, randomized study to compare recombinant human follicle stimulating hormone (FSH; Gonal-F) with highly purified urinary FSH (Metrodin) HP in women undergoing assisted reproductive techniques including intracytoplasmic sperm injection. The French Multicentre Trialists. Hum Reprod 2000;15: 520–525. [DOI] [PubMed] [Google Scholar]
- Gallego PE, Fernandez-Shaw S, Mayoral M, Rodriguez L, Grande C, Pons I, Martinez V, Real GD.. The treatment with recombinant FSH improvement the embryo quality in IVF cycles. Rev Iberoam Fertil Reprod Hum 2003;20:43–50. [Google Scholar]
- Gerli S, Bini V, Favilli A, Di Renzo GC.. Clinical efficacy and cost-effectiveness of HP-human FSH (Fostimon(R)) versus rFSH (Gonal-F(R)) in IVF-ICSI cycles: a meta-analysis. Gynecol Endocrinol 2013;29:520–529. [DOI] [PubMed] [Google Scholar]
- Germond M, De Palma R, Senn A, Inaudi P, Dessole S, De Grandi P.. Recombinant versus highly purified urinary FSH to induce ovulation induction and pregnancies in women over 35 years in an IVF/ICSI programme. Hum Reprod 2000;15(Special Issue):Abstract O-118. [Google Scholar]
- Ghosh S, Chattopadhyay R, Goswami S, Chakravarty BN. Recombinant FSH versus highly purified urinary FSH—our experience. Abstract book, 11th World Congress of In vitro Fertilization and Human Reproductive Genetics Vol. 264,1999. [Google Scholar]
- Hompes PG, Broekmans FJ, Hoozemans DA, Schats R.. Effectiveness of highly purified human menopausal gonadotropin vs. recombinant follicle-stimulating hormone in first-cycle in vitro fertilization-intracytoplasmic sperm injection patients. Fertil Steril 2008;89:1685–1693. [DOI] [PubMed] [Google Scholar]
- Hoomans EH, Andersen AN, Loft A, Leerentveld RA, van Kamp AA, Zech H.. A prospective, randomized clinical trial comparing 150 IU recombinant follicle stimulating hormone (Puregon((R))) and 225 IU highly purified urinary follicle stimulating hormone (Metrodin-HP((R))) in a fixed-dose regimen in women undergoing ovarian stimulation. Hum Reprod 1999;14:2442–2447. [DOI] [PubMed] [Google Scholar]
- Hugues JN, Bry-Gauillard H, Bstandig B, Uzan M, Cedrin-Durnerin I.. Comparison of recombinant and urinary follicle-stimulating hormone preparations in short-term gonadotropin releasing hormone agonist protocol for in vitro fertilization-embryo transfer. J Assist Reprod Genet 2001;18:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilani Z, Dakkak A, Ghunaim S, Cognigni GE, Tabarelli C, Parmegiani L, Filicori M.. A prospective, randomized, controlled trial comparing highly purified hMG with recombinant FSH in women undergoing ICSI: ovarian response and clinical outcomes. Hum Reprod 2003;18:1194–1199. [DOI] [PubMed] [Google Scholar]
- Lenton E, Soltan A, Hewitt J, Thomson A, Davies W, Ashraf N, Sharma V, Jenner L, Ledger W, McVeigh E.. Induction of ovulation in women undergoing assisted reproductive techniques: recombinant human FSH (follitropin alpha) versus highly purified urinary FSH (urofollitropin HP). Hum Reprod 2000;15:1021–1027. [DOI] [PubMed] [Google Scholar]
- Lispi M, Bassett R, Crisci C, Mancinelli M, Martelli F, Ceccarelli D, De Bellis C, Mendola D.. Comparative assessment of the consistency and quality of a highly purified FSH extracted from human urine (urofollitropin) and a recombinant human FSH (follitropin alpha). Reprod Biomed Online 2006;13:179–193. [DOI] [PubMed] [Google Scholar]
- Liu X, Hao C, Wang J. Efficacy of highly purified urinary FSH versus recombinant FSH in Chinese women over 37 years undergoing assisted reproductive techniques. Int J Fertil Steril 2015;8:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunenfeld B, Lunenfeld E. Gonadotropic preparations—lessons learned. Fertil Steril 1997;67:812–814. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Stouffer RL, Giudice LC, Fauser BC.. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev 2006;27:170–207. [DOI] [PubMed] [Google Scholar]
- Mohamed MA, Sbracia M, Pacchiarotti A, Micara G, Linari A, Tranquilli D, Espinola SM, Aragona C.. Urinary follicle-stimulating hormone (FSH) is more effective than recombinant FSH in older women in a controlled randomized study. Fertil Steril 2006;85:1398–1403. [DOI] [PubMed] [Google Scholar]
- Nardo LG, Bellanca SA, Messina K, Nardo F.. Efficacy of recombinant follicle stimulating hormone versus urinary follicle stimulating hormone in in-vitro fertilization: a prospective, randomized, assessor-blind study. Ital J Gynaecol Obstet 2000;12:53. [Google Scholar]
- O’Dea L, Loumaye E, Liu H. A randomized, comparative, multicenter clinical trial of recombinant and urinary human FSH in in vitro fertilization and embryo transfer (IVFET). The American Fertility Society and The Canadian Fertility and Andrology Society 1993 Annual Meeting, Program Supplement,1993, S50–S51, abstract O-106.
- Olsson H, Sandstrom R, Grundemar L. Different pharmacokinetic and pharmacodynamic properties of recombinant follicle-stimulating hormone (rFSH) derived from a human cell line compared with rFSH from a non-human cell line. J Clin Pharmacol 2014;54:1299–1307. [DOI] [PubMed] [Google Scholar]
- Out HJ, Mannaerts BM, Driessen SG, Bennink HJ.. A prospective, randomized, assessor-blind, multicentre study comparing recombinant and urinary follicle stimulating hormone (Puregon versus Metrodin) in in-vitro fertilization. Hum Reprod 1995;10:2534–2540. [DOI] [PubMed] [Google Scholar]
- Practice Committee of American Society for Reproductive Medicine, Birmingham, Alabama Gonadotropin preparations: past, present, and future perspectives. Fertil Steril 2008;90:S13–S20. [DOI] [PubMed] [Google Scholar]
- Schats R, Sutter PD, Bassil S, Kremer JA, Tournaye H, Donnez J.. Ovarian stimulation during assisted reproduction treatment: a comparison of recombinant and highly purified urinary human FSH. On behalf of The Feronia and Apis study group. Hum Reprod 2000;15:1691–1697. [DOI] [PubMed] [Google Scholar]
- Selman HA, De Santo M, Sterzik K, Coccia E, El-Danasouri I.. Effect of highly purified urinary follicle-stimulating hormone on oocyte and embryo quality. Fertil Steril 2002;78:1061–1067. [DOI] [PubMed] [Google Scholar]
- Smitz J, Wolfenson C, Chappel S, Ruman J.. Follicle-stimulating hormone: a review of form and function in the treatment of infertility. Reprod Sci 2016;23:706–716. [DOI] [PubMed] [Google Scholar]
- Wely M, Kwan I, Burt AL, Thomas J, Vail A, Van der Veen F, Al-Inany HG. Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles. Cochrane Database Syst Rev 2011;9:CD005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenson C, Groisman J, Couto AS, Hedenfalk M., Cortvrindt RG, Smitz JE, Jespersen S.. Batch-to-batch consistency of human-derived gonadotrophin preparations compared with recombinant preparations. Reprod Biomed Online 2005;10:442–454. [DOI] [PubMed] [Google Scholar]
- Ye H, Huang G, Pei L, Zeng P, Luo X.. Outcome of in vitro fertilization following stimulation with highly purified hMG or recombinant FSH in downregulated women of advanced reproductive age: a prospective, randomized and controlled trial. Gynecol Endocrinol 2012;28:540–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


