Abstract
STUDY QUESTION
Are routinely collected data from fertility populations adequately validated?
SUMMARY ANSWER
Of the 19 studies included, only one validated a national fertility registry and none reported their results in accordance with recommended reporting guidelines for validation studies.
WHAT IS KNOWN ALREADY
Routinely collected data, including administrative databases and registries, are excellent sources of data, particularly for reporting, quality assurance, and research. However, these data are subject to misclassification bias due to misdiagnosis or errors in data entry and therefore need to be validated prior to using for clinical or research purposes.
STUDY DESIGN, SIZE, DURATION
We conducted a systematic review by searching Medline, Embase, and CINAHL from inception to 6 October 2016 to identify validation studies of databases that contain routinely collected data in an ART setting. Webpages of international ART centers were also searched.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We included studies that compared at least two data sources to validate ART population data. Key words and MeSH terms were adapted from previous systematic reviews investigating routinely collected data (e.g. administrative databases and registries), measures of validity (including sensitivity, specificity, and predictive value), and ART (including infertility, IVF, advanced reproductive age, and diminished ovarian reserve). Only full-text studies in English were considered. Results were synthesized qualitatively. The electronic search yielded 1074 citations, of which 19 met the inclusion criteria.
MAIN RESULTS AND THE ROLE OF CHANCE
Two studies validated a fertility database using medical records; seven studies used an IVF registry to validate vital records or maternal questionnaires, and two studies failed to adequately describe their reference standard. Four studies investigated the validity of mode of conception from birth registries; two studies validated diagnoses or treatments in a fertility database; four studies validated a linkage algorithm between a fertility registry and another administrative database; one study created an algorithm in a single database to identify a patient population. Sensitivity was the most commonly reported measure of validity (12 studies), followed by specificity (9 studies). Only three studies reported four or more measures of validation, and five studies presented CIs for their estimates. The prevalence of the variable in the target population (pre-test prevalence) was reported in seven studies; however, only four of the studies had prevalence estimates from the study population (post-test prevalence) within a 2% range of the pre-test estimate. The post-test estimate was largely discrepant from the pre-test value in two studies.
LIMITATIONS, REASONS FOR CAUTION
The search strategy was limited to the studies and reports published in English, which may not capture validation studies from countries that do not speak English. Furthermore, only three specific fertility-based diagnostic variables (advanced reproductive age, diminished ovarian reserve, and chorionicity) were searched in Medline, Embase, and CINAHL. Consequently, published studies with other diagnoses or conditions relevant to infertility may not have been captured in our review.
WIDER IMPLICATIONS OF THE FINDINGS
There is a paucity of literature on validation of routinely collected data from a fertility population. Furthermore, the prevalence of the markers that have been validated are not being presented, which can lead to biased estimates. Stakeholders rely on these data for monitoring outcomes of treatments and adverse events; therefore, it is essential to ascertain the accuracy of these databases and make the reports publicly available.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by Canadian Institutes of Health Research (CIHR) (FDN-148438). There are no competing interests for any of the authors.
REGISTRATION NUMBER
International Prospective Register of Systematic Reviews ID: CRD42016048466.
Keywords: ART, infertility, database, quality assurance, validation, reproductive epidemiology
WHAT DOES THIS MEAN FOR PATIENTS?
The World Health Organization recognizes the inability to have a healthy child after 1 year of attempting pregnancy as a disease or a disability. The psychosocial implications of infertility are vast, including depression, discrimination, and ostracism, the latter being of particular importance in lower income countries.
Determining the prevalence and burden of infertility, as well as performing regular surveillance on ART treatments and outcomes, is essential to inform policy, conduct research, and counsel patients. For example, the International Committee for Monitoring Assisted Reproductive Technologies relies on large-population data from regional and national ART registries around the world. With these data, they are able to provide reports depicting trends in practice, utilization of health care, and pregnancy outcomes after treatment.
Accurate and robust data are paramount to providing such reports. While these reports are reliant on administrative databases, our systematic review demonstrated that the quality assurance practices to establish accurate and reliable data are lacking in the literature. Moreover, where reports were published, adherence to reporting guidelines for studies using administrative data was also insufficient. We have provided a comprehensive review of the current literature, describing current practices, various strategies, and guidelines for which a validation study should adhere to in order to ensure accurate data.
Introduction
Infertility burdens 1.9% to 10.5% of child-seeking women worldwide and was estimated to affect 48.5 million couples in 2010 (Mascarenhas et al., 2012). According to the International Committee for Monitoring Assisted Reproductive Technologies (ICMART), 1.4 to 1.6 million ART cycles were initiated per year from 2008 to 2010, resulting in approximately 800 000 babies born over this time period (Dyer et al., 2016). ART is a rapidly evolving field in medicine with new advances in research and technology. From freezing techniques for gametes and embryos (Loutradi et al., 2008; AbdelHafez et al., 2010; National Institute for Health and Care Excellence, 2013) to the number of embryos replaced (Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine, 2012; National Institute for Health and Care Excellence, 2013) and utilization of PGD (Harton et al., 2011), reproductive technologies and guidelines are changing regularly. It is, therefore, prudent to ensure we can adequately monitor treatment outcomes and adverse events. Studies from the USA and Europe estimate that the prevalence of live births born after IVF ranges from 1% to 6% (Sullivan et al., 2013; Sunderam et al., 2017). The risk of adverse obstetrical events is significantly higher in ART compared to naturally conceived pregnancies (McGovern et al., 2004; Sazonova et al., 2011; Qin et al., 2016). However, the prevalence of these complications attributed to ART, such as ectopic pregnancies, placenta previa, and congenital anomalies, is low, with estimates 1–2% (Perkins et al., 2015; Santos-Ribeiro et al., 2016), 1.6% (Romundstad et al., 2006), and ~8% (Davies et al., 2012), respectively. Similarly, other neonatal outcomes, including small for gestational age, preterm delivery, and admission to a critical unit, also occur infrequently (McGovern et al., 2004; Sazonova et al., 2011; Qin et al., 2016). Therefore, in order to adequately understand the implications of such treatment, studies using large sample sizes are required.
Collections of routinely collected data, such as administrative databases and registries, are excellent sources of population-level data. These databases often contain sociodemographic information, health care utilization, treatment, and diagnostic information affiliated with health care visits. However, these data are not collected for a specific research question and are prone to error resulting from clerical errors, illegible charts, and documentation problems (Hierholzer Jr, 1991). If not validated adequately, utilization of these data for surveillance, quality improvement, and research can lead to misclassification bias and unmeasured confounding due to missing data (Benchimol et al., 2015).
Many studies that use large administrative and registry databases to identify patients who undergo ART treatments indicate that they are using validated data (Fedder et al., 2013; Traeger-Synodinos et al., 2013; De Geyter et al., 2015). However, the literature is scarce on validation studies and measures performed to ensure accuracy among these databases. There is extensive literature indicating the importance of presenting measures of validity, including sensitivity, specificity, and positive predictive values (PPVs), to reflect whether these data can be reliably used for research and reporting (Sørensen et al., 1996; Herrett et al., 2010; Van Walraven et al., 2010; Benchimol et al., 2011).
Ideally, a validation study uses a gold standard as a measure to guide the accuracy and reliability of the validated variable. Based on the Standards for Reporting of Diagnostic Accuracy Studies guidelines for evaluating diagnostic tests, a gold standard should be the best available test in identifying the condition of interest (Bossuyt et al., 2003). To this end, the gold standard of determining accuracy of database variables or data elements has not been established (Sørensen et al., 1996; Juurlink et al., 2006; Lain et al., 2012; Benchimol et al., 2015). In the absence of a true gold standard, some argue that the medical record should serve as the reference standard (Widdifield et al., 2013; Frosst et al., 2015).
With this in mind, we conducted a systematic review to identify the validation studies of databases that contain these routinely collected data (including administrative data and registry data) in an ART setting. Our primary objective was to assess how ART centers (either databases maintained by a clinic or those managed by a region or country) validate and report their fertility data, their rationale for choosing specific data elements for validation activities, the extent to which a database is considered valid for use, and actions taken when validity was deemed poor. Our secondary objective was to investigate whether ART centers were reporting their validation studies in accordance with the published reporting guidelines for validation studies (Benchimol et al., 2011) with details pertaining to the method of validation and quality control, the variables chosen to validate the database, and the outcome measures.
Materials and Methods
This review was conducted in accordance with a protocol developed and registered a priori (International Prospective Register of Systematic Reviews ID: CRD42016048466). We selected studies that performed a validation of ART population data, which were based on comparison of at least two data sources (health administrative or registry databases, chart reabstraction, self-reported questionnaires). Large administrative or registry databases were defined as those collecting data routinely without an a priori research question. We included studies that validated specific data elements or variables (e.g. diagnoses and treatments), case-finding algorithms within fertility databases or registries, or linkage studies between two or more databases that include a fertility registry. In this setting, a data element or variable could include (but was not limited to) diagnosis, treatment, and patient characteristic. Case-finding algorithms were defined as combinations of data elements used to identify a patient population. Linkage studies were defined as studies that used two databases that are joined together to identify or create a study population. The outcomes of interest were measures of diagnostic validity including sensitivity, specificity, PPV, negative predictive value (NPV), likelihood ratio, kappa coefficient, area under the receiver operating characteristic curve or c-statistic, accuracy, or agreement of the selected data elements. Only full-text articles published in English were considered.
The search strategy was developed with the aid of an information specialist with expertise in clinical research, adapted from previous systematic reviews (Benchimol et al., 2011; Shiff et al., 2014). Electronic bibliographic databases, specifically Medline, Embase, and CINAHL, were searched using specific vocabulary and MeSH keywords (see Supplementary Data 1 for Medline search strategy). Reference lists of all included articles and relevant systematic reviews were screened to identify additional studies. Web pages for major international fertility surveillance systems were searched to account for validation activities presented within surveillance reports, which are typically not indexed in bibliographic databases. We also contacted these surveillance programs to request reports that were not publicly available. These programs included https://www.belrap.be/Public/Default.aspx?Lg=En (Belgium), https://www.sart.org/ (USA), https://www.asrm.org/about-us/contact-us/ (American Society of Reproductive Medicine), https://npesu.unsw.edu.au/data-collection/australian-new-zealand-assisted-reproduction-database-anzard (Australia and New Zealand), https://www.hfea.gov.uk/ (UK), https://www.eshre.eu/Home/Contact-us.aspx (ESHRE), and https://www.icmr.gov.in/icmrnews/art/contact_us.htm (India). Citations were imported into EndNote and managed within Covidence (www.covidence.org). This process was recorded using the preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram (Moher et al., 2009). We performed the final search on 6 October 2016. As validating the dataset or part of the dataset is often a secondary objective of studies using routinely collected data, our search strategy could not capture all relevant validation studies. For example, investigators performing a cohort or case-control study involving a specific diagnosis or treatment may validate that data element prior to its use. While that diagnosis or treatment may be relevant to an infertility population, these studies would not be identified as validation studies and thus would not be included.
Screening was performed in two steps by two independent reviewers (V.B. and M.R.) using the eligibility criteria. Title and abstract screening was performed initially, followed by full-text screening. Disagreements were resolved by consensus or through consultation with a senior expert where consensus could not be reached. Reasons for excluding studies in the full-text screening step were documented.
We extracted data from each included study on country of origin, year of publication, number of clinics involved, number of treatment records, sample size calculation, variables or algorithms used, method of validation (chart review versus survey of patients versus another validated database), whether datasets were linked, how datasets were linked (probabilistic versus deterministic), prevalence of the variable(s) under investigation estimated both prior to the study from the target population (pre-test prevalence) and from the study population (post-test prevalence), and validation outcome measures (listed above). Two independent reviewers extracted these data (i.e. in duplicate).
We used items from previously published reporting guidelines for validation studies as a guide to evaluate whether the included studies used rigorous methodology to conduct their validation (Benchimol et al., 2011). This checklist was implemented by two independent reviewers. We made a post-hoc decision after protocol registration to adapt the quality assessment tools used by two previously published systematic reviews to assess both reporting and quality of studies (Benchimol et al., 2011; Grams et al., 2011). All results were synthesized qualitatively.
Results
The electronic search yielded 1074 citations after removing duplicates. Upon applying the inclusion and exclusion criteria, we identified 65 studies for full-text screening after title and abstract screening. Seven additional studies were identified for full-text screening after reviewing the references of pertinent articles and searching web pages. Of these 72 studies, 53 did not meet inclusion criteria for various reasons including wrong study design, comparator, or patient population (details can be found in Supplementary Data 2). Nineteen studies were included for final analysis (Fig. 1), representing the USA (Sunderam et al., 2006; Molinaro et al., 2009; Zhang et al., 2010, 2012, Buck Louis et al., 2014, 2015; Cohen et al., 2014; Kotelchuck et al., 2014; Liberman et al., 2014; Centers for Disease Control and Prevention et al., 2016; Luke et al., 2016; Stern et al., 2016a, 2016b), Finland (Hemminki et al., 2003; Gissler et al., 2004), Denmark (Hvidtjorn et al., 2009), the Netherlands (Overbeek et al., 2013), Israel (Rosenfeld and Strulov, 2009a), and the UK (Williams et al., 2013). Of these studies, four did not use any reference standard (Hemminki et al., 2003; Gissler et al., 2004; Sunderam et al., 2006; Williams et al., 2013), and the reference was poorly described in two studies (Molinaro et al., 2009; Rosenfeld and Strulov, 2009a) (Table I). Two studies used medical records to validate a fertility database (Molinaro et al., 2009; Centers for Disease Control and Prevention et al., 2016), seven studies used an IVF registry as the reference standard to validate either vital records or maternal questionnaires (Hvidtjorn et al., 2009; Zhang et al., 2012; Williams et al., 2013; Cohen et al., 2014; Liberman et al., 2014; Buck Louis et al., 2015; Luke et al., 2016), one study utilized maternal report as the reference standard for validation (Buck Louis et al., 2014), and three studies used vital records (birth and death certificates) as the reference standard (Overbeek et al., 2013; Kotelchuck et al., 2014; Stern et al., 2016a). Finally, one study used both IVF registries and vital records as the reference standard depending on the data element validated (Stern et al., 2016b).
Figure 1.
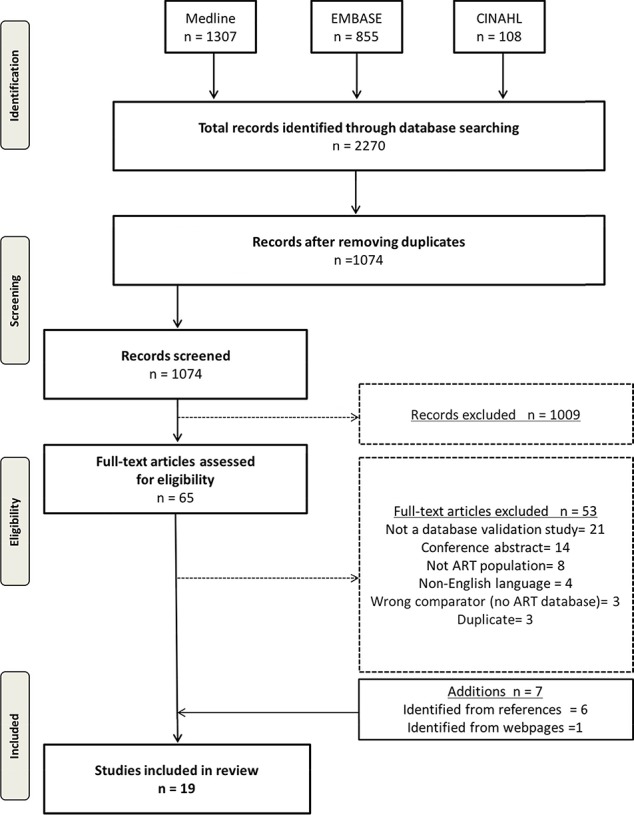
Preferred reporting items for systematic reviews and meta-analysis flow diagram.
Table I.
Descriptive characteristics of included studies.
| Authors | Year | Country | Data source being validated | Reference standard | Population | Sample size |
|---|---|---|---|---|---|---|
| Buck Louis GM and Druschel C | 2015 | USA | Questionnaire (Upstate New York Infant Development Screening Program Study) | IVF registry (SART CORS) | Mothers who had live births in Upstate New York between July 2008 and May 2010 in whom `Infertility treatment’ was checked on birth certificate and multiple births matched to singleton infants whose treatment box was not checked | 5034 |
| Buck Louis GM and Hediger ML | 2014 | USA | Administrative database (Perinatal Data System) | Questionnaire | Mothers who had live births in Upstate New York between July 2008 and May 2010 in whom `Infertility treatment’ was checked on birth certificate and multiple births matched to singleton infants whose treatment box was not checked | 4989 |
| Centers for Disease Control and Prevention | 2016 | USA | Fertility database (SART) | Medical record | ART cycle data from 458 fertility clinics in the US during the 2014 cycle year. A random selection of 34 clinics were selected | 1996 |
| Cohen B | 2014 | USA | Administrative database (birth certificates) | IVF registry (NASS) | Live births to Florida or Massachusetts resident mothers that occurred in state from March 2004 to December 2006 | 856 165 |
| Gissler M | 2004 | Finland | Administrative database (medical birth record) | NA (compared ad hoc IVF research and IVF statistics, no reference standard) | Newborns from fertility treatments from 1996 to 1998 | 176 698 |
| Hemminki E | 2003 | Finland | Administrative database (Drug Reimbursement Register) | Internal examination of data and linkage to Birth Register | Women exposed to ART between 1996 and 1998 | 24 318 |
| Hvidtjørn D | 2009 | Denmark | Administrative database | IVF Registry | Women who participated in the first Danish National Birth Cohort (study) interview with a pregnancy resulting in a live born child between October 2007 and June 2003 | 88 151 |
| Kotelchuck M | 2014 | USA | IVF registry (SART) | Administrative database (PELL) | Children born to Massachusetts resident women in MA hospitals from July 2004 to December 2008 conceived by ART | 10 138 |
| Liberman RF | 2014 | USA | Questionnaire (National Birth Defects Prevention Study) | IVF registry | Women who completed the NBDPS with in-state deliveries between September 2004 and December 2008 | 77 |
| Luke B | 2016 | USA | Administrative database (birth certificates) | IVF registry | Live births in Florida, Massachusetts, New York, Pennsylvania, Texas, California, Ohio, and Colorado between 2004 and 2009. IVF cycles from SART CORS were linked to birth certificates. | 716 103 |
| Molinaro TA | 2009 | USA | IVF registry | Medical records | IVF patients enrolled for other studies at the University of Pennsylvania between December 2003 and June 2006 | 590 |
| Overbeek A | 2013 | Netherlands | Questionnaire (DCOG LATER-VEVO Study—nationwide cohort study) | Administrative database (Netherlands Perinatal Registry) | Childhood cancer survivors who achieved pregnancy and their sibling controls | 524 |
| Rosenfeld Y | 2009a | Israel | IVF reporting system | Medical record | Women who receive fertility treatment in the District of Haifa and Western Galilee of the General Health Services | 108 |
| Stern JE andGopal D | 2016a | USA | IVF registry (SART) | Administrative database (Massachusetts BDMP Registry) | ART deliveries from 1 July 2004 to 31 December 2008 in Massachusetts | 9092 |
| Stern JE and McLain AC | 2016b | USA | Questionnaire (Upstate New York Infant Development Screening Program Study) | SART database for current cycle; Questionnaire for prior treatment information | Mothers who participated in Upstate KIDS Study linked with SART CORS | 617 |
| Sunderam S | 2006 | USA | Administrative database | IVF registry | Infants born in 1997 and 1998 in MA, RI, NH, CT to MA-resident mothers who used ART clinics in MA or RI | 2703 |
| Williams CL | 2013 | UK | Administrative database (National Registry of Childhood Tumours) | IVF registry (HFEA) | Children born between 1 January 1992 and 31 December 2008 | 106 013 |
| Zhang Y | 2012 | USA | Administrative database | IVF registry (NASS) | Live births to MA-resident mothers that occurred in MA during 1997-2000 | 6139 |
| Zhang Z | 2010 | USA | Administrative database (Massachusetts Registry of Vital Records and Statistics-MBC) | IVF registry (NASS) | Live births to MA-resident mothers that occurred in MA during 1997–2000 | 5190 |
BDMP, Birth Defects Monitoring Program; NASS, National ART Surveillance System; NBDP, National Birth Defects Prevention; NBPDS, National Birth Defects Prevention Study; PELL, Pregnancy to Early Life Longitudinal data system; SART CORS, Society for Assisted Reproductive Technology Clinical Outcomes Reporting System.
Four studies validated method of conception from birth registries (Gissler et al., 2004; Zhang et al., 2010; Cohen et al., 2014; Luke et al., 2016), two validated diagnoses or treatment variables within the fertility database (Molinaro et al., 2009; Centers for Disease Control and Prevention et al., 2016), one study created an algorithm to identify a patient population (Hemminki et al., 2003), and four studies validated linkage algorithms between a fertility and a second administrative database (Sunderam et al., 2006; Zhang et al., 2012; Williams et al., 2013; Kotelchuck et al., 2014).
Sensitivity was the most commonly reported validation measure. Twelve studies reported sensitivity (Hvidtjorn et al., 2009; Zhang et al., 2010, 2012; Overbeek et al., 2013; Buck Louis et al., 2014, 2015; Cohen et al., 2014; Kotelchuck et al., 2014; Liberman et al., 2014; Luke et al., 2016; Stern et al., 2016a, 2016b), nine reported specificity (Hvidtjorn et al., 2009; Zhang et al., 2010; Overbeek et al., 2013; Buck Louis et al., 2014, 2015; Cohen et al., 2014; Kotelchuck et al., 2014; Liberman et al., 2014; Luke et al., 2016), six reported PPV (Hvidtjorn et al., 2009; Zhang et al., 2010; Overbeek et al., 2013; Cohen et al., 2014; Kotelchuck et al., 2014; Buck Louis et al., 2015), one reported NPV (Buck Louis et al., 2015), five reported the Kappa coefficient (Gissler et al., 2004; Overbeek et al., 2013; Buck Louis et al., 2014; Kotelchuck et al., 2014; Stern et al., 2016a), and seven reported percentage agreement (Gissler et al., 2004; Hvidtjorn et al., 2009; Zhang et al., 2012; Overbeek et al., 2013; Buck Louis et al., 2014; Stern et al., 2016a, 2016b) (Table II). The data quality measures are presented in Supplementary Data 3. Only three studies reported four or more measures of validation (Hvidtjorn et al., 2009; Buck Louis et al., 2014, 2015). Nine studies presented 95% CIs with the estimates (Gissler et al., 2004; Zhang et al., 2010, 2012; Overbeek et al., 2013; Cohen et al., 2014; Liberman et al., 2014; Buck Louis et al., 2015; Centers for Disease Control and Prevention et al., 2016; Stern et al., 2016a), of which five reported CIs for all estimates (Zhang et al., 2012; Cohen et al., 2014; Liberman et al., 2014; Buck Louis et al., 2015; Centers for Disease Control and Prevention et al., 2016).
Table II.
Summary of reported validity measures.
| Study | Sensitivity | Specificity | PPV | NPV | Kappa | % Agreement | ICC | AUC/c-statistic | Likelihood ratios | Four or more measures of validity | Number of measures | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buck Louis et al. (2015) | 10/10 | 10/10 | 10/10 | 10/10 | No | No | No | No | No | 10/10 | 4 | 10/10 |
| Buck Louis et al. (2014) | 1/4 | 1/4 | No | No | 1/4 | 4/4 | No | No | No | 1/4 | 4 | 0/4 |
| Centers for Disease Control and Prevention et al. (2016) | No | No | No | No | No | No | No | No | No | No | 1 | 18/18 |
| Cohen et al. (2014) | 2/2 | 2/2 | 2/2 | No | No | No | No | No | No | No | 3 | 2/2 |
| Gissler et al. (2004) | No | No | No | No | 1/2 | 2/2 | No | No | No | No | 2 | 1/2 |
| Hemminki et al. (2003) | No | No | No | No | No | No | No | No | No | No | 0 | NA |
| Hvidtjørn et al. (2009) | 3/3 | 3/3 | 3/3 | No | No | 3/3 | No | No | No | 3/3 | 4 | 0/3 |
| Kotelchuck et al. (2014) | 3/3 | 1/3 | 3/3 | No | 3/3 | No | No | No | No | No | 4 | 0/3 |
| Liberman et al. (2014) | 5/5 | 5/5 | No | No | No | No | No | No | No | No | 2 | 5/5 |
| Luke et al. (2016) | 1/1 | 1/1 | No | No | No | No | No | No | No | No | 2 | No |
| Molinaro et al. (2009) | No | No | No | No | No | No | No | No | No | No | 0 | NA |
| Overbeek et al. (2013) | 10/26 | 10/26 | 10/26 | No | 16/26 | 16/26 | No | No | No | No | 4 | 16/26 |
| Rosenfeld and Strulov (2009) | No | No | No | No | No | No | No | No | No | No | 2 | No |
| Stern et al. (2016a) | 6/11 | No | No | No | 2/11 | 2/11 | No | No | No | No | 3 | 6/11 |
| Stern et al. (2016b) | 13/13 | No | No | No | No | 3/13 | No | No | No | No | 5 | No |
| Sunderam et al. (2006) | No | No | No | No | No | No | No | No | No | No | 0 | NA |
| Williams et al. (2013) | No | No | No | No | No | No | No | No | No | No | 1 | No |
| Zhang et al. (2012) | 1/1 | No | No | No | No | No | No | No | No | No | 2 | 1/2 |
| Zhang et al. (2010) | 1/1 | 1/1 | 1/1 | No | No | No | No | No | No | No | 3 | 3/3 |
AUC, area under the curve; ICC, intraclass correlation coefficient; NPV, negative predictive value; PPV, positive predictive value.
Numerator: number of validated variables; denominator: total number of variables considered for validation in each study.
The elements of data quality are summarized in Tables III and IV. Sixteen studies (84.2%) adequately described their data source, and all but one described the type of patient records from which data were extracted (Rosenfeld and Strulov, 2009a). The studies predominantly described inclusion and exclusion criteria and their methods for determining the validity of the data. Fifteen studies adequately described their method of patient sampling while 14 studies sampled the entire population in the database (Hemminki et al., 2003; Sunderam et al., 2006; Hvidtjorn et al., 2009; Zhang et al., 2010, 2012; Williams et al., 2013; Overbeek et al., 2013; Buck Louis et al., 2014, 2015; Cohen et al., 2014; Kotelchuck et al., 2014; Liberman et al., 2014; Luke et al., 2016; Stern et al., 2016a); one study performed a random sampling strategy (Centers for Disease Control and Prevention et al., 2016). Only one group performed their study using an a priori sample size (Centers for Disease Control and Prevention et al., 2016), and none provided statistical justification for their sample size.
Table III.
Reporting quality of methodology of included studies.
| Methods | Frequency | % |
|---|---|---|
| Describes the data source | ||
| Yes | 16/19 | 84.2 |
| Incomplete | 2/19 | 10.5 |
| Unclear | 1/19 | 5.3 |
| Describes type of records (inpatient, outpatient, linked records) | ||
| Yes | 18/19 | 94.7 |
| Unclear | 1/19 | 5.3 |
| Describes setting and locations where data were collected | ||
| Yes | 18/19 | 94.7 |
| Incomplete | 1/19 | 5.3 |
| Reports a priori sample size | ||
| Yes | 1/19 | 5.3 |
| Provides statistical justification for the sample size | ||
| Yes | 0/19 | 0.0 |
| Describe recruitment procedure of validation cohort (from a database, based on diagnostic codes) | ||
| Yes | 17/19 | 89.5 |
| Unclear | 2/19 | 10.5 |
| Describe patient sampling (Random, consecutive, all) | ||
| Random sampling | 1/19 | 5.3 |
| All | 14/19 | 73.7 |
| Unclear | 2/19 | 10.5 |
| Incomplete | 2/19 | 10.5 |
| Describe how participants were chosen for data collection and analysis | ||
| Yes | 15/19 | 78.9 |
| Unclear | 2/19 | 10.5 |
| Describes inclusion/exclusion criteria | ||
| Yes | 14/19 | 73.7 |
| Incomplete | 1/19 | 5.3 |
| Describes who identified patients (for patients identified from medical records) | ||
| Yes | 1/19 | 5.3 |
| Incomplete | 1/19 | 5.3 |
| Describes who collected data | ||
| Yes | 3/19 | 15.8 |
| Describes use of a priori data collection form | ||
| Yes | 13/19 | 68.4 |
| Unclear | 1/19 | 5.3 |
| Use of a split sample or an independent sample (revalidation using a separate cohort) | ||
| Yes | 1/19 | 5.3 |
| Describes the reference standard | ||
| Yes | 13/17 | 76.5 |
| Reports the number of persons reading the reference standard | ||
| Yes | 2/17 | 11.8 |
| Describes the training or expertise of persons reading reference standard | ||
| Yes | 1/17 | 5.9 |
| Readers of the reference standard were blinded to the results of the classification by routinely collected data for that patient (reference standard: medical records) | ||
| Yes | 1/17 | 5.9 |
| Reports a measure of concordance if >1 persons reading the reference standard | ||
| Yes | 0/17 | 0.0 |
| Describes the linkage procedure, if done (probabilistic/deterministic) | ||
| Yes | 8/15 | 50.0 |
| Incomplete | 6/15 | 37.5 |
| Describes the methods of linkage quality evaluation | ||
| Yes | 7/15 | 46.7 |
| Incomplete | 2/15 | 13.3 |
| Describes explicit methods for calculating or comparing measures of accuracy and statistical methods used to quantify uncertainty | ||
| Yes | 13/19 | 68.4 |
Table IV.
Reporting quality of the results of included studies.
| Frequency | % | |
|---|---|---|
| Reports the number of participants satisfying the inclusion/exclusion criteria | ||
| Yes | 13/18 | 68.4 |
| Incomplete | 1/18 | 5.6 |
| Describes the characteristics of misclassified patients (false positives and/or false negatives) | ||
| Yes | 13/18 | 68.4 |
| Unclear | 2/18 | 11.1 |
| Provides a study flow diagram | ||
| Yes | 4/19 | 21.1 |
| Reports the number of records unable to link | ||
| Yes | 11/12 | 91.7 |
| Incomplete | 1/12 | 8.3 |
| Reports missing medical records or reports the number of patients unwilling to participate | ||
| Yes | 10/19 | 52.6 |
| Reports incomplete records | ||
| Yes | 13/19 | 68.4 |
| Presents a cross tabulation of results of the validated source to the reference standard | ||
| Yes | 11/19 | 57.9 |
| Incomplete | 1/19 | 5.3 |
| Reports the pretest prevalence in the study sample | ||
| Yes | 5/19 | 26.3 |
| Incomplete | 2/19 | 10.5 |
| Tests and reports results of multiple algorithms | ||
| Yes | 6/15 | 40.0 |
| Reports estimates of test reproducibility of the split or independent sample if done | ||
| Yes | 0/19 | 0.0 |
Where multiple databases were linked using a common patient identifier, the linkage procedures were adequately described in eight (53.3%) of the studies (Sunderam et al., 2006; Zhang et al., 2010, 2012; Williams et al., 2013; Cohen et al., 2014; Kotelchuck et al., 2014; Stern et al., 2016a, 2016b). The quality of these procedures was described in only seven studies (46.7%) (Hemminki et al., 2003; Sunderam et al., 2006; Zhang et al., 2010, 2012; Williams et al., 2013; Kotelchuck et al., 2014; Stern et al., 2016a).
The pre-test prevalence of the validated variables was provided in seven studies (Sunderam et al., 2006; Zhang et al., 2010; Buck Louis et al., 2014; Cohen et al., 2014; Kotelchuck et al., 2014; Liberman et al., 2014; Luke et al., 2016) (Table V). The post-test prevalence of these variables was within a 2% range of the pre-test values for four of the studies (Zhang et al., 2010; Cohen et al., 2014; Kotelchuck et al., 2014; Liberman et al., 2014); however, in two studies, the post-test prevalence was largely discrepant from pre-test values (Buck Louis et al., 2014; Luke et al., 2016).
Table V.
Description of the pre- and post-test prevalence of measured estimates of validity in included studies.
| Study | Prevalence estimate reported | Pre-test prevalence (%) | Post-test prevalence*(%) |
|---|---|---|---|
| Buck Louis et al. (2015) | No | — | — |
| Buck Louis et al. (2014) | ART conceived infant | 1.40 | 14.0 |
| Centers for Disease Control and Prevention (2016) | No | — | — |
| Cohen et al. (2014) | ART conceived infant | 1.40 | 0.45 |
| Gissler et al. (2004) | No | — | — |
| Hemminki et al. (2003) | No | — | — |
| Hvidtjørn et al. (2009) | No | — | — |
| Kotelchuck et al. (2014) | ART conceived infant | 1.60 | 2.72 |
| Liberman et al. (2014) | ART conceived infant in MA | 4.30 | 5.30 |
| Luke et al. (2016) | ART conceived infant | 1.70 | 9.80 |
| Molinaro et al. (2009) | No | — | — |
| Overbeek et al. (2013) | No | — | — |
| Rosenfeld and Strulov (2009) | No | — | — |
| Stern et al. (2016a) | Incomplete | — | — |
| Stern et al. (2016b) | No | — | — |
| Sunderam et al. (2006) | Yes | 3.00 | — |
| Williams et al. (2013) | No | — | — |
| Zhang et al. (2012) | No | — | — |
| Zhang et al. (2010) | ART Live birth deliveries | 3.00 | 1.70 |
*Based on reference standard.
Discussion
This study demonstrates that there is a paucity of the literature on the validation of data elements within fertility databases and registries. There were numerous studies that validated ART information derived from maternal report or birth and death certificates by comparing those data to the reference standard of a fertility registry; however, we only identified one study that assessed the validity of a fertility registry by comparing data elements from the database to the reference standard of the patient record (Centers for Disease Control and Prevention et al., 2016). Furthermore, only seven studies published the baseline prevalence of the data element being validated (Sunderam et al., 2006; Zhang et al., 2010; Buck Louis et al., 2014; Cohen et al., 2014; Kotelchuck et al., 2014; Liberman et al., 2014; Luke et al., 2016), of which only four studies’ sample prevalence approximated that of the population (Buck Louis et al., 2014; Luke et al., 2016).
There are three commonly cited validation study designs: ecological studies, reabstraction studies, and gold standard studies (Van Walraven and Austin, 2012). Ecological studies compare measures of disease prevalence in the database to those obtained from more reliable methods, like those published elsewhere. Reabstraction studies compare the database variable or element to the medical record. Finally, gold standard studies compare the database variable to a case definition, either based on clinical or laboratory values or clinical consensus (Van Walraven and Austin, 2012).
Hemminki et al. (2003) and Gissler et al. (2004) both performed ecological studies using national statistics. Hemminki et al. (2003) and Gissler et al. (2004) created a case-finding algorithm using data from a drug reimbursement register and a physician examination and intervention register to identify an infertility population in Finland. They subsequently compared these data to national statistics to validate their algorithm. Gissler et al. (2004) compared prevalence estimates both from a birth registry and from aggregate IVF statistics to estimates generated from Hemminki’s study to assess the completeness and validity of these routinely collected data sources. Firstly, these reference standards rely on the accuracy of the national statistics, which were not established and should not be implicitly assumed. Secondly, as the comparison is based on aggregate data rather than patient-level data, identifying specific differences and agreements is impossible.
Of the 19 studies included in our review, only 2 used the medical record as the reference standard (Molinaro et al., 2009; Centers for Disease Control and Prevention et al., 2016), and only 1 presented measures of validation (Centers for Disease Control and Prevention et al., 2016). The others used either another database or patient report as the reference. Molinaro et al. (2009) attempted to validate diagnosis variables in The Society of American Reproductive Technologies (SART) using case definitions based on clinical values in the patients’ charts rather than relying on the expertise of clinicians. They did not report their measures of validity, making it challenging to determine if this method is superior. Using objective measures, such as laboratory tests and strict diagnostic criteria, for validation compared to documentation may be more reliable, though such approaches were not identified by our review of ART validation studies.
The study performed by SART assessed multiple patient variables at one time, comparing SART data to patient charts (Centers for Disease Control and Prevention et al., 2016). However, due to the presentation of discrepancy rates without other important measures of validity, such as sensitivity, kappa coefficients, or PPVs, it is difficult to determine how reliable these data are. A subgroup evaluation by the size of the clinic or geography would be useful to investigate whether specific variables are largely problematic or if there is an issue at a specific clinic.
A Canadian study investigating the validity of diagnostic codes in 10 major hospitals found that the sensitivity and specificity were highly dependent on the hospital, where some had a high accuracy and others demonstrated poor sensitivity (Juurlink et al., 2006). Clinics may have specific expertise with respect to their patient populations, and the prevalence of certain conditions or treatments may vary based on health care provider. Predictive tests (PPV, likelihood ratios) are highly dependent on the baseline prevalence of the specific treatment or disease (Altman and Bland, 1994). Furthermore, in certain cases, the sensitivity and specificity may vary with the prevalence (Brenner and Gefeller, 1997). While the accuracy of those records would not necessarily be influenced, the metrics such as PPV, NPV, and sensitivity will be affected. Only four of the included studies presented post-test prevalence estimates that approximated the reported pre-test prevalence; it, therefore, puts into question the degree of bias in the estimates presented. As such, it is essential to describe both the source of data and prevalence of the variable of interest to adequately interpret the results.
There is insufficient documentation in the literature with respect to how national fertility registries are validating their databases. SART publishes a publicly available report on an annual basis indicating which variables are discrepant between the medical chart and the database (Centers for Disease Control and Prevention et al., 2016). According to ICMART’s world report, there were 61 countries that submitted nationwide ART data for surveillance (Dyer et al., 2016). Unfortunately, none of the other national databases have generated such reports or have made them easily accessible. The Human Fertilisation and Embryology Authority in the UK, Australian & New Zealand Assisted Reproduction Database (ANZARD), and the Belgian Register for Assisted Procreation endorse strict adherence to quality assurance practices; however, no reports were available describing their data-validation processes (written communication with Belgium and ANZARD). As all stakeholders, including patients, health care practitioners, researchers, and policy makers, rely on these data to understand the implications of fertility treatments, including the prevalence of disease, practice patterns, and complications and outcomes of ART, it is essential that these reports are made publicly available (Butler, 2003; Chambers et al., 2009; Canadian Fertility Andrology Society, 2014; Harris et al., 2016; Human Fertilisation and Embryology Authority, 2016).
It is clear from this review that databases are audited, but tracking that process and determining which data elements are reliable are challenging. Therefore, a gold standard from this source should not be implicitly accepted. More studies investigating the accuracy of routinely collected data in local or national registries need to be performed and published, with adherence to reporting guidelines. Upon demonstration of data validity, research can be performed utilizing these databases with measures to reduce bias. Finally, patient report is subject to recall bias, particularly as increasing time has passed from the event to the survey (Leong et al., 2013).
Our review has several limitations. We restricted our inclusion criteria to published reports in English. As many of the internal processes are likely to occur in the primary language of the registry or organization, it is possible that we were unable to capture validation processes from registries. A comprehensive search on the internet did not yield any results, even in other languages, however. Moreover, only four studies were excluded from our database search due to language restriction (Lidegaard and Hammerum, 2002; Rosenfeld and Strulov, 2009b; Ameri and Alizadeh, 2014; Pierron et al., 2015). Our study was also limited by the search strategy developed for Medline, Embase, and CINAHL. While the strategy was quite general for routinely collected databases, the list was not exhaustive for specific diagnoses relevant to infertility. Consequently, it is probable that other published studies were not captured in our review.
In spite of these limitations, our study is strengthened by the systematic and comprehensive approach to searching the articles and analyzing the measures of validity. This is the first study to our knowledge to assess the utility of validation tools for fertility registries. Although many of these reports were not published in indexed bibliographic databases, numerous attempts were made to contact ART surveillance database managers in the UK, Denmark, Belgium, Australia, New Zealand, and the USA to obtain unpublished or ad hoc reports on data maintenance and quality assurance.
This review highlights an important gap in the field of fertility research where the validation of widely utilized databases has not been well described. Big data are increasingly used for research, quality assurance, and policy; therefore, the accuracy of these data is essential. Furthermore, during the validation process, the prevalence of the variables and the statistical estimates need to be adequately measured and compared to the prevalence from the drawn study population. This would allow the reader to assess the generalizability of the study population to the general population. As the prevalence of the condition varies based on health care provider or geographic location, so will these measures. Future studies need to be conducted and published using rigorous methodology that will allow for greater transparency and accuracy of research within this rapidly evolving field of medicine and research.
Supplementary Material
Acknowledgements
We would like to thank Risa Shorr who helped in the development of the search strategy.
Authors’ roles
V.B. developed the protocol, served as a reviewer and data abstractor in the data acquisition phase, performed the analysis, and drafted the article. M.R. served as a second reviewer and data abstractor in the data acquisition phase. He participated in critically revising the manuscript and approved the final version for publication. D.B.F. helped to develop the protocol, provided guidance in the data analysis, participated in critically revising the manuscript, and approved the final version for publication. H.S. helped to develop the protocol, participated in critically revising the manuscript, and approved the final version for publication. M.W. helped to develop the protocol, provided guidance in the data analysis, participated in critically revising the manuscript, and approved the final version for publication. L.M.G. helped to develop the protocol, provided guidance in the data analysis, participated in critically revising the manuscript, and approved the final version for publication.
Funding
Canadian Institutes of Health Research (CIHR) (FDN-148438).
Conflict of interest
None of the authors have any conflicts of interest to declare.
References
- AbdelHafez FF, Desai N, Abou-Setta AM, Falcone T, Goldfarb J.. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online 2010;20:209–222. [DOI] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Diagnostic tests 2: predictive values. BMJ 1994;309:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri H, Alizadeh S. Assessing the effects of infertility treatment drugs using clustering algorithms and data mining techniques. J Maz Univ Med Sci 2014;24:26–35. [Google Scholar]
- Benchimol EI, Manuel DG, To T, Griffiths AM, Rabeneck L, Guttmann A.. Development and use of reporting guidelines for assessing the quality of validation studies of health administrative data. J Clin Epidemiol 2011;64:821–829. [DOI] [PubMed] [Google Scholar]
- Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sørensen HT, von Elm E, Langan SM.. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HCW et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for reporting of diagnostic accuracy. Clin Chem 2003;49:1–6. [DOI] [PubMed] [Google Scholar]
- Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med 1997;16:981–991. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Druschel C, Bell E, Stern JE, Luke B, McLain A, Sundaram R, Yeung E.. Use of assisted reproductive technology treatment as reported by mothers in comparison with registry data: the upstate KIDS study. Fertil Steril 2015;103:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Bell EM, Kus CA, Sundaram R, McLain AC, Yeung E, Hills EA, Thoma ME, Druschel CM.. Methodology for establishing a population-based birth cohort focusing on couple fertility and children’s development, the upstate KIDS study. Paediatr Perinat Epidemiol 2014;28: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. Assisted reproduction in developing countries-facing up to the issues. Prog Hum Reprod Res 2003:1–8. http://www.who.int/reproductivehealth/publications/infertility/progress63.pdf(12 August 2017, date last accessed). [Google Scholar]
- Canadian Fertility Andrology Society CARTR Annual Reports 2014. https://cfas.ca/cartr-annual-reports/(12 August 2017, date last accessed).
- Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology . 2014 Assisted Reproductive Technology Fertility Clinic Success Rates Report. Centers for Disease Control and Prevention, Atlanta, 2016. [Google Scholar]
- Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD.. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril 2009;91:2281–2294. [DOI] [PubMed] [Google Scholar]
- Cohen B, Bernson D, Sappenfield W, Kirby RS, Kissin D, Zhang Y, Copeland G, Zhang Z, Macaluso M.. Accuracy of assisted reproductive technology information on birth certificates: Florida and Massachusetts, 2004–06. Paediatr Perinat Epidemiol 2014;28:181–190. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A.. Reproductive technologies and the risk of birth defects. N Engl J Med 2012;366:1803–1813. [DOI] [PubMed] [Google Scholar]
- De Geyter C, Fehr P, Moffat R, Gruber I, Von W.. Twenty years’ experience with the Swiss data registry for assisted reproductive medicine: outcomes, key trends and recommendations for improved practice. Swiss Med Wkly 2015;145:w14087 http://doi.emh.ch/smw.2015.14087(29 July 2016, date last accessed). [DOI] [PubMed] [Google Scholar]
- Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson GD.. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2008, 2009 and 2010. Hum Reprod 2016;31:1588–1609. [DOI] [PubMed] [Google Scholar]
- Fedder J, Loft A, Parner ET, Rasmussen S.. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: a controlled national cohort study. Hum Reprod 2013;28:230–240. [DOI] [PubMed] [Google Scholar]
- Frosst G, Hutcheon J, Joseph K, Kinniburgh B, Johnson C, Lee L.. Validating the British Columbia perinatal data registry: a chart re-abstraction study. BMC Pregnancy Childbirth 2015;15:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissler M, Klemetti R, Sevón T, Hemminki E.. Monitoring of IVF birth outcomes in Finland: a data quality study. BMC Med Inform Decis Mak 2004;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grams ME, Plantinga LC, Hedgeman E, Saran R, Myers GL, Williams DE, Powe NR.. Validation of CKD and related conditions in existing data sets: a systematic review. Am J Kidney Dis 2011;57:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K, Fitzgerald O, Macaldowie A, Lee E, Chambers G. Assisted reproductive technology in Australia and New Zealand 2014. Sydney: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales, 2016. https://npesu.unsw.edu.au/sites/default/files/npesu/surveillances/Assisted reproductive technology in Australia and New Zealand 2014.pdf (12 August 2017, last date accessed). [Google Scholar]
- Harton G, Braude P, Lashwood A, Schmutzler A, Traeger-Synodinos J, Wilton L, Harper JC.. European Society for Human Reproduction and Embryology (ESHRE) PGD Consortium. ESHRE PGD consortium best practice guidelines for organization of a PGD centre for PGD/preimplantation genetic screening. Hum Reprod 2011;26:14–24. [DOI] [PubMed] [Google Scholar]
- Hemminki E, Klemetti R, Rinta-Paavola M, Martikainen J.. Identifying exposures of in vitro fertilization from drug reimbursement files: a case study from Finland. Med Inform Internet Med 2003;28:279–289. [DOI] [PubMed] [Google Scholar]
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ.. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer WJ., Jr Health care data, the epidemiologist’s sand: comments on the quantity and quality of data. Am J Med 1991;91:21S–26S. [DOI] [PubMed] [Google Scholar]
- Human Fertilisation and Embryology Authority Annual report and accounts 2015/16. 2016. http://ifqtesting.blob.core.windows.net/umbraco-website/1183/56071_hc_380_web_v02.pdf (12 August 2017, date last accessed).
- Hvidtjørn D, Grove J, Schendel D, Schieve LA, Ernst E, Olsen J, Thorsen P.. Validation of self-reported data on assisted conception in the Danish National Birth Cohort. Hum Reprod 2009;24:2332–2340. [DOI] [PubMed] [Google Scholar]
- Juurlink D, Preyra C, Croxford R, Chong A, Austin P, Tu J, Laupacis A.. Canadian Institute for Health Information discharge abstract database: a validation study. Toronto Inst Clin Eval Sci 2006:1–69. [Google Scholar]
- Kotelchuck M, Hoang L, Stern J, Diop H, Belanoff C, Declercq E.. The MOSART Database: linking the SART CORS clinical database to the population-based Massachusetts PELL Reproductive Public Health Data System. Matern Child Health J 2014;18:2167–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lain SJ, Hadfield RM, Raynes-Greenow CH, Ford JB, Mealing NM, Algert CS, Roberts CL.. Quality of data in perinatal population health databases: a systematic review. Med Care 2012;50:e7–e20. [DOI] [PubMed] [Google Scholar]
- Leong A, Dasgupta K, Bernatsky S, Lacaille D, Avina-Zubieta A, Rahme E.. Systematic review and meta-analysis of validation studies on a diabetes case definition from health administrative records. PLoS One 2013;8:e75256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman RF, Stern JE, Luke B, Reefhuis J, Anderka M.. Validating assisted reproductive technology self-report. Epidemiology 2014;25:773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidegaard O, Hammerum MS. The National Patient Registry as a tool for continuous production and quality control. Ugeskr Laeger 2002;164:4420–4423. [PubMed] [Google Scholar]
- Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G,Bontis I, Tarlatzis BC.. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril 2008;90:186–193. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Spector LG. Validation of infertility treatment and assisted reproductive technology use on the birth certificate in eight states. Am J Obstet Gynecol 2016;215:126–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA.. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern PG, Llorens AJ, Skurnick JH, Weiss G, Goldsmith LT.. Increased risk of preterm birth in singleton pregnancies resulting from in vitro fertilization–embryo transfer or gamete intrafallopian transfer: a meta-analysis. Fertil Steril 2004;82:1514–1520. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (reprinted from Annals of Internal Medicine). Phys Ther 2009;89:873–880. [PubMed] [Google Scholar]
- Molinaro TA, Shaunik A, Lin K, Sammel MD, Barnhart KT.. A strict infertility diagnosis has poor agreement with the clinical diagnosis entered into the Society for Assisted Reproductive Technology registry. Fertil Steril 2009;92:2088–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence Fertility Problems: Assessment and Treatment. National Institute for Health and Care Excellence, 2013, https://www.nice.org.uk/guidance/cg156/resources/fertility-problems-assessment-and-treatment-35109634660549 (27 July 2016, last date accessed). [Google Scholar]
- Overbeek A, van den Berg MH, Hukkelhoven CWPM, Kremer LC, van den Heuvel-Eibrink MM, Tissing WJE, Loonen JJ, Versluys AB, Bresters D, Kaspers GJL et al. Validity of self-reported data on pregnancies for childhood cancer survivors: a comparison with data from a nationwide population-based registry. Hum Reprod 2013;28:819–827. [DOI] [PubMed] [Google Scholar]
- Perkins KM, Boulet SL, Kissin DM, Jamieson DJ.. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001-2011. Obstet Gynecol 2015;125:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierron A, Revert M, Goueslard K, Vuagnat A, Cottenet J, Benzenine E, Fresson J.. Evaluation of the metrological quality of the medico-administrative data for perinatal indicators: a pilot study in 3 university hospitals. Rev Epidemiol Sante Publique 2015;63: 237–246. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the Society for Assisted Reproductive Technology, Practice Committee of the American Society for Reproductive Medicine . Elective single-embryo transfer. Fertil Steril 2012;97:835–842.22196716 [Google Scholar]
- Qin J, Liu X, Sheng X, Wang H.. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril 2016;105:73–85e6. [DOI] [PubMed] [Google Scholar]
- Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjaerven R, Vatten LJ.. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod 2006;21:2353–2358. [DOI] [PubMed] [Google Scholar]
- Rosenfeld Y, Strulov A. Improvement of accuracy of clinical reports--the case of IVF cycle rank. J Assist Reprod Genet 2009a;26:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld Y, Strulov A. Clinical reports on IVF cycle rank--reliability and validity. Harefuah 2009b;148:22–24. [PubMed] [Google Scholar]
- Santos-Ribeiro S, Tournaye H, Polyzos NP. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12-year nationwide analysis including 160 000 pregnancies. Hum Reprod 2016;31: 393–402. [DOI] [PubMed] [Google Scholar]
- Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm U–B, Bergh C.. Obstetric outcome after in vitro fertilization with single or double embryo transfer. Hum Reprod 2011;26:442–450. [DOI] [PubMed] [Google Scholar]
- Shiff NJ, Jama S, Boden C, Lix LM, Virnig B, McBean M, van Walvaren C, Austin P, Bennett C et al. Validation of administrative health data for the pediatric population: a scoping review. BMC Health Serv Res 2014;14: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HT, Sabroe S, Olsen J. A framework for evaluation of secondary data sources for epidemiological research. Int J Epidemiol 1996;25: 435–442. [DOI] [PubMed] [Google Scholar]
- Stern JE, Gopal D, Liberman RF, Anderka M, Kotelchuck M, Luke B.. Validation of birth outcomes from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS): population-based analysis from the Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART). Fertil Steril 2016a;106:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, McLain AC, Buck Louis GM, Luke B, Yeung EH.. Accuracy of self-reported survey data on assisted reproductive technology treatment parameters and reproductive history. Am J Obstet Gynecol 2016b;215:219.e1–219.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EA, Zegers-Hochschild F, Mansour R, Ishihara O, De Mouzon J, Nygren KG, Adamson GD.. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: assisted reproductive technology 2004. Hum Reprod 2013;28:1375–1390. [DOI] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, Barfield WD.. Assisted reproductive technology surveillance — United States, 2014. MMWR Surveill Summ 2017;66:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderam S, Schieve LA, Cohen B, Zhang Z, Jeng G, Reynolds M, Wright V, Johnson C, Macaluso M.. Linking birth and infant death records with assisted reproductive technology data: Massachusetts, 1997–1998. Matern Child Health J 2006;10:115–125. [DOI] [PubMed] [Google Scholar]
- Traeger-Synodinos J, Coonen E, Goossens V. Data from the ESHRE PGD consortium. Hum Reprod 2013;28:i18. [DOI] [PubMed] [Google Scholar]
- Van Walraven C, Austin PC. Administrative database research has unique characteristics that can risk biased results. J Clin Epidemiol 2012;65:126–131. [DOI] [PubMed] [Google Scholar]
- Van Walraven C, Austin PC, Manuel D, Knoll G.. The usefulness of administrative databases for identifying disease cohorts is increased with a multivariate model. J Clin Epidemiol 2010;63:1332–1341. [DOI] [PubMed] [Google Scholar]
- Widdifield J, Labrecque J, Lix L, Paterson JM, Bernatsky S, Tu K, Ivers N, Bombardier C.. Systematic review and critical appraisal of validation studies to identify rheumatic diseases in health administrative databases. Arthritis Care Res (Hoboken) 2013;65:1490–1503. [DOI] [PubMed] [Google Scholar]
- Williams CL, Bunch KJ, Stiller CA, Murphy MFG, Botting BJ, Wallace WH, Davies M, Sutcliffe AG.. Cancer risk among children born after assisted conception. N Engl J Med 2013;369:1819–1827. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cohen B, Macaluso M, Zhang Z, Durant T, Nannini A.. Probabilistic linkage of assisted reproductive technology information with vital records, Massachusetts 1997-2000. Matern Child Health J 2012;16:1703–1708. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Macaluso M, Cohen B, Schieve L, Nannini A, Chen M, Wright V.. Accuracy of assisted reproductive technology information on the Massachusetts birth certificate, 1997-2000. Fertil Steril 2010;94:1657–1661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


