Abstract
Rationale
Systemic levels of C reactive protein (CRP), surfactant protein D (SPD), fibrinogen, soluble receptor of activated glycogen end-product (sRAGE) and club cell protein 16 (CC-16) have been associated with chronic obstructive pulmonary disease (COPD) outcomes. However, they require validation in different cohorts.
Objectives
Relate systemic levels of those proteins to forced expiratory volume in 1 s (FEV1) decline, exacerbations, hospitalisations and mortality in COPD patients (FEV1 of ≥50 and ≤70% predicted) and heightened cardiovascular risk in a substudy of the Study to Understand Mortality and MorbidITy trial.
Methods
Participants were randomised to daily inhalations of placebo, vilanterol 25 µg (VI), fluticasone furoate 100 µg (FF) or their combination (VI 25/FF 100) and followed quarterly until 1000 deaths in the overall 16 485 participants occurred. Biomarker blood samples were available from 1673 patients. The FEV1 decline (mL/year), COPD exacerbations, hospitalisations and death were determined. Associations between biomarker levels and outcomes were adjusted by age and gender.
Results
Systemic levels of CC-16, CRP, sRAGE, SPD and fibrinogen did not relate to baseline FEV1, FEV1 decline, exacerbations or hospitalisations. Fibrinogen and CRP were related to mortality over a median follow-up of 2.3 years. Only the CC-16 changed with study therapy (VI, FF and FF/VI, p<0.01) at 3 months.
Conclusions
In COPD, systemic levels of CC-16, CRP, sRAGE, SPD and fibrinogen were not associated with FEV1 decline, exacerbations or hospitalisations. These results cast doubts about the clinical usefulness of the systemic levels of these proteins as surrogate markers of these COPD outcomes. The study confirms that CRP and fibrinogen are associated with increased risk of death in patients with COPD.
Trial registration number
Keywords: chronic obstructive pulmonary disease, mortality, biomarkers, Lung function decline
Key messages.
There is intense interest in discovering systemic biomarkers that may relate to important patient related outcomes (PRO) in patients with COPD.
Using data and biological samples prospectively collected in the Study to Understand Mortality and MorbidITy (SUMMIT) study in 1,673 COPD patients with heightened cardiovascular risk the systemic levels of C-reactive protein (CRP), fibrinogen, surfactant protein-D (SPD), soluble receptor of glycation end-product (sRAGE) and club cell protein 16 (CC16) were related to rate of decline of the forced expiratory volume in the 1st second (FEV1), exacerbations, hospitalizations and mortality.
We found no relationship between serum levels of these 5 biomarkers and rate of FEV1 decline (ml/year) and COPD exacerbations and hospitalizations. The serum levels of CRP and fibrinogen, but not sRAGE, SPD or CC16 were related to increased risk of death. These results cast doubts about the clinical usefulness of the systemic levels of these proteins as surrogate markers of COPD related outcomes.
Strengths.
The relatively large sample size and multi-centre nature of the study overcome the usual limitations of smaller trials conducted in a single center.
The patients included had a careful clinical, functional and biological characterization. In addition, the study had a prospective design, significant follow-up time and a clinical adjudicating committee that validated the outcomes.
The selections of biomarkers was based on previous studies suggesting a relationship between those proteins and outcomes.
Limitations.
The SUMMIT study was an event-driven design and as such not all patients were followed over a period of several years.
An assumption was made that a few (or possibly only one) biomarkers would be valid for all COPD patients. Given the heterogeneity of patients with COPD, this assumption may not be right.
Introduction
Chronic obstructive pulmonary disease (COPD) is an important cause of morbidity and mortality worldwide.1 Accurate prediction of outcomes such as rate of lung function decline, exacerbations, healthcare utilisation of resources and risk of death are important because it helps identify patients in whom the implementation of therapeutic measures could improve those outcomes.2 COPD is also a complex and heterogeneous disease at the genetic, cellular and molecular level, and therefore, it is likely that the use of biomarkers that reflect diverse pathobiological pathways could help assess multiple dimensions of disease progression that could be modulated with specific therapeutic agents.3
Several systemic biomarkers, including C reactive protein (CRP), fibrinogen and surfactant protein D (SPD) have been associated with increased risk of death in patients with COPD.4–8 However, their relationship to other outcomes such as rate of decline of the forced expiratory volume in 1 s (FEV1) of a forced expiratory manoeuvre, exacerbations and hospitalisations remains unclear.9 10 The serum concentration of club cell protein 16 (CC-16) was inversely related to rate of FEV1 decline in observational studies,11 in pharmacological trials of patients with COPD12 and in smokers without clinical airflow limitation.13 The serum levels of soluble receptor of activated glycogen end-product (sRAGE) relate inversely to progression of emphysema,14 as determined by serially repeated CT of the lungs. In addition, genome-wide association studies (GWAS) do support the increased prevalence of single nucleotide polymorphisms associated to the RAGE,15 SPD and CC-16 genes.16 Importantly, the systemic baseline levels of troponin determined in the patients participating in the biomarker component of the Study to Understand Mortality and MorbidITy (SUMMIT) trial were highly predictive of subsequent cardiovascular (CV) events and death in this cohort.17
Based on these findings, we hypothesised that the serum levels of one or more of CC-16, CRP, sRAGE, SPD and fibrinogen would relate to the following outcomes: rate of FEV1 decline, exacerbations of COPD, hospitalisations due to these episodes and the risk of death in patients with COPD. We tested this hypothesis using data and biological samples prospectively collected in the subset of patients recruited in the USA who participated in the SUMMIT study. SUMMIT was an event-driven study that assessed the efficacy and safety of inhaled corticosteroids (ICSs) and long-acting beta agonists (LABA) in COPD patients with heightened CV risk. In the subset of patients, serum samples were taken at baseline and 3 months after randomisation, enabling us to also test whether pharmacotherapy modulated the serum levels of these biomarkers.
Methods
Study design and study population
SUMMIT was a prospective, multicentre, international randomised controlled trial to determine whether treatment with the inhaled LABA vilanterol (VI), the ICS fluticasone furoate (FF) or both in combination could improve clinical outcomes in patients with moderate COPD and increased CV risk. Details regarding study design and its primary results have been previously published.18 19 In brief, participants included former or current smokers (≥10 pack-years) between 40 and 80 years of age, with a history of COPD and a postbronchodilator FEV1 to forced vital capacity ≤0.70 and an FEV1 ≥50% and ≤70% of predicted. They had a score ≥2 on the modified Medical Research Council dyspnoea scale. Patients were additionally required to have a history, or be at increased risk, of CV disease defined as coronary artery or peripheral arterial disease, prior stroke or myocardial infarction, or diabetes mellitus with target organ disease. Patients were also included if they had increased CV risk defined as being ≥60 years and receiving medications for two or more of the following: hypercholesterolaemia, hypertension, diabetes mellitus or peripheral vascular disease. While ICSs and LABA treatments were discontinued before study entry, other COPD medications were permitted during the trial. Participants were randomly allocated to one of four treatments: placebo, FF (100 µg), VI (25 µg) or their combination (FF/VI, 100/25 µg) inhaled once daily as a dry powder. Of the total of 16 485 patients who participated in SUMMIT, 1673 of those enrolled in the USA had blood samples analysed for this biomarker substudy. All patients provided written informed consent. The study is registered on ClinicalTrials.gov.
Patient and public involvement
Patients were not involved in the design, conduct or interpretation of the study.
Measurements and endpoints
On-treatment spirometry was performed following the European Respiratory Society/American Thoracic Society guidelines. Spirometric reference values were those of National Health and Nutrition Examination Survey.20 On-treatment COPD exacerbations requiring treatment with antibiotics and/or oral corticosteroids were recorded as moderate exacerbations, and those requiring hospitalisation were recorded as severe. On-treatment and post-treatment deaths were adjudicated for cause by an endpoint committee.19
Biomarkers
Blood samples were collected at baseline and 3 months postrandomisation. Samples were stored at –80°C until analysed. SPD, CC-16 and sRAGE were measured in serum samples. Fibrinogen and CRP (high-sensitivity methods) were measured in plasma samples. All protein biomarkers were measured by validated immunoassays. Concentrations below lower limit of detection (LLD) were imputed to ½×LLD.
Statistical analysis
To examine the effect of treatment on the change in biomarker concentrations between baseline and 3 months, analyses of covariance were performed using the covariates of baseline biomarker concentration, age and gender. As all biomarkers except fibrinogen had positively skewed distributions, these were log transformed, and the changes were expressed as ratios, and geometric means presented rather than arithmetic means.
The biomarker concentrations were divided into groups according to tertiles at baseline and are presented in online supplementary table 1. To explore whether the biomarker concentrations affected rate of decline in FEV1, random coefficients models were fitted with terms of age, gender, baseline FEV1, time, treatment, treatment by time, baseline biomarker tertile groups and baseline biomarker tertile groups by time, with subjects fitted as a random effect. Associations between baseline biomarker tertile groups and endpoints including all-cause mortality, CV death, moderate or severe exacerbations and severe exacerbations were modelled using Cox proportional hazards including terms for age, gender and study treatment and also previous exacerbation history for the exacerbation endpoints.
bmjresp-2019-000431supp001.pdf (526.9KB, pdf)
Correlations between variables of interest were explored using Pearson’s correlation coefficient.
Two-sided tests were performed post hoc at the 0.05 level of significance, using SAS V.9.4. All p values are nominal, as no adjustment was made for multiple comparisons.
Results
Clinical data
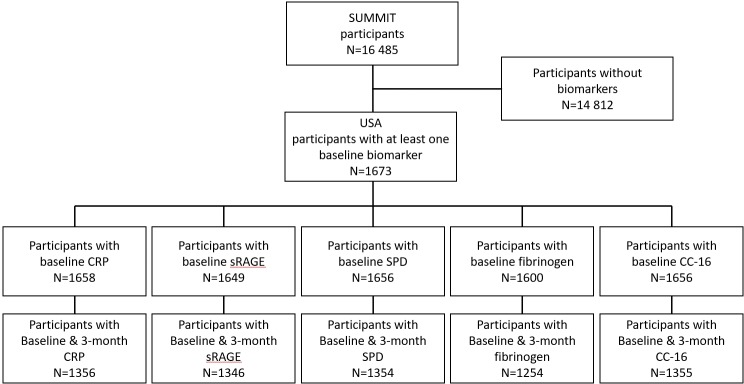
The published primary results of SUMMIT showed that there was no effect of any of the therapies on mortality, the primary outcome.19 The Consolidated Standards of Reporting Trials diagram of the SUMMIT systemic biomarker substudy enrolled in the USA is shown in figure 1. Of the 16 485 worldwide patients participating in SUMMIT, 1673 recruited in the USA had biomarker data at baseline.
Figure 1.
CONSORT diagram of the patients included in the systemic biomarker study completed only in the USA, as part of the SUMMIT international trial. CC-16, club cell protein 16; CONSORT, Consolidated Standards of Reporting Trials; CRP, C reactive protein; SPD, surfactant protein D; sRAGE, soluble receptor of activated glycogen end-product.
The clinical characteristics of the patients included in the study and those from the general SUMMIT population are shown in table 1. Most characteristics were similar in both groups, except for the biomarker population having more females, higher body mass index, fewer COPD exacerbations in the year prior to the study and some differences in CV history and CV therapy. In the biomarker substudy, the average age was 66 years, 62% of the patients were male and 49% were smoking at the time of enrolment. By design, they had moderate airflow limitation by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (FEV1 60% predicted) and had significant CV disease or risk. Patients were followed for mortality for a median of 2 years and 3 months and for all on-treatment endpoints for a median of 18 months and an average of seven spirometric measurements over this time.
Table 1.
Demographic characteristics of the patients included in the biomarker substudy conducted in the USA and that of the worldwide participants
| Biomarker substudy | SUMMIT population | |
| n=1673 | n=16 485 | |
| Age, years | 66 (8) | 65 (8) |
| Women | 635 (38%) | 4196 (25%) |
| BMI, kg/m2 | 31 (7) | 28 (6) |
| Smoking status | ||
| Current smoker | 828 (49%) | 7678 (47%) |
| Smoking history (pack-years) | 52 (29) | 41 (24) |
| Respiratory status | ||
| Postbronchodilator FEV1 (L) | 1.7 (0.4) | 1.7 (0.4) |
| Predicted postbronchodilator FEV1 (%) | 59 (7) | 60 (6) |
| Exacerbations in 12 months before study | ||
| 0 | 1215 (73%) | 10 021 (61%) |
| 1 | 290 (17%) | 4020 (24%) |
| 2+ | 168 (10%) | 2444 (15%) |
| Cardiovascular inclusion criteria | ||
| Manifest disease | ||
| Coronary artery disease | 818 (49%) | 8379 (51%) |
| Peripheral arterial disease | 384 (23%) | 3145 (19%) |
| Previous stroke | 172 (10%) | 1595 (10%) |
| Previous myocardial infarction | 413 (25%) | 2774 (17%) |
| Diabetes with target organ disease | 226 (14%) | 1503 (9%) |
| At risk | ||
| Hypercholesterolaemia | 1072 (64%) | 8479 (51%) |
| Hypertension | 1166 (70%) | 11 478 (70%) |
| Diabetes mellitus | 447 (27%) | 3480 (21%) |
| Peripheral arterial disease | 106 (6%) | 1154 (7%) |
| Concomitant medications | ||
| Antiplatelet | 1081 (65%) | 8517 (52%) |
| Beta-blocker | 787 (47%) | 5667 (34%) |
| ACE inhibitor | 779 (47%) | 7655 (46%) |
| Statin | 1263 (75%) | 10 721 (65%) |
| Long-acting muscarinic antagonist | 168 (10%) | 818 (5%) |
| Xanthine (including theophylline) | 78 (5%) | 3719 (23%) |
| Treatment allocation | ||
| Placebo | 439 (26%) | 4111 (25%) |
| Fluticasone furoate | 415 (25%) | 4135 (25%) |
| Vilanterol | 416 (25%) | 4118 (25%) |
| Combination therapy | 403 (24%) | 4121 (25%) |
Continuous variables are mean (SD); categorical variables are n and (%).
BMI, body mass index;FEV1, forced expiratory volume in 1 s;SUMMIT, Study to Understand Mortality and MorbidITy.
Effect of treatment on biomarker concentration at 3 months
Inhaled VI, FF and the combination resulted in a 6%–9% reduction in CC-16 serum levels as is shown in online supplementary table 2 (p<0.01 for all three compared with placebo). However, there was no effect of the inhaled therapy on systemic levels of CRP, sRAGE, SPD and fibrinogen when compared with placebo (online supplementary table 3).
Baseline biomarker data and outcomes
There was no relationship between baseline levels of CC-16, CRP, sRAGE, SPD and fibrinogen and baseline FEV1 expressed as % of predicted normal value (figure 2). The rate of decline in FEV1 was not different between the baseline tertile groups for any of the biomarkers (table 2). There was no association between any of the biomarker concentrations and the risk of a moderate or severe COPD exacerbation (online supplementary figure 1) or a hospitalised exacerbation (online supplementary figure 2).
Figure 2.
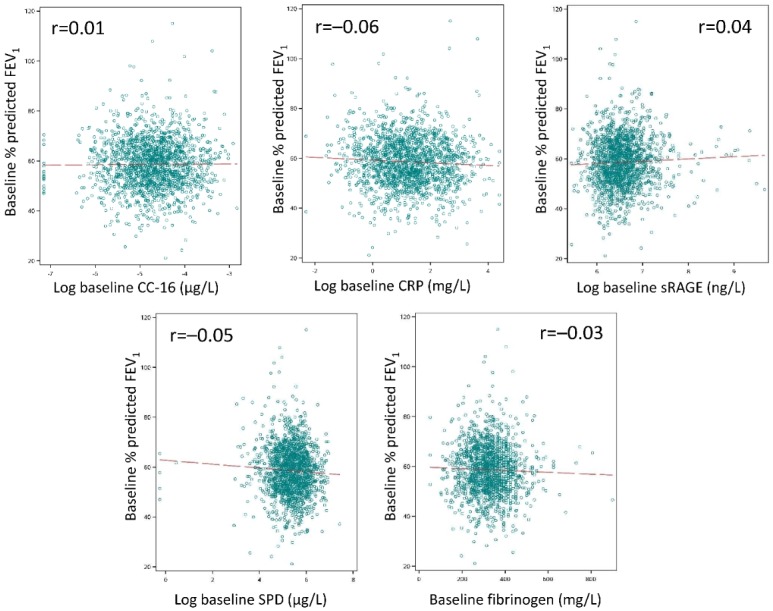
Relationship between baseline biomarker concentration and percent predicted FEV1. CC-16, club cell protein 16; CRP, C reactive protein; FEV1, forced expiratory volume in 1 s; SPD, surfactant protein D; sRAGE, soluble receptor of activated glycogen end-product.
Table 2.
Rate of FEV1 decline (mL/year) by systemic biomarker tertile groups
| Number of patients in analysis* | Lowest tertile group | Middle tertile group | Highest tertile group | |
| Mean rate of decline (mL/year) (SE) | ||||
| CRP† | 1462 | –38 (7) | –40 (7) | –33 (7) |
| sRAGE† | 1454 | –37 (7) | –37 (7) | –36 (7) |
| SPD† | 1461 | –30 (7) | –33 (7) | –48 (7) |
| Fibrinogen† | 1410 | –39 (7) | –35 (7) | –37 (8) |
| CC-16‡ | ||||
| Placebo FF 100 VI 25 FF/VI 100/25 |
371 365 362 363 |
33 (15) 32 (15) 62 (15) 23 (13) |
57 (14) 47 (15) 41 (15) 37 (14) |
27 (15) 21 (14) 56 (15) 11 (13) |
CC-16, club cell protein 16; CRP, C reactive protein; FEV1, forced expiratory volume in 1 s; FF, fluticasone furoate; SPD, surfactant protein D; sRAGE, soluble receptor of activated glycogen end-product; VI, vilanterol.
*To be included in the analysis a patient needed to have an on-treatment FEV1 measurement and the baseline biomarker measurement.
†All four treatment arms (placebo, FF, VI and FF/VI): random coefficients model fitted with terms of age, gender, baseline FEV1, time, treatment, treatment by time, baseline biomarker tertile groups and baseline biomarker tertile groups by time.
‡Presented by arm, because CC-16 concentrations at 3 months were affected by treatment (online supplementary table S2): random coefficients model fitted with terms of age, gender, baseline FEV1, time, baseline CC-16 tertile groups and baseline CC-16 tertile groups by time.
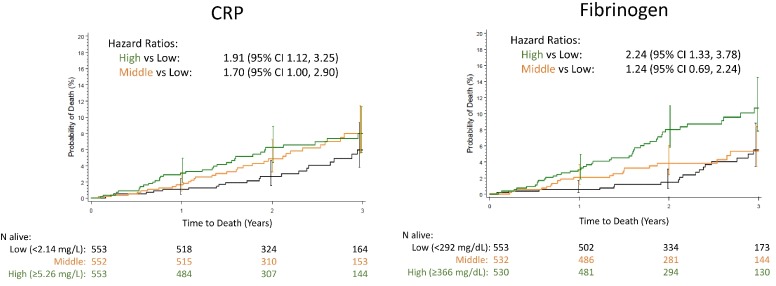
Higher systemic levels of CRP and fibrinogen were associated with higher risk of all-cause mortality as is shown in figure 3. The number of deaths by any cause for CRP were: highest tertile group 36 (7%) versus lowest tertile group 22 (4%), HR 1.91; middle tertile group 35 (6%) versus lowest tertile group 22 (4%), HR 1.70; for fibrinogen they were highest group 43 (8%) versus lowest group 21 (4%), HR 2.24; middle group 24 (5%) versus lowest group 21 (4%), HR 1.24. There were no apparent relationships between CC-16, sRAGE and SPD and all-cause mortality (online supplementary figure 3) or with any of the measured biomarkers and CV death (online supplementary figure 4).
Figure 3.
Kaplan–Meier plots of time to death by tertile groups for CRP and fibrinogen. HRs from Cox proportional hazard model adjusted for treatment, age and gender. Biomarker tertile group: Low in black colour. Middle in orange colour. High in green colour. CRP, C reactive protein.
Discussion
This prospective study of COPD patients with moderate airflow limitation and heightened CV risk provides two novel findings. First, the systemic levels of the five cytokines (CC-16, sRAGE, SPD, CRP and fibrinogen S), selected because of previous studies suggesting their potential value as predictors of outcomes in COPD, were unrelated to rate of FEV1 decline, exacerbation frequency and hospitalisations in these patients. The systemic levels of CRP and fibrinogen were associated with increased risk of all-cause mortality, confirming the predictive value for this outcome reported by others. Second, compared with placebo, once-daily inhalation of VI, FF and the combination resulted in small but significant decreases in the serum levels of CC-16, but not in the other biomarkers.
Previous studies
There has been a growing interest in the field of biomarkers in COPD as a tool to predict outcomes or as marker of them, thus making them theoretically useful as surrogate markers of response for therapeutic interventions.9 10 21–23 CRP has been the best studied biomarker in COPD. Most studies found that CRP levels are elevated in these patients,6 24 25 as compared with non-smokers and smokers without airflow obstruction, but the relationship between CRP levels and exacerbations as well as mortality remain inconsistent.25 26 Studies have also not consistently shown a relationship between CRP and rate of lung function decline.11 Similarly, several studies have documented a relationship between blood levels of fibrinogen and risk of death in patients in general27 and COPD in particular.5 7 28 Integration of all the data available by the COPD Biomarker Qualification Consortium initiative resulted in US Food and Drug Administration approval of this biomarker to stratify patients for studies of COPD.7 There is less information regarding the relationship between systemic levels of fibrinogen and other important outcomes in COPD, such as rate of lung function decline and hospitalisations.29
Of the many other systemic biomarkers studied in patients with COPD, the circulating levels of CC-16, SPD and sRAGE have shown the strongest association between their levels and COPD outcomes.15 30–34 CC-16 levels have been found to be reduced in patients with COPD and are inversely associated with rate of decline of FEV1 in some studies.13 35 The lung-derived protein SPD, however, is associated with presence of pulmonary inflammation and is elevated in smokers with or without COPD.31 The systemic levels of sRAGE are inversely related to the degree of emphysema as determined by CT scans of the lungs,14 while GWAS have documented the increased prevalence of single nucleotide polymorphisms associated with the RAGE gene.15 All of these proteins have been suggested as potential biomarkers of COPD outcomes, but not all of them have been studied simultaneously and prospectively while evaluating the effect of therapy on their levels.
Although statistically significant associations are important for studies and research, the ideal biomarker for the clinician is useful primarily if it helps a single patient, the one in front of the provider. So if a biomarker is to reach the bedside, it has to have a strong association with an outcome and provide information above and beyond that obtained by good clinical evaluation.
Current findings
The prospective SUMMIT study provided the opportunity to test the predictive ability of the selected circulating proteins on important respiratory outcomes. To our disappointment, none of the systemic levels of the five circulating proteins studied related to rate of FEV1 decline, exacerbations and hospitalisations (table 2, online supplementary figures 1 and 2). Although a true association might have been found if more patients had been recruited, this appears unlikely as several of the studies documenting an association between those biomarkers and the outcomes were smaller or similar in size to ours. In addition, it is known that the rate of FEV1 decline is larger in patients with higher FEV1,36 so if there was a relationship between any of the biomarkers selected and lung function decline, these moderately obstructed patients were the most likely individuals to have demonstrated such an association. Furthermore, a biomarker is most useful when it can help clinicians in their practice usually with a single patient, and certainly, the lack of precision documented in this study renders them of limited use in everyday patient care. However, the association observed between systemic levels of CRP and fibrinogen with mortality in this study (figure 3) supports their validity as predictors of risk of death in general and in patients with heightened CV risk, as were those included in SUMMIT.37 38 We acknowledge that there were no measurements of these biomarkers during exacerbations, where the changes might identify or grade the severity of these events, as has been suggested before.39 Importantly, the significant association observed between baseline serum levels of troponin and subsequent risk of CV events and death in this cohort17 indicates that methodological issues or the study design and completion are unlikely to have influenced the results here reported.
The difference between the negative results in COPD-related outcomes in this study, and the positive ones reported by previous authors may relate primarily to the populations included in the different studies.6–8 11–15 Whereas the majority of the positive results came from cohort studies such as ECLIPSE and COPDgene, which included patients with a wider range of lung function impairment, the population included in SUMMIT consisted of patients with a moderate degree of airflow limitation and heightened CV risk. However, for a biomarker to be useful in clinical practice, it has to help clinicians at any stage of the disease and most of all for patients with milder disease, such as those included in SUMMIT.
One potential use of biomarkers is their response to therapy specifically directed at the disease in question and if this change was associated to a modification in outcomes. In this study, the relative levels of CC-16 were decreased by the three active treatment arms of the study compared with placebo. The effects were small, likely of little clinical meaning, but nevertheless statistically significant (table S2). A decrease in CC-16 was not associated with FEV1 decline in this study, a fact that could have several explanations. First, that CC-16 bears little relation to FEV1 decline, as has been suggested by some studies,12 or second, that the small changes in serum levels of CC-16 carry little biological significance. However, the results do suggest that inhaled therapies may alter systemic levels of cytokines and that if associations with valid outcomes were to be found, one or more cytokines could be used as surrogate markers to evaluate effect of therapy. Interestingly, we found no effect of any of the therapies given in SUMMIT on CRP levels, providing contrast to the findings of an older study that demonstrated reduced CRP levels after the administration of ICSs.40 Conversely, however, our findings are in agreement with those of a further study, in which the administration of inhaled tiotropium did not affect inflammatory markers in patients with COPD.41 Finally, the stability of the selected biomarkers over 3 months is consistent with data previously reported in ECLIPSE.42
Strengths and limitations
The relatively large sample size and multicentre nature of the study, its careful clinical, functional and biological characterisation and its prospective design, follow-up time and clinical adjudicating committee are strengths of this study. However, there were several potential limitations. First, the study had an event-driven design and as such not all patients were followed over a period of several years. However, with an average of 18 months of on-treatment observation and of 27 months for mortality, the determination of two biosamples separated by 3 months and a mean number of 7 spirometric measurements per patient, we believe the observation time is sufficient for all of the outcomes selected. Second, the chosen panel of proteins did not include all possible biomarkers that have been suggested.9 43 However, the ones here measured included those that have the strongest support in the literature as being potentially applicable in clinical practice. Third, it could be argued that there was no derivative and validating cohort. However, this is customary for non-validated biomarkers, whereas in this study, we compared validated clinical and serum biomarkers modelled on studies in the respiratory and CV arena. The main weakness is likely to be the assumption that a few (or possibly only one) biomarkers will be valid for all patients with COPD. Given the heterogeneity of the disease this is unlikely; however, we currently are unable to identify subgroups where specific biomarkers would be of particular value.
Conclusions
In this substudy of the SUMMIT trial of patients with moderate COPD, the serum level of CC-16, sRAGE and SPD or their changes over 3 months were not predictive of rate of lung function decline and risk of exacerbations or hospitalisations. Although systemic levels of CRP and fibrinogen were associated with increased mortality risk, the levels were not associated with rate of FEV1 decline, exacerbations or hospitalisations. These results cast some doubts about the clinical usefulness of the systemic levels of CC-16, sRAGE and SPD as surrogate markers of disease in patients with moderate COPD.
Acknowledgments
Editorial assistance was provided by Matthew Hallam, MSc, and Carol A Richter, PhD, at Gardiner-Caldwell Communications (Macclesfield) and was funded by GlaxoSmithKline plc.
Footnotes
Contributors: Study design: BCR, JAA, RB, PC, CC, ID, FJM, DEN, JY and JV. Data collection: JAA, CC, NJC and JY. Database search: N/A. Data analysis/interpretation: BCR, JAA, RB, PC, NJC, CC, ID, VK, FJM, AM, DEN, JY and JV. Writing/reviewing of the manuscript: BCR, JAA, RB, PC, NJC, CC, ID, VK, FJM, AM, DEN, JY and JV. Final approval of the manuscript: all authors.
Funding: This work was supported by GlaxoSmithKline plc.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All patients provided written informed consent. The study was approved by local ethics committees and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Members of the Steering Committee Jørgen Vestbo (cochair, UK), Robert Brook (USA), Peter Calverley (UK), Bartolome Celli (USA), Fernando Martinez (USA), David Newby (UK), Courtney Crim, (cochair, GlaxoSmithKline plc, USA), Julie Anderson (GlaxoSmithKline plc, UK) and Julie Yates (GlaxoSmithKline plc, USA). Members of the Independent Data Monitoring Committee Peter Lange (chair, Denmark), Richard Kay (UK), Mark Dransfield (USA) and Sanjay Rajagopalan (USA). Members of the Clinical Endpoint Committee Robert Wise (chair, USA), Dennis Niewoehner (USA), Camilo Gomez (USA), Sheldon Madger (Canada), Martin Denvir (UK) and Pierre Amarenco (France).
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement: Information on GSK’s data sharing commitments and requesting access to anonymised individual participant data and associated documents can be found at www.clinicalstudydatarequest.com
References
- 1. López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016;21:14–23. 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 2. Celli BR, Decramer M, Wedzicha JA, et al. An official American thoracic Society/European Respiratory Society statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;191:e4–27. 10.1164/rccm.201501-0044ST [DOI] [PubMed] [Google Scholar]
- 3. Agusti A, Sobradillo P, Celli B. Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am J Respir Crit Care Med 2011;183:1129–37. 10.1164/rccm.201009-1414PP [DOI] [PubMed] [Google Scholar]
- 4. Man SFP, Xing L, Connett JE, et al. Circulating fibronectin to C-reactive protein ratio and mortality: a biomarker in COPD? Eur Respir J 2008;32:1451–7. 10.1183/09031936.00153207 [DOI] [PubMed] [Google Scholar]
- 5. Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012;7:e37483 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahl M, Vestbo J, Lange P, et al. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:250–5. 10.1164/rccm.200605-713OC [DOI] [PubMed] [Google Scholar]
- 7. Miller BE, Tal-Singer R, Rennard SI, et al. Plasma fibrinogen qualification as a drug development tool in COPD: perspective of the COPD biomarker qualification Consortium. Am J Respir Crit Care Med 2016;193:607–13. [DOI] [PubMed] [Google Scholar]
- 8. Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:1065–72. 10.1164/rccm.201110-1792OC [DOI] [PubMed] [Google Scholar]
- 9. Kostikas K, Bakakos P, Papiris S, et al. Systemic biomarkers in the evaluation and management of COPD patients: are we getting closer to clinical application? Curr Drug Targets 2013;14:177–91. 10.2174/1389450111314020005 [DOI] [PubMed] [Google Scholar]
- 10. Barnes PJ, Chowdhury B, Kharitonov SA, et al. Pulmonary biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:6–14. 10.1164/rccm.200510-1659PP [DOI] [PubMed] [Google Scholar]
- 11. Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011;365:1184–92. 10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 12. Park HY, Churg A, Wright JL, et al. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:1413–9. 10.1164/rccm.201305-0892OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen H, Leng S, Belinsky SA, et al. Low plasma CC16 levels in smokers are associated with a higher risk for chronic bronchitis. Eur Respir J 2015;46:1501–3. 10.1183/13993003.00682-2015 [DOI] [PubMed] [Google Scholar]
- 14. Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the eclipse study. Lancet Respir Med 2013;1:129–36. 10.1016/S2213-2600(13)70006-7 [DOI] [PubMed] [Google Scholar]
- 15. Cheng DT, Kim DK, Cockayne DA, et al. Systemic sRAGE is a biomarker of emphysema and associated with AGER genetic variants in COPD patients. Am J Respir Crit Care Med 2013;188:948–57. [DOI] [PubMed] [Google Scholar]
- 16. Kim DK, Cho MH, Hersh CP, et al. Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:1238–47. 10.1164/rccm.201206-1013OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adamson PD, Anderson JA, Cowans NJ, et al. Cardiac troponin I and risk of cardiovascular events in patients with COPD and heightened cardiovascular risk. J Am Coll Cardiol. In Press 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vestbo J, Anderson J, Brook RD, et al. The study to understand mortality and morbidity in COPD (Summit) study protocol. Eur Respir J 2013;41:1017–22. 10.1183/09031936.00087312 [DOI] [PubMed] [Google Scholar]
- 19. Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (Summit): a double-blind randomised controlled trial. Lancet 2016;387:1817–26. 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 20. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87. 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 21. Chen Y-WR, Leung JM, Sin DD. A systematic review of diagnostic biomarkers of COPD exacerbation. PLoS One 2016;11:e0158843 10.1371/journal.pone.0158843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bowler RP, Wendt CH, Fessler MB, et al. New strategies and challenges in lung proteomics and metabolomics. An official American Thoracic Society workshop report. Ann Am Thorac Soc 2017;14:1721–43. 10.1513/AnnalsATS.201710-770WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradford E, Jacobson S, Varasteh J, et al. The value of blood cytokines and chemokines in assessing COPD. Respir Res 2017;18 10.1186/s12931-017-0662-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinto-Plata VM, Müllerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006;61:23–8. 10.1136/thx.2005.042200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:867–74. 10.1164/rccm.200604-506OC [DOI] [PubMed] [Google Scholar]
- 26. de Torres JP, Cordoba-Lanus E, López-Aguilar C, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J 2006;27:902–7. 10.1183/09031936.06.00109605 [DOI] [PubMed] [Google Scholar]
- 27. Gabazza EC, Taguchi O, Yamakami T, et al. Evaluating prethrombotic state in lung cancer using molecular markers. Chest 1993;103:196–200. 10.1378/chest.103.1.196 [DOI] [PubMed] [Google Scholar]
- 28. Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013;309:2353–61. 10.1001/jama.2013.5732 [DOI] [PubMed] [Google Scholar]
- 29. Dahl M, Tybjaerg-Hansen A, Vestbo J, et al. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1008–11. 10.1164/ajrccm.164.6.2010067 [DOI] [PubMed] [Google Scholar]
- 30. Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the eclipse cohort. Respir Res 2010;11 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lomas DA, Silverman EK, Edwards LD, et al. Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J 2009;34:95–102. 10.1183/09031936.00156508 [DOI] [PubMed] [Google Scholar]
- 32. Lomas DA, Silverman EK, Edwards LD, et al. Evaluation of serum CC-16 as a biomarker for COPD in the eclipse cohort. Thorax 2008;63:1058–63. 10.1136/thx.2008.102574 [DOI] [PubMed] [Google Scholar]
- 33. Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol 2010;2010:1–11. 10.1155/2010/917108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miniati M, Monti S, Basta G, et al. Soluble receptor for advanced glycation end products in COPD: relationship with emphysema and chronic cor pulmonale: a case-control study. Respir Res 2011;12 10.1186/1465-9921-12-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laucho-Contreras ME, Polverino F, Tesfaigzi Y, et al. Club cell protein 16 (CC16) augmentation: a potential disease-modifying approach for chronic obstructive pulmonary disease (COPD). Expert Opin Ther Targets 2016;20:869–83. 10.1517/14728222.2016.1139084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scanlon PD, Connett JE, Waller LA, et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. the lung health study. Am J Respir Crit Care Med 2000;161:381–90. 10.1164/ajrccm.161.2.9901044 [DOI] [PubMed] [Google Scholar]
- 37. Klingenberg R, Aghlmandi S, Räber L, et al. Improved risk stratification of patients with acute coronary syndromes using a combination of hsTnT, NT-proBNP and hsCRP with the grace score. Eur Heart J Acute Cardiovasc Care 2018;7:129–38. 10.1177/2048872616684678 [DOI] [PubMed] [Google Scholar]
- 38. Kaptoge S, Di Angelantonio E, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012;367:1310–20. 10.1056/NEJMoa1107477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karadeniz G, Polat G, Senol G, et al. C-reactive protein measurements as a marker of the severity of chronic obstructive pulmonary disease exacerbations. Inflammation 2013;36:948–53. 10.1007/s10753-013-9625-z [DOI] [PubMed] [Google Scholar]
- 40. Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:760–5. 10.1164/rccm.200404-543OC [DOI] [PubMed] [Google Scholar]
- 41. Powrie DJ, Wilkinson TMA, Donaldson GC, et al. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J 2007;30:472–8. 10.1183/09031936.00023907 [DOI] [PubMed] [Google Scholar]
- 42. Dickens JA, Miller BE, Edwards LD, et al. COPD association and repeatability of blood biomarkers in the eclipse cohort. Evaluation of COPD longitudinally to identify surrogate endpoints (eclipse) Study Investigators. Respir Res 2011;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinto-Plata V, Toso J, Lee K, et al. Use of proteomic patterns of serum biomarkers in patients with chronic obstructive pulmonary disease: correlation with clinical parameters. Proc Am Thorac Soc 2006;3:465–6. 10.1513/pats.200603-030MS [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2019-000431supp001.pdf (526.9KB, pdf)