Abstract
The molecular mechanisms by which extracellular guidance cues regulate axonal morphology are not fully understood. Recent findings suggest that increased activity of the protein kinase Akt promotes dendritic branching and elongation in hippocampal neurons. We tested whether expression of constitutively active Akt (CA-Akt) in primary sensory neurons would promote axonal branching and whether targeting CA-Akt to lipid rafts, common sites of Akt function, would differentially regulate axonal morphology. Biolistic transduction of sensory neurons induced a rapid expression of CA-Akt, resulting in increased axonal branching, cell hypertrophy, and growth cone expansion. Additionally, we found that targeting of CA-Akt to lipid rafts significantly potentiated growth cone expansion compared with expression of CA-Akt throughout the neuron. Because lipid rafts are concentrated within the growth cone, this finding suggests that signaling of expansion is likely regulated locally. We found that CA-Akt-mediated growth cone expansion, but not axonal branching, was attenuated by coexpression of dominant-negative Rac1. In contrast, blockade of mammalian target of rapamycin (mTOR) prevented axonal branching and hypertrophy in response to CA-Akt, but not growth cone expansion. These data indicate that Akt activity can regulate growth cone expansion via localized Rac1 signaling and regulate axonal branching and soma size via activation of mTOR.
Keywords: Akt, constitutively active Akt (CA-Akt), Rho GTPases, localized signaling, axonal branching, growth cone expansion, lipid rafts
In response to multiple guidance molecules, intracellular signaling cascades are activated to mediate axonal branching and growth. One such intracellular signaling molecule that is required for localized regulation of axonal branching (Gallo and Letourneau, 1998) and growth cone guidance (Zhou et al., 2004) is phosphatidylinositol 3-kinase (PI-3K). The contribution of Akt, a protein kinase downstream of PI-3K, in neurite branching has been recently noted (Jaworski et al., 2005; Kumar et al., 2005). Traditionally studied in antiapoptotic signaling, Akt can also phosphorylate many proteins that may regulate neuronal morphology (Chan et al., 1999). There are two reports demonstrating that expression of constitutively active Akt (CA-Akt) increases dendritic branching in cultured hippocampal neurons (Jaworski et al., 2005; Kumar et al., 2005) and one report that CA-Akt increases axonal branching in cultured sensory neurons (Markus et al., 2002). We used dorsal root ganglion (DRG) neurons that have only axons (Kato et al., 2000) to elucidate the mechanisms regulating axonal morphology in response to increased Akt activity. We measured the soma area, axonal branching, and growth cone area of neurons expressing CA-Akt and concurrently blocked the activity of specific downstream targets of Akt to identify the molecular mechanisms regulating neuronal morphology.
We focused on two downstream targets of Akt that are most likely to play a role in mediating changes in morphology; the Rho-family GTPases and the mammalian target of rapamycin (mTOR). Rho GTPases (including Rac1, Cdc42) are key regulators of the actin cytoskeleton and play critical roles in regulation of axonal branching and growth cone dynamics (Gov and Gopinathan, 2006). Because all members of the Rho GTPases contain a consensus phosphorylation sequence for Akt, RxRxx(S/T) (Kwon et al., 2000), we predicted that they may regulate the actin cytoskeleton as a downstream effector of Akt. Through translational regulation, mTOR activity can modulate dendritic arborization (Jaworski et al., 2005; Kumar et al., 2005) and regeneration of growth cones following axotomy (Verma et al., 2005). Therefore, we propose that mTOR is also a candidate for Akt-mediated regulation of axonal morphology.
Spatial asymmetry of intracellular signaling molecules is required for a neuron to respond to guidance molecules (Song and Poo, 2001). When an axon is presented with a chemoattractant gradient, PI-3K, and likely Akt, is preferentially recruited to the plasma membrane on the side of higher concentration (Ming et al., 1999). In our experiments, addition of a myristoyl group on an Akt fusion protein recruits it to the plasma membrane, rendering it constitutively active. We used the myristoyl group from src (MS) to direct distribution of Akt throughout the plasma membrane (Mukherjee et al., 2003) and the myristoyl group from fyn (MF) to target Akt accumulation electively into lipid rafts (Park et al., 2006). Because lipid rafts are common sites of Akt function (Bauer et al., 2003), we tested whether concentrating CA-Akt in the lipid rafts (MFΔAkt) of sensory neurons would differentially regulate axonal branching and growth cone morphology compared with neurons uniformly expressing CA-Akt (MSΔAkt).
MATERIALS AND METHODS
Unless otherwise noted, chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO), and culture media and supplements were obtained from Gibco-Invitrogen (Carlsbad, CA). Antibodies were obtained from Cell Signaling Technologies (Boston, MA), and fluorochromes were obtained from Molecular Probes-Invitrogen (Carlsbad, CA).
Preparation of Plasmids
A plasmid containing cDNA encoding the gene for cytosolic EGFP-N1, under the control of the human cytomegalovirus (CMV) promoter, was used as a control plasmid. Two distinct plasmids were constructed, also under control of the CMV promoter, to express n-myristoylated murine Akt/EGFP fusion proteins with a hemagglutinin (HA) marker. One plasmid, p.MsrcEGFP-Akt-HA (p.MSΔAkt), contained cDNA (5′-tcgaggctgcggcgccgctggctggcatccttgggcttgctcttgttg ctacccatggtggccgc-3′) encoding the myristoyl group of src. The other plasmid, p.MfynEGFP-Akt-HA (p.MFΔAkt), incorporated cDNA (5′-ggccaccatgggctgtgtgcaatgtaaggataaagaagcaa caaaactgacggaggagc-3′) encoding the myristoyl group from fyn (Li et al., 2002). Src and fyn are nonreceptor tyrosine kinases that are differentially located within the plasma membrane (Geahlen et al., 2004). In each of the Akt plasmids, the region of cDNA encoding the Akt1 gene was altered so that the recombinant Akt transgene was lacking the regulatory pleckstrin homology (PH) domain (residues 1–102), allowing for PI-3K-independent Akt activity (Li et al., 2002; Park et al., 2006). The same two distinct myristoylation sequences were used to create p.MS.EGFP and p.MF.EGFP, two distinct constructs encoding membrane-targeted EGFP. These plasmids were used as controls for the expression of nonbiologically active myristoylated proteins. N-terminally GFP-tagged rac1N17 and cdc42N17 (dominant-negative forms of Rac1 and Cdc42, respectively) were constructed under the control of the CMV promoter (plasmids for these fusion proteins were provided by Dr. Michael Way, Lincoln’s Inn Fields Laboratories, London, United Kingdom).
Neuronal Cell Culture and Transduction
Embryonic chick dorsal root ganglion (DRG) neurons were isolated as previously described (Grider et al., 2005). Briefly, chick embryos (E9–E10) were removed from the egg, and the ganglia were dissected and transferred to ice-cold Hanks balanced salt solution supplemented with 0.01% (w/v) glucose. The ganglia were then incubated in 0.125% trypsin at 37°C for 25 min, at which time trypsin activity was blocked by addition of DMEM + 10% fetal bovine serum (growth media). The ganglia were dissociated by passage through a sterile 200-μl plastic pipette tip 10 times, passed through a 40-μm nylon cell strainer, and plated on plastic culture dishes in growth media supplemented with 20 ng/ml nerve growth factor (NGF; Sigma). After 1 hr at 37°C, the nonadherent cells, which consisted primarily of neurons, were resuspended in growth media supplemented with 1 ng/ml NGF and transferred to poly-lysine/laminin-coated glass coverslips (18 mm) at a density of 7,500–10,000 neurons/well. Six hours later, a Helios gene gun (Bio-Rad, Hercules, CA) attached to 150 psi ultrahigh-purity helium gas (Air Liquide) was used to accelerate plasmid-coated gold particles (2 mg plasmid/ng gold, 1.6-μm-diameter gold) into the neurons (Klimaschewski et al., 2002). A 40-μm nylon mesh (BD Bioscience, San Jose, CA) was placed between the gene gun and the target cells (approximately 3–4 cm) to minimize damage to the neurons (Klimaschewski et al., 2002). Where applicable, rapamycin (100 nM; Cell Signaling Technologies) was added to the culture media 6 hr after the neurons were plated. Cultures were maintained at 37°C in 5% CO2 for the remainder of the experiment.
Western Blot Detection
Neuronal cultures were transduced with p.EGFP-N1, p.MSΔAkt, or p.MFΔAkt by calcium phosphate-mediated transduction. After 48 hr, cells were lysed in a buffer containing 10 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 1% NP-40, 1 mM PMSF, 1 mM EDTA, 1 mM Na4P2O7, 2 mM Na3VO4, 10 mM NaF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin. The protein concentrations of the lysates were determined by using a BCA assay, and equal amounts of protein from each treatment group were loaded into each well of a denaturing SDS gel, separated by gel electrophoresis, and transferred to a PVDF membrane (Millipore, Billerica, MA). Membranes were rinsed in phosphate-buffered saline (PBS) and blocked against nonspecific binding for at least 1 hr with 5% dehydrated nonfat milk in PBS with 0.1% Tween-20 (PBST). The membranes were incubated overnight at 4°C in primary polyclonal antibodies (1:1,000 in PBST) against phospho-AktS473 or the HA epitope. After a 2-hr incubation with anti-rabbit peroxidase-conjugated secondary antibodies (1:20,000 in PBST) and a 5-min incubation with a chemiluminescent detection reagent (Amersham, Buckinghamshire, United Kingdom), the membrane was exposed to X-ray film. The time of exposure was varied to ensure that the linear range of the film was used.
Lipid Raft Isolation and Spectrophotometry of Sucrose Gradient Fraction
Lipid rafts were isolated as detergent-resistant membranes using mild detergent, followed by high-speed sucrose gradient fractionation (Mukherjee et al., 2003). To test for the presence of lipid rafts in each fraction, DRG neurons were collected and rinsed in ice-cold PBS, incubated in peroxidaseconjugated cholera toxin B (CTB) for 1 hr on ice, then rinsed three times in PBS. The neurons were resuspended in MES-buffered saline (MBS; 25 mM MES, pH 6.5, 150 mM NaCl, 1 mM EDTA) containing 10 μg/ml each of the protease inhibitors aprotinin and leupeptin and were lysed for 1 hr with 1% Triton X-100/MBS, either on ice or at 37°C. Incubation in 1% Triton X-100 on ice minimally affects lipid raft concentration (5% soluble), whereas incubation at 37°C solubilizes 99% of lipid rafts (Brown and Rose, 1992). The cell lysates were then combined with an equal volume of 80% sucrose/MBS, placed in the bottom of ultracentrifuge tubes, layered with a discontinuous 5–30% sucrose/MBS gradient, and subjected to high-speed centrifugation (gmax = 198,000g) for 22 hr at 4°C to separate the buoyant lipid raft complexes. Ten 1-ml fractions were collected, and the level of peroxidase-conjugated CTB was analyzed by measuring the absorbance of oxidized 2,2′-azinobis 3-ethylbenzthiazoline-6-sulfonic acid (ABTS) at 420 nm (Blank et al., 2002).
To test for the presence of Akt/EGFP fusion proteins in the lipid rafts, DRG were transduced with p.MSΔAkt, p.MFΔAkt, or mock transduction. Forty-eight hours after transduction, the neurons were harvested and resuspended in MES-buffered saline containing protease inhibitors and 1% Triton X-100 for 1 hr, on ice or at 37°C. The cell extracts were subjected to fractionation as described above. The intensity of EGFP/Akt-fusion fluorescent emissions (counts per second) from sucrose gradient fractions was measured with a Fluorolog-3 spectrophotometer (Jobin Yvon Inc., Edison, NJ). The samples were excited at 488 nm, and the peak emission wavelength, determined empirically, was measure at 508 nm.
Immunocytochemistry
Neuronal cultures were incubated for 1–2 hr in 4% paraformaldehyde/PBS (PFA) 48 hr after biolistic transduction with p.EGFP-N1, p.MSΔAkt, or p.MFΔAkt. Primary antibodies were directed against phospho-AktS473 or HA epitope to confirm that EGFP-positive neurons were expressing HA and elevated Akt activity. Neuronal cultures were blocked against nonspecific binding by incubation in 10% heat-inactivated horse serum (HIHS)/PBST, then incubated with primary antibodies (1:1,000 in PBST) plus 2% HIHS for 1–2 hr at room temperature (RT). Neurons were rinsed twice in PBS and then incubated with fluorochrome-conjugated anti-rabbit secondary antibody (1:1,000 in PBST) for an additional 1 hr. Cultures were rinsed twice in PBS, once in water, and then mounted on glass microscope slides for subsequent analysis.
Microscopy and Image Analysis
Forty-eight hours after biolistic transduction, glass coverslips with attached neuronal cultures were incubated for 1–2 hr in 4% PFA, rinsed once in PBS and once in water, and then mounted onto microscope slides with aqueous mounting media (Biomeda, Foster City, CA). Confocal micrographs of EGFP-positive or Akt/EGFP-positive neurons were captured with a Zeiss LSM510 microscope, and the morphology of each transduced neuron was measured in the included software (LSM Image Browser; Carl Zeiss). Growth cone and soma areas were measured by manually tracing the perimeter of the structures, including all filopodia and lamellipodia for determining growth cone area. Axonal branching was quantified by counting all branch points on a given cell. To confirm that the fluorescent signal accurately represented the neurons’ morphology, a subset of transduced neurons was independently measured from micrographs captured with both fluores-cent and phase optics. Data were analyzed by one-way ANOVA, followed by Dunnett’s t-tests. Data are displayed as the mean of at least four separate experiments (n = 5–54 neurons per group in each experiment).
RESULTS
Transduction of DRG Neurons With p.MFΔAkt, p.MSΔAkt, or p.EGFP-N1 Induces Rapid Expression of MFΔAkt, MSΔAkt, or EGFP-N1, Respectively
To confirm the ability of biolistic transduction to direct a rapid expression of recombinant proteins in sensory neurons, we transduced DRG neurons with plasmids containing cDNA encoding EGFP-N1, MsrcEGFP-Akt-HA (MSΔAkt), or MfynEGFP-Akt-HA (MFΔAkt). Using fluorescent microscopy, we identified transduced neurons as positive for EGFP or Akt/EGFP fusion protein fluorescence and immunoreactive-positive for the HA-epitope tag (only MSΔAkt and MFΔAkt treatment groups). At time points as early as 6 hr posttransduction, neurons transduced with MSΔAkt or MFΔAkt were identified as positive for the HA tag and for fluorescence derived from the EGFP control protein or Akt/EGFP fusion proteins. Fluorescence was bright and easily detected, demonstrating successful transduction and gene expression. Morphology of neurons transduced with EGFP-N1 did not differ from that of nontransduced neurons, and all transduced cells appeared healthy. Transduced neurons were robust at 96 hr, the longest time point at which we followed the cultures.
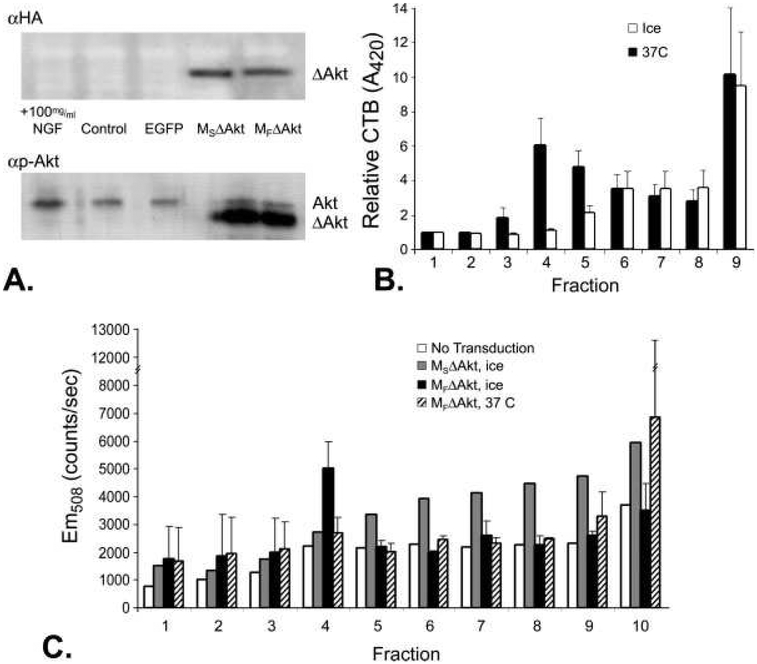
To measure the level of expression of myristoylated Akt in cultured neurons, we used Western blot analysis of lysates from neurons transduced with p.EGFP-N1, p.MSΔAkt, or p.MFΔAkt. We used calcium-phosphate-mediated transduction of DRG neurons instead of biolistic transduction to increase the transduction efficiency. Twenty-four hours after transduction, lysates from neuronal cultures were separated by gel electrophoresis. Subsequent immunodetection of the HA epitope and Akt phosphorylated at serine residue 473 (p-AktS473) revealed the presence of MSΔAkt and MFΔAkt in transduced neuronal cultures (Fig. 1A). The recombinant myristoylated Akt was distinguished from endogenous Akt by the slightly different molecular weight of the truncated Akt transgene, which lacks the regulatory PH domain but has EGFP and HA markers (Park et al, 2006).
Fig. 1.
DRG neurons transduced by p.MSΔAkt and p.MFΔAkt display different subcellular targeting of the Akt/EGFP fusion protein. A: Western blot detection of HA-epitope tag or phospho-AktS473 (p-Akt) of neuronal cultures transduced with MSΔAkt or MFΔAkt revealed efficient and similar levels of expression of the myristoylated transgene (ΔAkt). Addition of NGF (positive control) increased the levels of p-Akt, whereas cultures transduced with p.EGFP had levels of p-Akt similar levels in to control cultures. B: Peroxidase activity from CTB-HRP was detected by measuring the level of oxidized ABTS, which absorbs light at 420 nm. Neuronal cultures lysed at 0°C had high levels of peroxidase activity in fraction 4, indicating the presence of buoyant lipid rafts. Incubation of neuronal cultures in Triton X-100 at 37°C, known to disrupt lipid rafts, abolishes the peak in peroxidase activity in fraction 4. C: Detection of EGFP (Em508) from sucrose gradient fractions indicated lipid raft accumulation of MFΔAkt. In cultures incubated on ice, which maintains the integrity of the lipid rafts, greater amounts of EGFP in the lipid raft fraction (fraction 4) of neuronal cultures that expressed the MFΔAkt fusion protein were detected compared with cultures that expressed the MSΔAkt fusion protein. Disruption of lipid rafts with Triton X-100 at 37°C before fractionation decreased the amount of Akt/EGFP fusion protein in fraction 4 of neurons that expressed MFΔAkt fusion protein to control levels.
Expression of MFΔAkt Targets Akt to Lipid Rafts
The myristoyl group from fyn (MF) was used to target accumulation of Akt to lipid rafts within the membrane, whereas the myristoyl group from src (MS) was used to target a more general distribution of Akt throughout the plasma membrane (Park et al., 2006). With Akt constructs similar to those that we employed here, previous findings have demonstrated appropriate subcellular targeting of CA-Akt in cultured mammalian cells (Park et al., 2006). We initially attempted to use microscopy to colocalize CA-Akt with a marker of lipid rafts, cholera toxin B (CTB). However, we observed a uniform distribution of CBT throughout the plasma membrane. This difficulty in localizing lipid raft micro-domains has been previously reported (Brown and London, 1998; Jacobson and Dietrich, 1999) and may be due to the small size of rafts (Brown and London, 2000). We then used a biochemical assay to validate the differential subcellular localization of the CA-Akt constructs using sucrose gradient fractionation to isolate the lipid rafts. Lipid rafts were purified as detergent-resistant membranes by incubating cell homogenates with ice-cold nonionic detergent (1% Triton X-100), followed by fractionation with sucrose gradient centrifugation (Mukherjee et al., 2003). First, we detected lipid raft-containing fractions with CBT. Neurons were pre-incubated in peroxidase-conjugated CTB before fractionation, and the individual fractions were tested for peroxidase activity by measuring the chromagenic product of ABTS oxidation (Blank et al., 2002). In neurons lysed on ice, CTB-peroxidase was significantly higher in fraction 4, whereas, in neurons incubated at 37°C, peroxidase activity peaked in the heavier fractions (Fig. 1B). Incubation of neuronal cultures in 1% Triton X-100 on ice solubilizes only 5% of lipid rafts, whereas incubation at 37°C solubilizes 99% of lipid rafts (Brown and Rose, 1992). Therefore, fraction 4 contained lipid rafts in neurons lysed on ice, but not at 37°C. Fraction 10 (not shown) contained high variability for all treatment groups. This has been previously reported and is thought to be due to residual, nonbound CTB-HRP after rinses and nonspecific binding of CTB-HRP to cytoskeleton components (Blank et al., 2002).
Next, we used fluorescent spectrophotometry to detect Akt/EGFP fusion protein within the individual fractions. We found that the lipid raft fraction (fraction4) from cultures that expressed MFΔAkt had a greater amount of EGFP fluorescence compared with cultures that expressed MSΔAkt (Fig. 1C). When the neuronal homogenates were incubated at 37°C in 1% Triton X-100 before fractionation, EGFP fluorescence in fractions from neuronal cultures that expressed MFΔAkt were distributed similarly to controls (Fig. 1C). Hence, these data demonstrate that the myristoyl group from fyn directed accumulation of MFΔAkt fusion protein to lipid rafts. The intensity of EGFP emissions from each treatment group before sucrose gradient fractionation, a measurement of total transgene expression level, was not significantly different between cultures expressing MSΔAkt or MFΔAkt (not shown). These data indicate that Akt incorporating the myristoylation target sequence from fyn (MFΔAkt) targets Akt to the lipid rafts of DRG neurons, consistent with findings in other cellular model systems (Park et al., 2006).
Expression of CA-Akt Increases Axonal Branching
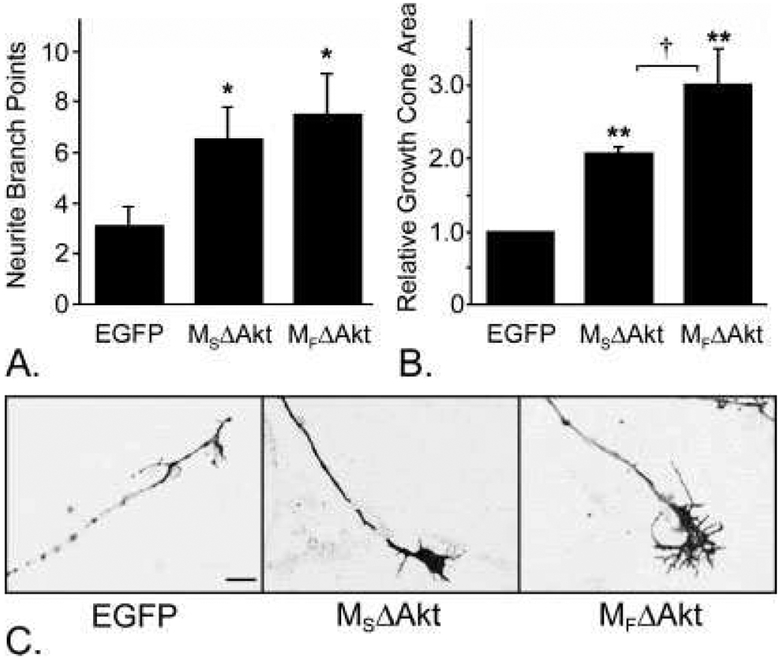
To determine the effects of Akt on axon branching, we tested whether expression of CA-Akt would promote axonal branching in cultured DRG neurons. Additionally, we tested whether targeting CA-Akt to the lipid rafts would differentially influence axonal branching compared with targeting CA-Akt throughout the plasma membrane. Forty-eight hours after biolistic transduction with p.EGFP-N1, p.MSΔAkt, or p.MFΔAkt (Fig. 2C), the number of axonal branch points was measured from all transduced neurons. Transduced neurons were identified by using morphological criteria and by the fluores cence of EGFP protein (control) or the Akt/EGFP fusion protein (MSΔAkt or MFΔAkt). Neurons that expressed either MSΔAkt or MFΔAkt had a significantly greater number of axonal branch points compared with neurons that expressed the control protein EGFP-N1 (P < 0.0001 each). However, the number of axonal branch points was not significantly different between the two CA-Akt constructs (P = 0.17; Fig. 2A). This observation suggests that subcellular location of Akt activity does not influence the degree of axonal branching in sensory neurons.
Fig. 2.
Expression of myristoylated Akt promoted axonal branching and growth cone expansion. A: DRG neurons that expressed MSΔAkt (n = 14) or MFΔAkt (n = 20) had significantly more axonal branch points than neurons that expressed the control protein, EGFP (n = 32; P < 0.0001 for each), but there was no significant difference in axonal branching between the two Akt constructs (P = 0.17). B: Neurons that expressed CA-Akt throughout the plasma membrane (MSΔAkt; n = 54) had significantly larger growth cone areas than control neurons that expressed EGFP (n = 97; P = 0.007). Neurons that expressed lipid raft-targeted CA-Akt (MFΔAkt; n = 92) had significantly larger growth cones than neurons that expressed either MSΔAkt or EGFP (P = 0.045 and P = 0.005, respectively). C: Representative photomicrographs growth cones from transduced DRG neurons.
*P < 0.05, **P < 0.01 compared with EGFP, †P < 0.05 between MSΔAkt and MFΔAkt. Scale bar = 10 μm.
Differential Targeting of CA-Akt Differentially Mediates Growth Cone Expansion
Similarly, we tested whether expression of CA-Akt would modify growth cone morphology and whether selectively targeting Akt activity to lipid rafts would differentially affect growth cone morphology compared with Akt activity throughout the plasma membrane. Because lipid rafts accumulate within the growth cone, we predicted that directing CA-Akt to the lipid rafts would potentiate the effects of Akt signaling in growth cone dynamics. Forty-eight hours after biolistic transduc tion of DRG neurons with p.MSΔAkt, p.MFΔAkt, or p.EGFP-N1, the growth cones of all transduced neurons were measured (Fig. 2C). We found that neurons that expressed MSΔAkt had significantly larger growth cone areas than control neurons that expressed EGFP-N1 (P = 0.007; Fig. 2B). More importantly, we also found that targeting of Akt activity to the lipid rafts significantly potentiated growth cone expansion. Growth cones from neurons that expressed MFΔAkt were significantly larger than growth cones from neurons that expressed either MSΔAkt (P = 0.045) or EGFP-N1 (P = 0.005; EGFP: 21.0 ± 1.9 μm2, MSΔAkt: 45.4 ± 2.1 μm2, MFΔAkt: 63.1 ± 10.4 μm2; Fig. 2B).
To verify that the growth cone expansion we observed was not due simply to the presence of differentially myristoylated proteins, we tested whether expression of myristoylated forms of EGFP in DRG neurons would affect neuronal morphology. DRG neurons were transduced with p.EGFP-N1, p.Ms.EGFP, or p.Mf.EGFP, and the morphology of transduced neurons was measured 48 hr later, in the same manner as for the experiment described above. There was no difference in the number of axonal branches, growth cone area, or soma area between neurons that expressed MS.EGFP, MF.EGFP, or cytosolic EGFP-N1 (Supp. Info. Fig. 1), indicating that presence of a myristoyl group does not have a generalized effect on neuronal morphology.
Whereas spatial restriction of Akt activity to the lipid rafts significantly potentiated growth cone expansion, the subcellular localization of CA-Akt did not have an affect on axonal branching. This may be due to separate, independent mechanisms of Akt-mediated regulation of growth cone size versus axonal branching.
Increased Axonal Branching and Cell Hypertrophy in Response to CA-Akt Is mTOR Dependent
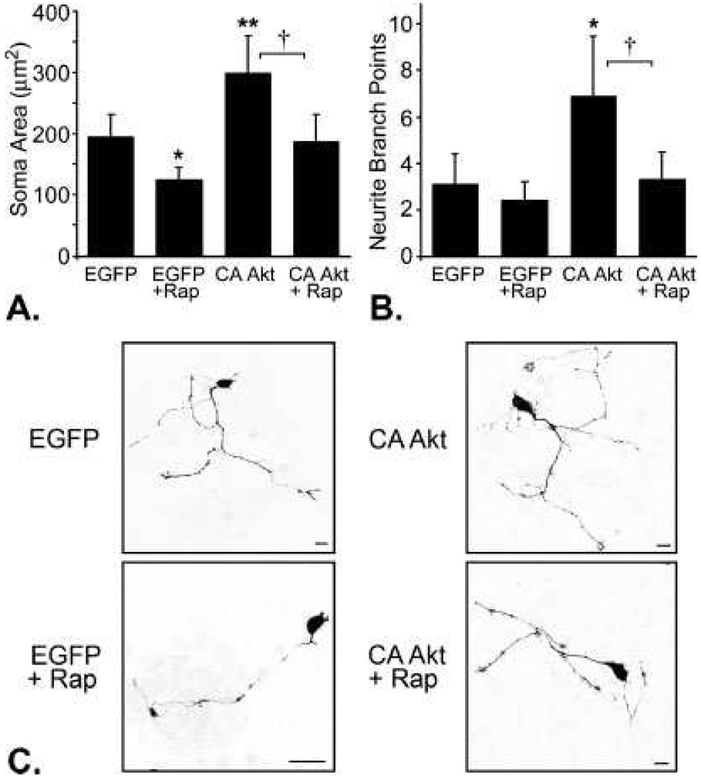
To determine whether the morphological changes we observe in response to expression of CA-Akt are regulated through activation of mTOR, we incubated DRG neurons expressing MSΔAkt, MFΔAkt, or EGFP in the presence or absence of rapamycin (100 nM), a pharmacological inhibitor of mTOR (Parsons et al., 2006). After 48 hr, the number of axonal branch points, soma area, and growth cone area were measured. In this experiment, we transduced neurons with p.MFΔAkt, but not p.MSΔAkt, because transduction of DRG neurons with p.MFΔAkt promoted an equally or more robust increase in axonal branching, soma area, and growth cone expansion in previous experiments, compared with expression of MSΔAkt. Incubation of neurons in rapamycin abolished cell hypertrophy in neurons that expressed CA-Akt (P = 0.03); neurons that expressed CA-Akt in the absence of rapamycin had cell bodies measuring 298 ± 62 μm2, whereas neurons that expressed CA-Akt in the presence of rapamycin had soma areas that averaged 187 ± 43 μm2, which is similar to soma areas of controls, 195 ± 36 μm2 (Fig. 3A,C). This result is consistent with other reports that mTOR activity regulates neuronal soma size (Kwon et al., 2003; Kumar et al., 2005). More notably, rapamycin also blocked the effects of CA-Akt on axonal branching. In the absence of rapamycin, neurons that expressed CA-Akt had more than twice the number of axonal branch points per neuron compared with control neurons that expressed EGFP-N1 (P = 0.048, Fig. 3B), but the number of axonal branches in neurons that expressed CA-Akt in the presence of rapamycin was not statistically different from controls (P = 0.83, Fig. 3B,C). Because rapamycin almost completely blocked CA-Akt-mediated axonal branching, mTOR is likely the predominant mediator of axonal branching in response to expression of CA-Akt. The presence or absence of rapamycin had no significant effect on growth cone area (Supp. Info. Fig. 2; EGFP: 20.3 ± 4.1 μm2 vs. EGFP/rap: 18. ± 6 5.5 μm2, P = 0.82, CA-Akt: 49.9 ± 7.8 μm2 vs. CA-Akt/rap: 44.7 ± 11.7 μm2, P = 0.63), suggesting that regulation of growth cone dynamics is mediated through a different mechanism, such as the Rho GTPases.
Fig. 3.
Inhibition of mTOR with rapamycin prevents CA-Akt-mediated increases in soma area and axonal branching. A: Neurons that expressed CA-Akt (n = 18) have significantly larger soma areas than control neurons that expressed EGFP (n = 14; P = 0.035). However, in the presence of rapamycin (+Rap), the soma areas of neurons that expressed CA-Akt (n = 12) were significantly smaller than in the absence of rapamycin (P = 0.03) and were similar to control soma size (n = 11; P = 0.82). B: Neurons that expressed CA-Akt in the absence of rapamycin (n = 18) had significantly more axonal branch points than control neurons that expressed EGFP (n = 14; P = 0.048); however, in the presence of rapamycin, neurons that expressed CA-Akt (n = 12) were no different from controls (n = 11; P = 0.83). C: Representative photomicrographs of transduced DRG neurons.
*P < 0.05, **P < 0.01 compared with EGFP, †P < 0.05 between CA-Akt and CA-Akt 1 rapamycin. Scale bar = 20 μm.
Growth Cone Expansion in Response to CA-Akt Expression Is Rac1 Dependent
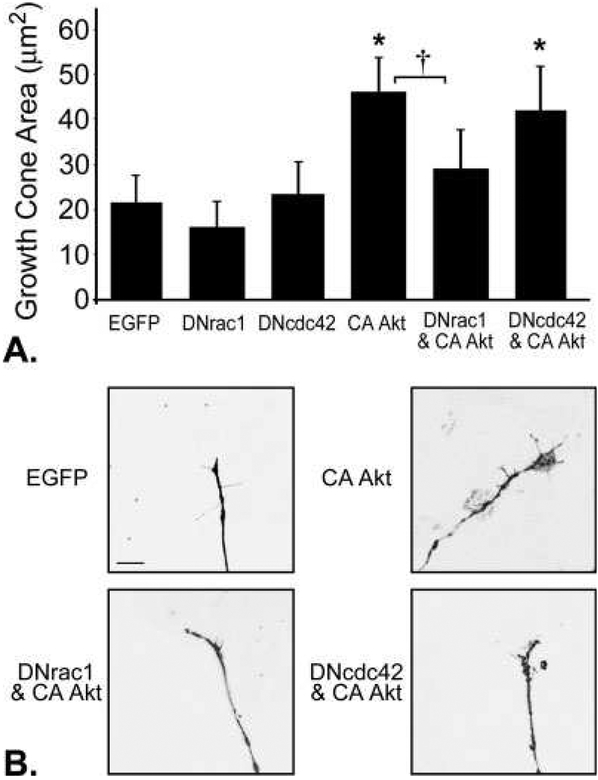
Actin dynamics within the growth cone are regulated in part by Rac1 and Cdc42 activity (Kuhn et al., 2000). We tested whether coexpression of EGFP-tagged, dominant negative (DN) forms of Rac1 or Cdc42 would inhibit the effects of CA-Akt expression on axonal branching, growth cone expansion, and soma hypertrophy. DRG cultures were assigned to one of the following treatment groups: DNrac1, DNcdc42, MFΔAkt, MFΔAkt and DNrac1, MFΔAkt and DNcdc42, or EGFP-N1 as a control. At 48 hr, we found no signifi-cant effect of DNrac1 or DNcdc42 on the number of axonal branches or soma area when expressed alone or in conjunction with CA-Akt (Supp. Info. Figs. 3, 4). However, growth cone expansion in neurons expressing CA-Akt was attenuated by coexpression of DNrac1, but not DNcdc42. Although the growth cone areas of neurons that expressed DNrac1 or DNcdc42 alone were not significantly different from controls, the growth cones of neurons that coexpressed CA-Akt and DNrac1 were significantly smaller than growth cones of neurons that expressed CA-Akt alone (P = 0.006; Fig. 4A,B). This finding suggests that decreasing Rac1 activity reduces the ability of CA-Akt to promote growth cone expansion in sensory neurons. There was no statistical difference in growth cone area between neurons that expressed CA-Akt and neurons that coexpressed CAAkt and DNcdc42 (P = 0.49; Fig. 4A,B). Insofar as Rac1 mediates lamellipodia formation and Cdc42 mediates filopodia formation (Causeret et al., 2004), the differential effects of DNrac1 and DNcdc42 in the presence of CA-Akt are likely due to their differential regulation of lamellipodia and filopodia, respectively.
Fig. 4.
Growth cone expansion in response to CA-Akt was attenuated in by coexpression of dominant negative (DN) Rac1 (n = 18), but not DNcdc42 (n = 67). A: Neurons that expressed CA-Akt (n = 38) had significantly larger growth cones than control neurons that expressed EGFP (n = 80; P < 0.001). The growth cones from neurons that coexpressed CA-Akt and DNrac1 (n = 18) were significantly smaller than growth cones of neurons that expressed CA-Akt alone (P = 0.006; n = 55 for DNcdc42 alone; n = 14 for DNrac1 alone). B: Representative photomicrographs of growth cones from transduced DRG neurons.
*P < 0.05 compared with EGFP, †P < 0.01 between CA-Akt and DNrac1 and CA-Akt alone. Scale bar 5 5 μm.
DISCUSSION
Understanding the molecular cascades that mediate axonal branching and growth downstream of guidance receptors is of importance to the fields of neurodevelopment and regeneration. Therefore, we investigated the role of Akt, a signaling molecule situated at the convergence point of multiple signaling cascades, in regulating axonal branching and growth cone dynamics. To investigate the effects of CA-Akt specifically on axonal morphology, we used DRG sensory neurons that extend only axons (Kato et al., 2000). We confirmed that expression of CA-Akt promoted axonal branching and further demonstrated, for the first time, that expression of CA-Akt in sensory neurons promoted growth cone expansion. Furthermore, we determined that targeted accumulation of CA-Akt to the lipid rafts significantly potentiated growth cone expansion compared with expression of CA-Akt throughout the plasma membrane.
To further elucidate the mechanisms by which Akt activity signals axonal branching and growth cone expansion, we expressed CA-Akt while simultaneously down-regulating the activity of important downstream targets of Akt, mTOR, or the Rho-family GTPases Rac1 and Cdc42. We found that blockade of mTOR prevented axonal branching and cell hypertrophy in response to increased Akt activity and that coexpression of dominant negative Rac1, but not dominant negative Cdc42, attenuated Akt-mediated growth cone expansion.
mTOR-Mediates Axonal Branching and Cell Hypertrophy
mTOR activity is increased in response to several neurotrophic factors and is required for local protein translation in response to BDNF (Takei et al., 2004). We observed that blockade of mTOR activity with rapamycin decreased cell body size in neurons that expressed the control protein EGFP and completely prevented cell hypertrophy in neurons that expressed CA-Akt. In addition, we found that blockade of mTOR activity for 48 hr with rapamycin prevented the ability of CA-Akt to promote axonal branching in cultured sensory neurons. This finding is in concurrence with two recent reports that describe mTOR-dependent Akt-mediated increases in dendritic branching with long-term (14 day) cultures of hippocampal neurons (Jaworski et al., 2005; Kumar et al., 2005).
Because we observed that blockade of mTOR activity with rapamycin abolished the ability of CA-Akt to promote axonal branching, we suggest that activation of mTOR is likely the predominant mechanism of axonal branching induced by expression of constitutively active Akt. Whether this is due to an increase in collateral branching or to increased bifurcations of growth cones is unknown. It has been suggested that mTOR in complex with rictor (mTOR complex 2; mTORC2) regulates actin dynamics through Rho GTPases (Jacinto et al., 2004) and PKC-alpha (Sarbassov et al., 2004). Although mTORC2 is generally thought to be insensitive to acute application of rapamycin, several studies suggest that long-term exposure (24+ hr) to 100 nM rapamycin, as we used in our experiments, can affect the phosphorylation and assembly of rictor with mTOR in vitro (Akcakanata et al., 2007; Rosner and Hengstschläger, 2008). This issue is still under debate, because some cell lines show rapamycin-sensitive dephosphorylation and disassembly of rictor, whereas other cell lines do not (Sarbassov et al., 2006).
Rac1-Mediates Growth Cone Expansion
Activation of guidance receptors on the axonal growth cone induces changes in the actin cytoskeleton that dictate filopodia and lamellipodia dynamics, consequences of which are the attraction, repulsion, or branching of the axon (Gallo and Letourneau, 2004). It is currently thought that Rac1 and Cdc42 activity promotes neurite growth and branching via lamellipodia and filopodia formation, respectively (Causeret et al., 2004). We predicted, based on reports that neurons from Rac1 knockout Drosophila models display significant branching defects (Ng et al., 2002) and dominant negative Cdc42 attenuates lateral neurite branching of retinal ganglion cells upon contact with posterior tectal neurons (Thies and Davenport, 2003), that expression of dominant negative forms of Rac1 or Cdc42 would attenuate axonal branching in response to expression of CA-Akt. Surprisingly, we observed that expression of DNrac1 or DNcdc42 had no significant effect on axonal branching in cultured DRG neurons whether or not they expressed CA-Akt.
Growth cone expansion is a primary growth response to chemoattractants such as netrin-1 (Dent et al., 2004), whereas growth cone collapse has been observed in response to soluble repulsive growth factors such as collapsin-1 (Dent et al., 2004; Chadborn et al., 2006). We found that expression of DNrac1, but not DNcdc42, attenuated growth cone expansion in neurons that expressed CA-Akt. Rac1 is known to regulate lamellipodia positively, whereas Cdc42 is involved in filopodia formation (Ridley et al., 1992). The considerably larger contribution of lamellipodia to the total growth cone size, compared with filopodia, was likely the reason we observed attenuation of growth cone expansion only in neurons that coexpressed DNrac1. Neurons that expressed DNcdc42 had a nonsignificant decrease in filopodia number, but it was not feasible to quantify growth cone lamellipodia because the boundary between lamellipodia and the central domain of the growth cone was frequently ambiguous.
Although we found that expression of DNrac1 attenuated growth cone expansion in response to CAAkt, we did not observe any decrease in growth cone size in neurons that expressed DNrac1 alone, compared with controls. This is in concurrence with other reports that, although the expression of constitutively active forms of Rac1 or Cdc42 can promote growth cone expansion, expression of DNrac1 or DNcdc42 does not decrease growth cone size (Brown et al., 2000; Matsuura et al., 2004). It follows that Rac1 and Cdc42 activity are not required for growth cone maintenance under normal conditions but that Rac1 is necessary for the signaling of growth cone expansion. For example, in hippocampal neurons, filopodia and lamellipodia extension in response to BDNF requires Rac1 activity (Fujitani et al., 2005).
Targeting of CA-Akt Expression to the Lipid Rafts Potentiates Growth Cone Expansion
Axons are one of the most sensitive biological structures to gradients of extracellular molecular cues. When an axon is presented with a chemoattractant gradient, spatially asymmetric activation of guidance receptors promotes turning of the axon toward the higher concentration. This spatial resolution is maintained in the transmission of the extracellular signals to the intracellular space, as PI-3K and Akt are recruited to the leading edge of growth cones in response to growth factors (Kamiguchi, 2006). Akt is recruited to lipid rafts upon activation of positive guidance receptors (Bauer et al., 2003), and intact lipid rafts are required for growth cone guidance in response to chemoattractants (Guirland et al., 2004). Because Akt signaling in response to guidance molecules is highly location dependant (Bauer et al., 2003), we tested whether subcellular localization of CA-Akt to the lipid rafts by association with the myristoyl group from fyn (Park et al., 2006) would differentially regulate axonal branching, growth cone expansion, and cell hypertrophy compared with localization of Akt throughout the plasma membrane by association with the myristoyl group from src (Park et al., 2006).
We found that targeted accumulation of CA-Akt in lipid rafts significantly potentiated growth cone expansion compared with expression of CA-Akt throughout the plasma membrane. We suggest two non-exclusive explanations for this observation. First, because lipid rafts are concentrated at the leading edge of growing axons (Guirland et al., 2004), spatially restricting CA-Akt to the lipid rafts could simply be increasing the local concentration of CA-Akt in the growth cones and thereby increasing the intensity of Akt signaling specifically within the growth cone. Second, because lipid rafts are thought to function in the assembly of guidance receptors with their downstream effectors (Dykstra et al., 2001), targeting of CA-Akt to the lipid rafts may position Akt in close proximity to its downstream targets and thereby augment Akt-mediated activity. In concurrence with this second hypothesis, we found that growth cone expansion in response to expression of CA-Akt is dependent on Rac1, which is recruited to lipid rafts upon activation (Fujitani et al., 2005). Intact lipid rafts are required for BDNF/Rac1-mediated growth cone spreading in cultured hippocampal neurons (Fujitani et al., 2005).
Akt-mediated expansion of growth cones via rac-1 likely is due to regulation of the actin cytoskeleton. However, Akt may also be increasing growth cone area by expansion of the cell membrane or regulation of microtubules. Laurino et al. (2005) found that blockade of Akt activity with LY294002 prevented IGF-1-stimulated plasmalemmal expansion in isolated growth cones from hippocampal pyramidal cells. Additionally, Akt can regulate microtubule dynamics during mitosis and determination of cell polarity through phosphorylation of glycogen synthase kinase-3 (GSK-3) or the microtubule-associated protein tau (Buttrick and Wakefield, 2008).
In summary, we show that expression of constitutively active Akt in cultured sensory neurons promoted axonal branching, cell hypertrophy, and growth cone expansion. Growth cone expansion was significantly potentiated in neurons expressing CA-Akt within the lipid rafts compared with neurons expressing CA-Akt throughout the plasma membrane. Additionally, we report that axonal branching and cell hypertrophy in response to increased Akt activity were regulated through activation of mTOR, whereas growth cone expansion in response to increased Akt activity was regulated, in part, via Rac1. To our knowledge, this is the first report to observe Rac1-dependent growth cone expansion in response to increased Akt activity or Akt location-dependent changes in growth cone morphology. These findings are important for understanding the signaling mechanisms controlling neuronal morphology and can be applied to the study of neurodevelopment, regeneration, and chemotaxis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Michael Way for kindly providing DNA plasmids and Dr. Qin Chen, Dr. Carlos Rivera, Dr. Gang-Yi Wu, Dr. Lyric Jorgenson, and Nikki Sunnen for technical assistance and helpful suggestions.
Contract grant sponsor: NIH; Contract grant number: NS39198;
Contract grant sponsor: The Christopher Reeve Paralysis Foundation;
Contract grant sponsor: Mission Connect a project of TIRR Foundation.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Akcakanata A, Singha G, Hunga MC, Meric-Bernstam F. 2007. Rapamycin regulates the phosphorylation of rictor. Biochem Biophys Res Commun 362:330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Jenny M, Fresser F, Uberall F, Baier G. 2003. AKT1/PKBalpha is recruited to lipid rafts and activated downstream of PKC isotypes in CD3-induced T cell signaling. FEBS Lett 541:155–162. [DOI] [PubMed] [Google Scholar]
- Blank N, Gabler C, Schiller M, Kriegel M, Kalden JR, Lorenz HM. 2002. A fast, simple and sensitive method for the detection and quantification of detergent-resistant membranes. J Immunol Methods 271:25–35. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. 1998. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164:103–114. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. 2000. Structure and function of sphingolipidand cholesterol-rich membrane rafts. J Biol Chem 275:17221–17224. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533–544. [DOI] [PubMed] [Google Scholar]
- Brown MD, Cornejo BJ, Kuhn TB, Bamburg JR. 2000. Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J Neurobiol 43:352–364. [DOI] [PubMed] [Google Scholar]
- Buttrick GJ, Wakefield JG. 2008. PI3-K and GSK-3: Akt-ing together with microtubules. Cell Cycle 7:2621–2625. [DOI] [PubMed] [Google Scholar]
- Causeret F, Hidalgo-Sanchez M, Fort P, Backer S, Popoff MR, Gauthier-Rouviere C, Bloch-Gallego E. 2004. Distinct roles of Rac1/Cdc42 and Rho/Rock for axon outgrowth and nucleokinesis of precerebellar neurons toward netrin 1. Development 131:2841–2852. [DOI] [PubMed] [Google Scholar]
- Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ. 2006. PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci 119:951–957. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinosi-tide-dependent phosphorylation. Annu Rev Biochem 68:965–1014. [DOI] [PubMed] [Google Scholar]
- Dent EW, Barnes AM, Tang F, Kalil K. 2004. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci 24:3002–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra M, Cherukuri A, Pierce SK. 2001. Rafts and synapses in the spatial organization of immune cell signaling receptors. J Leukoc Biol 70: 699–707. [PubMed] [Google Scholar]
- Fujitani M, Honda A, Hata K, Yamagishi S, Tohyama M, Yamashita T.2005. Biological activity of neurotrophins is dependent on recruitment of Rac1 to lipid rafts. Biochem Biophys Res Commun 327:150–154. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. 1998. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci 18:5403–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. 2004. Regulation of growth cone actin filaments by guidance cues. J Neurobiol 58:92–102. [DOI] [PubMed] [Google Scholar]
- Geahlen RL, Handley MD, Harrison ML. 2004. Molecular interdiction of Src-family kinase signaling in hematopoietic cells. Oncogene 23: 8024–8032. [DOI] [PubMed] [Google Scholar]
- Gov NS, Gopinathan A. 2006. Dynamics of membranes driven by actin polymerization. Biophys J 90:454–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider MH, Mamounas LA, Le W, Shine HD. 2005. In situ expression of brain-derived neurotrophic factor or neurotrophin-3 promotes sprouting of cortical serotonergic axons following a neurotoxic lesion. J Neurosci Res 82:404–412. [DOI] [PubMed] [Google Scholar]
- Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. 2004. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron 42:51–62. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6:1122. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Dietrich C. 1999. Looking at lipid rafts? Trends Cell Biol 9:87–91. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. 2005. Control of dendritic arborization by the phosphoinositide-30-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci 25:11300–11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H 2006. The region-specific activities of lipid rafts during axon growth and guidance. J Neurochem 98:330–335. [DOI] [PubMed] [Google Scholar]
- Kato H, Chen S, Kiyama H, Ikeda K, Kimura N, Nakashima K, Taga T. 2000. Identification of a novel WD repeat-containing gene predominantly expressed in developing and regenerating neurons. J Biochem (Tokyo) 128:923–932. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L, Nindl W, Pimpl M, Waltinger P, Pfaller K. 2002. Biolistic transfection and morphological analysis of cultured sympathetic neurons. J Neurosci Methods 113:63–71. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Meberg PJ, Brown MD, Bernstein BW, Minamide LS, Jensen JR, Okada K, Soda EA, Bamburg JR. 2000. Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases. J Neurobiol 44:126–144. [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. 2005. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci 25:11288–11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ. 2003. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A 100:12923–12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T, Kwon DY, Chun J, Kim JH, Kang SS. 2000. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J Biol Chem 275:423–428. [DOI] [PubMed] [Google Scholar]
- Laurino L, Wang XX, de la Houssaye BA, Sosa L, Dupraz S, Caceres A, Pfenninger KH, Quiroga S. 2005. PI3K activation by IGF-1 is essential for the regulation of membrane expansion at the nerve growth cone. J Cell Sci 118:3653–3662. [DOI] [PubMed] [Google Scholar]
- Li B, Desai SA, MacCorkle-Chosnek RA, Fan L, Spencer DM. 2002. A novel conditional Akt “survival switch” reversibly protects cells from apoptosis. Gene Ther 9:233–244. [DOI] [PubMed] [Google Scholar]
- Markus A, Zhong J, Snider WD. 2002. Raf and akt mediate distinct aspects of sensory axon growth. Neuron 35:65–76. [DOI] [PubMed] [Google Scholar]
- Matsuura R, Tanaka H, Go MJ. 2004. Distinct functions of Rac1 and Cdc42 during axon guidance and growth cone morphogenesis in Drosophila. Eur J Neurosci 19:21–31. [DOI] [PubMed] [Google Scholar]
- Ming G, Song H, Berninger B, Inagaki N, Tessier-Lavigne M, Poo M. 1999. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron 23:139–148. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Arnaud L, Cooper JA. 2003. Lipid-dependent recruitment of neuronal Src to lipid rafts in the brain. J Biol Chem 278:40806–40814. [DOI] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. 2002. Rac GTPases control axon growth, guidance and branching. Nature 416:442–447. [DOI] [PubMed] [Google Scholar]
- Park D, Lapteva N, Seethammagari M, Slawin KM, Spencer DM. 2006. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat Biotechnol 24:1581–1590. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. 2006. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci 26:12977–12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70:401–410. [DOI] [PubMed] [Google Scholar]
- Rosner M, Hengstschläger M. 2008. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet 17:2934. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14:1296. [DOI] [PubMed] [Google Scholar]
- Song H, Poo M. 2001. The cell biology of neuronal navigation. Nat Cell Biol 3:E81–E88. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. 2004. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci 24:9760–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies E, Davenport RW. 2003. Independent roles of Rho-GTPases in growth cone and axonal behavior. J Neurobiol 54:358–369. [DOI] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. 2005. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci 25:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. 2004. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42:897–912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.