Abstract
Introduction
Multimorbidity and polypharmacy are important risk factors for drug-related hospital admissions (DRAs). DRAs are often linked to prescribing problems (overprescribing and underprescribing), as well as non-adherence with drug regimens for different reasons. In this trial, we aim to assess whether a structured medication review compared with standard care can reduce DRAs in multimorbid older patients with polypharmacy.
Methods and analysis
OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people is a European multicentre, cluster randomised, controlled trial. Hospitalised patients ≥70 years with ≥3 chronic medical conditions and concurrent use of ≥5 chronic medications are included in the four participating study centres of Bern (Switzerland), Utrecht (The Netherlands), Brussels (Belgium) and Cork (Ireland). Patients treated by the same prescribing physician constitute a cluster, and clusters are randomised 1:1 to either standard care or Systematic Tool to Reduce Inappropriate Prescribing (STRIP) intervention with the help of a clinical decision support system, the STRIP Assistant. STRIP is a structured method performing customised medication reviews, based on Screening Tool of Older People’s Prescriptions/Screening Tool to Alert to Right Treatment criteria to detect potentially inappropriate prescribing. The primary endpoint is any DRA where the main reason or a contributory reason for the patient’s admission is caused by overtreatment or undertreatment, and/or inappropriate treatment. Secondary endpoints include number of any hospitalisations, all-cause mortality, number of falls, quality of life, degree of polypharmacy, activities of daily living, patient’s drug compliance, the number of significant drug–drug interactions, drug overuse and underuse and potentially inappropriate medication.
Ethics and dissemination
The local Ethics Committees in Switzerland, Ireland, The Netherlands and Belgium approved this trial protocol. We will publish the results of this trial in a peer-reviewed journal.
Main funding
European Union’s Horizon 2020 programme.
Trial registration number
NCT02986425, SNCTP000002183, NTR6012, U1111-1181-9400.
Keywords: clinical pharmacology, general medicine (see internal medicine), internal medicine, geriatric medicine
Strengths and limitations of this study.
This is the first multicentre randomised trial that examines the impact of a structured approach to optimise pharmacotherapy in multimorbid older people on drug-related hospital admissions.
This is one of the largest trials undertaken in the growing population of multimorbid older adults; a population, that is, to date poorly investigated and often excluded from large scale trials.
We chose a cluster randomisation design in order to limit potential contamination from a learning effect among the prescribing physicians; however, the cluster design of this trial entails some potential for selection bias.
A blinded adjudication committee (pharmacist and senior physician) for each site will judge whether a hospital admission during follow-up should be considered a drug-related hospital admission.
Introduction
Patients with multimorbidity have been excluded in >60% of the randomised controlled trials (RCTs) published in high-impact journals during the last 15 years due to their complexity and frailty.1 This finding is in contrast to the rapidly growing numbers of patients with coexisting chronic diseases, with associated increased mortality,2 decreased health-related quality of life (QoL), increased healthcare utilisation, increased hospital admissions3 4 and higher rates of drug prescriptions with subsequent polypharmacy.5 Polypharmacy refers to the concurrent use of multiple drugs, often defined as taking ≥5 long-term medications.6 Appropriate polypharmacy can improve health-related QoL and prevent consequences of diseases, whereas inappropriate polypharmacy is often harmful, particularly in multimorbid older people.6 Polypharmacy increases the risk of inappropriate prescribing, defined as misuse of medication or overtreatment and drug–drug interactions.7–9 With polypharmacy, there is also an increased risk of non-compliance, adverse drug reactions (ADRs), drug–drug and drug–disease interactions.10 Inappropriate prescribing can lead to a drug-related hospital admission (DRA),11 lower QoL and a higher number of falls.12 13 The incidence of DRAs in older people may be as high as 30% of all hospital admissions,13–15 and about half of DRAs are considered potentially preventable.13 16
Literature reviews recently suggested that interventions optimising polypharmacy could reduce inappropriate prescribing17 18 and the risk of ADRs.19 The Screening Tool of Older People’s Prescriptions/Screening Tool to Alert to Right Treatment (STOPP/START) criteria20 have been developed by geriatric medicine and pharmacotherapy experts based on review of up-to-date evidence and consensus validation to screen for inappropriate prescribing; they have also been shown to significantly improve medication appropriateness.21 This property of STOPP/START criteria therefore, has the potential to reduce DRAs.22 The Systematic Tool to Reduce Inappropriate Prescribing (STRIP) is a tool that combines STOPP/START criteria to increase appropriate prescribing for older people.23 However, until now, only a few RCTs have examined the impact of reducing inappropriate medications on clinical outcomes and they had several limitations, such as missing adjudication of DRAs by an independent adjudication committee, no cluster randomisation (contamination bias) or small sample size, young population and/or short follow-up time, leaving currently considerable uncertainty on the best ways to improve the appropriate use of polypharmacy.14 24 25
The OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people (OPERAM) trial will examine the effect of a structured medication review (STRIP) supported by the STRIP Assistant (STRIPA) clinical decision support software on DRAs (main endpoint) compared with usual care. Secondary endpoints include number of any hospitalisations, all-cause mortality, number of falls, QoL, degree of polypharmacy, activities of daily living (ADL), patient’s drug compliance, as well as the number of significant drug–drug interactions, drug overuse and underuse and potentially inappropriate medication.
Methods and analysis
General study design and setting
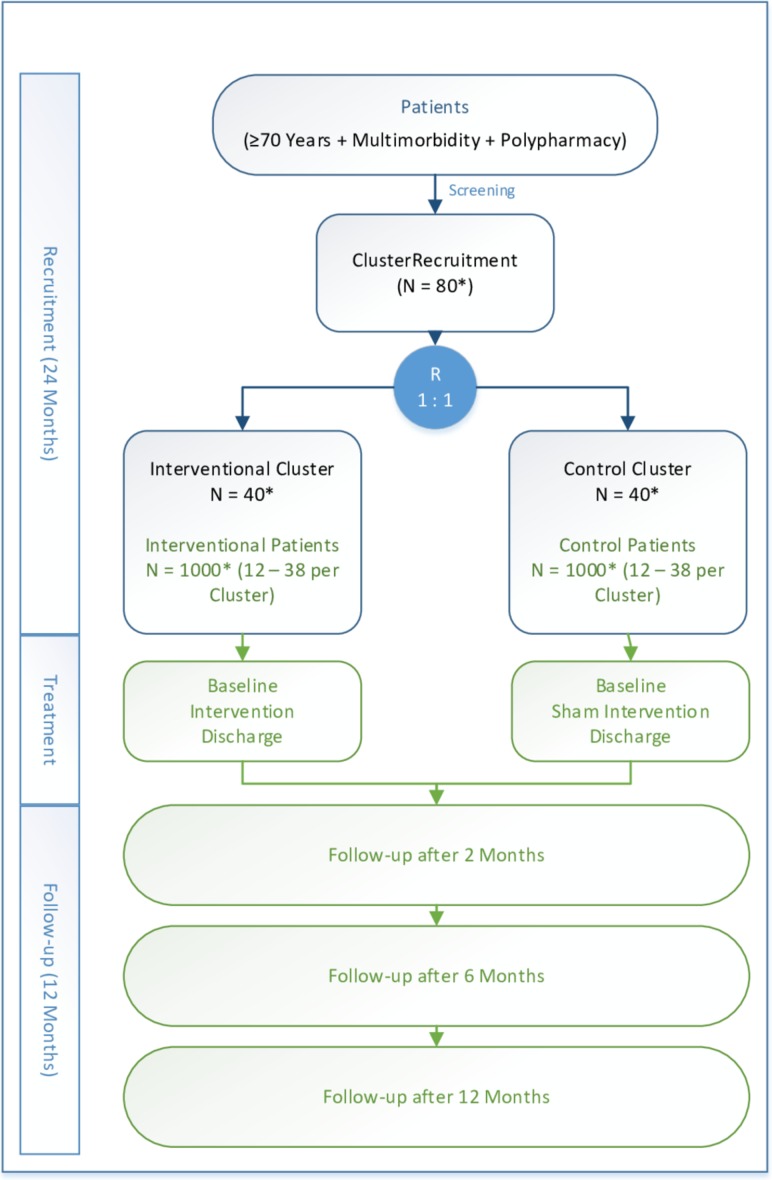
OPERAM is a European multicentre cluster RCT. A prescribing physician will define a cluster. Older hospitalised patients with multimorbidity and polypharmacy will receive either a structured drug review or usual care, according to the allocation of their prescribing physician. Clusters will be randomised 1:1 to either the intervention arm (drug review) or usual care. Patients will be followed-up by phone at 2, 6 and 12 months after inclusion; information may be provided by proxy persons (table 1).
Table 1.
Study population, intervention, control and outcomes
| Population | Consecutive older adults (≥70 years) with multimorbidity (≥3 chronic medical problems) and polypharmacy (≥5 regular drugs for >30 days). |
| Intervention | Pharmacotherapy optimisation based on the Systematic Tool to Reduce Inappropriate Prescribing through (1) systematic medication review by a physician and a pharmacist, with support of the STRIP Assistant, a software-based tool taking into account the predictable adverse medication effects, advising safe and appropriate therapy using established STOPP/START criteria, monitoring clinically relevant interactions and dosing appropriately in accordance with renal function, (2) drug discussion and adaptation with the prescribing physician, (3) shared decision-making with the patient and (4) generation of a report with specific recommendations for the patient’s general practitioner. |
| Control | Usual practice and a sham intervention using a questionnaire (Medication Adherence Measure Questionnaire, ©MMAS30–32 *) by a team member (the physician or the pharmacist) to mimic the intervention and improve blinding of the patient and other blinded team members. |
| Outcomes |
Primary: drug-related hospital admission within 1 year after enrolment Secondary: number of any hospitalisations, mortality, number of falls, quality of life, degree of polypharmacy, activities of daily living, patient’s drug compliance, as well as the number of significant drug–drug interactions, drug overuse and underuse and potentially inappropriate medication. |
*Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A licence agreement is available from: Donald E Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA Fielding School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90 095–1772, dmorisky@ucla.edu.
START/STOPP, Screening Tool of Older People’s Prescriptions/Screening Tool to Alert to Right Treatment.
This study protocol follows the Standard Protocol Items: Recommendations for Interventional Trials guidelines.26
Objective
This study aims to compare the effect of a structured medication review (supported by the STRIPA software) versus usual care on DRAs. Secondary endpoints include number of any hospitalisations, mortality, number of falls, QoL, degree of polypharmacy, ADL, all-cause mortality, patient’s drug compliance, as well as the number of significant drug–drug interactions, drug overuse and any rehospitalisation occurring within 1 year after the index-hospitalisation, underuse and potentially inappropriate medication.
Cluster definition
The trial is conducted in four University Hospital Centres in Europe (Bern, Switzerland; Utrecht, The Netherlands; Brussels, Belgium; Cork, Ireland). Clusters are defined by the prescribing physician, that is, the single physician who has the final responsibility for the pharmacotherapy and treatment of patients in the department/ward and also decides on the implementation of potential treatment suggestions made by involved specialists. Clusters are possible in any department/ward at each site with a relevant proportion of the appropriate patient population (multimorbid older people with polypharmacy, see above), where patients are not included in another trial aiming at optimising drug therapy. Recruitment of potential physicians follows a multilevel approach: first, eligible departments/wards are identified by the local principal investigator and then prescribing physicians are enrolled in order to each form a cluster within the trial. Enrolment and opening of clusters is distributed over the complete recruitment period to ensure an approximately similar number of open clusters in each site at any time. The recruitment target ranges from a minimum of 12 to a maximum of 38 patients per cluster in order to achieve recruitment of 2000 patients.
Inclusion criteria
Consecutive patients are screened for eligibility and recruited from the clusters at each site. Inclusion criteria are age 70 years or older, multimorbidity (defined as three or more chronic medical conditions) and polypharmacy (defined as use of five or more regular drugs for >30 days). Written informed consent by patients themselves or, in the case of cognitive impairment by a legal representative, is required before enrolment.
Exclusion criteria
Exclusion criteria are reduced to a minimum to allow for maximum generalisability. Only patients planned for direct admission to palliative care (<24 hours after admission), or patients undergoing a structured drug review other than the trial intervention, or who have passed a structured drug review within the last 2 months are deemed ineligible.
Randomisation
In this study design, each prescribing hospital physician defines a cluster. Physicians are allocated 1:1 to either the intervention arm or the control arm, using a probabilistic minimisation method implemented by a web-based clinical trial management system (WebSpirit hosted by the Clinical Trials Unit (CTU) Bern). Minimisation is done according to country in order to ensure a balanced distribution of hospitals. The minimisation algorithm is implemented using randomisation lists generated by an independent statistician in Stata (StataCorp., Stata Statistical Software Version 14). Only system administrators who are otherwise not involved in the conduct of the trial have access to the randomisation lists, to ensure concealment of allocation.
Blinding procedures
This study is partially blinded, with blinding implemented at each site as follows (table 2):
Table 2.
Blinding status and measures to assure blinding
| Blinding status | How to achieve blinding | |
| Recruitment team (study nurse/research physician) | Blinded | Randomisation status will be kept concealed from the recruiting team, no access is given to unblinded study information in the database or locally to the source data. |
| Intervention team (physician, pharmacist) | Unblinded | In order to perform a safe intervention, including a shared decision-making with the patient, blinding is impossible. |
| Follow-up team (study nurse) | Blinded | Randomisation status will be kept concealed from the team conducting follow-up calls, no access is given to unblinded study information in the database or locally to the source data. In case an event or serious adverse event has occurred, the unblinded study team will be informed. |
| Unblinded | This team is informed about the treatment allocation. They collect the necessary information about events and anonymise revealing information about allocation on the documents for the adjudications. Make safety assessments. | |
| Adjudication (pharmacist/physician) | Blinded, work independently from study team | Receives only blinded information on hospital admission and deaths after study inclusion. |
| Patients | Partially blinded | Will be seen by the study team in case of intervention or control allocation. Control patients undergo a sham intervention using the ©MMAS-8.30–32 |
| Prescribing physician | Partially blinded | Will receive only high-level information about the trial. Every prescribing physician who defines a cluster will sign a disclosure form in order not to share information about the approach of the study team with their colleagues. |
| General practitioner | Partially blinded | Will receive only high-level information about the trial using an information flyer that does not inform about the two different study arms. The GP will receive a form about study inclusion for each patient (regardless of study allocation), and in the case of the intervention group, the GP will also receive the STRIPA report. |
©MMAS, Medication Adherence Measure Questionnaire; GP, general practitioner; STRIPA, STRIP Assistant.
Screening and enrolment. A person blinded to the allocation of recruiting clusters screens and enrols patients in order to avoid selection bias. Coded information (gender, age, multimorbidity, degree of polypharmacy and so on) from all screened patients is collected and regularly monitored centrally to assess the risk of selection bias. The blinded person works separately from the rest of the trial team at that site and all team members signed a non-disclosure form to limit unblinding of this person.
Patients. A tailored informed consent procedure is implemented in which patients are given a ‘high-level description’ of the study objectives with only superficial information on the study intervention, as accepted by the ethics committees. They are informed that their prescribing physician is allocated to one of the study groups without revealing which study group it is in order to minimise performance and other reporting biases during follow-up. Patients randomised to the control arm undergo an attention sham intervention (figure 1).
Prescribing physician. Similarly, the cluster-defining physician is not informed about his/her study arm allocation, receiving only a minimum amount of required information regarding the study objectives, as described above. Furthermore, each cluster-defining physician signs a non-disclosure contract to limit unblinding.
Figure 1.
Study flowchart (*planned numbers).
To communicate study inclusion and results of the medication review to the patients’ general practitioner (GP), all GPs receive the same standardised high-level information about the study goals and a document indicating that one of their patients has been included. For those patients in the intervention arm, the GPs additionally receive a report about the recommendations from the intervention (STRIPA report).
Follow-up. A blinded trial team member assesses the trigger events for the primary outcome and all secondary outcomes by telephone interview. In case of hospital readmission, an unblinded team member is informed by the blinded follow-up interviewer. The unblinded team member then collects the necessary documentation and information about the hospitalisation and ensures any clue about the treatment allocation is concealed before giving the documents to the adjudication board.
Adjudicators. Blinded independent adjudication boards composed of an experienced pharmacist and physician at each study site adjudicate the primary outcome (DRA) using a standardised chart review method.27
Intervention
An unblinded, independent trial team composed of a research physician and pharmacist conducts the intervention during the hospital stay of the study participant. All team members conducting the intervention underwent training prior to the beginning of the study. The study intervention is a structured method to perform pharmacotherapy optimisation called STRIP. The STRIP intervention consists of nine steps and is administered early during the index hospitalisation. In order to enable healthcare providers to incorporate STRIP into daily practice the so-called STRIPA has been developed, a stand-alone web application of the STRIP. It is a software-based tool for the support of the pharmaceutical analysis (step 2 of STRIP) by means of (1) taking into account the predictable adverse medication effects, (2) advising safe and appropriate therapy using established STOPP/START criteria,20 (3) monitoring clinically relevant interactions and (4) dosing appropriately in accordance with renal function. With STRIPA, the number of correct medical decisions during a medication review was significantly increased, whereas the number of inappropriate medication decisions was reduced.28 The nine steps of STRIP are as follows.
Structured history taking of medication using a questionnaire for taking the medication history based on the medication taken at home: Structured History taking of Medication use questionnaire.29
Recording of medications and diagnoses in the decision-support software with implemented STOPP/START criteria (STRIPA).
Structured medication review, including evaluation of STRIPA recommendations, by a qualified physician and pharmacist.
Generation of a report with specific recommendations for the prescribing hospital physician.
Communication and discussion of the structured drug review report with the prescribing physician, with possible adaptation of recommendations. The prescribing physician remains responsible for final decisions on drug therapy.
Shared decision-making with the patient to take into account patient preferences, again with possible adaptation of recommendations.
Revision based on new data acquired during hospitalisation (eg, new diagnoses, occurrence of ADRs).
Generation of a report with specific recommendations for the patient’s GP (STRIPA report).
Mail delivery of the report to the GP with optional, additional direct communication.
Control intervention (standard/routine/comparator)
Participants in the control group receive medication review by their prescribing physicians in accordance with usual practice at each site. A sham intervention is conducted using the Medication Adherence Measure Questionnaire (©MMAS-8).30–32 The ©MMAS-8 sham intervention is administered by the team members who are also conducting the intervention, to mimic the intervention. This helps to maintain the blinding status of the patients and blinded team members.
Follow-up
Follow-up and outcome data will be gathered via telephone interviews at 2, 6 and 12 months after the index hospitalisation. Included patients or their proxy persons will be interviewed by a blinded trial team member to assess the trigger events for the primary outcome and all secondary outcomes. If necessary, the treating physician of the included participant will also be contacted to complete the missing data.
Assessment of primary outcome
The primary patient-level outcome of this trial is the first DRA, that is, rehospitalisation within 1 year after enrolment. Rehospitalisations are detected during the follow-up telephone interviews by asking the patient or proxy person directly about any hospital stays since discharge from the index hospitalisation. For all patient-reported rehospitalisations occurring after the initial discharge, detailed documentation will be requested from the respective hospital.
An independent and blinded adjudication committee (per site) composed of one physician and one pharmacist adjudicates the drug relatedness of each hospital admission using a standardised adjudication guideline.27 For each patient, reported hospitalisations are adjudicated consecutively until the first DRA is confirmed by the adjudication committee. We only consider hospitalisations for adjudication that are preceded by discharge from the hospital where the patient was enrolled in the trial and where the patient is managed in hospital for longer than 24 hours (but not hospitalisations for a diagnostic or elective procedure for a pre-existing condition). The hospital admission is assessed for its relationship with the medication taken by the study participant prior to rehospitalisation. To assess the inter-rater reliability of our standardised adjudication guideline to identify DRA, a certain amount of common cases will be evaluated by adjudicators from all study sites.
Assessment of secondary outcomes
We assess secondary outcomes after enrolment (to generate a baseline) and during the follow-up telephone calls.
Secondary endpoints include number of any hospitalisations, all-cause mortality, number of falls, QoL, degree of polypharmacy, ADL, patient’s drug compliance, as well as the number of significant drug–drug interactions, drug overuse and underuse and potentially inappropriate medication.
All follow-up information is assessed via telephone call by a blinded trial team member at 2, 6 and 12 months of follow-up. In case the patient cannot be reached, the team member conducting the telephone interviews attempts to identify the patient’s survival status and collect the required information at any of the follow-up calls by contacting family members/proxies, responsible person from a nursing home (if applicable) or the patient’s GP.
The team member asks specifically about recent hospitalisations and healthcare utilisation (including unscheduled physician consultations and visits to the emergency department without hospital admission). Furthermore, the medication currently taken by the patient is recorded, as are adverse medical events including adverse drug events and ADRs as well as falls. If necessary (eg, if the patient does not remember his/her current medication), this information is obtained by contacting patients’ GPs, pharmacists, proxies or responsible persons from a nursing home. The degree of polypharmacy is defined as the number of regular chronic medications (<10 vs ≥10). QoL is assessed using the five dimensional EuroQOL (EQ-5D) instrument.33 ADL are assessed using the Barthel Index basic ADL questionnaire.34
Drug compliance is measured using the ©MMAS-8.30–32 The numbers of drug–drug interactions, drug overuse and underuse, as well as potentially inappropriate medication are assessed for the intervention group at 2 months, based on STRIPA information including the medical diagnoses from the index hospitalisation and the updated medication list from the 2-month telephone follow-up. Assessment of safety outcome will be based serious adverse events. In addition, all recorded device deficiencies will be described.
Data management
All data will be entered electronically using a dedicated electronic data capturing system (WebSpirit) hosted by CTU Bern. Original study forms will be entered and kept on file at each participating study site. Only system administrators will have direct access to the server. For quality control of the study conduct and data retrieval, all study sites will be visited on-site by appropriately trained and qualified monitors of CTU Bern. Data management and monitoring works independently from study investigators. All principal investigators will be given access to the cleaned datasets.
Study duration
We plan to recruit participants over a period of about 2 years. Once recruited, participants will be followed-up for 1 year.
Sample size
Sample size calculation was done using Stata Statistical Software and the clustersampsi command V.st0286 2.35 We estimated that the event rate of experiencing at least one DRA over a 12-month follow-up (primary endpoint) is ~20% in the control group.15 36 We assumed that patient deaths and drop-outs would be increasing during the follow-up phase and that the DRA event rate would slightly decrease over time. Combining these assumptions with an overall mortality of 20%37 and a drop-out rate of 6% both at 1 year, we estimated the event rate in the control group at 19.5%. We aimed to detect a 30% relative risk reduction by the intervention at a two-sided alpha of 5%, assuming an intracluster correlation of 0.02 as typically found for binary outcomes in elderly individuals.38
This translates into an expected DRA event rate of 13.7% in the intervention group. We also performed a survey at all trial sites to realistically estimate the number of available clusters. All sites responded and the overall number of clusters available for the trial was estimated at ~80. The same survey revealed that a cluster size of 25 participants would be realistic but that this might vary from cluster to cluster. We therefore allowed for variable cluster size with a coefficient of variation of 0.25 (effectively allowing for a cluster size ranging from 12 to 38 participants). Based on these assumptions, 2000 patients, 1000 patients in each arm, need to be recruited over 24 months in order to have 80% power in order to show a statistically significant difference for the primary endpoint.
Statistical analysis
All statistical analyses including a description of all relevant derivations of variables will be described in a statistical analysis plan before the end of recruitment and without inspection of the data. A statistician at CTU Bern will perform the statistical analyses using R Statistical Software.39
In the primary analysis, all patients will be analysed using the FAS (full analysis set) according to the intention-to-treat principle. The primary outcome, first confirmed DRA after discharge, will be analysed by using a mixed-effects survival model with a fixed effect for the intervention group and random-effects for centre and treating physician to account for clustering. To deal with competing risks (ie, death as competing risk for DRA), we will use extensions of the Fine-Gray proportional hazards model that account for clustering in competing risk settings.
Secondary time-to-event outcomes will be analysed using Kaplan-Meier curves and a mixed-effects Cox proportional hazards model with a fixed effect for the intervention group and random effects for centre and treating physician. Binary outcomes will be analysed using a mixed-effects logistic regression model using a fixed effect for the intervention group and random effects for centre and treating physician. Continuous outcomes will be analysed using mixed-effects linear model with a fixed effect for the intervention group and random effects for centre and treating physician, adjusted for the baseline value as a covariate if available.
To deal with dropouts and losses to follow-up (expected to be mainly caused by death), we will employ two different strategies: in the first approach, we will impute missing data. In the second approach, we will explore whether data allow for joint modelling of repeated measures and survival data.
In a secondary per-protocol (PP) analysis, all outcomes will be evaluated as described above based on the PP analysis set. Since cluster randomisation may lack the excellent balancing in characteristics between groups seen in individual-level randomisation, we will also adjust each model for additional patient-level, physician-level and hospital-level variables in a sensitivity analysis.
Ethics and dissemination
The local ethics committee at each site has approved the study protocol and other documentation concerning the study conduct. Where needed, approval by a regulatory authority has been obtained before enrolment of the first patient. All participants and their data are handled according to the ethical principles of the Declaration of Helsinki.40 This study complies with all applicable standards of the International Conference on Harmonisation E6 Guideline for Good Clinical Practice (ICH-GCP 1996) guideline.41 The ethics committees and regulatory authorities receive annual safety reports and will be informed about study stop/end in agreement with local requirements. OPERAM embraces an open access policy and strives for complete dissemination of all resulting data, clinical results and publications.
Patient and public involvement
A patient organisation was involved in the trial design and the development of the research question and the choice and measure of the outcomes (WHO Patients for Patient Safety Advocate Group; http://www.who.int/patientsafety/patients_for_patient/en/), as well as in the conduct of the study by being member of the scientific advisory board. Patients were actively involved in several steps of the process of developing the core outcome sets. Specifically, semistructured interviews with older patients and caregivers were undertaken in order to identify the most relevant outcomes for older individuals and stakeholders.42 To limit the burden of the intervention, special adaptations were planned for very old and sick patients (printable versions of the questionnaires, filling the questionnaire with an interviewer, priority lists to reduce the burden of the intervention) based on pilot patients and first patients enrolled, as well as specific processes for patients with of cognitive impairment, with the support of their relatives. Patients have no role in the recruitment of study participants, but are actively involved in the study intervention through shared decision-making. The results of this study will be disseminated to the patients through newsletters and through the above-mentioned patient organisation.
Discussion
This is the first large multicentre randomised trial that examines the impact of a structured approach to optimising pharmacotherapy in hospitalised multimorbid older individuals on clinical outcomes, including DRAs, inappropriate medication use and QoL. It will also be one of the largest trials in the growing population of multimorbid older adults that are currently understudied, as they are often excluded from trials due to their age and multimorbidity1; as a result, clinical guidelines are based on evidence that might not relate to patients with multimorbidity, underlining the need of this trial among elderly patients with multimorbidity.
The above described trial has several important strengths: it is a multicentre, large trial with broad inclusion criteria and very few exclusion criteria in order to provide good external validity. Patients with cognitive impairment will not be excluded, as this population is particularly prone to polypharmacy and its negative consequences and may particularly benefit from medication optimisation.43 The studied intervention is well defined with clear steps. The primary outcome (DRA) is adjudicated according to well defined standard operating procedures by an adjudication committee consisting of experienced physicians and pharmacists.
Limitations to this trial are the cluster randomised design, which was chosen to avoid a learning effect of the prescribing physicians allocated to the control group. However, this design is susceptible to selection bias, which we will try to avoid through measures summarised below. Finally, the outcome assessment at follow-up is based on the patient’s or proxy’s self-report and might therefore miss some events. In this cohort of multimorbid older patients, the death rate is expected to be high and we will have to account for death in our analyses.
Potential problems and solutions
To avoid possible selection bias, we aim to keep the study personnel as blinded as possible. Due to the nature of the intervention, complete blinding of all the study staff (eg, the staff conducting the intervention) is not possible. However, recruiting study staff and outcome assessors are kept blinded as to the randomisation status of patients, at all times. Study source data with unblinding potential are stored in a secure place without investigator access. Patients are kept partially blinded from the allocation of their prescribing physician (table 2). All patients included in the study control arm undergo a sham intervention in order to mimic the procedure of the intervention arm.
Outcome assessors are blinded concerning the patient’s study group allocation. Adjudication is carried out based on completely anonymised information about deaths and rehospitalisations acquired by the unblinded study personnel. Due to legal regulations and language issues (hospital reports and follow-up notes during hospitalisations are in the local language), a central data adjudication is not possible. However, we cross-reference a limited number of events in a central manner in order to avoid site-specific bias.
In order to avoid bias from cluster contamination, clusters are temporarily closed in case of absence of the prescribing physician. There is the potential of contamination at the level of a patient’s GP; however, we used partial blinding of GPs who receive only standardised high-level information about the study goals. Also, the number of GPs with patients in both the intervention and control group is expected to be small, given that the number of GPs referring patients to the enrolling leading university leading centres is very large.
To assess the potential for selection of a specific subpopulation into the trial and better understand generalisability of the study results, we will compare differences in selected characteristics such as age and gender of consenting and non-consenting patients.
Conclusion
The cluster-randomised OPERAM trial aims to add to provide direct evidence of best care for the growing population of older and multimorbid persons among the wider European population. The trial exclusively includes patients aged ≥70 years with multimorbidity for whom few direct comparisons of pharmacotherapy strategies exist. In the long term, we hope to contribute to an improved health status for the rapidly growing older population with multimorbidity and polypharmacy.
Current status of the OPERAM trial
The OPERAM trial started recruitment in December 2016. The trial follow-up will be completed in the 2nd semester of 2019.
Supplementary Material
Acknowledgments
Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A licence agreement is available from: Donald E Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA Fielding School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1772, dmorisky@ucla.edu.
Footnotes
Contributors: Study concept and design: NR, ST, DO, AS, WK, SB, OD, BB, PJü, PJ, PMK, MSc, DA, CB and KM-B. Acquisition, analysis or interpretation of data: LA, EM, ALL, SJ, CS, CF, NS, SH, CJAH, SS, MSc, AVD, OD, SB, WK, AS, DO, ST and NR. Drafting of the manuscript: LA, EM and MF. Critical revision of the manuscript for important intellectual content: LA, EM, CB, ALL, MF, KM-B, NS, SH, CS, SJ, CF, AL, KTJ, CJAH, SS, MSc, MSp, AVD, JD, PMK, PJü, DA, PJ, BB, OD, SB, WK, AS, DO, ST and NR. Statistical analysis: ST and AL. Obtained funding: NR. Administrative, technical or material support: NS, KTJ, MSp, AVD and SH. Supervision: NR, ST, DO, AS and WK.
Funding: This work is supported by the European Union’s Horizon 2020 research and innovation program under the grant agreement No. 6342388, and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: The trial protocol has been peer-reviewed for ethical and funding approval prior to submission.
Patient consent for publication: Obtained.
References
- 1. Jadad AR, To MJ, Emara M, et al. Consideration of multiple chronic diseases in randomized controlled trials. JAMA 2011;306:2670–2. 10.1001/jama.2011.1886 [DOI] [PubMed] [Google Scholar]
- 2. Menotti A, Mulder I, Nissinen A, et al. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: The FINE study (Finland, Italy, Netherlands, Elderly). J Clin Epidemiol 2001;54:680–6. [DOI] [PubMed] [Google Scholar]
- 3. Cassell A, Edwards D, Harshfield A, et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2018;68:e245–e251. 10.3399/bjgp18X695465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. A G. Making the case for ongoing care. 2010. http://www.rwjf.org/en/research-publications/find-rwjf-research/2010/02/chronic-care.html.
- 5. Aubert CE, Streit S, Da Costa BR, et al. Polypharmacy and specific comorbidities in university primary care settings. Eur J Intern Med 2016;35:35–42. 10.1016/j.ejim.2016.05.022 [DOI] [PubMed] [Google Scholar]
- 6. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–95. 10.1016/j.jclinepi.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 7. Kuijpers MA, van Marum RJ, Egberts AC, et al. Relationship between polypharmacy and underprescribing. Br J Clin Pharmacol 2008;65:130–3. 10.1111/j.1365-2125.2007.02961.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalleur O, Boland B, De Groot A, et al. Detection of potentially inappropriate prescribing in the very old: cross-sectional analysis of the data from the BELFRAIL observational cohort study. BMC Geriatr 2015;15:156 10.1186/s12877-015-0149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jin H, Tang C, Wei Q, et al. Age-related differences in factors associated with the underuse of recommended medications in acute coronary syndrome patients at least one year after hospital discharge. BMC Cardiovasc Disord 2014;14:127 10.1186/1471-2261-14-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med 2012;28:173–86. 10.1016/j.cger.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 11. Chan M, Nicklason F, Vial JH. Adverse drug events as a cause of hospital admission in the elderly. Intern Med J 2001;31:199–205. 10.1046/j.1445-5994.2001.00044.x [DOI] [PubMed] [Google Scholar]
- 12. Cooper JA, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open 2015;5:e009235 10.1136/bmjopen-2015-009235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leendertse AJ, Egberts AC, Stoker LJ, et al. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med 2008;168:1890–6. 10.1001/archinternmed.2008.3 [DOI] [PubMed] [Google Scholar]
- 14. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med 2009;169:894–900. 10.1001/archinternmed.2009.71 [DOI] [PubMed] [Google Scholar]
- 15. Howard RL, Avery AJ, Slavenburg S, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007;63:136–47. 10.1111/j.1365-2125.2006.02698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004;329:15–19. 10.1136/bmj.329.7456.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2014;10:CD008165 10.1002/14651858.CD008165.pub3 [DOI] [PubMed] [Google Scholar]
- 18. Alldred DP, Raynor DK, Hughes C, et al. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev 2013;2:CD009095. [DOI] [PubMed] [Google Scholar]
- 19. Gray SL, Hart LA, Perera S, et al. Meta-analysis of interventions to reduce adverse drug reactions in older adults. J Am Geriatr Soc 2018;66:282–8. 10.1111/jgs.15195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213–8. 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 2011;89:845–54. 10.1038/clpt.2011.44 [DOI] [PubMed] [Google Scholar]
- 22. O’Connor MN, O’Sullivan D, Gallagher PF, et al. Prevention of hospital-acquired adverse drug reactions in older people using screening tool of older persons' prescriptions and screening tool to alert to right treatment criteria: a cluster randomized controlled trial. J Am Geriatr Soc 2016;64:1558–66. 10.1111/jgs.14312 [DOI] [PubMed] [Google Scholar]
- 23. Drenth-van Maanen AC, Leendertse AJ, Jansen PAF, et al. The Systematic Tool to Reduce Inappropriate Prescribing (STRIP): Combining implicit and explicit prescribing tools to improve appropriate prescribing. J Eval Clin Pract 2018;24:317–22. 10.1111/jep.12787 [DOI] [PubMed] [Google Scholar]
- 24. Rankin A, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2018;9:CD008165 10.1002/14651858.CD008165.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006;166:565–71. 10.1001/archinte.166.5.565 [DOI] [PubMed] [Google Scholar]
- 26. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thevelin S, Spinewine A, Beuscart JB, et al. Development of a standardized chart review method to identify drug-related hospital admissions in older people. Br J Clin Pharmacol 2018;84:2600–14. 10.1111/bcp.13716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meulendijk MC, Spruit MR, Drenth-van Maanen AC, et al. Computerized decision support improves medication review effectiveness: an experiment evaluating the STRIP Assistant’s Usability. Drugs Aging 2015;32:495–503. 10.1007/s40266-015-0270-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drenth-van Maanen AC, Spee J, van Hensbergen L, et al. Structured history taking of medication use reveals iatrogenic harm due to discrepancies in medication histories in hospital and pharmacy records. J Am Geriatr Soc 2011;59:1976–7. 10.1111/j.1532-5415.2011.03610_11.x [DOI] [PubMed] [Google Scholar]
- 30. Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure for hypertension control. Journal of clinical hypertension 2008;10:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Krousel-Wood M, Islam T, Webber LS, et al. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 32. Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: Final response. J Clin Epidemiol 2011;64:262–3. 10.1016/j.jclinepi.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–108. 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- 34. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 35. Hemming K, Marsh J. A menu-driven facility for sample-size calculations in cluster randomized controlled trials. Stata J 2013;13:114–35. 10.1177/1536867X1301300109 [DOI] [Google Scholar]
- 36. Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012;10:142–51. 10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tal S, Guller V, Shavit Y, et al. Mortality predictors in hospitalized elderly patients. QJM 2011;104:933–8. 10.1093/qjmed/hcr093 [DOI] [PubMed] [Google Scholar]
- 38. Smeeth L, Ng ES, Es N. Intraclass correlation coefficients for cluster randomized trials in primary care: data from the MRC Trial of the Assessment and Management of Older People in the Community. Control Clin Trials 2002;23:409–21. [DOI] [PubMed] [Google Scholar]
- 39. Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 40. General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 2014;81:14–18. [PubMed] [Google Scholar]
- 41. ICo H. E6 Guideline for Good Clinical Practice. Iso En 1996;14155. [Google Scholar]
- 42. Beuscart JB, Dalleur O, Boland B, et al. Development of a core outcome set for medication review in older patients with multimorbidity and polypharmacy: a study protocol. Clin Interv Aging 2017;12:1379–89. 10.2147/CIA.S135481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monastero R, Palmer K, Qiu C, et al. Heterogeneity in risk factors for cognitive impairment, no dementia: population-based longitudinal study from the Kungsholmen Project. Am J Geriatr Psychiatry 2007;15:60–9. 10.1097/01.JGP.0000229667.98607.34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.