Abstract
Background
The objective of this study was to study the anti-inflammatory effect and possibly involved molecular mechanisms of matrine on oxidized low-density lipoprotein (ox-LDL)-exposed macrophages.
Material/Methods
Cultured human macrophages (THP-1 cell line) were exposed to ox-LDL at final concentrations of 0, 25, 50, and 100 μg/mL. Several cells were then treated with matrine at serial diluted concentrations. 2,7-Dichlorodi-hydrofluorescein diacetate (DCFH-DA) staining was used to evaluate reactive oxygen species (ROS) production; a colorimetric method was used to determine the cellular antioxidant capacity; production of pro-inflammatory cytokines interleukin (IL)18 and tumor necrosis factor (TNF)α were determined by enzyme-linked immunosorbent assay (ELISA); and immunoblot assay was used to assess the relative protein phosphorylation and expression.
Results
ox-LDL exposure significantly elevated intracellular ROS level and supernatant IL18 and TNFα concentrations, but impaired total antioxidant capacity (TAC) of macrophages. The relative phosphorylations of MAPK kinase kinases (MKK)6, MKK3, and p38 mitogen-activated protein kinases (MAPK) were increased by ox-LDL exposure. The expression levels of IL18 and TNFα were also increased in ox-LDL-treated macrophages. The matrine treatment reduced intracellular ROS level and supernatant IL18 and TNFα concentrations and increased TAC in a concentration- dependent manner. The relative phosphorylations of MKK6, MKK3, and p38 MAPK were reduced after matrine administration. Moreover, the expression levels of IL18 and TNFα were also decreased by matrine treatment, in a concentration-dependent manner.
Conclusions
ox-LDL increases inflammatory response in macrophages by activating the ROS-mediated MKKs/p38 MAPK-induced inflammatory signaling pathway. Matrine suppresses ox-LDL-induced inflammatory by inhibiting the MKKs/p38 MAPK signaling pathway.
MeSH Keywords: Inflammation, Macrophages, MAP Kinase Signaling System, Reactive Oxygen Species
Background
Atherosclerosis is a chronic inflammatory disease of the arterial vascular wall and is one of the common pathophysiological features of many cardiovascular diseases such as coronary arterial disease and stroke. Many cell types were involved [1]. Macrophages are the dominant immune cell type in atherosclerotic lesions, and it is believed that are critical for the initiation and development of atherosclerosis [2]. The inflammatory responses in macrophages result in local pro-inflammatory cascade activation, leading to foam cell formation, platelet activation, and plaque rupture [3].
It is well accepted that hyperlipidemia is an independent risk factor of atherosclerosis. Low-density lipoprotein (LDL) infiltrates into the vascular intima and turns into ox-LDL after oxidative modification [4]. The characterized pathological changes of atherosclerosis are highly associated with ox-LDL. Challenged by certain harmful stimuli, macrophages are polarized and exhibit 2 main phenotypes – M1 and M2. M1 macrophages are related to inflammation due to their ability to produce pro-inflammatory cytokines [5]. Activated by ox-LDL, the macrophages initiate and exacerbate inflammation response by producing pro-inflammatory cytokines and further promoting the destabilization of the atherosclerotic plaques [6].
Mitogen-activated protein kinases (MAPKs) are a protein family characterized by a structure composed of p38 MAPK, c-Jun N-terminal kinases, and extracellular signal-regulated protein kinases. The p38 MAPK signaling pathway is activated when encountering harmful stimuli such as reactive oxygen species (ROS), leading to increasing inflammatory responses [7]. Activation of the up-stream kinase MAPK kinase kinases (MKKs) – MKK6 and MKK3 – leads to further activation of p38 MAPK [8].
Matrine is one of the active components extracted from the natural herb Sophora flavescens, which has been utilized in the treatments of cardiovascular diseases in Traditional Chinese Medicine from ancient times [9]. Several previous investigations found that ox-LDL triggers excessive intracellular ROS production [4,10,11]. The vascular protective effects of matrine were reported by our group and others [7,12]. Moreover, one of our recent studies indicated that matrine and its derivatives affect activation the MKKs/MAPK signaling by reducing intracellular ROS production [9]. In the present study, we investigated the inhibitory effects of matrine on ox-LDL-induced inflammatory response in macrophages. The involvement of ROS- mediated MKKs/p38 MAPK inflammatory signaling was also investigated.
Material and Methods
Cell culture, grouping, and treatments
Human monocytic THP-1 cells (purchased from China Center for Type Culture Collection, CCTCC) were used in this study. Cells were maintained in RPMI 1640 medium (Hyclone) supplemented with fetal bovine serum (10%, FBS, Hyclone) in a humidified incubator providing an atmosphere of 5%CO2/95%O2 at 37°C. Serially diluted ox-LDL (Solarbio) at final concentrations of 0, 25, 50 and 100 μg/mL were used to treat the cells for 48 h. Concentrations of matrine were chosen according to the results from our pilot study. Cells exposed to ox-LDL at 100 μg/mL were also treated with serially diluted matrine at final concentrations of 0.0, 0.5, 1.0, and 2.0 mmol/L for 48 h after exposure to ox-LDL.
In situ ROS determination
The intracellular generation of ROS was evaluated by a fluorescent staining method using the ROS probe DCFH-DA (Beyotime). Briefly, cells were harvested and washed with PBS. Then, the DCFH-DA was loaded to cells by incubating the cells for 30 min at 37°C in the dark. An inverted microscope was used to observe and capture the fluorescent images. The intracellular ROS level was indicated with the mean fluorescent intensity of DCFH-DA, which was calculated with Image-Pro Plus (version 5.0).
Antioxidant capability evaluation
The cell homogenates were prepared after the cells were collected. At 4°C, the homogenates were centrifuged for 5 min at 12 000 g. The total antioxidant capacity (TAC) was measured in the resulting supernatant by using the T-AOC Assay Kit (Beyotime) according to the protocols provided by the manufacturer. The absorbance of ABTS+ at 734 nm was detected by a plate reader.
ELISA
The resulting supernatants of collected cells were also used for ELISA assay. The concentrations of IL-18 and TNF-α were measured with the Quantkine human IL-18 kit (R&D) and Quantkine human TNF-α kit (R&D) according to the protocols provided by the manufacturer. The concentrations of IL-6 and TNF-α were calculated with absorbance values and standard curves.
RT-PCR
The total RNA was extracted and isolated by using Beyozol total RNA extraction kits (Beyotime) according to the protocols provided by the manufacturer. SYBR green PCR master mix was used. Primers for IL18 were forward 5′-AAGAAAGCCGCCTCAAACCT-3′; reverse 5′-TCTGACATGGCAGCCATTGT-3′. Primers for TNF were forward 5′-ATCCGCGACGTGGAACTG-3′; reverse 5′-ACCGCCTGGAGTTCTGGAA-3′. The Mastercycler RT-PCR detection system (Eppendorf) was used to carry out the RT-PCR assay. GAPDH was used as the internal reference to normalize the mRNA expression levels of target genes by using the formula 2ΔCT(GAPDH-target).
Western blotting
Cultured macrophages were collected and further lysed in cell lysis buffer for Western and IP (Beyotime), which was supplemented with PSMF (Beyotime). The total protein of the cells was acquired with the Protein Extraction Kit (Beyotime). The protein sample concentrations were detected with bicinchoninic acid (BCA) assay with the BCA Assay Kit (Pierce). Then, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was utilized to separate the proteins, and the separated proteins were then electronically transferred to NC or PVDF membranes. Specific primary antibodies of phosphor-MKK3 (1: 4000, p-MKK3, Abcam), MKK3 (1: 4000, Abcam), p-MKK6 (1: 2000, CST), MKK6 (1: 2000, CST), p-p38 MAPK (1: 4000, Abcam), p38 MAPK (1: 1000, Abcam), IL-18 (1: 4000, Sigma), TNF-α (1: 4000, CST) and GAPDH (1: 4000, Santa Cruz) were used to incubate the membranes at 4°C for 12 h. After TBST washing, the membranes were then incubated with corresponding HRP-conjugated secondary antibodies (Abcam) at 20°C for 2 h. After being developed with Signal West Pico Chemiluminescent Substrate (Pierce), the membranes were exposed and the immunoblots were visualized. Image-Pro Plus (version 5.0) software was used to analyze the intensities of immunoblots.
Statistical analysis
Data are presented as mean ±SD. SPSS software was used to analyze the differences between groups with one-way analysis of variance (ANOVA) and t tests. At p<0.05, the differences were considered statistically significant.
Results
Matrine treatment reduced ROS production in ox-LDL-incubated macrophages
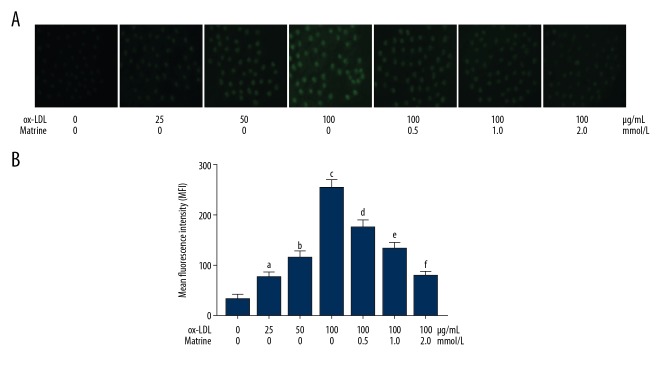
The results are demonstrated in Figure 1. The produced intracellular ROS was tagged by DCFH-DA fluorescent staining. The captured images of DCFH-DA are demonstrated. ROS level was dramatically increased after treatment with ox-LDL, in a concentration-dependent manner. The matrine administration significantly decreased ROS levels in ox-LDL exposed macrophages in a concentration-dependent manner.
Figure 1.
(A) The upper part demonstrates the captured fluorescent images of DCFH-DA staining of macrophages stimulated with ox-LDL at 0, 25, 50, and 100 μg/mL. Several cells incubated with ox-LDL at 100 μg/mL were also treated with matrine at 0.5, 1.0, and 2.0 mmol/L. (B) Columns on the lower panel indicate the detected mean fluorescence intensities (MFI) of DCFH-DA in each group. [a differences were significant when compared with macrophages incubated with ox-LDL at 0 μg/mL; b differences were significant when compared with macrophages incubated with ox-LDL at 25 μg/mL; c differences were significant when compared with macrophages incubated with ox-LDL at 50 μg/mL; d differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/mL and matrine at 0.0 mmol/L; e differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/mL and matrine at 0.5 mmol/L; f differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/ml and matrine at 1.0 mmol/L].
Matrine incubation restored antioxidant capacity in ox-LDL incubated macrophages
The antioxidant capacity of macrophages was indicated by measured TAC. As demonstrated in Figure 2, ox-LDL exposure significantly impaired TAC in macrophages in a concentration-dependent manner. The matrine administration, however, recovered the TAC in ox-LDL-exposed macrophages in a concentration-dependent manner.
Figure 2.
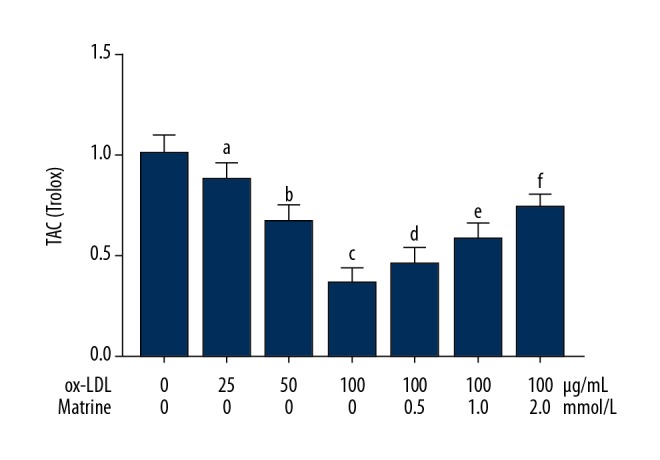
Columns in this figure indicate the calculated TAC of cultured macrophages stimulated with ox-LDL at 0, 25, 50, and 100 μg/mL. Several cells incubated with ox-LDL at 100 μg/mL were also administered matrine at 0.5, 1.0, and 2.0 mmol/L. [a differences were significant when compared with macrophages incubated with ox-LDL at 0 μg/mL; b differences were significant when compared with macrophages incubated with ox-LDL at 25 μg/mL; c differences were significant when compared with macrophages incubated with ox-LDL at 50 μg/mL; d differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/mL and matrine at 0.0 mmol/L; e differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/mL and matrine at 0.5 mmol/L; f differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/ml and matrine at 1.0 mmol/L].
Matrine treatment inhibited inflammatory cytokine production in ox-LDL-incubated macrophages
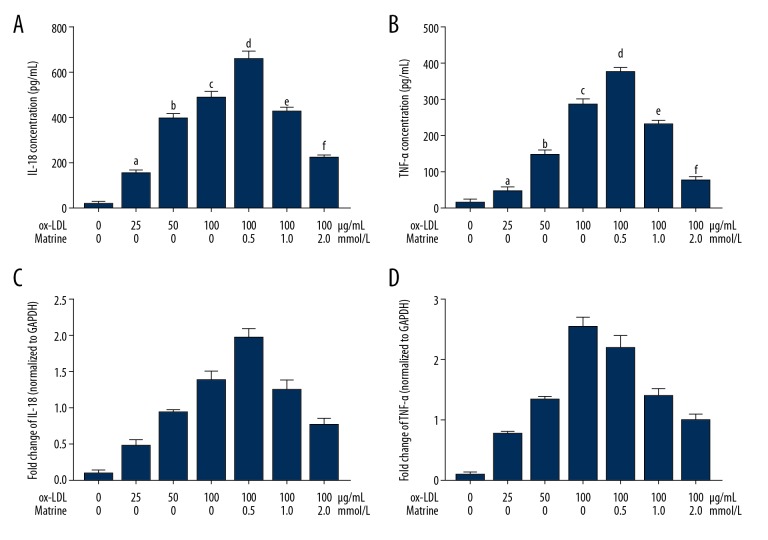
The concentrations and mRNA expression levels of inflammatory cytokines IL18 and TNFα were determined by ELSIA and RT-PCR, respectively. As shown in Figure 3, ox-LDL significantly increased the concentrations mRNA expression levels of IL18 and TNFα in macrophages in a concentration-dependent manner. However, the administration of matrine dramatically reduced the concentrations as well as the mRNA expression levels of IL18 and TNFα in a concentration-dependent manner.
Figure 3.
(A) Columns indicate the detected concentration of IL18 in supernatant from each group. (B) Columns indicate the detected concentration of TNFα in supernatant from each group. (C) Columns indicate the relative expression level of IL18 mRNA in macrophages. (D) Columns indicate the relative expression levels of TNFα mRNA in macrophages. [a differences were significant when compared with macrophages incubated with ox-LDL at 0 μg/mL; b differences were significant when compared with macrophages incubated with ox-LDL at 25 μg/mL; c differences were significant when compared with macrophages incubated with ox-LDL at 50 μg/mL; d differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/mL and matrine at 0.0 mmol/L; e differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/mL and matrine at 0.5 mmol/L; f differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/ml and matrine at 1.0 mmol/L].
Matrine incubation suppressed activation of MKKs/p38 MAPK inflammation signaling in ox-LDL-incubated macrophages
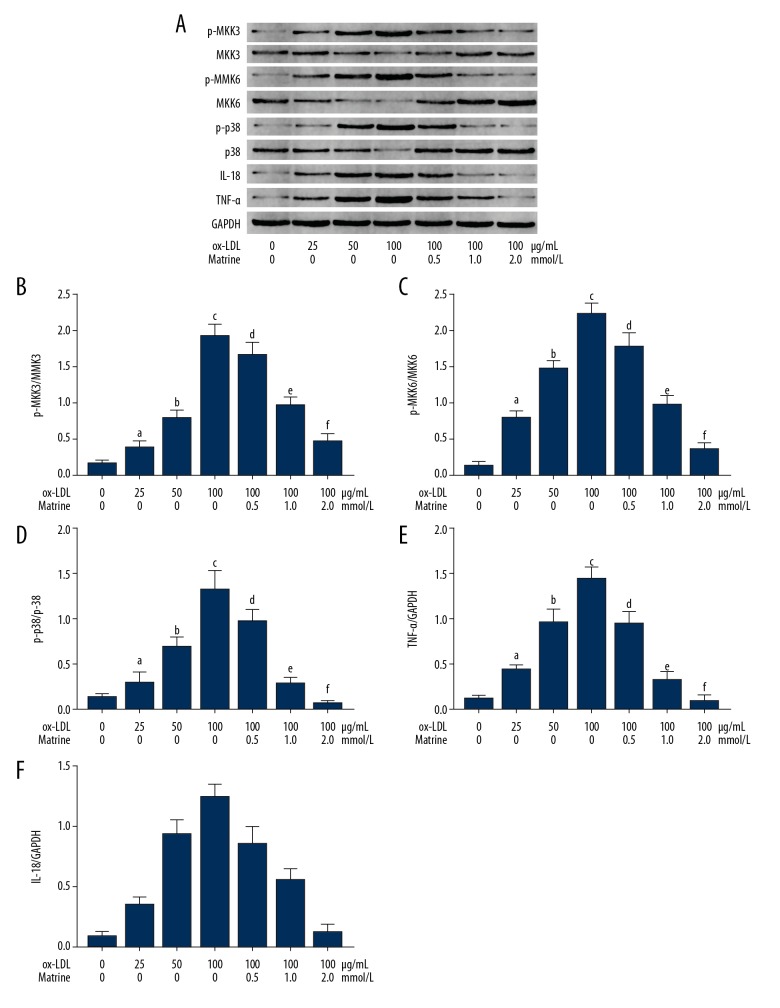
The immunoblots are demonstrated in Figure 4. The phosphorylation levels of MKK3, MKK6, and p38 MAPK and the expression levels of IL18 and TNFα were found significantly upregulated in ox-LDL-exposed macrophages. Treatment with matrine, however, dramatically suppressed the phosphorylation levels of MKK3, MKK6, and p38 MAPK in ox-LDL-exposed macrophages in a concentration-dependent manner. The expression levels of IL18 and TNFα were also suppressed by matrine in ox-LDL- exposed cultured macrophages.
Figure 4.
(A) The upper panel demonstrates the immunoblots of p-MKK3, MKK3, p-MKK6, MKK6, p-p38, p38, IL18, TNFα, and GAPDH of cultured macrophages. (B) Columns indicate the phosphorylation level of MKK3 (p-MKK3/MKK3). (C) columns indicate the phosphorylation level of MKK6 (p-MKK6/MKK6). (D) Columns indicate the phosphorylation level of p38 MAPK (p-p38/p38). (E) Columns indicate the expression level of TNFα (TNFα/GAPDH). (F) Columns indicate the expression level of IL18 (IL18/GAPDH). [a differences were significant when compared with macrophages incubated with ox-LDL at 0 μg/mL; b differences were significant when compared with macrophages incubated with ox-LDL at 25 μg/mL; c differences were significant when compared with macrophages incubated with ox-LDL at 50 μg/mL; d differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/mL and matrine at 0.0 mmol/L; e differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/mL and matrine at 0.5 mmol/L; f differences were significant when compared with macrophages incubated with ox-LDL at 100 μg/ml and matrine at 1.0 mmol/L].
Discussion
Myocardial infarction and stroke are among the serious complications of arterial atherosclerosis, contributing to the leading cause of death worldwide. The mechanisms underlying the occurrence and development of atherosclerosis are very complicated. LDL plays roles as initiator and accelerator in the pathogenesis of atherosclerosis. Under condition of hyperlipidemia, accumulated LDL infiltrates into the arterial intima through the dysfunctional endothelium, where it receives oxidation modification [12]. The resulting ox-LDL recruits circulating monocytes, which further infiltrate into the arterial intima and differentiate into macrophages [13].
Atherosclerosis is regarded as an inflammatory disease [14]. Many immune cells play roles in the inflammatory response [14]. The immediate inflammatory response is derived from the interaction between white blood cells and tissue damage, such as ruptured plaques. Activated lymphocytes become inflammatory cytokine-producing units, which further recruit more immune cells and platelets [15]. Activated macrophages found in atherosclerotic lesions, which are responsible for local vascular inflammation by producing pro-inflammatory cytokines. These cytokines are believed to further promote the recruitment and activation of immune cells [16]. In the present study, human macrophages were exposed to ox-LDL. The results show that ox-LDL exposure increased inflammatory cytokines TNFα and IL-18 in a concentration- dependent manner.
The antioxidant system maintains the intracellular antioxidant-oxidant balance, which is critical for normal physiological functions. When encountering pathological stimuli such as ox-LDL, excessive ROS is generated and overwhelms the antioxidants [17]. In this study, dramatically increased ROS generation was found in ox-LDL-exposed human macrophages. As shown by impaired TAC, the antioxidant capacities were significantly reduced by ox-LDL in a concentration-dependent manner. In this situation, many signaling pathways are activated, leading to cell injury, inflammation, and death.
The P38 MAPK signaling pathway was reported to induce inflammatory response in many cell types [18]. It was reported that p38 MAPK activation increases inflammation by upregulating inflammatory cytokines [19]. According studies by our group and others, the activation of p38 MAPK is dependent on phosphorylation of its up-stream kinases, MKK3 and MKK6 [9,20]. Previous investigations [21,22] found that TNFα and IL-18, which are typical inflammatory cytokines produced by macrophages, play a pro-atherogenic role. Disruption of TNFα impedes the development of atherosclerosis. In this investigation, we found that ox-LDL exposure significantly facilitated the phosphorylation of MKK3, MKK6, and p38, activating the p38 MAPK inflammatory signaling pathway.
Matrine is one of the effective quinolizidine alkaloids extracted from the Chinese medical herb S. flavesents. Matrine has been reported to exhibit multiple biological activities including anti-oxidative, anti-inflammatory, anti-viral, anti-cancer, and anti-fibrotic properties [23,24]. Our team has been focusing on studying the mechanisms of its cardioprotective effects. According to our previous investigations, matrine has potent anti-oxidative activity [11]. Moreover, matrine administration affects activation of the MKKs/p38 MAPK signaling pathway [9]. In this study, matrine was used to treat human macrophages stimulated by ox-LDL. The results showed that matrine treatment significantly suppressed the ox-LDL-induced inflammatory response in macrophages. We also observed that matrine administration dramatically reduced intracellular ROS generation by strengthening the antioxidant capacity in macrophages. Our further investigation indicated that matrine administration reduced activation of MKKs/p38 MAPK inflammatory signaling.
Conclusions
Results from this study indicate the ox-LDL exposure triggers excessive ROS generation, which further induces inflammatory response in macrophages by activating the MKKs/p38 MAPK signaling pathway. The administration of matrine significantly reduces intracellular ROS production and further suppresses activation of MKKs/p38 MAPK inflammatory signaling. The present results add to understanding of the role of ox-LDL in initiating and exacerbating atherosclerosis, and also provide evidence and clues for the potential utility of matrine and matrine- containing compounds in clinical treatments of atherosclerotic diseases.
Limitations
In the current study, we show the protective effect of matrine on ox-LDL-induced inflammation in macrophages. There are several limitations of this study. Firstly, the present study was carried out in vitro, and results from in vivo study would fortify the conclusions. Secondly, further study should be carried out investigating the phenotype and polarization of the macrophages. Thirdly, a macrophage cell line was used in this study, and it would be more persuasive to have used primary macrophages.
Footnotes
Source of support: This study was supported by National Scientific Foundation of China (81600646); Health Research Foundation of Shaanxi Province (2018E011); Innovative Talents Promotion Project of Shaanxi Province (2019KJXX-019)
References
- 1.Pant S, Deshmukh A, Gurumurthy GS, et al. Inflammation and atherosclerosis – revisited. J Cardiovasc Pharmacol Ther. 2014;19:170–78. doi: 10.1177/1074248413504994. [DOI] [PubMed] [Google Scholar]
- 2.Bories GFP, Leitinger N. Macrophage metabolism in atherosclerosis. FEBS Lett. 2017;591:3042–60. doi: 10.1002/1873-3468.12786. [DOI] [PubMed] [Google Scholar]
- 3.Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153–66. doi: 10.1111/imr.12218. [DOI] [PubMed] [Google Scholar]
- 4.Yao Y, Wang Y, Zhang Y, Liu C. Klotho ameliorates oxidized low density lipoprotein (ox-LDL)-induced oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids Health Dis. 2017;16:77. doi: 10.1186/s12944-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quero L, Hanser E, Manigold T, et al. TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype. Arthritis Re Ther. 2017;19:245. doi: 10.1186/s13075-017-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavurma MM, Rayner KJ, Karunakaran D. The walking dead: macrophage inflammation and death in atherosclerosis. Curr Opin Lipidol. 2017;28:91–98. doi: 10.1097/MOL.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo M, Luo P, Zhang Z, et al. Zinc delays the progression of obesity-related glomerulopathy in mice via down-regulating P38 MAPK-mediated inflammation. Obesity (Silver Spring, Md) 2016;24:1244–56. doi: 10.1002/oby.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huth HW, Albarnaz JD, Torres AA, et al. MEK2 controls the activation of MKK3/MKK6-p38 axis involved in the MDA-MB-231 breast cancer cell survival: Correlation with cyclin D1 expression. Cell Signal. 2016;28:1283–91. doi: 10.1016/j.cellsig.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Lv Y, Zhang Y, et al. Matrine-type alkaloids inhibit advanced glycation end products induced reactive oxygen species-mediated apoptosis of aortic endothelial cells in vivo and in vitro by targeting MKK3 and p38MAPK signaling. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007441. pii: 007441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Zhao Z, Pang X, et al. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol Lett. 2017;277:115–22. doi: 10.1016/j.toxlet.2017.07.216. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Yang X, Qiu C, et al. Matrine suppresses AGE-induced HAEC injury by inhibiting ROS-mediated NRLP3 inflammasome activation. Eur J Pharmacol. 2018;822:207–11. doi: 10.1016/j.ejphar.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrman B, Partoush A, Volkova N, Aviram M. Ox-LDL induces monocyte-to-macrophage differentiation in vivo: Possible role for the macrophage colony stimulating factor receptor (M-CSF-R) Atherosclerosis. 2008;196:598–607. doi: 10.1016/j.atherosclerosis.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Tousoulis D, Oikonomou E, Economou EK, et al. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur Heart J. 2016;37:1723–32. doi: 10.1093/eurheartj/ehv759. [DOI] [PubMed] [Google Scholar]
- 15.Legein B, Temmerman L, Biessen EA, et al. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci. 2013;70:3847–96. doi: 10.1007/s00018-013-1289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobryshev YV, Nikiforov NG, Elizova NV, Orekhov AN. Macrophages and their contribution to the development of atherosclerosis. Results Probl Cell Differ. 2017;62:273–98. doi: 10.1007/978-3-319-54090-0_11. [DOI] [PubMed] [Google Scholar]
- 17.Amir Aslani B, Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016;146:163–73. doi: 10.1016/j.lfs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 18.O’Neil JD, Ammit AJ, Clark AR. MAPK p38 regulates inflammatory gene expression via tristetraprolin: Doing good by stealth. Int J Biochem Cell Biol. 2018;94:6–9. doi: 10.1016/j.biocel.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz L, Yeung YT, Grewal T. Oncogenic Ras modulates p38 MAPK-mediated inflammatory cytokine production in glioblastoma cells. Cancer Biol Ther. 2016;17:355–63. doi: 10.1080/15384047.2016.1139249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stramucci L, Pranteda A, Bossi G. Insights of crosstalk between p53 protein and the MKK3/MKK6/p38 MAPK signaling pathway in cancer. Cancers (Basel) 2018;10(5) doi: 10.3390/cancers10050131. pii: E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Sun C, Gerdes N, et al. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat Med. 2015;21:820–26. doi: 10.1038/nm.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Yang X, Bian F, et al. TNF-alpha promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: Crosstalk between NF-kappaB and PPAR-gamma. J Mol Cell Cardiol. 2014;72:85–94. doi: 10.1016/j.yjmcc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YJ, Guo YJ, Yang XL, Ou ZL. Anti-cervical cancer role of matrine, oxymatrine and sophora flavescens alkaloid gels and its mechanism. J Cancer. 2018;9:1357–64. doi: 10.7150/jca.22427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Xu H. Matrine: Bioactivities and structural modifications. Curr Top Med Chem. 2016;16:3365–78. doi: 10.2174/1568026616666160506131012. [DOI] [PubMed] [Google Scholar]