Abstract
Objectives
To evaluate if obesity is associated with increased angiotensin II (Ang II) and decreased angiotensin-(1–7) [Ang-(1–7)] in the circulation and urine among adolescents born prematurely.
Study design
In a cross-sectional analysis of 175 14-year-olds born preterm with very low birth weight, we quantified plasma and urinary Ang II and Ang-(1–7) and compared their levels between subjects with overweight/obesity (body mass index ≥85th percentile, n = 61) and those with body mass index <85th percentile (n = 114) using generalized linear models, adjusted for race and antenatal corticosteroid exposure.
Results
Overweight/obesity was associated with higher systolic blood pressure and a greater proportion with high blood pressure. After adjustment for confounders, overweight/obesity was associated with an elevated ratio of plasma Ang II to Ang-(1–7) (β: 0.57, 95% CI 0.23 to 0.91) and higher Ang II (β: 0.21 pmol/l, 95% CI 0.03 to 0.39) but lower Ang-(1–7) (β: −0.37 pmol/l, 95% CI −0.7 to −0.04). Overweight/obesity was associated with a higher ratio of urinary Ang II to Ang-(1–7) (β: 0.21, 95% CI −0.02 to 0.44), an effect that approached statistical significance.
Conclusions
Among preterm-born adolescents, overweight/obesity was associated with increased Ang II but reduced Ang-(1–7) in the circulation and the kidney as well as higher blood pressure. Obesity may compound the increased risk of hypertension and cardiovascular disease in individuals born prematurely by further augmenting the prematurity-associated imbalance in the renin-angiotensin system.
Keywords: cardiovascular disease, hypertension, perinatal programming, renin-angiotensin system
The survival of infants born preterm has increased due to advances in prenatal and neonatal care, but premature birth and other early life events increase the lifetime risk for chronic health conditions such as hypertension and cardiovascular disease.1–3 The mechanisms behind this elevated risk are described incompletely but may include programmed alterations in various hormonal pathways. Perinatal events have been shown to induce changes in the renin-angiotensin system (RAS), a critical regulator of blood pressure and overall cardiovascular function4. In addition to the traditional angiotensin-converting enzyme (ACE) / angiotensin II (Ang II) / Ang II type 1 receptor pathway, the RAS also consists of the regulatory ACE2 / Ang-(1–7) / Mas receptor pathway which acts in part to counteract Ang II’s actions by promoting vasodilation and sodium excretion as well as inhibiting inflammation and fibrosis5, 6. Alterations in the RAS that promote Ang II and suppress Ang-(1–7) are now recognized to be linked to hypertension7, 8.
Obesity is also associated with increased Ang II expression which may lead to higher blood pressure but its effect in individuals born prematurely is unknown9. Race and antenatal corticosteroid exposure are associated with alterations in the RAS and may affect obesity’s influence10, 11. We recently demonstrated that adolescents born preterm with very low birth weight have higher blood pressure and a higher ratio of plasma Ang II relative to reduced Ang-(1–7) as compared with term-born peers, an association that is stronger in subjects with overweight/obesity as compared with subjects without overweight/obesity12. Therefore, we further evaluated the effect of obesity on the RAS among adolescents born preterm. We hypothesized that obesity is associated with higher Ang II and lower Ang-(1–7) in the circulation and kidney of adolescents born preterm compared with those without obesity.
Methods
Subjects were recruited from a prospective birth cohort of 193 patients born prematurely with very low birth weight (birth weight <1500 grams) between January 1, 1992 and June 30, 1996 at a regional perinatal center (Forsyth Medical Center in Winston Salem, NC). As part of a broader study of cardiovascular outcomes in adolescents born prematurely, we evaluated the cohort at 14 years of age (Figure 1; available at www.jpeds.com). Inclusion criteria were singleton birth, birth weight < 1500 grams, follow-up clinical information through at least 1 year of age corrected for gestation, and successful contact at age 14 years. Exclusion criteria were major congenital anomalies or genetic syndromes and being a ward of the state. Subjects were assessed at three study visits; we report measurements conducted at the third study visit. The Institutional Review Boards of Wake Forest School of Medicine and Forsyth Medical Center approved the study. Written parental or legal guardian informed consent and subject assent were obtained. Subjects were compensated upon completion of all study visits, and parents/guardians received compensation for travel expenses.
Figure 1;
online only Study cohort
A research nurse reviewed medical records and research databases to obtain maternal and subjects’ birth characteristics, including antenatal corticosteroid exposure, maternal hypertensive pregnancy, maternal smoking during pregnancy, cesarean delivery, sex, gestational age, and birth weight. Subjects were categorized as small-for-gestational age if their birth weight was less than the 10th percentile for gestational age.13 At the 14-year visit, demographic data were recorded including parental-reported race (black or non-black), current Medicaid use, and patient smoking status. Measurements of the subjects’ height, weight, and waist circumference were obtained, and the waist-to-height ratio and the body mass index (BMI) were calculated. Subjects were categorized as having overweight/obesity if their BMI was ≥85th percentile for age and sex according to established pediatric recommendations14. Subjects rated their sexual maturity in private using a self-reported questionnaire on a scale of 1–5, and we reported the percentage of subjects with a score of 5 in either of the two determinants of secondary sexual characteristics (pubic hair in all subjects plus breast development in females and external genitalia development in males)15.
Blood pressure was measured according to established pediatric guidelines by trained staff using a mercury manometer and an appropriately sized blood pressure cuff16. Each subject was seated quietly for a minimum of five minutes with their arm fully supported. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded as the average of three measurements (taken 1 minute apart), and blood pressure z-scores were calculated according to age, sex, and height-specific values17. According to pediatric recommendations, we defined high blood pressure as SBP or DBP ≥ 120/80 mmHg and further classified the stages of hypertension as i) elevated blood pressure if SBP was 120 to 129 mmHg but DBP was < 80 mmHg; ii) stage 1 hypertension if SBP was 130 to 139 mmHg or DBP was 80 to 89 mmHg; and iii) stage 2 hypertension if SBP was ≥ 140 mmHg or DBP was ≥ 90 mmHg.
Detailed descriptions of RAS measurements have been extensively described in previous publications12. Briefly, blood samples were collected with subjects seated for measurement of plasma renin activity, aldosterone, Ang II, and Ang-(1–7). Peptide samples were immediately collected in a tube containing a cocktail of inhibitors. Spot urine samples were immediately collected in a tube with HCl to prevent peptide degradation. Ang II, Ang-(1–7), plasma renin activity, and aldosterone were analyzed using radioimmunoassays. ACE and ACE2 were assayed in non-acidified urine samples, and ACE and ACE2 protein content were based on human standards (R&D Systems, Minneapolis, Minnesota, U.S.A.). Urine creatinine levels were determined by a modified Jaffe assay traceable to isotope dilution mass spectrometry. We calculated the ratio of Ang II to Ang-(1–7) in the plasma and urine, calculated the ratio of ACE to ACE2 in the urine, and corrected the urinary concentrations of Ang II, Ang-(1–7), ACE, and ACE2 for urine creatinine. For sample results that were below the laboratory’s lower limit of detection, the sample’s measurement was assigned a value calculated as the lower limit of detection divided by the square root of two18.
Statistical Analyses
We used descriptive statistics including measures of central tendency and dispersion and frequencies. We reported continuous variable distributions as mean with standard deviation or median with interquartile range. We used Chi-square test, Fisher exact test, t-test, and Wilcoxon rank-sum test for between-group comparisons and Pearson or Spearman correlation coefficients to test for correlations between continuous variables. A 2-sided alpha level less than 0.05 was considered statistically significant.
We utilized generalized linear models to estimate the relationship between overweight/obesity and the RAS outcomes. Distributional characteristics of the outcomes were improved with natural logarithmic transformation in the models. We employed directed acyclic graphs to determine potentially confounding factors based on the literature a priori and identified race and antenatal corticosteroid exposure in our minimally sufficient set of confounders for inclusion in the models10, 11, 19. We used Enterprise Guide software, Version 7.11 of the SAS System for Windows (SAS Institute Inc, Cary, NC) for all analyses.
Results
Within the cohort of 193 enrolled subjects, 5 were excluded (3 had significant medical problems and 2 were twins). One hundred and seventy-seven subjects participated in the third study visit; 175 subjects provided urine samples and 121 subjects provided blood specimens. Subjects’ perinatal and adolescent characteristics are noted in Table I. Thirty-five percent of subjects had overweight/obesity at age 14 years. Among the perinatal characteristics, subjects with overweight/obesity had higher rates of maternal hypertensive pregnancy and maternal smoking during pregnancy compared with those with BMI <85th percentile. At age 14 years, subjects with overweight/obesity had greater median values for weight, BMI, and waist-to-height-ratio, and were more likely to have a sexual maturity rating of 5. The overweight/obesity group had higher mean systolic blood pressure (109.9 ± 9.1 vs 104.4 ± 9.7 mmHg) and mean systolic blood pressure z-score (0.04 ± 0.8 vs. −0.43 ± 0.9) at age 14 years. Subjects with overweight/obesity also had a greater rate of high blood pressure (20% vs. 8%).
TABLE 1.
Clinical characteristics by body mass index status
| Overweight/Obesity, n = 61 | BMI <85th %ile, n = 114 | |
|---|---|---|
| Perinatal | ||
| Female | 34 (56%) | 63 (55%) |
| Black | 29 (48%) | 45 (39%) |
| Antenatal corticosteroid exposure | 28 (46%) | 64 (56%) |
| Maternal hypertensive pregnancy | 34 (56%)* | 41 (36%) |
| Maternal smoking | 16 (26%)* | 15 (13%) |
| Cesarean section | 35 (57%) | 57 (50%) |
| Gestational age (weeks) | 28.2 (2.6) | 27.6 (2.6) |
| Birth weight (g) | 1066 (285) | 1051 (256) |
| Small for gestational age | 10 (16%) | 9 (8%) |
| Adolescent | ||
| Height (cm) | 162.7 (8.7) | 161.0 (9.5) |
| Weight (kg) | 74.5 [68.5, 83.5]* | 50.4 [44.4, 56.0] |
| BMI (kg/m2) | 28.7 [26.2, 30.8]* | 19.5 [17.7, 21.1] |
| Waist-to-height ratio | 0.59 [0.53, 0.63]* | 0.43 [0.41, 0.46] |
| Sexual maturity rating of 5 | 46 (77%)* | 59 (52%) |
| Medicaid | 26 (45%) | 43 (39%) |
| Current smoker | 2 (3%) | 3 (3%) |
| SBP (mmHg) | 109.9 (9.1)* | 104.4 (9.7) |
| DBP (mmHg) | 60.3 (9.5) | 61.9 (8.6) |
| SBP z-score | 0.04 (0.8)* | −0.43 (0.9) |
| DBP z-score | −0.35 (0.85) | −0.18 (0.79) |
| High blood pressure | 12 (20%)* | 9 (8%) |
| Elevated blood pressure | 9 (15%)* | 6 (5%) |
| Stage 1 hypertension | 3 (5%) | 2 (2%) |
| Stage 2 hypertension | 0 (0%) | 1 (1%) |
| Plasma RAS (N = 121) | n = 42 | n = 79 |
| Ang II / Ang-(1–7) | 6.09 [2.96, 12.22]* | 3.46 [1.59, 6.16] |
| Ang II (pmol/l) | 26.4 [18.9, 33.9]* | 20.15 [16.2, 30.5] |
| Ang-(1–7) (pmol/l) | 3.25 [1.98, 7.9]* | 7.9 [2.6, 14.6] |
| PRA (pmol Ang I/l/h) | 2.3 [1.1, 3.02] | 2.31 [1.2, 3.3] |
| Aldosterone (pmol/l) | 11.15 [6.16, 18.19] | 8.77 [4.85, 13.29] |
| Urinary RAS | ||
| Ang II / Ang-(1–7) | 0.16 [0.1, 0.31] | 0.15 [0.1, 0.2] |
| Ang II / Cr (pmol/g) | 0.06 [0.04, 0.1] | 0.05 [0.04, 0.1] |
| Ang-(1–7) / Cr (pmol/g) | 0.41 [0.31, 0.56] | 0.42 [0.3, 0.6] |
| ACE / Cr (ng/mg)a | 3.73 [2.07, 7.86] | 3.43 [1.04, 7.3] |
| ACE2 / Cr (ng/mg)a | 0.53 [0.33, 1.46] | 0.55 [0.18, 1.23] |
| ACE / ACE2 | 6.68 [3.65, 14.82] | 6.34 [3.01, 15.73] |
N (%), mean (SD), or median [IQR]. Cr, creatinine.
N = 123 (overweight/obesity 44, BMI <85th percentile 79).
P < 0.05 for comparison by BMI status via chi-square test, Fisher’s exact test, t-test, or Wilcoxon rank-sum test.
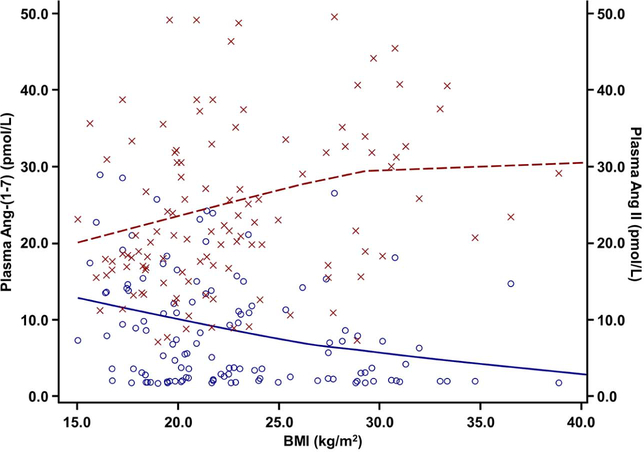
BMI correlated positively with plasma Ang II but inversely with Ang-(1–7) (Figure 2). Subjects with overweight/obesity had a higher median ratio of plasma Ang II to Ang-(1–7), higher median Ang II, and lower median Ang-(1–7) compared with subjects with BMI <85th percentile (Table 1). There were no differences in median plasma renin activity or aldosterone. The differences in the Ang II to Ang-(1–7) ratio, Ang II, and Ang-(1–7) in the plasma persisted after adjustment for the potentially confounding factors race and antenatal corticosteroid exposure (Table 2). Compared with those with a BMI <85th percentile, overweight/obesity was associated with a higher Ang II to Ang-(1–7) ratio (adjusted β: 0.57, 95% CI 0.23 to 0.91) as well as higher Ang II (adjusted β: 0.21 pmol/l, 95% CI 0.03 to 0.39) and lower Ang-(1–7) (adjusted β: −0.37 pmol/l, 95% CI −0.7 to −0.04).
Figure 2.
Body mass index is associated with higher plasma angiotensin II and lower plasma angiotensin-(1–7).
Plasma angiotensin-(1–7) on the left Y-axis and in black with circles; plasma angiotensin II on the right Y-axis and in gray with x’s; BMI on the X-axis. Loess fitted curves with cubic interpolation. Angiotensin-(1–7) – BMI Spearman correlation coefficient r = −0.29, P = .002. Angiotensin II – BMI Spearman correlation coefficient r = 0.29, p = 0.001.
TABLE 2.
Differences in the plasma and urinary renin-angiotensin system profiles according to body mass index status
| Crude | Adjusteda | |
|---|---|---|
| β (95% CI) | β (95% CI) | |
| Plasma Ang II / (1–7) | 0.63 (0.29, 0.97) | 0.57 (0.23, 0.91) |
| Plasma Ang II (pmol/l) | 0.17 (−0.01, 0.35) | 0.21 (0.03, 0.39) |
| Plasma Ang-(1–7) (pmol/l) | −0.46 (−0.8, −0.13) | −0.37 (−0.7, −0.04) |
| Urinary Ang II / (1–7) | 0.18 (−0.04, 0.41) | 0.21 (−0.02, 0.44) |
Generalized linear models with natural log-transformed outcomes.
Adjusted for race and antenatal corticosteroid exposure.
On initial bivariate analyses there were no between-group differences in the median values of the components of the RAS in the urine, including the ratio of Ang II to Ang-(1–7), Ang II/creatinine, Ang-(1–7)/creatinine, ACE/creatinine, ACE2/creatinine, and the ratio of ACE to ACE2 (Table 1). However, after controlling for race and antenatal corticosteroid exposure, overweight/obesity was associated with a higher ratio of urinary Ang II to Ang-(1–7) compared with those with a BMI <85th percentile (β: 0.21, 95% CI −0.02 to 0.44), a result that approached statistical significance (Table 2).
Discussion
Among adolescents born preterm with very low birth weight, overweight/obesity was associated with increased blood pressure, a greater proportion of high blood pressure, and alterations in the circulatory and renal RAS compared with subjects with BMI <85th percentile. Subjects with overweight/obesity had higher Ang II and lower Ang-(1–7) as well as a higher ratio of Ang II relative to Ang-(1–7) in the plasma. Compared with term-born peers, adolescents born preterm have a higher ratio of Ang II relative to Ang-(1–7) in the plasma, and obesity magnifies this effect wherein preterm-born adolescents with overweight/obesity have a higher ratio of plasma Ang II relative to Ang-(1–7) compared with term-born adolescents with overweight/obesity12. In the current study we confirmed that within subjects born preterm, obesity has additional detrimental effects on the circulatory RAS that may compound those attributed to perinatal programming events.
A shift in the circulatory RAS towards higher Ang II expression, or tone, and lower Ang-(1–7) tone may partially explain the higher blood pressure in adolescent subjects with overweight/obesity and could serve as a mechanism for further increasing the risk of hypertension and cardiovascular disease during adulthood that is known to be associated with prematurity. Upregulation of Ang II at the expense of Ang-(1–7) is associated with persistently elevated blood pressure as well as inflammation and fibrosis in various organ systems such as the vasculature, kidneys, and heart that occur in part via vasoconstriction and reduced nitric oxide production leading to increased oxidative stress6, 20, 21. Obesity is associated with dysregulation of the RAS which may contribute to the increased risk of hypertension and cardiovascular disease seen in patients with obesity22, 23. Our finding that overweight/obesity is associated with higher blood pressure in adolescents born preterm supports similar findings reported by Rotteveel et al, although this study did not assess the Ang II or Ang-(1–7) pathways in the plasma and urine24. Thus, obesity may provide an additive effect on the development of hypertension and cardiovascular disease in individuals born preterm that augments prematurity-induced perinatal programming of the RAS.
Subjects with overweight/obesity also had a higher ratio of urinary Ang II to Ang-(1–7) that approached statistical significance, indicating a possible shift in the renal RAS that increases the deleterious Ang II pathway but reduces the beneficial Ang-(1–7) pathway. Evidence from experimental models in rodents indicates that urinary angiotensin peptide levels are independent of glomerular filtration of peptides within the circulation and thus indicate generation within the tubules25. The exact mechanisms are unclear but could be due to increased tubular formation or reduced metabolism of Ang II or decreased formation or increased metabolism of Ang-(1–7). We found no differences in urinary ACE or ACE2 in the current study, but other peptidases such as neprilysin, thimet oligopeptidase, or dipeptidyl peptidase 3 may contribute to the tubular processing of angiotensins26. Additional study is warranted to better define this shift in the renal RAS by expanding our analysis of these peptidases in the urine.
The shift in the renal RAS that we describe could further contribute to the development of hypertension and cardiovascular disease by stimulating sodium retention, raising blood pressure, and ultimately promoting renal inflammation and fibrosis in part through lower nitric oxide production and increased oxidative stress27, 28. Experimental models and human studies of the fetal-programming effect of antenatal corticosteroid exposure on the renal RAS also demonstrate this shift in the kidney towards Ang II, leading to elevated blood pressure and, in sheep, decreased renal function7, 11. Our findings suggest that among adolescents born prematurely, a group already at increased risk for disease29, having overweight/obesity may augment this risk in part through the shift in the renal RAS towards Ang II and away from Ang-(1–7).
A limitation of our study is that although plasma renin activity and aldosterone were measured, neither levels of the precursor protein angiotensinogen nor the peptidases neprilysin or, in the circulation, ACE and ACE2 that can generate and metabolize Ang II and Ang-(1–7) were assessed30. Further study is warranted to establish whether alterations in the synthesizing components of the RAS are associated with higher Ang II or lower Ang-(1–7) in the overweight/obesity group. We also did not fully examine additional factors that may influence the RAS, including dietary salt or potassium intake or genetic profiles. Furthermore, the sex hormones including estrogen and testosterone are known to influence the RAS, and we did not measure their levels.
Potential strategies to prevent or treat disease in individuals born preterm could target the RAS to inhibit Ang II and augment expression of Ang-(1–7).
Supplementary Material
Figure 3.
Plasma ratio of angiotensin II to angiotensin-(1–7) according to body mass index status. Overweight/obesity on left, BMI <85th percentile on right. Bar denotes median, diamond denotes mean, box indicates IQR, and whiskers include ≤1.5x IQR. P < 0.05 via Wilcoxon rank-sum test. IQR, interquartile range.
Acknowledgements
We thank the participants and their families, Patricia Brown, RN, research nurse, and Alice Scott, RN, research study coordinator.
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01 HD047584; HD084227), the American Heart Association (AHA 14GRNT20480131), the Clinical Research Unit of Wake Forest Baptist Medical Center (MCRR/NIH M01-RR07122), the Wake Forest Clinical and Translational Science Award (NIH UL1 TR001420), and Forsyth Medical Center and Wake Forest School of Medicine Department of Pediatrics research funds. The authors declare no conflicts of interest. Portions of this study were presented as an abstract at the American Heart Association Council on Hypertension, September 17, 2016, Orlando, Florida.
Abbreviations
- ACE
angiotensin-converting enzyme
- ACE2
angiotensin-converting enzyme 2
- Ang II
angiotensin II
- Ang-(1–7)
angiotensin-(1–7)
- BMI
body mass index
- DBP
diastolic blood pressure
- RAS
renin-angiotensin system
- SBP
systolic blood pressure
Footnotes
Data Statement: Data sharing statement available at www.jpeds.com.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaiser JR, Tilford JM, Simpson PM, Salhab WA, Rosenfeld CR. Hospital survival of very-low-birth-weight neonates from 1977 to 2000. J Perinatol 2004; 24:343–50. [DOI] [PubMed] [Google Scholar]
- 2.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012; 59:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr H, Cnattingius S, Granath F, Ludvigsson JF, Edstedt Bonamy A-K. Preterm birth and risk of heart failure up to early adulthood. J Am Coll Cardiol 2017; 69:2634–42. [DOI] [PubMed] [Google Scholar]
- 4.McMullen S, Gardner DS, Langley-Evans SC. Prenatal programming of angiotensin II type 2 receptor expression in the rat. Br J Nutr 2004; 91:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX-J, et al. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol 2005; 289:H2356–H63. [DOI] [PubMed] [Google Scholar]
- 6.Chappell MC, Al Zayadneh EM. Angiotensin-(1–7) and the regulation of anti-fibrotic signaling pathways. J Cell Signal 2017; 2:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension 2009; 53:404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simões e Silva AC, Diniz JS, Regueira Filho A, Santos RA. The renin-angiotensin system in childhood hypertension: Selective increase of angiotensin-(1–7) in essential hypertension. J Pediatr 2004; 145:93–8. [DOI] [PubMed] [Google Scholar]
- 9.Chung S, Park CW, Shin SJ, Lim JH, Chung HW, Youn D-Y, et al. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol Dial Transplant 2010; 25:389–99. [DOI] [PubMed] [Google Scholar]
- 10.Yu Z, Eckert GJ, Liu H, Pratt JH, Tu W. Adiposity has unique influence on the renin-aldosterone axis and blood pressure in black children. J Pediatr 2013; 163:1317–22.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Snively BM, et al. Antenatal corticosteroids and the renin-angiotensin-aldosterone system in adolescents born preterm. Pediatr Res 2017; 81:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Jensen ET, et al. Association between preterm birth and the renin−angiotensin system in adolescence: Influence of sex and obesity. J Hypertens 2018; 10.1097/hjh.0000000000001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell BK. Babies who are small for gestational age. Pediatr Rev 1995; 16:354-. [Google Scholar]
- 14.Barlow SE. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary Report. Pediatrics 2007; 120 Suppl 4:S164–92. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SJC, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: A preliminary study. Paediatr Perinat Epidemiol 2001; 15:88–94. [DOI] [PubMed] [Google Scholar]
- 16.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: A Working Group Report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics 1996; 98 (4 Pt 1):649–58. [PubMed] [Google Scholar]
- 17.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: Some methodological issues. Am J Epidemiol 2008; 167:653–66. [DOI] [PubMed] [Google Scholar]
- 18.Croghan C, Egeghy PP. Methods of dealing with values below the limit of detection using SAS. Southeastern SAS User Group; 2003. September 22-24, 2003; St. Petersburg, FL. [Google Scholar]
- 19.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008; 8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampaio WO, Henrique de Castro C, Santos RAS, Schiffrin EL, Touyz RM. Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 2007; 50:1093–8. [DOI] [PubMed] [Google Scholar]
- 21.Varagic J, Ahmad S, VonCannon JL, Moniwa N, Brosnihan KB, Wysocki J, et al. Predominance of AT1 blockade over Mas–mediated angiotensin-(1–7) mechanisms in the regulation of blood pressure and renin–angiotensin system in mRen2.Lewis rats. Am J Hypertens 2013; 26:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension 2004; 43:41–7. [DOI] [PubMed] [Google Scholar]
- 23.Giacchetti G, Faloia E, Sardu C, Camilloni MA, Mariniello B, Gatti C, et al. Gene expression of angiotensinogen in adipose tissue of obese patients. Int J Obes Relat Metab Disord 2000; 24:S142. [DOI] [PubMed] [Google Scholar]
- 24.Rotteveel J, van Weissenbruch MM, Twisk JWR, Delemarre-Van de Waal HA. Infant and childhood growth patterns, insulin sensitivity, and blood pressure in prematurely born young adults. Pediatrics 2008; 122:313–21. [DOI] [PubMed] [Google Scholar]
- 25.Wilson BA, Marshall AC, Alzayadneh EM, Chappell MC. The ins and outs of angiotensin processing within the kidney. Am J Physiol Regul Integr Comp Physiol 2014; 307:R487–R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz-Diaz N, Wilson BA, Pirro NT, Brosnihan KB, Marshall AC, Chappell MC. Identification of dipeptidyl peptidase 3 as the Angiotensin-(1–7) degrading peptidase in human HK-2 renal epithelial cells. Peptides 2016; 83:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, et al. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 2007; 18:1558–65. [DOI] [PubMed] [Google Scholar]
- 28.Hollenberg NK, Moore T, Shoback D, Redgrave J, Rabinowe S, Williams GH. Abnormal renal sodium handling in essential hypertension: Relation to failure of renal and adrenal modulation of responses to angiotensin II. Am J Med 1986; 81:412–8. [DOI] [PubMed] [Google Scholar]
- 29.Kandasamy Y, Smith R, Wright IMR, Lumbers ER. Extra-uterine renal growth in preterm infants: Oligonephropathy and prematurity. Pediatr Nephrol 2013; 28:1791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chappell MC. Biochemical evaluation of the renin-angiotensin system: The good, bad, and absolute? Am J Physiol Heart Circ Physiol 2016; 310:H137–H52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.