Abstract
Importance:
Since 2000, the incidence and severity of Clostridium difficile infection (CDI) have increased.
Objective:
We reviewed current evidence regarding best practices for the diagnosis and treatment of CDI in adults (age ≥18 years).
Evidence Review:
Ovid Medline and Cochrane databases were searched using keywords relevant to the diagnosis and treatment of CDI in adults. Articles published between January 1978 and October 31 2014 were selected for inclusion based on targeted keyword searches, manual review of bibliographies, and whether the article was a guideline, systematic review, or meta-analysis published within the past 10 years. 4682 articles were initially identified; 196 were selected for full review. The most clinically pertinent 116 articles were included.
Findings:
Laboratory testing cannot distinguish between asymptomatic colonization and symptomatic infection with C. difficile. Diagnostic approaches are complex due to the availability of multiple testing strategies. Multistep algorithms using polymerase chain reaction (PCR) for the toxin gene(s) or single step PCR on liquid stool samples have the best test performance characteristics (multistep: sensitivity 0.68 to 1.00 / specificity 0.92 to 1.00; single step: sensitivity 0.86–0.92 / specificity 0.94–0.97). Vancomycin and metronidazole are first line therapies for most patients, although treatment failures have been associated with metronidazole in severe or complicated cases of CDI. Recent data demonstrates clinical success rates of 66.3% for metronidazole versus 78.5% for vancomycin for severe CDI. Newer therapies show promising results, including fidaxomicin (similar clinical cure rates to vancomycin, with lower recurrence rates for fidaxomicin, 15.4% vs. vancomycin, 25.3%, P = 0.005) and fecal microbiota transplantation (response rates of 83%−94% for recurrent CDI).
Conclusions and Relevance:
Diagnostic testing for CDI should be performed only in symptomatic patients. Treatment strategies should be based on disease severity, history of prior CDI, and the individual patient’s risk of recurrence. Vancomycin is the treatment of choice for severe or complicated CDI, with or without other adjunctive therapies. Metronidazole is appropriate for mild disease. Fidaxomicin is a therapeutic option for those with recurrent CDI or a high risk of recurrence. Fecal microbiota transplantation is associated with symptom resolution of recurrent CDI but its role in primary and severe CDI is not established.
Keywords: Clostridium difficile, diarrhea, infection
INTRODUCTION
Clostridium difficile was first identified as the major infectious cause of antibiotic-associated diarrhea in 19781. However since the emergence of the epidemic BI/NAP1/027 strain of C. difficile in 20002, C. difficile infections (CDI) have increased in prevalence and become less responsive to treatment2–4.
In the United States, the number of CDI hospital discharge diagnoses more than doubled from 2001(~148,900 discharges) to 2005 (~301,200 discharges) 5. CDI incidence has increased from 4.5/ 1000 adult discharges in 2001 to 8.2/1000 discharges in 2010 6. Patients with CDI have higher healthcare costs than patients without CDI. Annual attributable costs exceed $1.5 billion in the U.S.7.
CDI requires both acquisition of C. difficile and disruption of the gut microbiota. The exact mechanism by which C. difficile causes symptomatic infection is unclear. C. difficile is not invasive and toxin production is the key to pathogenesis (non-toxigenic strains of C. difficile do not cause diarrhea). The toxin disrupts epithelial integrity via microtubules and cell-cell tight junctions, resulting in cytokine release such as IL-88. These actions promote an inflammatory infiltrate in the colonic mucosa, fluid shifts leading to diarrhea, and epithelial necrosis. Antibiotics alter normal microbiota, increasing CDI risk9. Other factors associated with CDI include older age, recent hospitalization, longer hospitalization duration, receipt of multiple antibiotics, longer antibiotic use duration, proton pump inhibitors, chemotherapy, chronic kidney disease, and feeding-tubes10–14. This review focuses on the diagnosis and treatment of CDI in adults, including new diagnostic and therapeutic modalities.
METHODS
A literature search of the Ovid Medline and Cochrane databases was conducted using search terms and synonyms for Clostridium difficile (Appendix A). We searched for studies of diagnostic testing and treatment of CDI published between Jan 1978 to October 31, 2014. Studies published in non-English languages and studies involving animals or children were excluded. We identified 4,682 articles. Bibliographies of the retrieved studies and previous reviews were searched for other relevant studies. 196 articles were initially identified and were reduced to the most clinically relevant 116 (Appendix B). Meta-analyses, systematic reviews, and references cited in published clinical practice guidelines from the past 10 years were also reviewed.
Diagnosing C. difficile Infection: Who Should Be Tested
Laboratory testing alone cannot distinguish between asymptomatic colonization and clinical symptoms of infection. The diagnosis of CDI requires: 1) presence of diarrhea, defined as three or more unformed stools in 24-hours, and 2) positive stool test for toxigenic C. difficile or its toxins, or colonoscopic/histopathologic findings demonstrating pseudomembranous colitis15–17. The definitive gold standard for CDI is detection of toxigenic C. difficile in stool along with colonic histopathology showing pseudomembranes in a patient with clinical symptoms.18 Many laboratories will only test diarrheal stool for C. difficile15,16,19–21.
In one study, 56% of patients who responded to treatment asymptomatically shed C. difficile spores for up to six weeks22,23. Thus a “test of cure” is not recommended15. Studies have documented chronic shedding and an increased prevalence of asymptomatic colonization in healthcare facilities, consistent with the hypothesis that long-term asymptomatic colonization following CDI occurs24,25. Recurrent symptoms can occur in association with a transient functional bowel disorder in up to 35% of patients during the first two weeks following resolution of CDI. However, only 4.3% of patients have symptoms more than three months after the infection due to a post-infectious irritable bowel syndrome.26 The 2010 Society for Healthcare Epidemiology of America and Infectious Disease Society of America Clinical Practice Guidelines advise against treating asymptomatic carriage with C. difficile,15 thus, it is important to distinguish between symptoms due to recurrent CDI and transient functional bowel disorder or persistent irritable bowel syndrome. However, presently there are no validated approaches to distinguish between these conditions.
C. difficile Testing
Organism Detection
The gold standard for detecting toxigenic C. difficile in stool is toxigenic culture (TC)(Table 1).19 Stool specimens are cultured anaerobically on special media27 for 24–48 hours. After colony selection and confirmation of taxonomy (usually with an antigen detection strategy with latex agglutination or enzyme immunoassay (EIA) or real-time PCR),27,28 isolates are incubated for 48 hours followed by testing using a cell cytotoxicity assay (CCA)(Table 1). The independent performance of this method is unclear, since most studies compare other diagnostic modalities to TC or CCA,19 and there are differences in choice of media and sample pretreatment.
Table 1.
Diagnostic tests for toxigenic C. difficilea
| Testing Method | Target(s) | Notes |
|---|---|---|
| Gold Standard Tests | ||
| Toxigenic Culture | Toxigenic C. difficile | • Reference standard |
| • Difficult to perform | ||
| • Time consuming (24–48 hours) | ||
| Cell Cytotoxicity Assay | Toxins A or Bb | • Reference standard |
| • Highly sensitive for toxin compared to EIA | ||
| • Difficult to perform | ||
| • Time consuming (24–48 hours) | ||
| Rapid Diagnostic Tests | ||
| EIA | GDH | • GDH alone insufficient for diagnosis (must be paired with a test for toxin) |
| • Rapid | ||
| • Variable sensitivity and specificity | ||
| EIA | Toxins A or Bb | • Rapid |
| • Variable sensitivity and specificity | ||
| NAAT | • Rapid but more expensive than EIA | |
| • Highly sensitive and specific for presence of toxigenic C. difficile | ||
| • May increase detection of colonization and not true CDI | ||
| RT-PCR | tcdB or tcdC genes | • tcdA- / tcdB+ strains can cause disease |
| LAMP | tcdA or tcdB genes | • tcdA+ / tcdB- not well-described in human disease |
| • Caution required in interpreting negative results based on tcdA testing alone by LAMP |
Abbreviations: CDI, Clostridium difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; LAMP, loop-mediated isothermal amplification; NAAT, nucleic acid amplification testing; RT-PCR, real-time polymerase chain reaction.
Refer to the text or Table 2 / Appendix C for sensitivity / specificity of the diagnostic tests
C. difficile can produce toxin A and/or toxin B. Although both play a role in clinical disease, it is not known if strains producing only toxin A are associated with symptomatic infection in humans.
Although a reference standard, TC is time-intensive, requires specialized equipment and trained personnel. Diagnostic delays have implications for treatment decisions and infection control.29,30 Rapid testing overcomes these limitations. One method focuses on detecting a product of C. difficile, glutamate dehydrogenase (GDH), usually performed via EIA. Studies examining the performance characteristics of GDH EIA show substantial variability (Table 2). Because GDH is present in both toxigenic and non-toxigenic strains of C. difficile and data on asymptomatic colonization suggest up to 46% of C. difficile isolates are non-toxigenic31, GDH testing must be paired with a test that detects toxin.
Table 2.
Systematic reviews and meta-analyses examining the performance characteristics of rapid diagnostic tests for Clostridium difficile infection
| Test | Source | Number of Included Studies | Sensitivity | Specificity |
|---|---|---|---|---|
| Organism Detection | ||||
| GDH EIA | Crobach et al., 200919 | 11 | 0.88 (0.6–0.97)ae | 0.89 (0.75–0.97)ae |
| Shetty et al., 2011111 | 13 | 0.92 (0.8–1)ae | 0.93 (0.83–1)ae | |
| NAAT | Crobach et al., 200919 | 4 | 0.91 (0.86–1)ae | 0.96 (0.94–1)ae |
| Deshpande et al., 2011112 | 19 | 0.9 (0.88–0.91)be | 0.96 (0.96–0.97)be | |
| O’Horo et al., 2012113 | 25 | 0.92 (0.91–0.94)bc | 0.94 (0.94–0.95)bc | |
| 0.87 (0.84–0.9)bd | 0.97 (0.97–0.98)bd | |||
| Toxin Detection | ||||
| Toxin A/B EIA | Crobach et al., 200919 | 60 | 0.73 (0.32–0.99)ae | 0.98 (0.65–1)ae |
| Planche et al., 2008114 | 18 | 0.87 (0.69–0.99)ae | 0.97 (0.92–1)ae |
Abbreviations: EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification testing.
Mean (range)
Pooled (95% confidence interval)
Compared to TC
Compared to CCA
Compared to TC+CCA or another mixed reference standard
Nucleic acid amplification testing (NAAT), including RT-PCR and loop-mediated isothermal amplification (LAMP), can detect the tcdA/tcdB genes (regulate toxin A/B production) or the tcdC gene (a negative regulator of toxin A and B production) and identify the presence of toxigenic C. difficile in a single step (Table 1).19,21,32,33. NAAT testing shows sensitivity and specificity in the >0.90 range (Table 2). However, this higher sensitivity also identifies toxigenic C. difficile in asymptomatic patients. This underscores the importance of only testing symptomatic patients, leading some experts to argue against NAAT-based testing alone.16,19,34
Toxin Detection
The gold standard for detecting toxins A and/or B is CCA,27 which is performed directly on stool or as part of TC. Filtrates of stool suspensions or culture supernatants are inoculated into a cell culture and assessed for cytopathic effect after 24 or 48 hours.27 This test identifies as little as 3 picograms of toxin and is highly sensitive (0.94–1) and specific (0.99), especially if combined with antiserum.27,35 The main disadvantage is turnaround time and complexity.
Sensitivity and specificity of EIA for toxin A and/or B are variable (Table 2). Repeat testing does not improve sensitivity. A recent systematic review found that 91% of positive EIA results occur after one test and the probability of a second or third test becoming positive after 2 previous negative test(s) was <2.5%.36
Multistep Algorithms for Diagnosis of CDI
Given the suboptimal sensitivity of some toxin EIA kits combined with increased detection of asymptomatic colonization with single-step algorithms (NAAT), many experts and some guidelines have advocated approaches that use multiple tests (multistep algorithms) for rapid diagnosis.15,16,19,34 One example is shown in Figure 1; sensitivity of 0.91, specificity of 0.98, and negative predictive value of 0.9937.
Figure 1. Sample multistep algorithm for the rapid diagnosis of C. difficile infection.
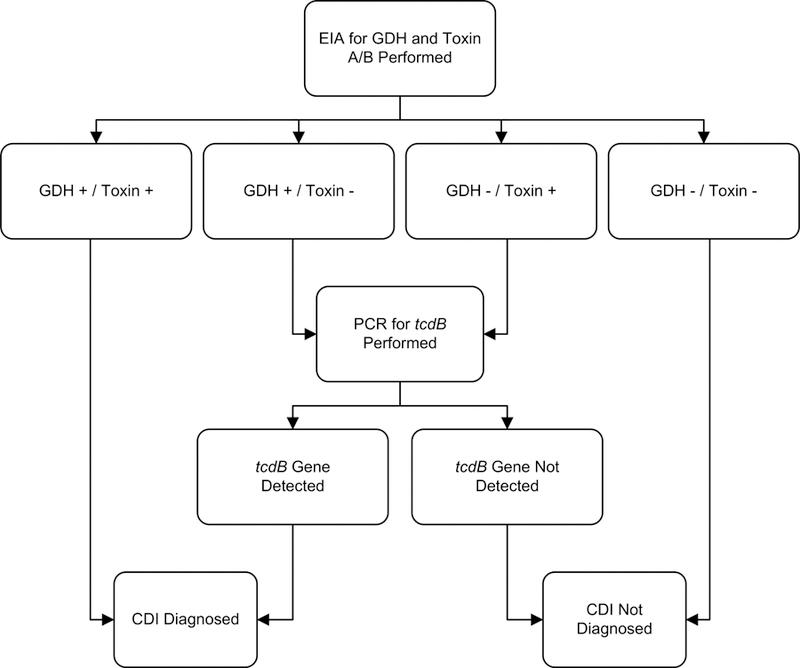
Abbreviations: CDI, Clostridium difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; PCR, polymerase chain reaction.
Footnotes: Adapted under Creative Commons License from Rao K, Erb-Downward JR, Walk ST, et al. The Systemic Inflammatory Response to Clostridium difficile Infection. PLoS ONE. 2014;9(3):e92578.
We reviewed studies using rapid testing algorithms with at least one gold standard comparator (Appendix C). In general, multistep algorithms using NAAT had excellent sensitivity (0.68–1) and specificity (0.92–1), but algorithms using only GDH or toxin EIA testing performed worse with greater variability. A large, multicenter study by Planche et al.38 reported that a GDH/NAAT based algorithm yielded the highest sensitivity (0.91–0.98) and specificity (0.96–0.98) (Appendix C).
Treating C. difficile Infection (CDI)
Since 2000, CDI treatment failures and recurrences have increased2–4. Treatment failures are likely related to a complex interplay of host factors, bacterial pathogenicity, and the ability to deliver therapeutic levels of drug to the colon. Strains with higher minimum inhibitory concentrations to metronidazole have been described and may contribute to treatment failures39 Guidelines recommend that CDI should be treated according to disease severity, and risk of recurrence or complications15,16.
Markers of Disease Severity
Clinical manifestations of C. difficile infections (CDI) range from mild diarrhea to life-threatening illness. Prediction rules have been developed to predict recurrences, complications, and mortality40. Many of these studies had small sample sizes, with significant heterogeneity40. One prospective study of 746 patients with CDI proposed the following risk scoring system to predict risk of fulminant CDI: age >70 years (2 points), WBC ≥20,000 cells/mL or ≤2,000/mL (1 point), cardiorespiratory failure (7 points), and diffuse abdominal tenderness (6 points). High risk patients had a score ≥641. Another scoring system study used age, treatment with systemic antibiotics, leukocyte count, albumin, serum creatinine to predict response to vancomycin or fidaxomicin42.
The 2010 Society for Healthcare Epidemiology of America and Infectious Disease Society of America Clinical Practice Guidelines categorize mild CDI as WBC < 15 X 109/L and serum creatinine < 1.5 times premorbid level; severe CDI as WBC ≥ 15 X 109/L, or serum creatinine ≥ 1.5 times premorbid level; and severe, complicated CDI as hypotension or shock, ileus, or megacolon15. Guidelines from the European Society of Clinical Microbiology and Infectious Diseases define severe CDI as an episode of CDI with a complicated disease course or one or more signs or symptoms of severe colitis, with significant systemic toxin effects and shock, resulting in intensive care unit admission, colectomy or death. Key findings included WBC >15 X 109/L, serum albumin <30 g/L and an increase in serum creatinine level ≥1.5 times premorbid level16. The term “fulminant” is sometimes used to describe severe, complicated CDI42–44. (Table 3)
Table 3.
C. difficile Infection (CDI) Classification based on Disease Severity
| Disease Category | Clinical and Laboratory Signs | Associated Risk Factors |
|---|---|---|
| Mild to moderate CDI | Diarrhea without systemic signs of infection, WBC< 15,000 cells/mL, and serum creatinine < 1.5 times baseline15 | Antibiotic use, previous hospitalization, longer duration of hospitalization, use of proton pump inhibitors, receipt of chemotherapy, chronic kidney disease, and presence of a feeding-tube10–14. |
| Severe CDI | Systemic signs of infection, and/or WBC ≥ 15,000 cells/mL, or serum creatinine ≥ 1.5 times the premorbid level 15 | Advanced age, infection with BI/NAP1/027 strain 115,116. |
| Severe, complicated CDI | Systemic signs of infection including hypotension, ileus, or megacolon 15 | See above, plus recent surgery, history of inflammatory bowel disease and intravenous immunoglobulin treatment43 |
| Recurrent CDI | Recurrence within 8 weeks of successfully completing treatment for CDI 16,20 | Patient age ≥65 years, concomitant antibiotic use, presence of significant comorbidities, concomitant use of proton pump inhibitors, and increased initial disease severity 16 |
Asymptomatic Carriers
Asymptomatic carriage of C. difficile affects 10 to 52% of defined populations45–49,25. Asymptomatic fecal shedding of C. difficile may be transient and one study showed that vancomycin therapy may temporarily interrupt shedding, but increased the risk of C. difficile carriage following therapy completion50. Asymptomatic colonization does not increase the risk of symptomatic CDI, and may protect against later development of symptomatic disease31,47,51 Shim et al studied 618 non-colonized patients and 192 asymptomatic carriers with two or more weekly follow up rectal swabs and reported that 3.6% of the non-colonized patients and only 1% of the asymptomatic carriers developed symptomatic CDI 31.
Withdrawing Precipitating Antibiotics
The human gut microbiota protects against pathogen overgrowth, including C. difficile. Any antibiotic can disrupt microbiota, although penicillins, cephalosporins and clindamycin are particularly associated with risk of CDI52–54. A systematic review on antibiotic use and CDI risk reported odds ratios ranging from 2.12–42 for clindamycin, and 3.84–26 for third-generation cephalosporins53, while a more recent meta-analysis found an odds ratio of 3.2 for third-generation cephalosporins and 2.86 for clindamycin52. Fluoroquinolones are associated with increased risk of the BI/NAP1/027 strain12.
Historically, antibiotic withdrawal was sometimes a stand-alone treatment55. Olson et al evaluated 908 patients with CDI from 1982–1991and found that 15% had symptom resolution without antibiotic therapy56. Whether antibiotic withdrawal remains effective for mild CDI is unclear, although some evidence exists to support this approach in combination with standard C. difficile therapy.57 Failure to stop offending antibiotics is associated with CDI recurrence58.
Metronidazole versus Vancomycin
Metronidazole and vancomycin have been primary therapies for CDI since the 1980s. Early studies suggested that oral metronidazole and oral vancomycin had equivalent efficacy, with similar tolerability and relapse rates56,59,60. Newer data suggest higher treatment failure rates when metronidazole is used in severe or complicated CDI3,61–64.
A large retrospective study found that oral metronidazole treatment failures increased (10% to 26%), and the 60-day probability of recurrence increased (21% to 47%), before vs. after emergence of BI/NAP1/0274. Other studies have not demonstrated increased metronidazole failures after BI/NAP1/027 emergence65,66.
Zar et al conducted a randomized trial evaluating response to metronidazole versus vancomycin in 150 patients stratified by CDI severity. Among patients with mild CDI, cure rates for metronidazole and vancomycin were not different (90% vs. 98% respectively). However, among patients with severe CDI, cure rates were better for vancomycin (76% vs. 97%)63. A systematic review from 2001–2010 reported higher treatment failures with metronidazole than vancomycin (22.4% vs. 14.2%; P = 0.002), while recurrence rate were similar (27.1% vs. 24.0%; P = 0.26). Metronidazole treatment failures were more frequent in North America than Europe3. A large clinical trial comparing tolevamer, a toxin-binding polymer, with vancomycin and metronidazole, found that while tolevemer was inferior to both metronidazole and vancomycin, metronidazole was inferior to vancomycin (success rates of 44.2%, 72.7% and 81.1% respectively). These differences were more pronounced in severe CDI (66.3% for metronidazole,78.5% for vancomycin)64.
Factors associated with metronidazole failures include age>60 years, fever, hypoalbuminemia, peripheral leukocytosis, ICU stay and abnormal abdominal CT imaging 61–63. Patients with hematologic malignancies and CDI respond more poorly to metronidazole and vancomycin (53.7% and 50% respectively) 67.
Patients receiving metronidazole have a longer time to symptomatic improvement than patients receiving vancomycin60,68. A retrospective study of 102 patients after emergence of the BI/NAP1/027 strain, found that only 71% of patients responded to metronidazole within 6 days. The overall response rate was 91% and failures were associated with higher severity of illness62.
Oral vancomycin is typically well-tolerated. However both oral and rectal administration of vancomycin may rarely be systemically absorbed69. Metronidazole is associated with gastrointestinal side effects a disulfiram-like reaction when ingested with alcohol, and peripheral neuropathy with prolonged therapy70.
Treatment by Disease Severity
Table 3 lists definitions of CDI severity, definitions for recurrent disease, and factors associated with recurrence15,16,20. Figure 2 provides a possible approach for CDI treatment according to disease severity. However, the approach in Figure 2 has not been validated 71–73,74,75.
Figure 2. Possible Approach for the Treatment of C. difficile Infection (CDI).
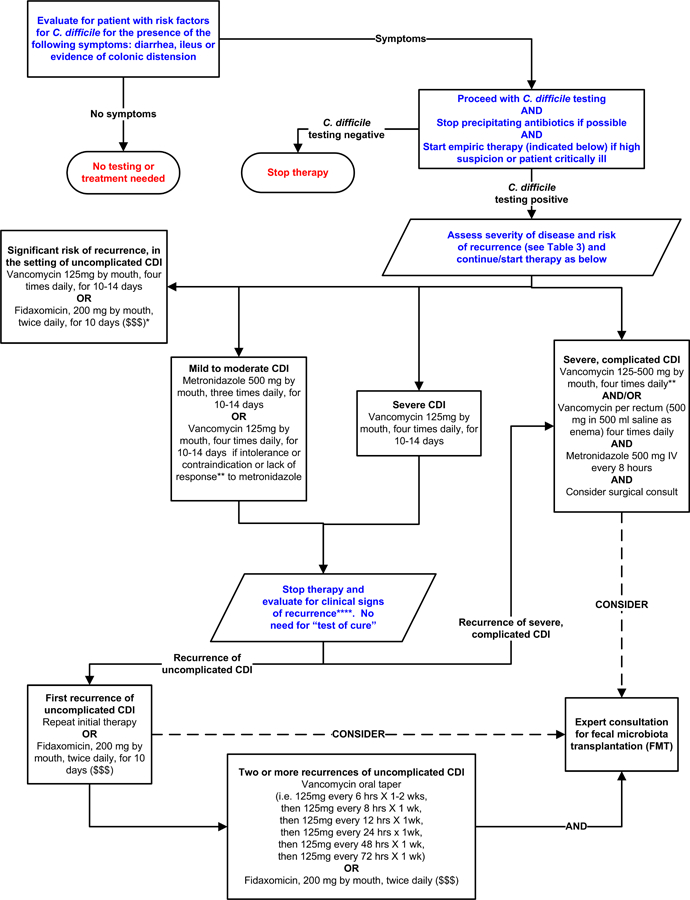
Footnotes: *Suggested approach for CDI treatment according to disease severity based on current guidelines, recent reviews/meta-analyses of fecal microbiota transplantation and randomized controlled trials of fidaxomicin. This approach is not validated. There are no data supporting the use of fidaxomicin for complicated CDI. **Treatment response is defined by clinical improvement in diarrhea or other signs of infection; response may require 3–5 days after starting therapy, but therapy escalation can be considered sooner based on disease severity. ***Duration of therapy depends on treatment response. ****Consider post-infectious irritable bowel syndrome rather than recurrent CDI for mild symptoms. “$ $ $” indicates that costs are substantially higher. References: 15,16,71–73,75
Treating Mild to Moderate CDI
For mild to moderate CDI, oral metronidazole remains the preferred therapy in part because of its low cost 15,16,63. The standard dose is 500mg orally, three times daily for 10–14 days. For patients unable to take oral medications, metronidazole can be administered intravenously at the same dose, although metronidazole is not recommended as monotherapy when administered intravenously. 15,16. Based on a recent study64 that showed a lower clinical success rate for metronidazole vs. vancomycin, it may be reasonable to consider vancomycin for mild to moderate CDI.
Treating Severe or Complicated CDI
Vancomycin is the preferred therapy for severe or complicated CDI15,16,63. Vancomycin 125 mg orally four times daily for 10–14 days is non-inferior to higher doses, in the absence of complicated infection22. However, expert opinion often favors higher doses in severe or complicated disease15,16.
Vancomycin may also be administered rectally in the setting of ileus, as an adjunctive therapy, although evidence is limited to case reports15,76,77. Rectally administered vancomycin is not typically used alone, because rectally administered vancomycin may not reach the entire affected area78. Intravenous metronidazole achieves detectable levels throughout the colon79, and may be an adjunctive therapy for ileus or severe/complicated CDI, typically with oral and/or rectal vancomycin. However, there are no randomized trials supporting this practice15,16. Treatment failures have occurred in patients with ileus administered IV metronidazole monotherapy56,77.
Prompt surgical evaluation should be obtained in patients with complicated CDI. Early intervention can reduce mortality80,81. Subtotal or total colectomy with end ileostomy is often performed when surgery is required, although there are newer colon-preserving techniques80,81.
Treating Recurrent C. difficile Infection
Recurrent CDI is more common in older patients and in those with concomitant antibiotic use, presence of comorbidities, concomitant use of proton pump inhibitors, and worse initial disease severity 11,16. Inadequate antibody response after an episode of CDI is associated with increased recurrence rates82,83.
Guidelines recommend oral metronidazole or vancomycin for the first recurrence of mild-moderate CDI15,16. Vancomycin is recommended therapy for any subsequent recurrences. Pulsed or tapering courses are often employed 84. Randomized trials are lacking but case series and case reports support this practice23,84,85. McFarland et al enrolled 163 patients with recurrent CDI, with an overall subsequent recurrence rate of 44.8%; while tapering and pulsed courses of vancomycin resulted in fewer recurrences (31%, p=0.01 and 14.3%, p=0.02 respectively), although the number of patients was small (29 and 7 respectively) 23.
Fidaxomicin was approved for treating CDI in 2011. Randomized studies demonstrated similar cure rates between fidaxomicin and oral vancomycin74,86. In a double-blinded randomized trial, Cornely et al reported that 221/252 (87.7%) of patients receiving fidaxomicin for CDI achieved clinical cure, versus 223/257 (86.8%) of patients receiving vancomycin. These results achieved criteria for non-inferiority between fidaxomicin and vancomycin74. Louie et al reported clinical cure rates with fidaxomicin that were noninferior to vancomycin (88.2% versus 85.8%) in 629 patients, with fewer recurrences with fidaxomicin (15.4% vs. 25.3%, P = 0.005) 86.
When antibiotics cannot be discontinued because of ongoing infection, clinical cure rates for concomitant CDI are higher with fidaxomicin than with vancomycin58. Fidaxomicin may preserve the human gut microbiota better than alternative treatments 75. Fidaxomicin is not considered first-line therapy for mild or uncomplicated disease, because of its higher costs87 No data support its use in complicated or fulminant disease 16. Fidaxomicin may be used for recurrent CDI, for the treatment of an initial CDI episode, when there is a high risk of recurrence, or when administered immediately after a course of vancomycin, for patients with multiple CDI recurrences 16,84,88.
Anecdotal evidence supports rifaximin as an adjunctive therapy for recurrent CDI, usually after a course of standard therapy for CDI89,90. Monotherapy should be avoided, given the propensity for resistance89. Nitazoxinide is not a first-line therapy for an initial episode of CDI but may be used as an adjunctive therapy for recurrent CDI. However, data are limited15.
Probiotics and Fecal Microbiota Transplantation
Recurrent CDI can occur, as relapse of infection, or as reinfection with another strain. Preserving normal gut microbiota diversity may prevent or treat recurrences91.
Probiotics are live microorganisms that can restore normal gut microbiota. The role of probiotics in CDI treatment is poorly defined, although evidence suggests probiotics may prevent initial episodes, as well as recurrence92–94. Probiotic-associated bacteremia and fungemia have been described, primarily in immunocompromised or critically-ill patients95. However, probiotics are generally well tolerated without major side effects96. A recent case series suggested that daily administration of kefir, a probiotic made from fermented milk, with staggered, tapered doses of either vancomycin or metronidazole, was beneficial for recurrent CDI 97
Fecal microbiota transplantation restores gut microbiota diversity, with the instillation of donor stool into the gastrointestinal tract of an infected patient. This procedure has had good clinical response without reports of adverse events, for refractory or recurrent CDI71–73. The first systematic review was published in 2011 and included 317 patients with recurrent CDI treated with fecal microbiota transplantation via enema, nasojejunal-tube/gastroscope or colonoscopy. Clinical resolution occurred in 92% of patients (89% after a single treatment), without serious adverse effects73. A recent review of 536 patients reported a 87% clinical response rate72.
A randomized trial of fecal microbiota transplantation demonstrated symptom resolution in 94% of patients who received vancomycin for 5 days followed by either one or two treatments with fecal microbiota transplantation, versus 31% in those receiving vancomycin alone for 14 days, and 23% for those receiving vancomycin for 14 days plus bowel lavage. This study was stopped early after interim analyses demonstrated superiority of fecal microbiota transplantation. Among 18 patients in the other treatment groups who received subsequent fecal microbiota transplantation 83% had symptom resolution98.
In 2013 a stool substitute preparation, made from purified fecal cultures, from a single healthy donor was used to treat two patients with recurrent CDI who had failed repeated courses of antibiotics and resulted in symptom resolution99. A 1989 study used a rectal administration of ten facultatively aerobic and anaerobic bacteria to successfully treat five patients with CDI100. A recent feasibility study used frozen fecal capsules, prepared from prescreened unrelated donors, to treat 20 patients with recurrent CDI, resulting in a 90% response rate after one or two treatment courses101. Pre-screened, filtered, and frozen donor stool for fecal microbiota transplantation is also available102 However, the FDA considers fecal microbiota transplantation investigational, requiring an Investigational New Drug application. There are also anecdotal reports supporting fecal microbiota transplantation for treating refractory or complicated CDI in the setting of ileus or megacolon103.
Other Therapies for the Treatment of CDI
Other Antibiotics
Teicoplanin was demonstrated to be noninferior to vancomycin, but teicoplanin is unavailable in the U.S.59. Case reports suggest efficacy of tigecycline for severe or recurrent CDI 104, however the role of tigecycline for CDI remains unclear. Phase III trials are ongoing for surotomycin and cadazolid.
Toxin Binders
Randomized trial data show that nonabsorbable anionic polymers including colestipol and cholestyramine are not effective for CDI. Tolevamer is an anionic polymer that binds C. difficile toxins A and B. However recent data show that tolevamer is inferior to vancomycin and metronidazole for CDI64. Polymers can bind other agents such as vancomycin and should not be administered concomitantly with standard therapy15.
Immunotherapy
Serum antibody response to toxin A may protect against recurrent symptomatic CDI45,82. A C. difficile vaccine is in development for both primary and recurrent CDI 105 )106,107.
Pooled immunoglobulin neutralizes C. difficile toxins in vitro but there are limited data supporting intravenous immunoglobulin for recurrent CDI108, although its role in severe CDI remains unclear. In a randomized, double-blind, placebo-controlled study, two neutralizing, human monoclonal antibodies against C. difficile toxins A (CDA1) and B (CDB1) combined with standard therapy resulted in a lower recurrent infection rate (7% vs. 25%) 109. Phase III trials are evaluating MK-3415 (human monoclonal antibody to C. difficile toxin A), MK-6072 (human monoclonal antibody to C. difficile toxin B), and MK-3415A (human monoclonal antibodies to C. difficile toxins A and B) to prevent recurrent CDI in patients receiving other recommended therapies110.
Discussion
Manifestations of C. difficile vary from asymptomatic colonization to fulminant disease. Laboratory testing does not distinguish between asymptomatic colonization versus CDI, therefore testing should be limited to symptomatic individuals15. Many testing strategies exist for CDI diagnosis. Many experts and some guidelines recommend multistep algorithms15,16,19,34.
Whether and how to treat C. difficile should be based on disease severity and relapse risk. Oral vancomycin is recommended for severe, complicated or recurrent CDI, while oral metronidazole is recommended for mild to moderate disease, although recommendations may change if further studies demonstrate that metronidazole is inferior to vancomycin15,16,64. Fidaxomicin may be used when risk of recurrence is high, however cost may be prohibitive. Data supporting the use of FMT for recurrent CDI are growing,71–73,98 however the regulation and standardization of FMT is evolving. Studies are ongoing to develop synthetic stool for treating CDI99 or capsules for administrating FMT101.
Conclusion
C. difficile remains an important cause of morbidity and mortality. Treatment strategies should be based on disease severity and recurrence risk. Fecal microbiota transplantation is associated with symptom resolution in recurrent CDI, and its role may be expanded in the future.
Supplementary Material
BOX: “Key messages regarding diagnosis and treatment of Clostridium difficile infection in adults.
DIAGNOSIS
Clostridium difficile infection (CDI) requires diarrhea (three or more unformed stools in 24-hours), AND a positive stool test for toxigenic C. difficile or its toxins, or colonoscopic/histopathologic evidence of pseudomembranous colitis. Laboratory testing cannot distinguish between colonization and infection. CDI testing should be performed only in symptomatic patients.
Diagnostic testing strategies for CDI vary. Multistep approaches using polymerase chain reaction (PCR) for the toxin gene(s) or single step PCR on liquid stool samples have the highest sensitivity and specificity.
“Test of cure” is not recommended after CDI treatment
TREATMENT
CDI should be treated according to disease severity, and risk of recurrence or complications
Vancomycin and metronidazole are first line therapy.
Vancomycin is preferred for severe or complicated disease.
Recurrent CDI is more common in older patients, and those with concomitant antibiotic use, presence of comorbidities, concomitant use of proton pump inhibitors, and worse initial disease severity
Oral metronidazole or vancomycin are recommended for the first recurrence of mild-moderate CDI.
Vancomycin is recommended for patients with 2 or more recurrences.
Fidaxomicin may be considered for recurrent CDI.
Fecal microbiota transplantation is associated with symptom resolution in recurrent CDI.
Acknowledgements:
The authors thank Mrs. Whitney Townsend, MLIS (University of Michigan) for assistance with our literature search. Ms. Townsend was not compensated for her specific contributions beyond her usual salary.
Funding/Support: This work was supported in part by the National Institutes of Health grant 1U19AI090871-01 (Drs. Rao and Malani), the Claude D. Pepper Older Americans Independence Center grant AG-024824 (Dr. Rao), and the Michigan Institute for Clinical and Health Research grant 2UL1TR000433 (Dr. Rao).
Footnotes
Conflicts of Interest Disclosures: All authors have completed and submitted the ICJME Form for Disclosure of Potential Conflicts of Interest: None reported. JAMA Associate Editor, Dr Malani had no role in the review of the paper or decision to accept for publication.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med 1978;298(10):531–534. [DOI] [PubMed] [Google Scholar]
- 2.Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 2004;171(5):466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vardakas KZ, Polyzos KA, Patouni K, Rafailidis PI, Samonis G, Falagas ME. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. Int. J. Antimicrob. Agents 2012;40(1):1–8. [DOI] [PubMed] [Google Scholar]
- 4.Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis 2005;40(11):1591–1597. [DOI] [PubMed] [Google Scholar]
- 5.Elixhauser A, Jhung M. Clostridium Difficile-Associated Disease in U.S. Hospitals, 1993–2005: Statistical Brief #50. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville MD: 2006. [Google Scholar]
- 6.Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2011–2010. Am J Infect Control 2014;42(10):1028–1032. [DOI] [PubMed] [Google Scholar]
- 7.Zimlichman E, Henderson D, Tamir O, et al. Health Care-Associated Infections: A Meta-analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern Med 2013;173(22):2039–2046. [DOI] [PubMed] [Google Scholar]
- 8.Pothoulakis C Effects of Clostridium difficile toxins on epithelial cell barrier. Ann. N. Y. Acad. Sci 2000;915:347–356. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol 2002;51(5):448–454. [DOI] [PubMed] [Google Scholar]
- 10.Asha NJ, Tompkins D, Wilcox MH. Comparative analysis of prevalence, risk factors, and molecular epidemiology of antibiotic-associated diarrhea due to Clostridium difficile, Clostridium perfringens, and Staphylococcus aureus. J. Clin. Microbiol 2006;44(8):2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J. Hosp. Infect 2008;70(4):298–304. [DOI] [PubMed] [Google Scholar]
- 12.Vardakas KZ, Konstantelias AA, Loizidis G, Rafailidis PI, Falagas ME. Risk factors for development of Clostridium difficile infection due to BI/NAP1/027 strain: a meta-analysis. Int. J. Infect. Dis 2012;16(11):e768–773. [DOI] [PubMed] [Google Scholar]
- 13.Mullane KM, Cornely OA, Crook DW, et al. Renal impairment and clinical outcomes of Clostridium difficile infection in two randomized trials. Am. J. Nephrol 2013;38(1):1–11. [DOI] [PubMed] [Google Scholar]
- 14.Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin. Infect. Dis 2011;53(1):42–48. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect. Control Hosp. Epidemiol 2010;31(5):431–455. [DOI] [PubMed] [Google Scholar]
- 16.Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect 2014;20 Suppl 2:1–26. [DOI] [PubMed] [Google Scholar]
- 17.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infect. Control Hosp. Epidemiol 2007;28(2):140–145. [DOI] [PubMed] [Google Scholar]
- 18.Keighley MR, Burdon DW, Alexander-Williams J, et al. Diarrhoea and pseudomembranous colitis after gastrointestinal operations. A prospective study. Lancet 1978;2(8101):1165–1167. [DOI] [PubMed] [Google Scholar]
- 19.Crobach MJT, Dekkers OM, Wilcox MH, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): Data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin. Microbiol. Infect 2009;15(12):1053–1066. [DOI] [PubMed] [Google Scholar]
- 20.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am. J. Gastroenterol 2013;108(4):478–498. [DOI] [PubMed] [Google Scholar]
- 21.Brecher SM, Novak-Weekley SM, Nagy E. Laboratory diagnosis of Clostridium difficile infections: there is light at the end of the colon. Clin. Infect. Dis 2013;57(8):1175–1181. [DOI] [PubMed] [Google Scholar]
- 22.Fekety R, Silva J, Kauffman C, Buggy B, Gunner Deery H. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: Comparison of two dosage regimens. Am. J. Med 1989;86(1):15–19. [DOI] [PubMed] [Google Scholar]
- 23.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am. J. Gastroenterol 2002;97(7):1769–1775. [DOI] [PubMed] [Google Scholar]
- 24.Sethi Ajay K, Al-Nassir Wafa N, Nerandzic Michelle M, Bobulsky Greg S, Donskey Curtis J. Persistence of Skin Contamination and Environmental Shedding of Clostridium difficile during and after Treatment of C. difficile Infection. Infect. Control Hosp. Epidemiol 2010;31(1):21–27. [DOI] [PubMed] [Google Scholar]
- 25.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin. Infect. Dis 2007;45(8):992–998. [DOI] [PubMed] [Google Scholar]
- 26.Piche T, Vanbiervliet G, Pipau FG, et al. Low risk of irritable bowel syndrome after Clostridium difficile infection. Can. J. Gastroenterol 2007;21(11):727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delmee M Laboratory diagnosis of Clostridium difficile disease. Clin. Microbiol. Infect 2001;7(8):411–416. [DOI] [PubMed] [Google Scholar]
- 28.Rinttila T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol 2004;97(6):1166–1177. [DOI] [PubMed] [Google Scholar]
- 29.Tenover FC, Baron EJ, Peterson LR, Persing DH. Laboratory diagnosis of Clostridium difficile infection can molecular amplification methods move us out of uncertainty? J Mol Diagn 2011;13(6):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbut F, Surgers L, Eckert C, et al. Does a rapid diagnosis of Clostridium difficile infection impact on quality of patient management? Clin. Microbiol. Infect 2014;20(2):136–144. [DOI] [PubMed] [Google Scholar]
- 31.Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet 1998;351(9103):633–636. [DOI] [PubMed] [Google Scholar]
- 32.Norén T, Alriksson I, Andersson J, Åkerlund T, Unemo M. Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for Clostridium difficile Detection Challenges Cytotoxin B Cell Test and Culture as Gold Standard. J. Clin. Microbiol 2011 2011;49(2):710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drudy D, Fanning S, Kyne L. Toxin A-negative, toxin B-positive Clostridium difficile. Int. J. Infect. Dis 2007;11(1):5–10. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox M Overcoming barriers to effective recognition and diagnosis of Clostridium difficile infection. Clin. Microbiol. Infect 2012;18(s6):13–20. [DOI] [PubMed] [Google Scholar]
- 35.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N. Engl. J. Med 1994;330(4):257–262. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal R, Garimella P, Katz A. The utility of repeat enzyme immunoassay testing for the diagnosis of Clostridium difficile infection: a systematic review of the literature. J. Postgrad. Med 2012;58(3):194. [DOI] [PubMed] [Google Scholar]
- 37.Brown NA, Lebar WD, Young CL, Hankerd RE, Newton DW. Diagnosis of Clostridium difficile infection: comparison of four methods on specimens collected in Cary-Blair transport medium and tcdB PCR on fresh versus frozen samples. Infect. Dis. Rep 2011;3(1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Planche TD, Davies KA, Coen PG, et al. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C. difficile infection. Lancet Infect. Dis 2013;13(11):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brazier JS, Raybould R, Patel B, et al. Distribution and antimicrobial susceptibility patterns of Clostridium difficile PCR ribotypes in English hospitals, 2007–08. Euro Surveill 2008;13(41). [DOI] [PubMed] [Google Scholar]
- 40.Abou Chakra CN, Pepin J, Valiquette L. Prediction tools for unfavourable outcomes in Clostridium difficile infection: a systematic review. PLoS One 2012;7(1):e30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Wilden GM, Chang Y, Cropano C, et al. Fulminant Clostridium difficile colitis: Prospective development of a risk scoring system. J Trauma Acute Care Surg 2014;76(2):424–430. [DOI] [PubMed] [Google Scholar]
- 42.Miller MA, Louie T, Mullane K, et al. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect. Dis 2013;13:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenstein AJ, Byrn JC, Zhang LP, Swedish KA, Jahn AE, Divino CM. Risk factors for the development of fulminant Clostridium difficile colitis. Surgery 2008;143(5):623–629. [DOI] [PubMed] [Google Scholar]
- 44.Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch. Surg 2009;144(5):433–440. [DOI] [PubMed] [Google Scholar]
- 45.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med 2000;342(6):390–397. [DOI] [PubMed] [Google Scholar]
- 46.Ryan J, Murphy C, Twomey C, et al. Asymptomatic carriage of Clostridium difficile in an Irish continuing care institution for the elderly: prevalence and characteristics. Ir. J. Med. Sci 2009. [DOI] [PubMed]
- 47.Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet 1990;336(8707):97–100. [DOI] [PubMed] [Google Scholar]
- 48.McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J. Infect. Dis 1990;162(3):678–684. [DOI] [PubMed] [Google Scholar]
- 49.Privitera G, Scarpellini P, Ortisi G, Nicastro G, Nicolin R, de Lalla F. Prospective study of Clostridium difficile intestinal colonization and disease following single-dose antibiotic prophylaxis in surgery. Antimicrob. Agents Chemother 1991;35(1):208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson S, Homann SR, Bettin KM, et al. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann. Intern. Med 1992(4):297–302. [DOI] [PubMed] [Google Scholar]
- 51.Samore MH, DeGirolami PC, Tlucko A, Lichtenberg DA, Melvin ZA, Karchmer AW. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin. Infect. Dis 1994;18(2):181–187. [DOI] [PubMed] [Google Scholar]
- 52.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J. Antimicrob. Chemother 2014;69(4):881–891. [DOI] [PubMed] [Google Scholar]
- 53.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J. Antimicrob. Chemother 2003;51(6):1339–1350. [DOI] [PubMed] [Google Scholar]
- 54.Owens RC Jr., Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin. Infect. Dis 2008;46 Suppl 1:S19–31. [DOI] [PubMed] [Google Scholar]
- 55.Bartlett JG. Treatment of antibiotic-associated pseudomembranous colitis. Rev. Infect. Dis 1984;6 Suppl 1:S235–241. [DOI] [PubMed] [Google Scholar]
- 56.Olson MM, Shanholtzer CJ, Lee JT Jr., Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infect. Control Hosp. Epidemiol 1994;15(6):371–381. [DOI] [PubMed] [Google Scholar]
- 57.Modena S, Gollamudi S, Friedenberg F. Continuation of antibiotics is associated with failure of metronidazole for Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol 2006;40(1):49–54. [DOI] [PubMed] [Google Scholar]
- 58.Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin. Infect. Dis 2011;53(5):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin. Infect. Dis 1996;22(5):813–818. [DOI] [PubMed] [Google Scholar]
- 60.Wilcox MH, Howe R. Diarrhoea caused by Clostridium difficile: response time for treatment with metronidazole and vancomycin. J. Antimicrob. Chemother 1995;36(4):673–679. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez A, Anand G, Friedenberg F. Factors associated with failure of metronidazole in Clostridium difficile-associated disease. J. Clin. Gastroenterol 2004;38(5):414–418. [DOI] [PubMed] [Google Scholar]
- 62.Belmares J, Gerding DN, Parada JP, Miskevics S, Weaver F, Johnson S. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J. Infect 2007;55(6):495–501. [DOI] [PubMed] [Google Scholar]
- 63.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis 2007;45(3):302–307. [DOI] [PubMed] [Google Scholar]
- 64.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin. Infect. Dis 2014;59(3):345–354. [DOI] [PubMed] [Google Scholar]
- 65.Hu MY, Maroo S, Kyne L, et al. A prospective study of risk factors and historical trends in metronidazole failure for Clostridium difficile infection. Clin. Gastroenterol. Hepatol 2008;6(12):1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pepin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am. J. Gastroenterol 2007;102(12):2781–2788. [DOI] [PubMed] [Google Scholar]
- 67.Parmar SR, Bhatt V, Yang J, Zhang Q, Schuster M. A retrospective review of metronidazole and vancomycin in the management of Clostridium difficile infection in patients with hematologic malignancies. J. Oncol. Pharm. Pract 2014;20(3):172–182. [DOI] [PubMed] [Google Scholar]
- 68.Al-Nassir WN, Sethi AK, Nerandzic MM, Bobulsky GS, Jump RL, Donskey CJ. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin. Infect. Dis 2008;47(1):56–62. [DOI] [PubMed] [Google Scholar]
- 69.Spitzer PG, Eliopoulos GM. Systemic absorption of enteral vancomycin in a patient with pseudomembranous colitis. Ann. Intern. Med 1984;100(4):533–534. [DOI] [PubMed] [Google Scholar]
- 70.Horlen CK, Seifert CF, Malouf CS. Toxic metronidazole-induced MRI changes. Ann. Pharmacother 2000;34(11):1273–1275. [DOI] [PubMed] [Google Scholar]
- 71.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am. J. Gastroenterol 2013;108(4):500–508. [DOI] [PubMed] [Google Scholar]
- 72.Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J. Clin. Gastroenterol 2014;48(8):693–702. [DOI] [PubMed] [Google Scholar]
- 73.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis 2011;53(10):994–1002. [DOI] [PubMed] [Google Scholar]
- 74.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis 2012;12(4):281–289. [DOI] [PubMed] [Google Scholar]
- 75.Louie TJ, Cannon K, Byrne B, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin. Infect. Dis 2012;55 Suppl 2(Suppl.2):S132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Musgrave CR, Bookstaver PB, Sutton SS, Miller AD. Use of alternative or adjuvant pharmacologic treatment strategies in the prevention and treatment of Clostridium difficile infection. Int. J. Infect. Dis 2011;15(7):e438–448. [DOI] [PubMed] [Google Scholar]
- 77.Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin. Infect. Dis 2002;35(6):690–696. [DOI] [PubMed] [Google Scholar]
- 78.Bublin JG, Barton TL. Rectal use of vancomycin. Ann. Pharmacother 1994;28(12):1357–1358. [PubMed] [Google Scholar]
- 79.Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 1986;27(10):1169–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann. Surg 2011;254(3):423–427; discussion 427–429. [DOI] [PubMed] [Google Scholar]
- 81.Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis 2013;15(7):798–804. [DOI] [PubMed] [Google Scholar]
- 82.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001;357(9251):189–193. [DOI] [PubMed] [Google Scholar]
- 83.Leav BA, Blair B, Leney M, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine 2010;28(4):965–969. [DOI] [PubMed] [Google Scholar]
- 84.O’Horo JC, Jindai K, Kunzer B, Safdar N. Treatment of recurrent Clostridium difficile infection: a systematic review. Infection 2014;42(1):43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tedesco FJ, Gordon D, Fortson WC. Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am. J. Gastroenterol 1985;80(11):867–868. [PubMed] [Google Scholar]
- 86.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med 2011(5):422–431. [DOI] [PubMed] [Google Scholar]
- 87.Bartsch SM, Umscheid CA, Fishman N, Lee BY. Is fidaxomicin worth the cost? An economic analysis. Clin. Infect. Dis 2013;57(4):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson S, Gerding DN. Fidaxomicin “chaser” regimen following vancomycin for patients with multiple Clostridium difficile recurrences. Clin. Infect. Dis 2013;56(2):309–310. [DOI] [PubMed] [Google Scholar]
- 89.Johnson S, Schriever C, Patel U, Patel T, Hecht DW, Gerding DN. Rifaximin Redux: treatment of recurrent Clostridium difficile infections with rifaximin immediately post-vancomycin treatment. Anaerobe 2009;15(6):290–291. [DOI] [PubMed] [Google Scholar]
- 90.Garey KW, Ghantoji SS, Shah DN, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J. Antimicrob. Chemother 2011;66(12):2850–2855. [DOI] [PubMed] [Google Scholar]
- 91.Figueroa I, Johnson S, Sambol SP, Goldstein EJC, Citron DM, Gerding DN. Relapse Versus Reinfection: Recurrent Clostridium difficile Infection Following Treatment With Fidaxomicin or Vancomycin. Clin. Infect. Dis 2012 2012;55(suppl 2):S104–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD006095. [DOI] [PubMed] [Google Scholar]
- 93.Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann. Intern. Med 2012;157(12):878–888. [DOI] [PubMed] [Google Scholar]
- 94.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 2012;307(18):1959–1969. [DOI] [PubMed] [Google Scholar]
- 95.Segarra-Newnham M Probiotics for Clostridium difficile-associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann. Pharmacother 2007;41(7):1212–1221. [DOI] [PubMed] [Google Scholar]
- 96.Safdar N, Barigala R, Said A, McKinley L. Feasibility and tolerability of probiotics for prevention of antibiotic-associated diarrhoea in hospitalized US military veterans. J. Clin. Pharm. Ther 2008;33(6):663–668. [DOI] [PubMed] [Google Scholar]
- 97.Bakken JS. Staggered and tapered antibiotic withdrawal with administration of kefir for recurrent Clostridium difficile infection. Clin. Infect. Dis 2014;59(6):858–861. [DOI] [PubMed] [Google Scholar]
- 98.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med 2013;368(5):407–415. [DOI] [PubMed] [Google Scholar]
- 99.Petrof EO, Gloor GB, Vanner SJ, et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 1989;1(8648):1156–1160. [DOI] [PubMed] [Google Scholar]
- 101.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. JAMA 2014;312(17):1772–1778. [DOI] [PubMed] [Google Scholar]
- 102.OpenBiome. OpenBiome.org. Accessed 10/22/14.
- 103.Brandt LJ. American Journal of Gastroenterology Lecture: Intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am. J. Gastroenterol 2013;108(2):177–185. [DOI] [PubMed] [Google Scholar]
- 104.Larson KC, Belliveau PP, Spooner LM. Tigecycline for the treatment of severe Clostridium difficile infection. Ann. Pharmacother 2011;45(7–8):1005–1010. [DOI] [PubMed] [Google Scholar]
- 105.Gerding DN. Clostridium difficile infection prevention: biotherapeutics, immunologics, and vaccines. Discov. Med 2012;13(68):75–83. [PubMed] [Google Scholar]
- 106.clinicaltrials.gov. Vaccine trials Available at: http://clinicaltrials.gov/ct2/results?term=clostridium+difficile+vaccine.
- 107.clinicaltrial.gov. C. difficile Vaccine. http://clinicaltrials.gov/ct2/results?term=clostridium+difficile+vaccine.
- 108.O’Horo J, Safdar N. The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int. J. Infect. Dis 2009;13(6):663–667. [DOI] [PubMed] [Google Scholar]
- 109.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med 2010;362(3):197–205. [DOI] [PubMed] [Google Scholar]
- 110.clinicaltrials.gov. MK-6072 and MK-3415A http://clinicaltrials.gov/show/NCT01241552.
- 111.Shetty N, Wren MWD, Coen PG. The role of glutamate dehydrogenase for the detection of Clostridium difficile in faecal samples: a meta-analysis. J. Hosp. Infect 2011;77(1):1–6. [DOI] [PubMed] [Google Scholar]
- 112.Deshpande A, Pasupuleti V, Rolston DDK, et al. Diagnostic Accuracy of Real-time Polymerase Chain Reaction in Detection of Clostridium difficile in the Stool Samples of Patients With Suspected Clostridium difficile Infection: A Meta-Analysis. Clin. Infect. Dis 2011;53(7):e81–e90. [DOI] [PubMed] [Google Scholar]
- 113.O’Horo JC, Jones A, Sternke M, Harper C, Safdar N. Molecular Techniques for Diagnosis of Clostridium difficile Infection: Systematic Review and Meta-analysis. Mayo Clin. Proc 2012;87(7):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Planche T, Aghaizu A, Holliman R, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. The Lancet infectious diseases 2008;8(12):777–784. [DOI] [PubMed] [Google Scholar]
- 115.Miller M, Gravel D, Mulvey M, et al. Health care-associated Clostridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin. Infect. Dis 2010;50(2):194–201. [DOI] [PubMed] [Google Scholar]
- 116.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg. Infect. Dis 2009;15(3):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


