Abstract
Hypothesis:
Acoustic stimulation generates measurable sound pressure levels in the semicircular canals.
Background:
High-intensity acoustic stimuli can cause hearing loss and balance disruptions. To examine the propagation of acoustic stimuli to the vestibular end-organs, we simultaneously measured fluid pressure in the cochlea and semicircular canals during both air- and bone-conducted sound presentation.
Methods:
Five full-cephalic human cadaveric heads were prepared bilaterally with a mastoidectomy and extended facial recess. Vestibular pressures were measured within the superior, lateral and posterior semicircular canals and referenced to intracochlear pressure within the scala vestibuli with fiber-optic pressure probes. Pressures were measured concurrently with laser Doppler vibrometry measurements of stapes velocity during stimulation with both air- and bone-conduction. Stimuli were pure tones between 100 Hz and 14 kHz presented with custom closed-field loudspeakers for air-conducted sounds and via commercially available bone-anchored device for bone-conducted sounds.
Results:
Pressures recorded in the superior, lateral and posterior semicircular canals in response to sound stimulation were equal to or greater in magnitude than those recorded in the scala vestibuli (up to 20 dB higher). The pressure magnitudes varied across canals in a frequency-dependent manner.
Conclusions:
High sound pressure levels were recorded in the semicircular canals with sound stimulation, suggesting that similar acoustical energy is transmitted to the semicircular canals and the cochlea. Since these intralabyrinthine pressures exceed intracochlear pressure levels, our results suggest that the vestibular end-organs may also be at risk for injury during exposure to high-intensity acoustic stimuli known to cause trauma in the auditory system.
Introduction
While high-intensity sound exposure is a well-documented cause of hearing loss, its effects on the vestibular system and resulting balance disorders remain largely unknown.1 Several factors suggest a risk of coincident vestibular damage associated with noise-induced hearing loss: the vestibular labyrinth’s close anatomic proximity to the stapes footplate delivering acoustic energy to the cochlea, the structural similarities between cochlear and vestibular hair cells, the shared membranous labyrinth, and the common vascular supply to cochlear and vestibular end-organs.
Five end-organs comprise the peripheral vestibular system. The two otolithic organs - the utricle and saccule - sense linear acceleration, and the three semicircular canals detect rotational acceleration. Altogether, these end-organs provide information to the central nervous system allowing individuals to maintain posture and to stabilize their visual field. Within each end-organ, head movements are detected by vestibular hair cells, converted into hair cell electrical receptor potentials, and further propagated as action potentials in vestibular nerve afferent fibers.
Clinically, acoustical blast exposure can cause significant vestibular trauma, manifesting with signs and symptoms such as dizziness, imbalance, vertigo, oscillopsia and headache.2,3 After noise exposure, abnormal cervical vestibular-evoked myogenic potential (cVEMP) responses, a measure of saccule function, are seen in 38–75% of patients.4–6 Studies have also documented new-onset spontaneous or positional nystagmus,7 increased body sway,1 and decreased vestibulo-ocular reflex (VOR) gain.8 These and other clinical reports of balance disorders following noise exposure have so far given contradictory results including very wide ranges of abnormal VOR, cVEMP, and caloric tests, and therefore remain controversial.1,7–11 Abnormal vestibular testing is more common in patients with asymmetric noise-induced hearing loss than patients with symmetric hearing losses.11 Thus, the lower incidence of clinically evident noise-induced vestibulopathy relative to auditory dysfunction may be due to central vestibular compensation or redundancy within the vestibular system. In general, peripheral vestibular trauma is less well understood and can be difficult to separate from central effects of dizziness, vertigo, and headache from mild traumatic brain injury.
Energy propagation from the auditory to the vestibular system is supported by existing literature. Acoustic stimulation of the vestibular system occurs in both normal human and animal labyrinths and in pathologically dehiscent or fenestrated bony semicircular canals.12 The auditory and vestibular end-organs are derived from common embryologie structures, and are closely related in aquatic species, including fish and amphibians, in which the saccule serves as an acoustic organ.13–15 In higher order species, the vestibular system maintains some sensitivity to sound, as evidenced by the Tullio phenomenon and the ability to stimulate vestibular responses with acoustic energy, as with VEMP measurements.
There is conflicting evidence regarding the relative sound sensitivity of the five vestibular end-organs. Some authors report auditory stimulation of all five vestibular end-organs,15–17 whereas others report sensitivity of only the saccule.13,18 The semicircular canals are thought to be less sensitive than the saccule to impulse noise, even at high intensities.16,17
Stronger and more widespread vestibular response to sound is seen when the labyrinth is opened; fenestration of the bony labyrinth increases utricular and semicircular canal sound sensitivity as evidenced by enhanced vestibulo-ocular reflex (VOR) gain16 and lower VEMP thresholds in the presence of canal dehiscence.19 Wit et al. (1981) demonstrated in pigeons that vestibular organs are more sensitive to loud auditory stimuli after fenestration of one semicircular canal.20 In humans, this observation has been harnessed to enhance hearing capabilities of profoundly deaf patients. Ribaric et al (1992) fenestrated the lateral semicircular canal in profoundly deaf patients with normal vestibular function and demonstrated improved perception of bone-conducted sounds.21,22 Like fenestration, semicircular canal dehiscence also effectively opens the bony labyrinth and can lead to sound-evoked vestibular symptoms including vertigo and oscillopsia. Mechanistically, the dehiscence serves as a mobile third window so that endolymphatic motion causes excitatory cupula deflection within the superior semicircular canal stimulating vestibular nerve afferents that cause a vertical torsional nystagmus.19 Without a window in the vestibular end-organs, the cochlea has lower effective acoustic impedance than the vestibule; hence, less sound energy propagates to the vestibule.
Despite the variable clinical picture, literature supports the assertion that acoustical blast injury sometimes causes concomitant vestibular dysfunction,2 though the mechanism of injury remains unclear. We hypothesize that vestibular damage occurs through the same mechanism as acoustical blast injury via energy transmission to the vestibular end-organs. That is, high-intensity sound exposure, which generates intracochlear pressure waves at levels that can mechanically damage the cochlea,23,24 transmits sufficient acoustical energy from the cochlea to the vestibular end-organs to cause injury. In this study, we measured the sound pressure levels in the vestibular system in otherwise healthy ears during stimulation with high-intensity sound.
Materials and Methods
Data was collected and analyzed from nine ears in fresh-frozen whole cadaveric heads. All specimens were confirmed to have intact temporal bones and verified to have no history of middle ear disease or otologic surgery (Lone Tree Medical, Littleton, CO, USA). The use of cadaveric human tissue was in compliance with the University of Colorado Anschutz Medical Campus Institutional Biosafety Committee and Review Board (COMIRB EXEMPT #14-1464).
Temporal Bone Preparation
Temporal bone specimens were prepared as previously described by our laboratory,24–31 and similar to methods employed by other groups.23,32 Briefly, intact whole cadaveric heads were thawed overnight in cool water and inspected to rule out temporal bone, external or middle ear injury or disease. A canal-wall-up mastoidectomy with an extended facial recess approach was performed. The cochlear promontory was thinned overlying the scala vestibuli, and the bone overlying the superior semicircular canal (SSCC), lateral semicircular canal (LSCC) and posterior semicircular canal (PSCC) was blue-lined (Fig. 1A). Bone-conduction stimuli were generated using a commercially available bone-anchored sound processor affixed to an implanted titanium fixture. A 4-mm titanium implant (Cochlear Ltd., Centennial, CO, U.S.A.) was situated 50 mm from the external auditory canal. The specimens were acoustically isolated from other testing equipment via suspension by a Mayfield Clamp (Integra Lifesciences Corp., Plainsboro, NJ, U.S.A.) attached to a stainless steel baseplate. Cochleostomy into the scala vestibuli (SV) and canalostomies into the SSCC, LSCC and PSCC were created using a fine pick under a droplet of water, and fiber-optic pressure sensors (FOP-M260-ENCAP, FISO Inc., Quebec, QC, Canada) were inserted (Fig. 1B). Probes were sealed in place with alginate dental impression material (Jeltrate; Dentsply International Inc., York, PA). The fiber-optic sensors were factory calibrated and sensitivity was verified by normalizing the sensor response to laser Doppler vibrometry displacement measurements made while generating a known motion in a cup of fluid controlled by a B&K mini-shaker (Bruel & Kjær Type 4810, Nærum, Denmark).
Figure 1.
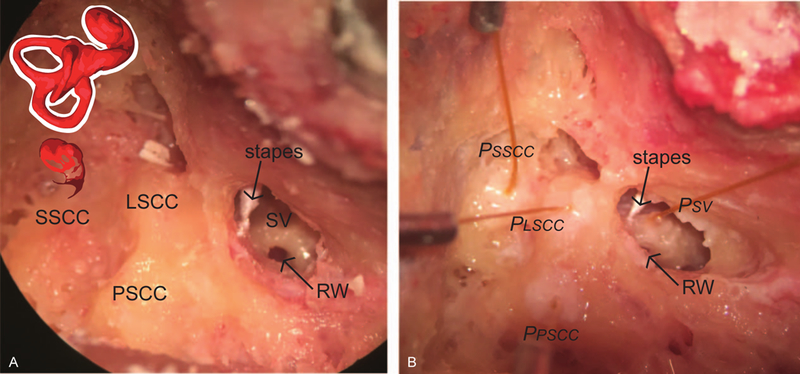
Photomicrographs of the specimen preparation. Temporal bones of intact cephali underwent mastoidectomy with wide facial recess. Scala vestibuli near the oval window (SV), lateral semicircular canal (LSCC), posterior semicircular canal (PSCC) and superior semicircular canal (SSCC) were blue lined in preparation for the intracochlear pressure probes (A). Pressure probes (PSV, PLSCC, PPSCC, PSSCC) were placed in cochleostomies in SV and canals, retro-reflective glass beads placed on the stapes for LDV measurements (B).
Out-of-plane velocity (i.e. velocity parallel to the laser beam) of the stapes was measured with a single-axis laser Doppler velocimeter (LDV) (OFV-534 & OFV-5000; Polytec Inc., Irvine, CA) mounted to a dissecting microscope (Carl Zeiss AG, Oberkochen, Germany). Microscopic retro-reflective glass beads (P-RETRO 45–63 μm dia., Polytec Inc., Irvine, CA) were placed on the stapes to enhance the reflected signal.33,34 Any remaining water was suctioned from the middle ear prior to data collection.
Stimuli Presentation and Data Acquisition
All experiments were performed in a double-walled sound-attenuating chamber (IAC Inc., Bronx, NY). Generation of sound stimuli and recording of responses was performed as described previously.24,29–31 Briefly, stimuli were generated digitally, and presented via a bone conduction oscillator (Cochlear Ltd., Centennial, CO, U.S.A.) or a closed-field magnetic speaker (MF1; Tucker-Davis Technologies Inc., Alachua, FL) coupled directly to the ear with an ear speculum altered to accommodate flexible speaker tubing and to reduce sound escape. Both transducers were driven by an external sound card (Hammerfall Multiface II, RME, Haimhausen, Germany), and output directed to the loudspeaker was amplified with one channel of a stereo amplifier (TDT SA1). The sound intensity in the ear canal was measured with a probe-tube microphone (type 4182; Bruel & Kjær, Nærum, Denmark), which was also placed through the modified ear speculum. Baseline acoustic transfer functions were generated from presentation of short (1 second duration) tone pips between 100 and 14,000 Hz ramped on and off with one half (5 ms) of a Hanning window. Tones were presented at 70 to 120 dB SPL. Input from the microphone, LDV, and pressure sensors were simultaneously captured via the sound card analog inputs.
Data Analysis
Temporal bone measurements are shown as transfer functions, which consist of measured signal output normalized to stimulus input for each frequency, representing system gain. Differential pressure is calculated as the difference in pressures between scala vestibuli and a given semicircular canal and represents the pressure transmitted across the membrane limitans into each semicircular canal. All measurements are in response to acoustic or bone-conducted stimulation with pure tones at different frequencies. All were acquired at 96 kHz sampling rate, then down sampled to 44.1 kHz, and band-pass filtered between 15 Hz and 15 kHz with a second-order Butterworth filter for further analysis.
The magnitude of the LDV signal was adjusted using a cosine correction (1/cos(9)) based on an estimate of the difference in angle between the primary axis of the stapes and the orientation of the LDV laser (usually approximately 45°). The magnitude of pressure signals was also adjusted using the manufacturer calibration.
Signal magnitude in all recordings (pressures and velocities) was computed by taking the value of the Fourier transform of the recording at the frequency corresponding to the pure tone stimulus being presented. Pressure and stapes velocity recordings are inherently very noisy; we thus designed the following method to exclude recordings that fall within the noise floor. First, we assessed the noise spectrum of each signal independently (LDV and pressure recordings in four channels). For each frequency, the noise distribution at that frequency was obtained by tallying all Fourier transform values recorded at that frequency when the presented stimulus was a different frequency. Then, we computed the mean and standard deviation of the noise spectra for all channels and frequencies. All reported data had magnitudes two standard deviations higher than the average noise value for this frequency and channel.
Results
Baseline Transfer Functions in the Cochlea
Baseline responses of stapes velocities and scala vestibuli (SV) pressure were assessed after placement of intralabyrinthine pressure probes to verify the condition of each temporal bone. Closed field acoustic transfer function magnitudes for the nine ears are shown in the upper panels of Figure 2. Responses were overlaid onto the 95% confidence interval for stapes velocity,35 and the mean +/−standard deviation of responses observed for scala vestibuli transfer functions reported previously by Nakajima et al.23 One specimen (160523) had intracochlear pressures outside the expected range and was determined to have abnormal middle ear function as verified by stapes velocity measurements. The data from this specimen has been excluded from further analysis, while data from the remaining specimens was consistent with what has been reported previously in the literature.23,24,29-32 Consistency in pressure measurements reflect the quality of the specimen, indicating a normal middle ear conductive mechanism and normal cochlea without pneumocochlea.
Figure 2.
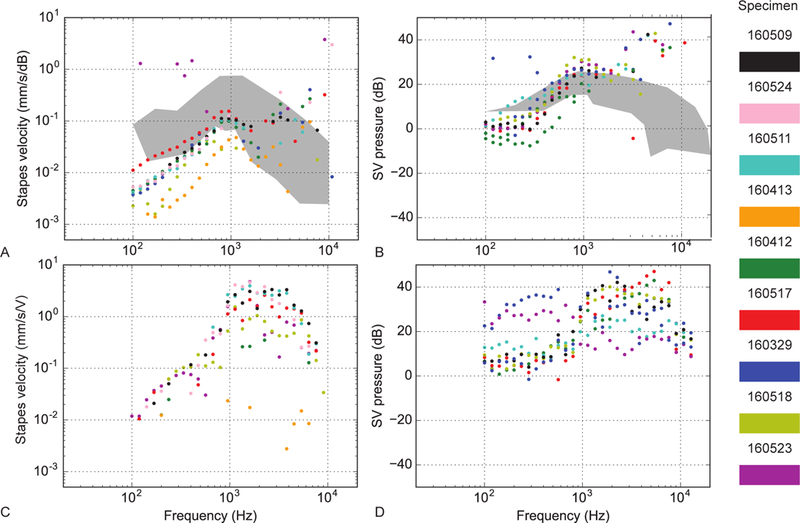
Baseline stapes velocity and scala vestibuli transfer magnitudes for air conduction (upper panels) and bone conduction (lower panels) in 9 specimens. A Stapes velocity normalized to the SPL recorded in the ear canal for air-conducted stimuli at different frequencies. Different color points are different specimens (see right panel). B Scala vestibuli pressures normalized to the SPL recorded in the ear canal for air-conducted stimuli at different frequencies. C Stapes velocity normalized to the input voltage of the bone conduction oscillator for bone-conducted stimuli. D Scala vestibuli pressures normalized to the input voltage of the oscillator. In A and B, responses are superimposed onto the 95% CI and range of responses (gray bands) observed previously.23,35 Right panel: specimen color scheme.
Baseline bone conduction transfer function magnitudes for the specimens are shown in the lower panels of Figure 2. Stapes and scala vestibuli transfer functions to bone-conducted stimuli were similar to those observed with air-conducted stimuli, as well as data collected previously in our laboratory.29,30
Acoustic Closed-Field Transfer Functions in the Vestibular System
Acoustic transfer function magnitudes measured in each of the semicircular canals for the eight ears are shown in Figure 3, with the pressures for each canal (from left to right: SSCC, LSCC and PSCC) referenced to the pressure measured simultaneously within the scala vestibuli of the cochlea in the upper panels. The lower panels show the averages of each canal’s pressure transfer function values across three different frequency bands (f < 600 Hz, 600 < f < 2000 Hz, and f > 2000 Hz). The colored bars represent each specimen and the dark gray bars represent the mean value across specimen. Only bands in which 10 or more data points above noise are averaged. A transfer function near zero would indicate that pressure was neither lost nor gained with sound energy transfer from the cochlea to the semicircular canal. The pressures were the highest within the PSCC, while pressures recorded in the SSCC were the most comparable to those measured within the SV particularly at frequencies less than 600 Hz. Pressures also varied across frequencies within the semicircular canals. Pressures were highest in the SSCC between 600 Hz and 2 kHz and in the LSCC and PSCC greater than 2 kHz. There do appear to be some differences between specimens, perhaps even showing bimodal responses, particularly in the SSCC and the LSCC. There were some specimens with positive gain at all frequencies and some that show negative gain between 300 Hz to 2 kHz.
Figure 3.
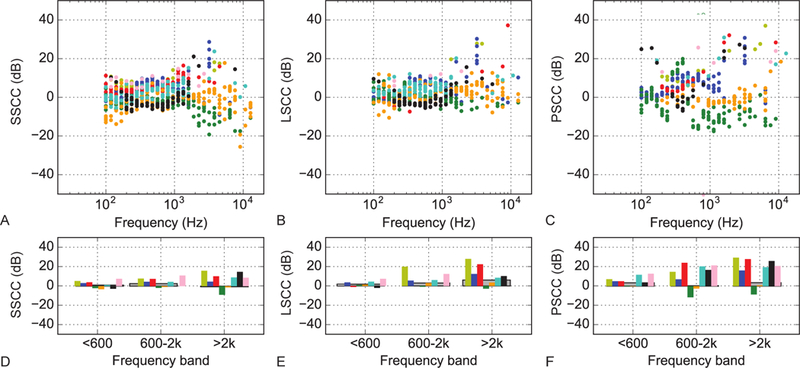
Acoustic closed-field transfer function magnitudes for semicircular canal pressure measurements normalized to the pressure in scala vestibuli. A Superior semicircular canal pressure transfer function. B same as A for lateral semicircular canal and C for the posterior semicircular canal. D Averages of the superior semicircular canal pressure transfer function values in three different frequency bands for each specimen (colored bars) and across specimens (dark gray bars). Only bands in which 10 or more data points above noise are averaged. E same as D for lateral semicircular canal and F for the posterior semicircular canal.
A single specimen (160412) was noted to have lower canal pressures to air-conducted stimuli when compared with results from other specimens. Normal stapes velocities verify normal middle ear function and suggest iatrogenic damage during preparation is unlikely. Higher than expected pressure measurements to bone-conducted stimuli - particularly in the PSCC - suggest the possibility of a canal dehiscence or incomplete sealing of the canalostomy site with dental amalgam.
Bone Conduction Transfer Functions in the Vestibular System
Transfer function magnitudes to bone-conducted stimuli for each of the semicircular canals in the eight ears are shown in Figure 4. The pressures for each canal (from left to right: SSCC, LSCC and PSCC) are normalized to pressure in the SV in the upper panels. The lower panels show the averages of each canal’s pressure transfer function values across the same three frequency bands as reported for air-conducted stimuli. Like with air-conducted stimuli, the energy transferred was most similar between the SV and the SSCC. Overall, the intralabyrinthine pressures were higher with bone-conducted high-intensity stimuli than with air-conducted stimuli. There was a 10 dB gain in the LSCC at frequencies greater than 2 kHz and a 20 dB gain in the PSCC at frequencies less than 600 Hz, though these larger mean differences may be influenced by the single specimen thought to have a canal dehiscence, as noted above.
Figure 4.
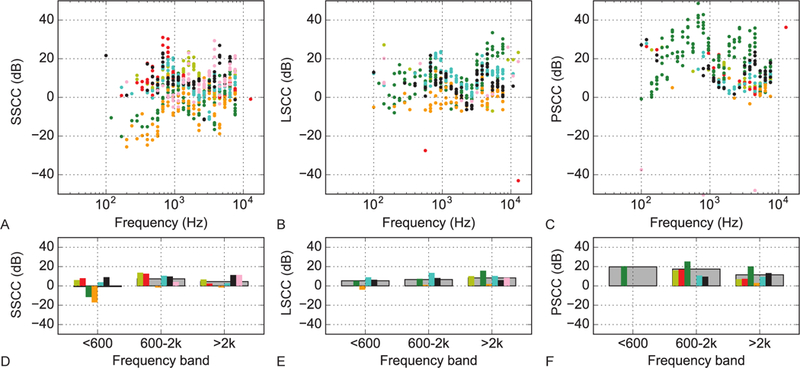
Bone-conducted transfer function magnitudes for semicircular canal pressure measurements normalized to the pressure in scala vestibuli. A Superior semicircular canal pressure transfer function. B same as A for lateral semicircular canal and C for the posterior semicircular canal. D Averages of the superior semicircular canal pressure transfer function values in three different frequency bands for each specimen (colored bars) and across specimens (dark gray bars). Only bands in which 10 or more data points above noise are averaged. E same as D for lateral semicircular canal and F for the posterior semicircular canal.
Discussion
This study demonstrates that large sound-induced pressure shifts are present and recordable in the semicircular canals. At present, these pressures have never before been reported to our knowledge.
Mechanisms of Vestibular Injury from Acoustic Trauma
Increased fluid pressure in the semicircular canals relative to the scala vestibuli noted in this study is likely the result of the lower cross sectional area (A) in the semicircular canals than in the cochlea. Knowing pressure (p) is defined as force (F) per unit area:
if we assume the force is equal in the two spaces:
then:
and the ratio of pressures is:
Therefore, the change in pressures is directly proportional to the change in cross sectional areas. The cross-sectional area of scala vestibuli has been measured in several studies, and is dependent upon the distance from the base of the cochlea.36–38 Near the base, where pressure probes are placed for our investigation, cross-sectional area is estimated to be between 0.97 mm2 and 1.7 mm2, depending on the study and calculation method used. In the semicircular canals, the probes are placed near to the middle of the canal along its length, where the diameter is at its narrowest, and surface area can be estimated to be about 0.5 mm2.39 Using this ratio of direct proportionality of change, we could expect to see between 2 to 3.5-fold increase in pressure from the scala vestibuli to the semicircular canals, which is consistent with our data. Deviation from this prediction could be explained by variation in the exact cross-sectional area at the precise placement of each probe, which can be difficult to control not only across specimen, but across placement within a single specimen.
Given the similar pressures with high-level incident sound, vestibular hair cells in the otolithic organs and the canals are likely subject to the same patterns of injury as cochlear hair cells; however, prior studies investigating this damage are contradictory. McCabe and Lawrence (1958) found that high-intensity acoustic sound resulted in saccular collapse, otoconia destruction and otolithic membrane disruption, but saw no effect on the utricle or semicircular canals.40 Ylikoski (1987) found that impulse noise from firearm exposure in guinea pigs (90–300 rifle shots, peak 158 dB SPL with maximal energy content at 1.1 kHz) induced permanent severe damage to ampullary cristae of the semicircular canals and minimal damage to the saccular and utricular maculae. Light microscopy demonstrated sensory epithelial destruction, hair cell edema, sterocilia damage and disappearance and a decrease in myelinated fibers in the ampullary cristae.41 Akdogen et al (2009) demonstrated degenerated epithelial cells, separation of layers, marked crystolysis and stromal cell apoptosis in guinea pig saccular maculae following continuous high-intensity noise (120 dB SPL 4 kHz octave band for 6 hours), but no effect at shorter durations.42 Fetoni (2009) showed that intense noise exposure (120 dB SPL 6 kHz pure tone for 60 minutes) causes severe noise-induced hearing loss, but only minor vestibular damage with temporary decreases in VOR gain, a measure of semicircular canal function, and over-expression of markers of oxidative stress.43 These discrepancies may be due to differences in sound levels and duration of exposure among studies.
Structures embryologically derived from the pars inferior - the cochlea and saccule - are thought to be more sensitive to noise exposure than those derived from the pars superior - the utricle and semicircular canals - likely due to protective effects of the intervening membrane limitans.44,45 Following chronic noise exposure, patients are most likely to develop auditory changes, followed by abnormal measures of saccular, utricular then semicircular canal function.44 Amongst the semicircular canals, Zhu et al. (2011 and 2014) demonstrated in an animal model that the anterior semicircular canal (a corollary to the human superior canal) is the most sensitive followed by the horizontal then the posterior semicircular canals.46,47
In the results presented here, pressures measured within the superior semicircular canal were most similar to cochlear pressures, while the posterior semicircular canal demonstrated more variable pressures with a low signal-to-noise ratio. Recently, Stewart et al. (2016) demonstrated the variable sensitivity of the cochlea and vestibular end organs to continuous broad-band noise exposure in rats. Hearing loss was induced with an approximately 40 decibel increase in auditory brainstem response (ABR) over baseline. In the vestibular system, 30–60% stereocilia bundle loss was seen in the saccule, utricle, anterior and horizontal semicircular canals, but no loss was seen within the posterior canal. This correlated with decreased baseline firing rates of otolith afferents and anterior semicircular canal afferents, as well as gain and phase changes of the anterior and horizontal semicircular canal afferent responses to head rotation, but no change in gain or phase of rotational or translational VOR. Authors speculated that the discrepancy between the large decrease in stereocilia but only minimal to moderate change in afferent firing rates and head rotation was due to vestibular system redundancy. VOR preservation was likewise thought to be a reflection of convergence of connected VOR pathways, maintenance of an intact contralateral labyrinth, and/or central compensation.12
Effect of Bone-Conducted Stimuli on Vestibular End-Organs
Our results showed that bone conduction appears to be a more efficient stimulator of the vestibular system than air-conduction. Welgampola et al. (2003) demonstrated that bone-conducted VEMP has lower threshold of activation at 97 dB SPL than air-conducted clicks at 132 dB SPL and tones at 114 dB SPL. For a given intensity, therefore, bone-conducted sound stimulates the vestibular system more effectively than air-conducted sound.48 In this study, we found sound pressure levels within the SSCC that were similar to or larger than the scala vestibuli with both air- and bone-conducted stimuli. In the LSCC, sound pressures were maximal at the highest frequencies: 10 dB above those in the cochlea with bone-conducted stimuli compared to a 5 dB gain with acoustic stimuli. In the PSCC, maximal sound pressures existed at the lowest frequencies with a 20 dB gain and 15 dB gain with the mid-frequencies. These values are somewhat confounded by the low signal-to-noise ratio measured within the PSCC resulting in the exclusion of many data points and may show some bias towards results from a single specimen with concern for dehiscence.
These direct measurements reflect previously published reports of vestibular frequency tuning determined clinically by measuring VEMP responses. With air-conduction in normal individuals, cervical VEMP is maximal at 400–800 Hz, and the ocular VEMP has similar tuning.49–52 With bone conduction in normal individuals, cVEMP and oVEMP have lower preferred frequency, near 100 Hz.53–54 Patients with superior semicircular canal dehiscence have broader tuning curves for both reflexes. Cervical VEMPs tune downwards and ocular VEMPs tune upwards, altered frequency-tuning responses that return to normal after superior semicircular canal resurfacing.49
Limitations
Limitations to this study include the fact that pressures were only directly measured within the semicircular canals and not within the otolithic organs. The semicircular canals are easily accessible via a mastoidectomy approach for insertion of fiber-optic pressure probes, while accessing the otolithic organs lie much closer to the oval window where probe placement would disrupt ossicular function. Additionally, fenestration of the semicircular canals is required for insertion of the fiber-optic probes to obtain these direct pressure measurements. As previously discussed, fenestration of the bony canals is known to increase the vestibular system’s sound sensitivity. We attempted to mitigate this effect by sealing the canals with alginate dental impression material. Finally, to better understand the clinical significance of these measured vestibular sound pressure level changes, correlation between these pressure levels and histologic evidence of vestibular damage is warranted.
Conclusions
Recordable pressure waves present in the semicircular canals with sound stimulation were equal to or higher than those recorded in the cochlea. Pressures were 10–40 dB greater with bone-conducted stimuli than with air-conducted stimuli. Results suggest that the patterns of injury sustained to the vestibular system during high-level sound exposure may be predictable based on the frequency content and source of the incident sound.
Footnotes
The authors disclose no conflicts of interest.
References
- 1.Ylikoski J, Juntunen J, Matikainen E, Ylikoski M, Ojala M. Subclinical vestibular pathology in patients with noise-induced hearing loss from intense impulse noise. Acta Otolaryngol. 1988. May-Jun;105(5–6):558–63. [DOI] [PubMed] [Google Scholar]
- 2.Hoffer ME, Balaban C, Gottshall K, et al. Blast exposure: vestibular consequences and associated characteristics. Otol Neurotol. 2010. February;31(2):232–6. [DOI] [PubMed] [Google Scholar]
- 3.Scherer MR, Burrows H, Pinto R, et al. Evidence of central and peripheral vestibular pathology in blast-related traumatic brain injury. Otol Neurotol. 2011. June;32(4):571–80. [DOI] [PubMed] [Google Scholar]
- 4.Akin FW, Murnane OD, Tampas JW, Clinard C, Byrd S, Kelly JK. The effect of noise exposure on the cervical vestibular evoked myogenic potential. Ear Hear. 2012. Jul-Aug;33(4):458–65. [DOI] [PubMed] [Google Scholar]
- 5.Wang YP, Hsu WC, Young YH. Vestibular evoked myogenic potentials in acute acoustic trauma. Otol Neurotol. 2006. October;27(7):956–61. [DOI] [PubMed] [Google Scholar]
- 6.Wu CC, Young YH. Ten-year longitudinal study of the effect of impulse noise exposure from gunshot on inner ear function. Int J Audiol. 2009;48(9):655–60. [DOI] [PubMed] [Google Scholar]
- 7.Oosterveld WJ, Polman AR, Schoonheyt J. Vestibular implications of noise-induced hearing loss. Br J Audiol. 1982. November;16(4):227–32. [DOI] [PubMed] [Google Scholar]
- 8.Shupak A, Bar-El E, Podoshin L, Spitzer O, Gordon CR, Ben-David J. Vestibular findings associated with chronic noise induced hearing impairment. Acta Otolaryngol. 1994. November;114(6):579–85. [DOI] [PubMed] [Google Scholar]
- 9.Akin FW, Murnane OD. Head injury and blast exposure: vestibular consequences. Otolaryngol Clin North Am. 2011. April;44(2):323–34 [DOI] [PubMed] [Google Scholar]
- 10.Kilburn KH, Warshaw RH, Hanscom B. Are hearing loss and balance dysfunction linked in construction iron workers? Br JIndMed. 1992. February;49(2):138–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golz A, Westerman ST, Westerman LM, Goldenberg D, Netzer A, Wiedmyer T, Fradis M, Joachims HZ. The effects of noise on the vestibular system. Am J Otolaryngol. 2001. May-Jun;22(3):190–6. [DOI] [PubMed] [Google Scholar]
- 12.Stewart C, Yu Y, Huang J, Maklad A, Tang X, Allison J, Mustain W, Zhou W, Zhu H. Effects of high intensity noise on the vestibular system in rats. Hear Res. 2016. May;335:118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCue MP, Guinan JJ Jr. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994. October;14(10):6058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCauley RD, Fewtrell J, Popper AN. High intensity anthropogenic sound damages fish ears. JAcoustSoc Am. 2003. January;113(1):638–42. [DOI] [PubMed] [Google Scholar]
- 15.Young ED, Fernández C, Goldberg JM. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol. 1977. Nov-Dec;84(5–6):352–60. [DOI] [PubMed] [Google Scholar]
- 16.Halmagyi GM, Curthoys IS, Colebatch JG, Aw ST. Vestibular responses to sound. Ann NY Acad Sci. 2005. April;1039:54–67. [DOI] [PubMed] [Google Scholar]
- 17.Hsu WC, Wang JD, Lue JH, Day AS, Young YH. Physiological and morphological assessment of the saccule in Guinea pigs after noise exposure. Arch Otolaryngol Head Neck Surg. 2008. October;134(10):1099–106. [DOI] [PubMed] [Google Scholar]
- 18.Cazals Y, Aran JM, Erre JP, Guilhaume A, Aurousseau C. Vestibular acoustic reception in the guinea pig: a saccular function? Acta Otolaryngol. 1983. Mar-Apr;95(3–4):211–7. [DOI] [PubMed] [Google Scholar]
- 19.Minor LB. Superior canal dehiscence syndrome. Am JOtol. 2000. January;21(1):9–19. [PubMed] [Google Scholar]
- 20.Wit HP, Bleeker JD, Segenhout JH. Vestibular and cochlear responses to acoustic transients. Some properties of whole-nerve action potentials in pigeons. Acta Otolaryngol. 1981. Nov-Dec;92(5–6):409–22. [DOI] [PubMed] [Google Scholar]
- 21.Ribarić K, Bleeker JD, Wit HP. Perception of audio-frequency vibrations by profoundly deaf subjects after fenestration of the vestibular system. Acta Otolaryngol. 1992;112(1):45–9. [DOI] [PubMed] [Google Scholar]
- 22.Ribarić K, Kekic B, Dergenc R. On the capability of the vestibular apparatus to perceive sound stimuli. Acta Otolaryngol. 1992;112(2):221–4. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima HH, Dong W, Olson ES, et al. Differential intracochlear sound pressure measurements in normal human temporal bones. J Assoc Res Otolaryngol. 2009. March;10(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene NT, Mattingly JK, Jenkins HA, et al. Cochlear Implant Electrode Effect on Sound Energy Transfer Within the Cochlea During Acoustic Stimulation. Otol Neurotol. 2015. September;36(9):1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devèze A, Koka K, Tringali S, et al. Active middle ear implant application in case of stapes fixation: a temporal bone study. Otol Neurotol 2010;31:1027–34. [DOI] [PubMed] [Google Scholar]
- 26.Tringali S, Koka K, Devèze A, et al. Round window membrane implantation with an active middle ear implant: a study of the effects on the performance of round window exposure and transducer tip diameter in human cadaveric temporal bones. Audiol Neurootol. 2010;15:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devèze A, Koka K, Tringali S, Jenkins HA, Tollin DJ. Techniques to improve the efficiency of a middle ear implant: effect of different methods of coupling to the ossicular chain. OtolNeurotol. 2013. January;34(1):158–66. [DOI] [PubMed] [Google Scholar]
- 28.Lupo JE, Koka K, Jenkins HA, et al. Vibromechanical Assessment of Active Middle Ear Implant Stimulation in Simulated Middle Ear Effusion: A Temporal Bone Study. Otology & Neurotology 2014;35:470–5. [DOI] [PubMed] [Google Scholar]
- 29.Mattingly JK, Greene NT, Jenkins HA et al. Effects of Skin Thickness on Cochlear Input Signal Using Transcutaneous Bone Conduction Implants. Otol Neurotol 2015;36:1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banakis Hartl RM, Mattingly JK, Greene NT, Jenkins HA, Cass SP, Tollin DJ. A Preliminary Investigation of the Air-Bone Gap: Changes in Intracochlear Sound Pressure With Air- and Bone-conducted Stimuli After Cochlear Implantation. Otol Neurotol. 2016. October;37(9):1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene NT, Mattingly JK, Banakis Hartl RM, Tollin DJ, Cass SP. Intracochlear Pressure Transients During Cochlear Implant Electrode Insertion. Otol Neurotol. 2016. October 5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson ES. Direct measurement of intra-cochlear pressure waves. Nature 1999;402:526–9. [DOI] [PubMed] [Google Scholar]
- 33.Stenfelt S, Hato N, Goode RL. Round window membrane motion with air conduction and bone conduction stimulation. Hear Res 2004;198:10–24. [DOI] [PubMed] [Google Scholar]
- 34.Stenfelt S, Hato N, Goode RL. Fluid volume displacement at the oval and round windows with air and bone conduction stimulation. JAcoust Soc Am 2004;115:797–812. [DOI] [PubMed] [Google Scholar]
- 35.Rosowski JJ, Chien W, Ravicz ME, et al. Testing a method for quantifying the output of implantable middle ear hearing devices. Audiol Neurootol 2007;12:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biedron S, Prescher A, Ilgner J, Westhofen M. The internal dimensions of the cochlear scalae with special reference to cochlear electrode insertion trauma. Otol Neurotol. 2010. July;31(5):731–7. [DOI] [PubMed] [Google Scholar]
- 37.Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW Jr, Gewalt SL. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 1999. October;109(10):1661–8. [DOI] [PubMed] [Google Scholar]
- 38.Wysocki J Dimensions of the human vestibular and tympanic scalae. Hear Res. 1999. September;135(1–2):39–46. [DOI] [PubMed] [Google Scholar]
- 39.Baloh RW, Honrubia V. Clinical Neurophysiology of the Vestibular System. Oxford University Press, 2001. [PubMed] [Google Scholar]
- 40.McCabe BF, Lawrence M. The effects of intense sound on the non-auditory labyrinth. Acta Otolaryngol. 1958. Mar-Apr;49(2):147–57. [DOI] [PubMed] [Google Scholar]
- 41.Ylikoski J Impulse noise induced damage in the vestibular end organs of the guinea pig. A light microscopic study. Acta Otolaryngol. 1987. May-Jun;103(5–6):415–21. [PubMed] [Google Scholar]
- 42.Akdogan O, Selcuk A, Take G, Erdoğan D, Dere H. Continuous or intermittent noise exposure, does it cause vestibular damage? An experimental study. Auris Nasus Larynx. 2009. February;36(1):2–6. [DOI] [PubMed] [Google Scholar]
- 43.Fetoni AR, Ferraresi A, Picciotti P, Gaetani E, Paludetti G, Troiani D. Noise induced hearing loss and vestibular dysfunction in the guinea pig. Int J Audiol. 2009. November;48(11):804–10. [DOI] [PubMed] [Google Scholar]
- 44.Tseng CC, Young YH. Sequence of vestibular deficits in patients with noise-induced hearing loss. Eur Arch Otorhinolaryngol. 2013. July;270(7):2021–6. [DOI] [PubMed] [Google Scholar]
- 45.Sazgar AA, Dortaj V, Akrami K, Akrami S, Karimi Yazdi AR. Saccular damage in patients with high-frequency sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2006. July;263(7):608–13. [DOI] [PubMed] [Google Scholar]
- 46.Zhu H, Tang X, Wei W, Mustain W, Xu Y, Zhou W. Click-evoked responses in vestibular afferents in rats. J Neurophysiol. 2011. August;106(2):754–63. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H, Tang X, Wei W, Maklad A, Mustain W, Rabbitt R, Highstein S, Allison J, Zhou W. Input-output functions of vestibular afferent responses to air-conducted clicks in rats. J Assoc Res Otolaryngol. 2014. February;15(1):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welgampola MS, Rosengren SM, Halmagyi GM, Colebatch JG. Vestibular activation by bone conducted sound. J Neurol Neurosurg Psychiatry. 2003. June;74(6):771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor RL, Bradshaw AP, Halmagyi GM, Welgampola MS. Tuning characteristics of ocular and cervical vestibular evoked myogenic potentials in intact and dehiscent ears. AudiolNeurootol. 2012;17(4):207–18. [DOI] [PubMed] [Google Scholar]
- 50.Winters SM, Berg IT, Grolman W, Klis SF. Ocular vestibular evoked myogenic potentials: frequency tuning to air-conducted acoustic stimuli in healthy subjects and Mènière’s disease. Audiol Neurootol. 2012; 17(1): 12–9. [DOI] [PubMed] [Google Scholar]
- 51.Zhang AS, Govender S, Colebatch JG. Tuning of the ocular vestibular evoked myogenic potential to bone-conducted sound stimulation. JAppl Physiol. 2012. April; 112(8): 1279–90. [DOI] [PubMed] [Google Scholar]
- 52.Park HJ, Lee IS, Shin JE, Lee YJ, Park MS. Frequency-tuning characteristics of cervical and ocular vestibular evoked myogenic potentials induced by air-conducted tone bursts. Clin Neurophysiol. 2010. January;121(1):85–9. [DOI] [PubMed] [Google Scholar]
- 53.Todd NP, Rosengren SM, Colebatch JG. A utricular origin of frequency tuning to low-frequency vibration in the human vestibular system? Neurosci Lett. 2009. February 27;451(3):175–80. [DOI] [PubMed] [Google Scholar]
- 54.Zhang AS, Govender S, Colebatch JG. Tuning of the ocular vestibular evoked myogenic potential (oVEMP) to air- and bone-conducted sound stimulation in superior canal dehiscence. Exp Brain Res. 2012. November;223(1):51–64. [DOI] [PubMed] [Google Scholar]


