Abstract
Objectives
Whether unintended discontinuation of common, evidence-based, long-term medication occurs after hospitalisation; what factors are associated with unintended discontinuation; and whether the presence of documentation of medication at hospital discharge is associated with continuity of medication in general practice.
Design
Retrospective cohort study between 2012 and 2015.
Setting
Electronic records and hospital supplied discharge notifications in 44 Irish general practices.
Participants
20 488 patients aged 65 years or more prescribed long-term medication for chronic conditions.
Primary and secondary outcomes
Discontinuity of four evidence-based medication drug classes: antithrombotic, lipid-lowering, thyroid replacement drugs and respiratory inhalers in hospitalised versus non-hospitalised patients; patient and health system factors associated with discontinuity; impact of the presence of medication in the hospital discharge summary on continuity of medication in a patient’s general practitioner (GP) prescribing record at 6 months follow-up.
Results
In patients admitted to hospital, medication discontinuity ranged from 6%–11% in the 6 months posthospitalisation. Discontinuity of medication is significantly lower for hospitalised patients taking respiratory inhalers (adjusted OR (AOR) 0.63, 95% CI (0.49 to 0.80), p<0.001) and thyroid medications (AOR 0.62, 95% CI (0.40 to 0.96), p=0.03). There is no association between discontinuity of medication and hospitalisation for antithrombotics (AOR 0.95, 95% CI (0.81 to 1.11), p=0.49) or lipid lowering medications (AOR 0.92, 95% CI (0.78 to 1.08), p=0.29). Older patients and those who paid to see their GP were more likely to experience increased odds of discontinuity in all four medicine groups. Less than half (39% to 47.4%) of patients had medication listed on their hospital discharge summary. Presence of medication on hospital discharge summary is significantly associated with continuity of medication in the GP prescribing record for lipid lowering medications (AOR 1.64, 95% CI (1.15 to 2.36), p=0.01) and respiratory inhalers (AOR 2.97, 95% CI (1.68 to 5.25), p<0.01).
Conclusion
Discontinuity of evidence-based long-term medication is common. Increasing age and private medical care are independently associated with a higher risk of medication discontinuity. Hospitalisation is not associated with discontinuity but less than half of hospitalised patients have medication recorded on their hospital discharge summary.
Keywords: transitions of care, medication reconciliation, continuity of patient care, cohort study
Strengths and limitations of this study.
This study includes prescribing data from a diverse group of general practices that includes non-fee and fee-paying patients.
We examined the impact of hospitalisation on continuity of evidence-based, long term medication after discharge using a novel data collection technique accessing general practitioner prescribing records (as opposed to pharmacy dispensing records), codified chronic disease information and hospital provided discharge summary information.
We had no information on reasons for hospitalisation or therapeutic intent in terms of discontinuing medication.
We examined a limited number of medication groups and did not report on patient related-outcomes.
Introduction
Older patients are more likely to be prescribed multiple medications, have multiple chronic conditions and experience increasing number of transitions of care.1–3 Adherence to clinically appropriate, evidence-based therapies is important for lowering the risk of progression and complications related to their underlying chronic conditions.
Poor coordination of transitions of care is associated with adverse drug events, rehospitalisation and discrepancies in medication lists.4–9 Disruptions in medication continuity following hospitalisation have been reported.10–13 In particular, omission of medication with known benefit has been noted in prescribing errors at discharge.14–18 Previous studies have primarily examined large dispensing and/or administrative databases post hospitalisation to record the outcome of ‘discontinuity’.10–13 19 Hospitalisation giving rise to discontinuity may be attributable to prescribing errors at discharge (eg, omissions, communication issues), disruption in the prescribing process at the general practitioner (GP) level, failure or error in dispensing at the pharmacy level or the multitude of reasons for patient non-adherence. It is unclear where and why this discontinuity arises. There has been limited assessment of the immediate impact of hospitalisation on medication omission at hospital discharge which in turn, influences general practice repeat prescribing records.20–24
Aim and objectives
The aim of this study was to determine whether the potentially unintentional discontinuation of common, evidence-based medications for chronic diseases occurs after hospitalisation among older community dwelling adults. The medicine groups considered are: antithrombotics (antiplatelet or anticoagulants), lipid-lowering medications, thyroid medications and respiratory inhalers. These medications are commonly prescribed in older populations, have a strong evidence base in terms of efficacy and once started are usually recommended to be continued on a long-term basis. Furthermore, the continuity of these medications in prescribing and dispensing records has been the subject of study internationally—allowing for comparison of results.11 25–32
We compare discontinuity of medication for each of the four medicine groups listed above in the GP prescribing record over a 6-month period between patients who had been admitted to hospital and a group of patients who had not been admitted to hospital. Second, we examine whether other patient and health-system factors are associated with discontinuity of medication. A third objective is to assess whether documentation of prescribing of the specific medication in the hospital discharge summary record is associated with the presence of the same medication in the GP’s prescribing record in the following 6 months.
Methods
Study design
We conducted a retrospective cohort study, adhering to the Strengthening the Reporting of Observational Studies in Epidemiology statement.33 Anonymous data were gathered using the general practice patient management system which includes prescribing, demographic and clinical records and hospital supplied hospitalisation records.
Practice recruitment
A data extraction tool was developed with Socrates (providers of electronic health record (EHR) software to a majority of GP practices in Ireland). Following piloting of the extraction tool, a convenience sample of practices using Socrates EHR and receiving electronic hospital discharge communication (n=48) were invited to participate. Forty-four GP practices (response rate 91%) provided consent to take part in the study. Thirty practices were in the catchment area of the Dublin hospitals, with one in the North-East of Ireland. Eleven practices were in the catchment area of the Galway hospitals and two in the catchment area of the Cork hospitals. Participating GPs were awarded continuing professional development points for their participation.
Medication classes
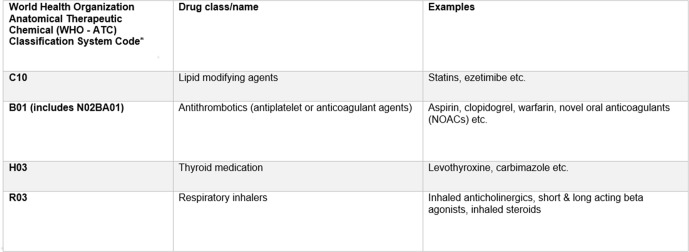
Four distinct patient cohorts were created based on the four medication classes: antithrombotics, lipid-lowering medications, thyroid medications and respiratory inhalers (figure 1). These medications are commonly prescribed in older populations and once commenced, are usually continued on a long-term basis.
Figure 1.
Medication classes. *Anatomical Therapeutic Chemical (ATC) code groupings were used to ensure all component drugs within a class were included (eg, prasugrel, tecagrelor, etc). This chapter refers to each cohort by the first three figures of the ATC group.
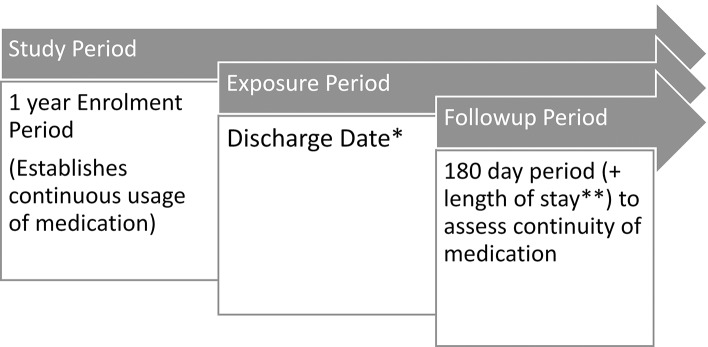
Study, enrolment and follow-up period criteria
The study period for each patient ranged from 1 January 2012 to the date when the data were extracted from the GP practice; this varied between practices, with the median time being 1 year and 180 days (figure 2). The study period included a 1-year enrolment period, and a 6-month follow-up period. The enrolment period for each medication class was the earliest 1-year period post 1 January 2012 over which a patient was continuously prescribed medication from that medication class. Continuously prescribed was defined as two prescriptions issued at least 5 months apart. No hospitalisations were allowed during the enrolment period to avoid misclassifying patients according to exposure. Patients could not be enrolled before 65 years of age and could be enrolled into more than one of the medication groups.
Figure 2.
Study enrolment and follow-up. *Discharge date was a random date applied to those not hospitalised. **Median length of stay of those hospitalised was added to those not hospitalised.
The start of the follow-up period, the period of time where discontinuity of medication was estimated, was marked by an index date. For patients who had been hospitalised, this was assigned as the day following discharge from hospital. For those individuals not experiencing hospitalisation, the index date was randomly assigned following the enrolment period. This method of generating a comparison group has been used previously and is in line with assuming the medications are long-term and unlikely to be discontinued.11
The follow-up period comprised a 6-month period following the index date. For patients who were readmitted to hospital during this 6-month period, the start of the follow-up period was reset until after the next discharge until a 6-month period free from further hospitalisation was established. For all hospitalised patients the 180-day follow-up period was extended to take account of their length of stay of the relevant admission (reflecting the possibility that patients may have supplies of long-term medication at home). A median length of stay for those hospitalised was added to the unexposed group follow-up period.
Patients who were categorised as deceased/inactive at the extraction date or who had no consultations after each follow-up period were excluded from the analyses. This avoided misclassifying a patient who may, for example, have died in hospital or was discharged to a long-term care facility and were not under the care of their previous GP.
Explanatory variables of interest
For the first two objectives, hospitalisation was the main explanatory variable of interest. The electronic messaging system Healthlink provided discharge messages in 41 practices to signal a hospitalisation (inpatient stay, not emergency department attendances). Hospitalisation was coded manually by research centre trained coders in four practices by examining the clinical records directly (one practice provided both Healthlink electronic discharge information and manually-coded discharge information). For the third objective, the main exposure variable was presence of medication in the hospital discharge summary note. This analysis was limited to hospitalised patients only. For all analyses, we examined whether patient and health-system variables might be associated with absence (primary analysis) or presence (secondary analysis) of medication in the GP prescribing—age, gender, public/private status, number of GP consultations, polypharmacy or multimorbidity.34–39 Medication burden was calculated using RxRisk.34–40 All covariates were measured during the enrolment period.
Outcomes
The primary outcome was discontinuity of medication (failure to renew medication) in one of the four, pre-specified medication classes in the GP record over the follow-up period. Changes within Anatomical Therapeutic Chemical (ATC) class were allowed (eg, between different brands of inhalers). For each medication class, discontinuity of medication was compared between those who had been hospitalised and those who had not. We calculated univariable associations across the four medication classes and adjusted for important confounders and other explanatory variables of interest. The secondary outcome was presence of relevant medication in the patient’s general practice prescribing record following discharge from hospital. Again, this was estimated for each medication cohort.
Sample size
The pilot phase and previous international studies in this area informed the calculation.11 12 Sample size calculation was based on 90% power to detect a 3% difference in the proportion of patients experiencing discontinuity. We assumed 11% of non-hospitalised patients have medications unintentionally discontinued. Additionally, a 4:1 ratio of non-hospitalised to hospitalised patients (based on experience from the pilot phase) with a statistical significance of 5% was used. This gave a total requirement of 8410 participants in any one medication cohort group.
Plan of analysis
The number of patients at each stage of the study is reported, including those potentially eligible for enrolment, those enrolled into each of the four cohorts and those available for analysis in the follow-up period. Reasons for removal are documented at each stage.
Descriptive statistics for the primary exposure (hospitalisation) and other explanatory variables are reported. For all statistical analyses, multilevel modelling was used to examine the association between each exposure and outcome of interest, adjusting for patient and health-system variables. In these models, individual patient, are nested within GP practices, giving rise to a (two level) multilevel model. Multilevel modelling allows for the fact that patients within any given practice could reasonably be expected to have more in common with each other than with those from a different practice—for instance in terms of prescriber patterns.
For the primary outcome, a multilevel logistic multivariate model was fitted to estimate the association between hospitalisation and discontinuity of medication for each medication class in turn, adjusted for patient and health system variables: age, gender, public/private status, Charlson score (comorbidity), number of repeat drug classes (polypharmacy) and number of enrolment period GP consultations. Results are reported as adjusted ORs (AOR) with 95% CI. These analyses were repeated using the number of hospital admissions (count variable) between the end of the enrolment period and the beginning of the follow-up period as the main exposure, in order to assess the impact of repeated hospital admissions on discontinuity of medication in the GP prescribing record.
For the secondary analyses, multilevel logistic multivariate regression was again used to examine, for each medication group, the association between prescribing of the specified medication at discharge from hospital and presence of the medication in the subsequent GP prescribing history over the next 6 months. Models were adjusted for the same patient and health-service variables listed above. Unadjusted analyses, examining the association between each explanatory variable and outcome in turn are reported for comparative purposes All analyses were performed using Stata V.14.41
Patient and public involvement
Patients were not involved in the conception, design or conduct of this research. We plan to disseminate the findings to the public and patients through our contacts in patient representative bodies, the popular media and through the participating general practices.
Results
Cohort flow
A total of 92 048 patients had their records extracted from the 44 recruited practices, of which 53 921 (58.6%) were removed immediately due to insufficient data (patients with sociodemographic data only, or who had no prescriptions or consultations with the GP after 1 January 2012) (figure 3). A further 11 871 patients were removed due to not being prescribed any medications from the four drug groups of interest or having less than 12 months of follow-up data available to enable enrolment. The enrolment criteria were applied to the 26 256 remaining patients, creating four cohorts—antithrombotics (ATC classification system, B01) (n=13 684), lipid-lowering medications (ATC C10) (n=14 427), thyroid medications (ATC H03) (n=3484) and respiratory inhalers (ATC R03) (n=5227). Out of the whole group of patients, 7896 (38.5%) were enrolled in one medicine group, 9184 (44.8%) in two groups, 3074 (15.0%) in three groups and 334 (1.6%) in all four groups.
Figure 3.
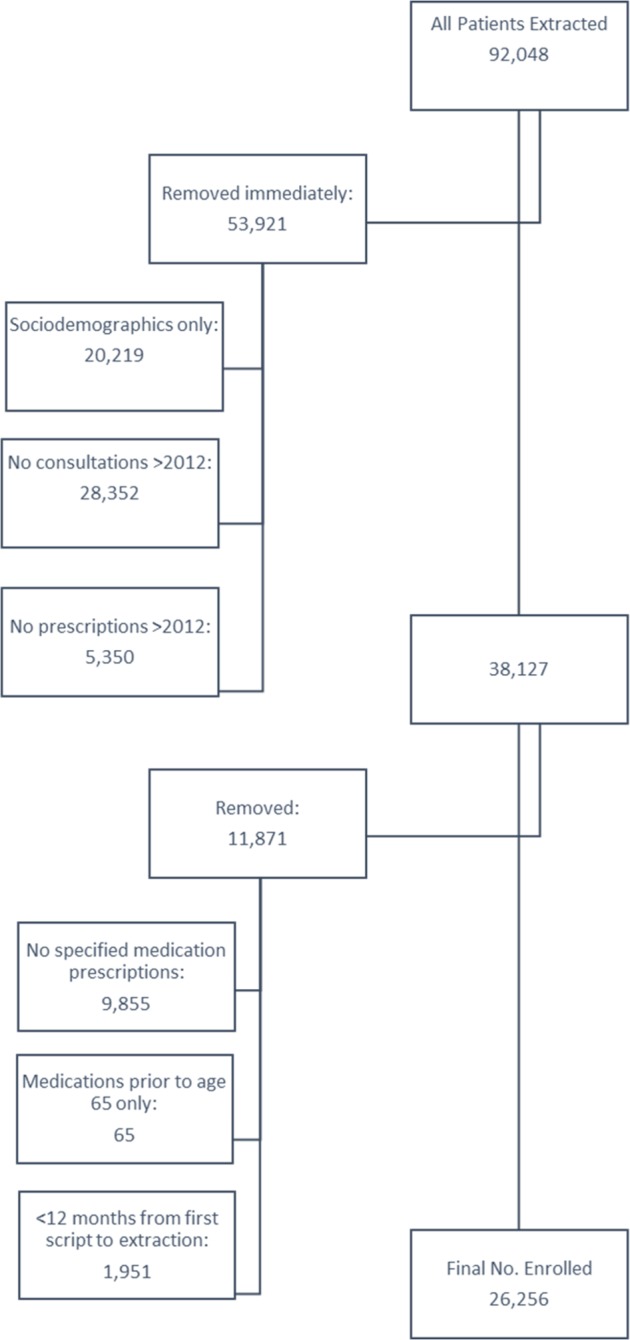
Participant flow chart.
Descriptive statistics
The demographics of the participants within the four cohorts of those available at the follow-up period are presented in table 1 (participant descriptives). Patients admitted to hospital tended to be slightly older, have more consultations with their GP and higher levels of polypharmacy and co-morbidity during the enrolment period than patients who remained out of hospital.
Table 1.
Descriptive statistics for participants in four evidence-based drug classes (ATC code)
| Medication Group (no patients enrolled) |
Antithrombotics (B01) (n=13 684) |
Lipid-lowering (C10) (n=14 427) |
Thyroid meds (H03) (n=3484) |
Respiratory inhalers (R03) (n=5227) |
||||
| No. patients at end of follow-up period | Hospitalised (n=2707) |
Non-hospitalised (n=6152) |
Hospitalised (n=2622) |
Non-hospitalised (n=6944) |
Hospitalised (n=586) |
Non-hospitalised (n=1641) |
Hospitalised (n=1067) |
Non-hospitalised (n=2110) |
| Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | |
| Age (years) | 78.38 (7.06) | 75.32 (6.95) | 77.05 (6.77) | 73.78 (6.45) | 78.34 (7.25) | 74.59 (7.18) | 76.88 (7.02) | 74.29 (6.90) |
| No of consultations in enrolment period | 18.28 (10.40) | 14.80 (9.66) | 17.50 (10.09) | 13.71 (8.79) | 18.76 (10.29) | 14.81 (9.10) | 19.64 (11.09) | 16.07 (10.57) |
| No of repeat drug classes during enrolment period | 8.04 (3.72) | 7.01 (3.45) | 7.77 (3.75) | 6.44 (3.41) | 8.59 (4.30) | 6.67 (3.87) | 9.26 (4.24) | 7.99 (4.13) |
| RxRisk during enrolment period | 5.07 (2.05) | 4.55 (1.89) | 4.99 (2.09) | 4.26 (1.97) | 5.37 (2.42) | 4.36 (2.09) | 4.79 (2.18) | 4.29 (2.12) |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Female | 1414 (52.23) | 3176 (51.63) | 1423 (54.27) | 3957 (56.98) | 468 (79.86) | 1349 (82.21) | 626 (58.67) | 1276 (60.47) |
| Insurance type: GMS/DVC | 2495 (92.17) | 5495 (89.32) | 2429 (92.64) | 6194 (89.20) | 537 (91.64) | 1445 (88.06) | 998 (93.53) | 1898 (89.95) |
| Charlson index of 1 or more | 1400 (51.72) | 2638 (42.88) | 1357 (51.75) | 2736 (39.40) | 290 (49.49) | 543 (33.09) | 690 (64.67) | 1120 (53.08) |
| Patients experiencing one hospitalisation only during first follow-up period | 2011 (74.29) | – | 1958 (74.68) | – | 457 (77.99) | – | 761 (71.32) | – |
| Patients discontinued during 1st follow-up period | 288 (10.64) | 693 (11.26) | 282 (10.76) | 727 (10.47) | 35 (5.97) | 139 (8.47) | 118 (11.06) | 359 (17.01) |
ATC, Anatomical Therapeutic Chemical classification system; DVC, doctor visit card; GMS, general medical services.
Among patients who were not hospitalised, the percentage of participants experiencing discontinuation of medication at follow-up ranged from 8.5% (thyroid medications) to 17.0% (respiratory inhalers); and from 5.9% (thyroid medications) to 11.1% (respiratory inhalers) in those who were hospitalised. Levels of discontinuity were higher among those who had not been hospitalised in three of the four drug classes that were examined (table 1).
Over two-thirds of patients did not experience a hospital admission during follow-up across the four medication groups (table 2). Of those admitted to hospital, the percentage of patients experiencing a single admission ranged between 20.4% and 23.9% across the four medication groups. A minority of patients experienced multiple medical admissions (table 2).
Table 2.
Number of hospital admissions following enrolment for patients assessed for medication discontinuity at follow-up
| Medication group (no patients enrolled) |
Antithrombotics (B01) (n=13 684) |
Lipid-lowering (C10) (n=14 427) |
Thyroid meds (H03) (n=3484) |
Respiratory inhalers (R03) (n=5227) |
| No patients at end of follow-up period | ||||
| 0 | 6152 (69.44%) | 6944 (72.59%) | 1641 (73.69%) | 2110 (66.41%) |
| 1 | 2011 (22.70%) | 1958 (20.45%) | 457 (20.52%) | 761 (23.95%) |
| 2 | 448 (5.06%) | 419 (4.38%) | 90 (4.04%) | 200 (6.30%) |
| 3 | 140 (1.58%) | 139 (1.45%) | 26 (1.17%) | 60 (1.89%) |
| 4 | 25 (0.28%) | 50 (5.23%) | 5 (0.23%) | 27 (0.85%) |
| 5 | 8 (0.09%) | 24 (0.25%) | 6 (0.27%) | 5 (0.16%) |
| 6 | 7 (0.08%) | 8 (0.09%) | 1 (0.04%) | 5 (0.16%) |
| >6 | 23 (0.26%) | 24 (0.25%) | 1 (0.04%) | 14 (0.44%) |
Univariable and multivariable associations
There is no difference in terms of likelihood of discontinuity for lipid-lowering and antithrombotic drugs between hospitalised and non-hospitalised patients. Hospitalisation is associated with less odds of discontinuity of long term medication on those prescribed thyroid medications and respiratory inhalers after adjustment for important confounders (table 3—analysis of primary outcome). For all four medication groups, older patients are more likely to experience discontinuity of medication than younger patients, with the odds of discontinuity increasing by between 3% and 6% per year (p<0.001). Private patients (those who paid for their own prescriptions and their GP visits out of pocket) have the strongest association with discontinuity across all four medicine groups with AOR varying between 3.75, (95% CI 2.84 to 4.96) for respiratory inhalers and 11.67, (95% CI 8.02 to 16.96) for thyroid medications (table 3). Number of consultations, multimorbidity, number of repeat medications and gender are not associated with an increased odds of discontinuity.
Table 3.
Univariable and multivariable associations in four evidence-based drug classes (ATC code)
| Antithrombotics (B01) | Lipid-lowering (C10) | Thyroid meds (H03) | Respiratory inhalers (R03) | |||||
| Unadjusted OR (95% CI, p value) | Adjusted OR (95% CI, p value) | Unadjusted OR (95% CI, p value) | Adjusted OR (95% CI, p value) | Unadjusted OR (95% CI, p value) | Adjusted OR (95% CI, p value) | Unadjusted OR (95% CI, p value) | Adjusted OR (95% CI, p value) | |
| Hospitalised vs non-hospitalised | 0.95 (0.82 to 1.10), p=0.49 | 0.95 (0.81 to 1.11), p=0.49 | 1.04 (0.89 to 1.20), p=0.64 | 0.92 (0.78 to 1.08), p=0.29 | 0.68 (0.46 to 1.00), p=0.05 | 0.62 (0.40 to 0.96), p=0.03 | 0.62 (0.49 to 0.78), p=0.001 | 0.63 (0.49 to 0.80), p<0.001 |
| Age (years) | 1.02 (1.01 to 1.03), p<0.001 | 1.03 (1.02 to 1.04), p<0.001 | 1.04 (1.03 to 1.05), p<0.001 | 1.05 (1.04 to 1.06), p<0.001 |
1.03 (1.01 to 1.05), p=0.002 | 1.06 (1.04 to 1.09), p<0.001 | 1.02 (1.01 to 1.03), p=0.004 | 1.04 (1.02 to 1.05), p<0.001 |
| Gender Female vs male |
1.02 (0.89 to 1.17), p=0.79 | 1.00 (0.87 to 1.15), p=0.99 | 0.85 (0.74 to 0.96), p=0.01 | 0.82 (0.72 to 0.95), p=0.01 | 0.84 (0.57 to 1.24), p=0.38 | 0.85 (0.56 to 1.30), p=0.46 | 1.04 (0.85 to 1.28), p=0.68 | 1.03 (0.83 to 1.27), p=0.79 |
| Insurance type Private vs GMS/DVC patients | 5.10 (4.31 to 6.04), p<0.001 | 5.35 (4.50 to 6.34), p<0.001 | 4.78 (4.06 to 5.62), p<0.001 | 5.68 (4.48 to 6.73), p<0.001 | 9.79 (6.90 to 13.89), p<0.001 | 11.67 (8.02 to 16.96), p<0.001 | 3.66 (2.78 to 4.82), p<0.001 | 3.75 (2.84 to 4.96), p<0.001 |
| Number of repeat drug classes | 0.99 (0.98 to 1.01), p=0.56 | 0.99 (0.97 to 1.01), p=0.28 | 1.01 (1.00 to 1.04), p=0.04 | 1.01 (0.99 to 1.04), p=0.24 | 0.98 (0.95 to 1.02), p=0.41 | 0.98 (0.94 to 1.03), p=0.44 | 0.97 (0.94 to 0.99), p=0.01 | 0.97 (0.94 to 0.99), p=0.02 |
| Charlson score (≥1 vs 0) |
0.93 (0.80 to 1.07), p=0.31 | 0.94 (0.80 to 1.09), p=0.41 | 1.05 (0.91 to 1.21), p=0.48 | 0.98 (0.84 to 1.14), p=0.78 | 0.78 (0.56 to 1.08), p=0.15 | 0.80 (0.54 to 1.15), p=0.22 | 0.66 (0.53 to 0.81), p<0.001 | 0.71 (0.58 to 0.88), p=0.002 |
| No of consultations in enrolment period | 1.00 (0.99 to 1.01), p=0.62 | 1.00 (0.99 to 1.01), p=0.63 | 1.00 (0.99 to 1.01), p=0.69 | 1.00 (0.99 to 1.01), p=0.75 | 0.99 (0.97 to 1.00), p=0.11 | 1.00 (0.98 to 1.02), p=0.83 | 0.99 (0.98 to 1.00), p=0.02 | 1.00 (0.99 to 1.01), p=0.72 |
Adjusted model includes gender, age, insurance type, Charlson Index, number of repeat drugs, number of consultations in the enrolment period.
ATC, Anatomical Therapeutic Chemical classification system; GMS, general medical services; DVC, doctor visit card.
In a sub-group analysis of the antithrombotics (B01) category, we found that antiplatelets were independently associated with increased discontinuation after hospitalisation (AOR 1.30, 95 % CI 1.12, 1.52), while for warfarin and new oral anticoagulants (NOACs), no association between hospitalisation and discontinuation was observed (AOR 0.97, 95% CI 0.68 to 1.39). For both antiplatelets and NOACs, older age and private patients were independently associated with discontinuation (online supplementary table 1).
bmjopen-2018-024747supp001.pdf (117.7KB, pdf)
Repeated hospital admissions
To assess the impact of repeated hospital admissions, models were re-estimated with the hospital exposure defined as the number of hospital admissions (count) between the end of the enrolment period and the beginning of the follow-up period. For antithrombotics, lipid-lowering medications and thyroid medications, there was no evidence of a statistically significant association between the number of admissions to hospital and discontinuity of medication in the 6-month follow-up period. However, for respiratory inhalers, the odds of discontinuity of medication fell by an estimated 13% per additional admission to hospital after adjusting for confounders (AOR 0.87, (95% CI 0.76 to 0.99), p=0.03). For further details see online supplementary table 2 (Repeated admissions analysis).
bmjopen-2018-024747supp002.pdf (81.6KB, pdf)
Impact of medication specified in patient’s hospital discharge summary
Recording of medication on the hospital discharge summary was relatively poor, with only 39.2% to 47.4% of patients having the relevant medication group documented across the four medication groups. Medication recording had improved at 6 months postdischarge, being present in 89.2% to 94.7% of patient’s GP clinical records across medication groups (table 4—documentation of medication at discharge and in the GP record). Having medication listed on hospital discharge summary was independently associated with medication being present on the GP record as 6 months follow-up for both lipid-lowering drugs and respiratory inhalers. Private patients were significantly less likely to have the relevant medication in their GP prescribing record in the 6-month period following discharge from hospital than public patients (table 5—analysis of secondary outcome).
Table 4.
Cross-tabulation of patients by presence of medication on hospital discharge summary and in the GP prescribing record at 6 months following hospitalisation
| Medication group | GP record | GP record | GP record | GP record | |||||
| Antithrombotics (B01) (n=1991)* |
Lipid-lowering (C10) (n=1954) * |
Thyroid meds (H03) (n=456) * |
Respiratory inhalers (R03) (n=757) * |
||||||
| Absent | Present | Absent | Present | Absent | Present | Absent | Present | ||
| Hospital discharge | Absent | 113 (10.55%) | 958 (89.45%) | 123 (10.35%) | 1065 (89.65%) | 16 (6.67%) | 224 (93.33%) | 65 (14.19%) | 393 (85.81%) |
| Hospital discharge | Present | 78 (8.48%) | 842 (91.52%) | 63 (8.22%0 | 703 (91.78%) | 8 (3.70%) | 208 (96.30%) | 17 (5.69%) | 282 (94.31%) |
*Patients with medication discontinued at hospital discharge excluded.
GP, general practitioner.
Table 5.
Multivariable association of required medication appearing in GP clinical record following discharge from hospital
| Antithrombotics (B01) (n=1991)* |
Lipid-lowering (C10) (n=1954)* |
Thyroid meds (H03) (n=456)* |
Respiratory inhalers (R03) (n=757)* |
|||||
| Unadjusted OR (95% CI, p value) | Adjusted OR (95% CI, p value) | Unadjusted OR (95% CI, p value) | Adjusted OR (95% CI, p value) | Unadjusted OR (95% CI, p value) | Adjusted OR (95% CI, p value) | Unadjusted OR (95% CI, p value) | Adjusted OR (95% CI, p value) | |
| Medication listed on discharge summary | 1.29 (0.95 to 1.76), p=0.11 | 1.34 (0.97 to 1.87), p=0.08 | 1.40 (0.99 to 1.97), p=0.06 | 1.64 (1.15 to 2.36), p=0.01 | 1.86 (0.77 to 4.43), p=0.16 | 1.76 (0.70 to 4.42), p=0.23 | 2.74 (1.57 to 4.78), p<0.001 | 2.97 (1.68 to 5.25), p<0.001 |
| Age (years) | 0.98 (0.96 to 1.00), p=0.03 | 0.98 (0.96 to 1.00), p=0.08 | 0.96 (0.94 to 0.98), p<0.001 | 0.95 (0.93 to 0.98), p<0.001 | 0.96 (0.91 to 1.02), p=0.16 | 0.96 (0.91 to 1.02), p=0.16 | 0.97 (0.94 to 1.01), p=0.12 | 0.96 (0.93 to 1.00), p=0.03 |
| Female vs male | 1.02 (0.76 to 1.38), p=0.90 | 0.97 (0.70 to 1.33), p=0.84 |
1.14 (0.84 to 1.56), p=0.39 | 1.15 (0.83 to 1.59), p=0.41 | 1.34 (0.52 to 3.49), p=0.54 | 1.35 (0.49 to 3.73), p=0.57 | 0.93 (0.58 to 1.50), p=0.77 | 0.87 (0.53 to 1.43), p=0.59 |
| Insurance type Private vs GMS/DVC patients | 0.18 (0.13 to 0.26), p<0.001 | 0.18 (0.12 to 0.27), p<0.001 | 0.19 (0.12 to 0.28), p<0.001 | 0.17 (0.11 to 0.27), p<0.001 | 0.10 (0.04 to 0.26), p<0.001 | 0.10 (0.04 to 0.26), p<0.001 | 0.26 (0.14 to 0.50), p<0.001 | 0.26 (0.13 to 0.49), p<0.001 |
| Number of repeat drug classes | 1.04 (1.00 to 1.09), p=0.06 | 1.04 (0.99 to 1.09), p=0.11 | 0.99 (0.94 to 1.03), p=0.49 | 1.00 (0.96 to 1.06), p=0.86 | 1.06 (0.95 to 1.18), p=0.30 | 1.10 (0.96 to 1.26), p=0.18 | 1.07 (10.01 to 1.13), p=0.03 | 1.08 (1.00 to 1.15), p=0.06 |
| Charlson score (≥1 vs 0) |
1.14 (0.84 to 1.54), p=0.40 | 1.08 (0.79 to 1.49), p=0.63 | 0.76 (0.55 to 1.04), p=0.09 | 0.79 (0.56 to 1.11) p=0.18 | 1.06 (0.46 to 2.40), p=0.90 | 0.82 (0.33 to 2.03), p=0.67 | 0.98 (0.61 to 1.58), p=0.94 | 0.86 (0.52 to 1.45), p=0.55 |
| No of consultations in enrolment period | 1.01 (0.99 to 1.03), p=0.19 | 1.00 (0.99 to 1.02), p=0.74 | 0.99 (0.97 to 1.01), p=0.22 | 0.99 (0.97 to 1.01), p=0.16 | 1.01 (0.97 to 1.06), p=0.63 | 0.99 (0.94 to 1.04), p=0.63 | 1.02 (1.00 to 1.05), p=0.07 | 1.02 (0.98 to 1.04), p=0.41 |
Adjusted model includes gender, age, insurance type, Charlson Index, number of repeat drugs, number of consultations in the enrolment period.
DVC, doctor visit card; GMS, general medical services; GP, general practitioner.
Discussion
Principal findings
Discontinuation of medication in patients who had been recently hospitalised ranged from 6% to 11% for commonly prescribed, evidence-based medicines, compared with 5%–17% for non-hospitalised patients. Patients prescribed thyroid medications and respiratory inhalers, who experienced hospitalisation, actually had a lower risk of discontinuity. Public or private care played a significant role in the likelihood of medication being discontinued with the odds of discontinuation significantly higher for private patients than non-private patients in all medication groups. Increasing age is independently associated with an increased odds of discontinuation of medication. Lastly, recording of mediation on hospital discharge summaries is incomplete, being present in less than 50% of discharged patients for all four medication groups. Presence of medication on hospital discharge summaries is associated with continuity on the GP prescribing record at 6 months for lipid lowering medication and respiratory inhalers.
Previous research
Findings from this observational study differs from similar studies in the USA, both in the magnitude of discontinuation: reported to be between 12% and 19% for thyroid and antithrombotic medications; and in terms of the impact of hospitalisation, with hospitalisation being independently associated with discontinuation, when assessed using pharmacy dispensing data.8–10 41 The impact of hospitalisation appears to be context and health system-specific, with some studies not finding a relationship between discontinuity and hospitalisation.42–44 We found that increased number of medications was not associated with discontinuation; in the respiratory inhalers group patients were less likely to be discontinued if they had increased numbers of medications.34 37–39 45–47 Like other studies we found that increasing age was independently associated with an increased discontinuity post discharge.19
A particularly interesting finding in our study is the marked difference between publicly funded and privately funded patients. Private patients were found to have a consistent pattern of discontinuity independent of other patient and health system factors (table 3). Similarly, in hospitalised patients, being a private patient was associated with discontinuity of medication recording in their GP record and significantly more likely at 6 months follow-up. There are possible explanations for this finding. Private patients are not required to have their hospital discharge prescription transcribed by their GP and may proceed directly to the pharmacy, thereby appearing as if their medication has been discontinued by our method of outcome calculation. Nevertheless, lack of continuity in the GP record raises concerns about completeness of the information a GP in relation to a patient’s medication file, monitoring requirements, potential drug-to-drug interactions and other potential prescribing errors.
In keeping with findings from other studies, the quality of prescribing information contained in hospital discharge summaries was incomplete for over half of discharged patients, with the omission of essential medications common.18 35 Furthermore, lack of medication reconciliation on hospital discharge appeared to persist for at least 6 months in general practice medication records.21 The hospital discharge summary used to determine discharge medication in this study is only one element of the information normally provided to patients at discharge from hospital. A supplementary discharge prescription may also be provided.35 Therefore, a discrepancy may arise between the hospital discharge summary and additional discharge prescription, as hospital doctors make judgements about what to include/exclude from discharge prescriptions.48 These parallel methods of providing post-discharge medication information is a cause for concern and likely enhance risks of medication discontinuity.
While lack of medication reconciliation following hospital discharge may be one possible explanation for the reported discontinuity, there are other possible explanations, most commonly poor patient adherence. A recent UK study of statin adherence reported discontinuation rates of 27% at 1 year in those prescribed statins. Notably this was examining primary non-adherence (failure to fill an initial prescription) as distinct from what may be secondary non-adherence (inadequate medication possession over a defined period of time) in this cohort).49 50 The factors that influence adherence may be patient, therapy, physician or health system related.51 While this study was able to control for some of these factors (demographics, comorbidities, public/private care status) others were not recorded (socioeconomic status, side-effects, individual physician behaviour and access to healthcare).
Lastly, inadequate adherence (and the related terms non-compliance and non-concordance) may take many forms, for example, non-filling of prescriptions, altering doses, stopping/starting. This study reported a varying discontinuity rate across the four drug classes (lower in antithrombotics and higher in respiratory inhalers). The variation between medication classes observed here may be explained by disease-specific issues (eg, altering doses of thyroxine replacement due to undulating severity of disease meaning repeat prescriptions are not required; asymptomatic asthma patients not needing to take bronchodilator inhalers), evolving or clinical considerations such as the changing risk benefit profile of an antithrombotic in a patient with a high risk of falls.52
Strengths and limitations of study
This is the largest Irish study to date to examine the effect of hospitalisation on the continuity of evidence-based medication in the GP prescribing record. It is also the first study to systematically use GP prescribing records (as opposed to pharmacy dispensing records) and includes details of both private and public patients, unique features of the mixed public/private health system in Ireland. The recruitment of GP practices was not limited to one geographically area/hospital catchment and the inclusion of multiple hospitals allowed comparison of messaging standards and their impact on prescribing continuity, enhancing the generalisability of the findings.
There are several limitations to this study. The medication groups were specifically chosen to be evidence-based and long-term in their usage and the establishment of an enrolment period of continuous usage over 1 year further ensures the pattern of ongoing use. However, the primary outcome of discontinuation of medication was applied to a prescribing database and does not contain information about indication or therapeutic intent, for example intentional discontinuation of statins in end-of-life patients. In addition, the nuances between different medications (eg, warfarin and aspirin) is lost by grouping in larger ATC classes. Differential discontinuation within the antithrombotic (B01) class of drugs was observed in a sub-group analysis, with antiplatelet discontinuation associated with hospitalisation, while for NOACs hospitalisation was not associated with discontinuation. These findings need to be treated with caution, as they were not pre-specified and the magnitude of association with antiplatelets is relatively modest.
The nature of data collection and the dataset itself also incur limitations. Hand-written prescriptions were not captured by this data collection technique. The follow-up of participants from enrolment through to outcome calculation also required assumptions to be made in preparing the data for analysis. However, the methods have been used previously, and are in line with the underlying assumption that there should be no difference between groups with both having 100% persistence of the medication in the GP record. These findings reflect the Irish healthcare system and may not be applicable in other systems with greater or lesser usage of electronic communication between primary/secondary care or developed reconciliation systems. Lastly, the recording of hospitalisation is likely to be variable within practices, with the Healthlink service employed differently by hospitals with the possibility of misclassification of exposed individuals. These methodological and data issues were explored in the sensitivity analysis with no change in the overall findings.
Clinical and healthcare policy implications
Medication reconciliation, the process of creating the most accurate list of medications at transition points, has been advocated by a number of different professional and accrediting bodies internationally. Ensuring the accuracy of medication information at transitions is reliant on good communication. The quality of electronic discharge communication received by general practices and the possible association with inappropriate discontinuation of evidence-based medication suggests more emphasis needs to be placed on improving the quality of discharge communication. The health service executive’s ePrescribing initiative and eScript pilot projects are efforts to improve the transfer of medication information.53 54
Future efforts should focus on identifying high-risk individuals who are receiving medications that would be the best targets for reconciliation studies and interventions. Recent efforts have been made to develop a consensus about high risk medications and methods of assessing the potential severity of medication omission.55
Conclusions
Discontinuity of evidence-based long-term medication is common. Increasing age and private medical care are independently associated with a higher risk of medication discontinuity. Hospitalisation was not associated with discontinuity but less than half of hospitalised patients had medication recorded on their hospital discharge summary. System based solutions that include ePrescribing are needed to enhance the transfer of medication information across the primary/secondary care interface.
Supplementary Material
Acknowledgments
We would like to thank Kevin O’Flannagan, Paul Dillon, Atieh Zarabzadeh, Khalid Munir, Oludare Alabi and Ronan McDonnell for their help in cleaning and categorising prescribing data. Healthlink, IPCRN and Socrates in design and data collection. We would also like to acknowledge the GP practices who participated in this study.
Footnotes
Contributors: PR initiated the project, designed data collection tools, monitored data collection, wrote the statistical analysis plan, cleaned and analysed the data, and drafted and revised the paper. RMcDowell wrote the statistical analysis plan, cleaned and analysed the data and revised the paper. TCG designed the data collection tools, wrote the statistical analysis plan and revised the paper. FB designed the data collection tools, wrote the statistical analysis plan and revised the paper. RMcDonnell designed the data collection tools and revised the paper. CH initiated the project, advised on the statistical analysis plan and revised the paper. TF initiated the project, monitored data collection, advised on the analysis plan and revised the paper, and is the guarantor.
Funding: This study was funded by the Health Research Board of Ireland, HRC-2014-1. This work was conducted as part of the HRB Scholar Programme in Health Services Research under Grant No. PHD/2007/16.
Competing interests: None declared.
Ethics approval: Ethical approval was granted from the Irish College of General Practitioners’ Research Ethics Committee. GPs as individual practice data controllers gave informed consent to participate.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data is available. A data sharing provision was not included in the application to the research ethics committee for approval of this study.
References
- 1. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 2. Moriarty F, Hardy C, Bennett K, et al. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross-sectional study. BMJ Open 2015;5:e008656 10.1136/bmjopen-2015-008656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coleman EA, Smith JD, Raha D, et al. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005;165:1842–7. 10.1001/archinte.165.16.1842 [DOI] [PubMed] [Google Scholar]
- 4. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003;18:646–51. 10.1046/j.1525-1497.2003.20722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Linden CM, Kerskes MC, Bijl AM, et al. Represcription after adverse drug reaction in the elderly: a descriptive study. Arch Intern Med 2006;166:1666–7. 10.1001/archinte.166.15.1666 [DOI] [PubMed] [Google Scholar]
- 6. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc 2003;51:549–55. 10.1046/j.1532-5415.2003.51185.x [DOI] [PubMed] [Google Scholar]
- 7. Hammad EA, Wright DJ, Walton C, et al. Adherence to UK national guidance for discharge information: an audit in primary care. Br J Clin Pharmacol 2014;78:1453–64. 10.1111/bcp.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boockvar KS, Liu S, Goldstein N, et al. Prescribing discrepancies likely to cause adverse drug events after patient transfer. Qual Saf Health Care 2009;18:32–6. 10.1136/qshc.2007.025957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boockvar K, Fishman E, Kyriacou CK, et al. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med 2004;164:545–50. 10.1001/archinte.164.5.545 [DOI] [PubMed] [Google Scholar]
- 10. Grimmsmann T, Schwabe U, Himmel W. The influence of hospitalisation on drug prescription in primary care--a large-scale follow-up study. Eur J Clin Pharmacol 2007;63:783–90. 10.1007/s00228-007-0325-1 [DOI] [PubMed] [Google Scholar]
- 11. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA 2011;306:840–7. 10.1001/jama.2011.1206 [DOI] [PubMed] [Google Scholar]
- 12. Stall NM, Fischer HD, Wu CF, Cf W, et al. Unintentional Discontinuation of Chronic Medications for Seniors in Nursing Homes: Evaluation of a National Medication Reconciliation Accreditation Requirement Using a Population-Based Cohort Study. Medicine 2015;94:e899 10.1097/MD.0000000000000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bell CM, Bajcar J, Bierman AS, et al. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med 2006;166:2525–31. 10.1001/archinte.166.22.2525 [DOI] [PubMed] [Google Scholar]
- 14. Latimer SL, Chaboyer W, Hall T. Non-Therapeutic Medication Omissions: Incidence and Predictors at an Australian Hospital. Journal of Pharmacy Practice and Research 2011;41:188–91. 10.1002/j.2055-2335.2011.tb00859.x [DOI] [Google Scholar]
- 15. Perren A, Previsdomini M, Cerutti B, et al. Omitted and unjustified medications in the discharge summary. Qual Saf Health Care 2009;18:205–8. 10.1136/qshc.2007.024588 [DOI] [PubMed] [Google Scholar]
- 16. Belda-Rustarazo S, Cantero-Hinojosa J, Salmeron-García A, et al. Medication reconciliation at admission and discharge: an analysis of prevalence and associated risk factors. Int J Clin Pract 2015;69:1268–74. 10.1111/ijcp.12701 [DOI] [PubMed] [Google Scholar]
- 17. Elliott RA, Tran T, Taylor SE, et al. Gaps in continuity of medication management during the transition from hospital to residential care: an observational study (MedGap Study). Australas J Ageing 2012;31:247–54. 10.1111/j.1741-6612.2011.00586.x [DOI] [PubMed] [Google Scholar]
- 18. Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother 2008;42:1373–9. 10.1345/aph.1L190 [DOI] [PubMed] [Google Scholar]
- 19. Stuffken R, Heerdink ER, de Koning FH, et al. Association between hospitalization and discontinuity of medication therapy used in the community setting in the Netherlands. Ann Pharmacother 2008;42:933–9. 10.1345/aph.1L062 [DOI] [PubMed] [Google Scholar]
- 20. Cochrane RA, Mandal AR, Ledger-Scott M, et al. Changes in drug treatment after discharge from hospital in geriatric patients. BMJ 1992;305:694–6. 10.1136/bmj.305.6855.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Riordan C, Grimes T. Medication reconciliation on discharge to primary care following an acute hospital admission. Int J Clin Pharm 2014;36:836. [Google Scholar]
- 22. Mansur N, Weiss A, Hoffman A, et al. Continuity and adherence to long-term drug treatment by geriatric patients after hospital discharge: a prospective cohort study. Drugs Aging 2008;25:861–70. 10.2165/00002512-200825100-00005 [DOI] [PubMed] [Google Scholar]
- 23. Viktil KK, Blix HS, Eek AK, et al. How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open 2012;2:e001461 10.1136/bmjopen-2012-001461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hammad E, Cadman B, Bale A, et al. Medication errors: Do they persist in primary care and can they be identified? Royal Pharmaceutical Society (RPS) Annual Conference. Birminghan, UK. [Google Scholar]
- 25. Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA 1998;279:1458–62. [DOI] [PubMed] [Google Scholar]
- 26. Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–61. 10.1001/jama.288.4.455 [DOI] [PubMed] [Google Scholar]
- 27. Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy 2008;28:437–43. 10.1592/phco.28.4.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ganz DA, Glynn RJ, Mogun H, et al. Adherence to guidelines for oral anticoagulation after venous thrombosis and pulmonary embolism. J Gen Intern Med 2000;15:776–81. 10.1046/j.1525-1497.2000.91022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hart RG, Halperin JL, Pearce LA, et al. Lessons from the Stroke Prevention in Atrial Fibrillation trials. Ann Intern Med 2003;138:831–8. 10.7326/0003-4819-138-10-200305200-00011 [DOI] [PubMed] [Google Scholar]
- 30. Izquierdo JL, Paredero JM, Piedra R. Relevance of dosage in adherence to treatment with long-acting anticholinergics in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016;11:289–93. 10.2147/COPD.S96948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Clinical Guideline Centre(UK). Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. Guidance: National Institute for Health and Clinical Excellence, 2014. [PubMed] [Google Scholar]
- 32. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease-2003. Can Respir J 2003;10 Suppl A:11A–65. 10.1155/2003/567598 [DOI] [PubMed] [Google Scholar]
- 33. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med 2007;147:573–8. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 34. Hu SH, Capezuti E, Foust JB, et al. Medication discrepancy and potentially inappropriate medication in older Chinese-American home-care patients after hospital discharge. Am J Geriatr Pharmacother 2012;10:284–95. 10.1016/j.amjopharm.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 35. Grimes TC, Duggan CA, Delaney TP, et al. Medication details documented on hospital discharge: cross-sectional observational study of factors associated with medication non-reconciliation. Br J Clin Pharmacol 2011;71:449–57. 10.1111/j.1365-2125.2010.03834.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feldman LS, Costa LL, Feroli ER, et al. Nurse-pharmacist collaboration on medication reconciliation prevents potential harm. J Hosp Med 2012;7:396–401. 10.1002/jhm.1921 [DOI] [PubMed] [Google Scholar]
- 37. Cornu P, Steurbaut S, Leysen T, et al. Discrepancies in medication information for the primary care physician and the geriatric patient at discharge. Ann Pharmacother 2012;46(7-8):983–91. 10.1345/aph.1R022 [DOI] [PubMed] [Google Scholar]
- 38. Stitt DM, Elliott DP, Thompson SN. Medication discrepancies identified at time of hospital discharge in a geriatric population. Am J Geriatr Pharmacother 2011;9:234–40. 10.1016/j.amjopharm.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 39. Hellström LM, Bondesson Å, Höglund P, et al. Errors in medication history at hospital admission: prevalence and predicting factors. BMC Clin Pharmacol 2012;12:9 10.1186/1472-6904-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sloan KL, Sales AE, Liu CF, et al. Construction and characteristics of the RxRisk-V: a VA-adapted pharmacy-based case-mix instrument. Med Care 2003;41:761–74. 10.1097/01.MLR.0000064641.84967.B7 [DOI] [PubMed] [Google Scholar]
- 41. StataCorp. Stata Statistical Software. College Station, TX. [Google Scholar]
- 42. Nelson LA, Graham MR, Schaefer MG. Characterization of Medication Discrepancies Occurring at the Time of Discharge from an Adult State Psychiatric Inpatient Facility. Hosp Pharm 2011;46:254–61. 10.1310/hpj4604-254 [DOI] [Google Scholar]
- 43. Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med 2005;165:424–9. 10.1001/archinte.165.4.424 [DOI] [PubMed] [Google Scholar]
- 44. Climente-Martí M, García-Mañón ER, Artero-Mora A, et al. Potential risk of medication discrepancies and reconciliation errors at admission and discharge from an inpatient medical service. Ann Pharmacother 2010;44:1747–54. 10.1345/aph.1P184 [DOI] [PubMed] [Google Scholar]
- 45. Forster AJ, Murff HJ, Peterson JF, et al. Adverse drug events occurring following hospital discharge. J Gen Intern Med 2005;20:317–23. 10.1111/j.1525-1497.2005.30390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grimes TC, Deasy E, Allen A, et al. Collaborative pharmaceutical care in an Irish hospital: uncontrolled before-after study. BMJ Qual Saf 2014;23:574–83. 10.1136/bmjqs-2013-002188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dedhia P, Kravet S, Bulger J, et al. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc 2009;57:1540–6. 10.1111/j.1532-5415.2009.02430.x [DOI] [PubMed] [Google Scholar]
- 48. Tully M, Cantrill J. What Hospital Doctors Think GPs Need In A Discharge Summary. London: WONCA Conference (London, UK), 2002. [Google Scholar]
- 49. Vinogradova Y, Coupland C, Brindle P, et al. Discontinuation and restarting in patients on statin treatment: prospective open cohort study using a primary care database. BMJ 2016;353:i3305 i3305 10.1136/bmj.i3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raebel MA, Schmittdiel J, Karter AJ, et al. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 2013;51:S11–S21. 10.1097/MLR.0b013e31829b1d2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mauskop A, Borden WB. Predictors of statin adherence. Curr Cardiol Rep 2011;13:553–8. 10.1007/s11886-011-0221-2 [DOI] [PubMed] [Google Scholar]
- 52. Jin J, Sklar GE, Min Sen Oh V, et al. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther Clin Risk Manag 2008;4:269–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Health Service Executive (HSE). eHealth strategy for Ireland [Internet]. 2013. http://www.ehealthireland.ie/Knowledge-Information-Plan/eHealth-Strategy-for-Ireland.pdf (cited 7 Jun 2016).
- 54. Health Information & Quality Authority (HIQA). National Standard for Patient Discharge Summary Information, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doerper S, Godet J, Alexandra JF, et al. Development and multi-centre evaluation of a method for assessing the severity of potential harm of medication reconciliation errors at hospital admission in elderly. Eur J Intern Med 2015;26:491–7. 10.1016/j.ejim.2015.07.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024747supp001.pdf (117.7KB, pdf)
bmjopen-2018-024747supp002.pdf (81.6KB, pdf)