Abstract
Introduction
Bariatric surgery is increasingly recognised as an effective treatment option for subjects with type 2 diabetes and obesity; however, there is no conclusive evidence on the superiority of Roux-en-Y gastric bypass or sleeve gastrectomy. The Oseberg study was designed to compare the effects of gastric bypass and sleeve gastrectomy on remission of type 2 diabetes and β-cell function.
Methods and analysis
Single-centre, randomised, triple-blinded, two-armed superiority trial carried out at the Morbid Obesity Centre at Vestfold Hospital Trust in Norway. Eligible patients with type 2 diabetes and obesity were randomly allocated in a 1:1 ratio to either gastric bypass or sleeve gastrectomy. The primary outcome measures are (1) the proportion of participants with complete remission of type 2 diabetes (HbA1c≤6.0% in the absence of blood glucose-lowering pharmacologic therapy) and (2) β-cell function expressed by the disposition index (calculated using the frequently sampled intravenous glucose tolerance test with minimal model analysis) 1 year after surgery.
Ethics and dissemination
The protocol of the current study was reviewed and approved by the regional ethics committee on 12 September 2012 (ref: 2012/1427/REK sør-øst B). The results will be disseminated to academic and health professional audiences and the public via publications in international peer-reviewed journals and conferences. Participants will receive a summary of the main findings.
Trial registration number
NCT01778738;Pre-results.
Keywords: type 2 diabetes, gastric bypass, sleeve gastrectomy, morbid obesity, randomised controlled trial, β-cell function
Strengths and limitations of this study.
Study design—randomised, triple-blinded superiority trial.
Numerous clinically relevant secondary endpoints including gastro-oesophageal reflux disease, fatty liver disease, cardiovascular risk factors, bone metabolism and patient-reported outcome measures covering health-related quality of life, psychological distress, eating behaviour and gastrointestinal symptoms.
Predefined algorithms for optimising medications and supplementations related to primary and secondary outcomes.
Insulin secretion and action are calculated using both oral and intravenous glucose tolerance tests.
The sample size is limited and may, thus, not provide sufficient statistical power to detect clinically relevant differences in secondary outcomes.
Introduction
Bariatric surgery is associated with long-term weight reduction and improvement of comorbidities, but also with adverse events and side effects.1 2 Roux-en-Y gastric bypass was for many years considered the ‘gold standard’ of bariatric surgery, but recently sleeve gastrectomy, a technically easier and faster to perform procedure, has gained popularity and is now the most common bariatric procedure in the USA.3
For subjects with type 2 diabetes and obesity, bariatric surgery is a particularly effective treatment option, and a number of randomised trials have demonstrated the superiority of surgery over medical care for glycaemic control and remission of diabetes.4–9 The improved glycaemic homoeostasis following bariatric surgery is to a large extent explained by the hypocaloric state and weight reduction. However, as improvements often are observed even before changes in body weight occur, some of the effects appear to be independent of weight loss and possibly related to the specific surgical procedure. Both gastric bypass and sleeve gastrectomy reduce the size of the stomach, but only gastric bypass includes a bypass of the duodenum and proximal small intestine. Thus, particularly, after gastric bypass, there is a rapid delivery of undigested food to the small intestine that enhances the release of gut-derived incretin hormones, such as glucagon-like peptide-1 (GLP-1), which further stimulate insulin secretion from pancreatic β-cells. Indeed, greater enhancement in postprandial GLP-1 levels have been observed after gastric bypass compared with sleeve gastrectomy,10 while others have reported no significant differences.11–13 The higher postprandial incretin hormone levels may be causally linked with the improved β-cell function observed after gastric bypass14–16 and sleeve gastrectomy.17 However, bariatric surgery is also accompanied by changes in other gut-derived and pancreatic-derived hormones, which directly or indirectly influence glycaemic control.18
A limited number of high-quality studies have compared the effect of gastric bypass and sleeve gastrectomy on remission of type 2 diabetes. Prior to the initiation of the present study, only one randomised controlled study had addressed glycaemic control in subjects with type 2 diabetes and obesity after gastric bypass and sleeve gastrectomy.19 The STAMPEDE trial showed similar reduction in HbA1c 1 year after the two surgical procedures. In the following years, results from comparable randomised controlled trials have been published20–23 including 3 and 5 years follow-up data from the STAMPEDE trial.4 24 The remission rates of type 2 diabetes between the two procedures have not been statistically different in these trials. Thus, there is currently no conclusive evidence showing the superiority of gastric bypass or sleeve gastrectomy for patients with type 2 diabetes and obesity. Also, the impact of altered insulin secretion and action, gut microbiota, hepatic steatosis and gastric emptying on glycaemia after gastric bypass compared with sleeve gastrectomy is not clear. Additional relevant outcomes, including changes in body weight, obesity-related cardiovascular risk factors, symptoms and findings of gastro-oesophageal reflux disease, health-related quality of life, psychosocial status, eating behaviour, bone health, vitamin and mineral deficiencies, surgical complications and side effects, need further examination.
Objectives
Primary objectives
The primary objectives of this study are to assess the effects of gastric bypass and sleeve gastrectomy on glycaemic control, diabetes remission and β-cell function.
Secondary objectives
Secondary objectives are to explore changes in variables that are related to the primary endpoints of the study (ie, body weight and composition, insulin sensitivity, liver fat content, energy intake, stomach emptying rate and intestinal microbiota). Moreover, we will explore cardiovascular risk factors influenced by weight reduction and possibly by changes in gut hormones (ie, blood pressure, arterial stiffness, albuminuria and lipids). Finally, we will examine possible differences in vitamin and mineral deficiencies, bone density, gastro-oesophageal reflux disease, hypoglycaemia, dumping syndrome, health-related quality of life, psychological distress, obesity-related symptoms, eating behaviour, nutrient intake, gastrointestinal symptoms and surgical and medical complications related to the two operations.
Trial design
This study is a single-centre, triple-blinded, two-armed superiority trial randomising patients with type 2 diabetes and obesity in a 1:1 allocation ratio to either gastric bypass or sleeve gastrectomy.
Methods and analysis
Study setting
The study is carried out at the Morbid Obesity Centre at Vestfold Hospital Trust in Norway, a tertiary healthcare centre. The centre performs between 250 and 300 bariatric procedures (gastric bypass and sleeve gastrectomy) annually and about 25% of the patients have type 2 diabetes.2 In 2012, when the Oseberg study was initiated, sleeve gastrectomy accounted for approximately 10% of bariatric procedures at our centre, increasing to approximately 40% in 2017.
Patient and public involvement
The steering committee includes a patient representative (MHK) to make sure that the patients’ voices are heard. The burden of the interventions will be assessed using several patient-reported outcome measures questionnaires, regarding several aspects of health-related quality of life as well as gastrointestinal symptoms. The patients will receive summaries of published findings throughout the study period.
Inclusion and exclusion criteria
Inclusion criteria
Previously verified body mass index (BMI)≥35.0 kg/m2 and current BMI≥33.0 kg/m2.
HbA1c≥6.5% or use of antidiabetic medications with HbA1c≥6.1%.
Age≥18 years.
Exclusion criteria
Not able to give informed consent.
Previously major abdominal surgery including bariatric surgery (appendectomy, laparoscopic cholecystectomy or gynaecological procedures not included).
Severe endocrine, heart, lung, liver and kidney disease, cancer and other medical conditions associated with significantly increased risk of perioperative and postoperative complications.
Drug or alcohol addiction.
Reduced compliance due to severe mental and psychiatric conditions.
Pregnancy.
Serum autoantibodies against glutamic acid decarboxylase (GAD) or tyrosine phosphatase (IA2).
Regular use (a total of 3 months cumulative use in the last 12 months) or treatment the past 2 months with systemic corticosteroids.
Severe gastro-oesophageal reflux disease defined as Los Angeles classification grade>B, Barrett’s oesophagus and/or hiatus hernia>5 cm.
Elevated oesophageal pressure (DCI>5000 mm Hg×s×cm) and symptoms of dysphagia and/or painful swallowing.
Interventions
All procedures were performed laparoscopically by experienced surgeons, and anaesthetists, familiar with obesity surgery, were responsible for the general anaesthesia. Anaesthesia was induced using propofol and rocuronium, while anaesthesia maintenance was achieved by combining desflurane gas and remifentanil infusion. Oral trimethoprim-sulpha was used as preoperative antibiotic prophylaxis.
The surgical procedures were standardised and similar in both gastric bypass and sleeve gastrectomy. Four trocars and one Nathanson’s retractor were placed using identical skin incisions. Pneumoperitoneum was maintained at an intra-abdominal pressure of 15 mm Hg using CO2 insufflation. Dissection and haemostasis were conducted using ultrasonic shears (Harmonic Ace Ethicon EndoSurgery), and Sonicison, Medtronic (formerly Covidien) Echelon Flex (Ethicon EndoSurgery) or EndoGIA Ultra Universal Stapler (Covidien) was used for stapling. After reducing the intra-abdominal pressure to 10 mm Hg, all trocars were removed under visual guidance. Drains were not used.
Roux-en-Y gastric bypass
The left crus was dissected free and any hiatal hernia left in place. The minor curvature was opened at the second vessel and the lesser sac entered. A 25 mL gastric pouch was created by firing one horizontal and two vertical 45 mm blue (Ethicon) or tan (Medtronic, formerly Covidien) staple loads. The ligament of Treitz was identified and a proximal loop of small intestine anastomosed to the pouch 60 cm from the ligament of Treitz with one firing of a 45 mm linear stapler (white or tan load) using the full length of the stapler, creating an antecolic, antegastric alimentary limb. The opening was closed using a single row, running absorbable suture. An entero-enteroanastomosis was then made 120 cm distal of the gastro-enteroanastomosis by firing one 45 mm white load (Ethicon) or tan (Medtronic, formerly Covidien). The introductory opening was closed with a single row, running absorbable suture. Finally, the small intestine was divided with one 45 mm white load (Ethicon) or tan (Medtronic, formerly Covidien) between the gastro-entero-enteroanastomosis and the entero-enteroanastomosis in order to complete a bypass with an alimentary limb of 120 cm and a bileopancreatic limb of 60 cm25. The mesentery was not divided, and the omentum was only divided if indicated. A leak test with methylene blue was performed and the pouch inspected on both sides. The mesenteric defects under the entero-enteroanastomosis and at Peterson’s space were closed with a running, non-absorbable suture or non-absorbable clips.
Sleeve gastrectomy
The greater curvature was dissected free starting 4–5 cm from the pylorus up to the angle of Hiss. The left crus was visualised and inspected for hiatal hernia. Small sliding hernias and wide hiatus were left in situ. The ventricle was then lifted and any adhesions in the lesser sac divided. A 35 Fr bougie was placed down to the pylorus guiding the creation of a tubular sleeve with linear staplers. The first two loads were always green (Ethicon) or purple (Covidien), while blue (Ethicon) or tan (Medtronic, formerly Covidien) loads were used for the rest of the ventricle. The last stapler was placed 5 mm laterally to the angle of Hiss. The staple line was then inspected and secured with clips for additional haemostasis, no oversewing or buttressing material was routinely used. A leak test was performed with 100 mL methylene blue. The specimen was retrieved from one of the trocar sites and visually examined for pathology, no routine histological assessment was performed.
Concomitant care
The two intervention groups receive identical concomitant care. Both groups completed a low-calorie diet (<1200 kcal/day) in the 2 weeks preceding surgery, and, at all visits throughout the study, the patients are informed about the importance of lifestyle behaviour change, diet and physical activity. All patients receive advanced medical therapy defined as the implementation of current (2012) international guidelines to optimise management of hyperglycaemia, elevated blood pressure and dyslipidaemia.26–28 Specific algorithms for antidiabetic treatment, blood pressure management, statin therapy, as well as management of reflux disease and vitamin and mineral supplementations, were implemented in order to achieve the recommended treatment targets (online supplementary file 1).
bmjopen-2018-024573supp001.pdf (219.1KB, pdf)
Outcome measure
Primary outcome measures
Secondary outcome measures
Secondary outcome measures are listed in box 1. The summary measures for the two surgical groups include mean/median and proportions as appropriate. Outcome variables include changes from baseline, final values and time to events, and will be measured at baseline, 5 weeks, 16 weeks, 34 weeks, 1 year and annually for 4 more years (see table 1).
Box 1. Secondary outcome measures.
Glucose homoeostasis
HbA1c
Insulin secretion and action
Fasting and stimulated levels of glucose and insulin
Use of antidiabetic medication
Gastrointestinal hormone levels
Gastric emptying rate
Body weight and composition
Body weight, body mass index, waist circumference and hip circumference
Body composition
Obesity-related cardiovascular comorbidities
Resting systolic and diastolic blood pressure
Twenty-four hours ambulatory blood pressure
Use of blood pressure lowering drugs
Pulse wave velocity
Cholesterol and triglyceride levels
Use of lipid-lowering agents
Obstructive sleep apnoea score
Microalbuminuria
Gastrointestinal tract and liver
Gastro-oesophageal reflux disease and oesophageal motility
Fatty liver disease
Gut microbiota and microbiome
Self-reported gastrointestinal symptoms
Physical activity
Measured physical activity
Self-reported physical activity
Energy intake and eating behaviour
Food frequency
Food tolerance
Eating patterns
Obesity specific well-being
Health-related quality of life
Obesity-related symptoms
Psychological distress
Harms
Surgical and medical complications
Hypoglycaemic episodes and early dumping
Vitamin and mineral deficiencies
Bone mineral density and metabolism
Length of hospital stay
Readmissions
Table 1.
Patient visit schedule
| Time (accepted variation) |
Screening | Baseline | Operation 0 |
Follow-up period | |||||
| −3 weeks (−52 to −2) |
5 weeks (4–8) |
16 weeks (12–24) |
34 weeks (28–40) |
52 weeks (46–60) |
2, 3 and 4 years±2 months |
5 years±4 months |
|||
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8–10 | 11 |
| Demographic data | x | ||||||||
| Comorbidities | x | ||||||||
| Regular medication | x | x | x | x | x | x | x | ||
| Clinical examination | x | x | x | x | x | x | x | x | |
| Physician consultation | x | x | x | x | x | x | x | x | |
| Inclusion and exclusion criteria | x | ||||||||
| Signed informed consent | x | ||||||||
| Blood samples* | x | x | x | x | x | x | x | x | x |
| Urine samples* | x | x | x | x | |||||
| Faecal sample* | x | x | x | x | |||||
| Pulse wave velocity | x | x | x | x | |||||
| Bioelectrical impedance analysis | x | x | x | x | x | x | x | ||
| ECG | x | x | x | ||||||
| OGTT and FSIGT | x | x | x | x | |||||
| PROMs questionnaires (web) | x | x | x | x | x | ||||
| Food frequency questionnaire | x | x | x | ||||||
| DEXA | x | x | x | ||||||
| MRI | x | x | x | x | |||||
| Upper endoscopy | x | x | x | ||||||
| Manometry | x | x | x | ||||||
| pH measurement | x | x | x | ||||||
| Twenty-four hours ambulatory blood pressure | x | x | x | x | |||||
| SenseWear | x | x | x | x | |||||
| ApneaLink | x | x | x | x | |||||
| Hypoglycaemia/dumping | x | x | x | x | x | x | x | ||
| Adverse events | x | x | x | x | x | x | x | ||
*See table 2.
DEXA, dual-energy X-ray absorptiometry; FSIGT, frequently sampled glucose tolerance test; OGTT, oral glucose tolerance test; PROMs, patient-reported outcome measures.
Participant timeline
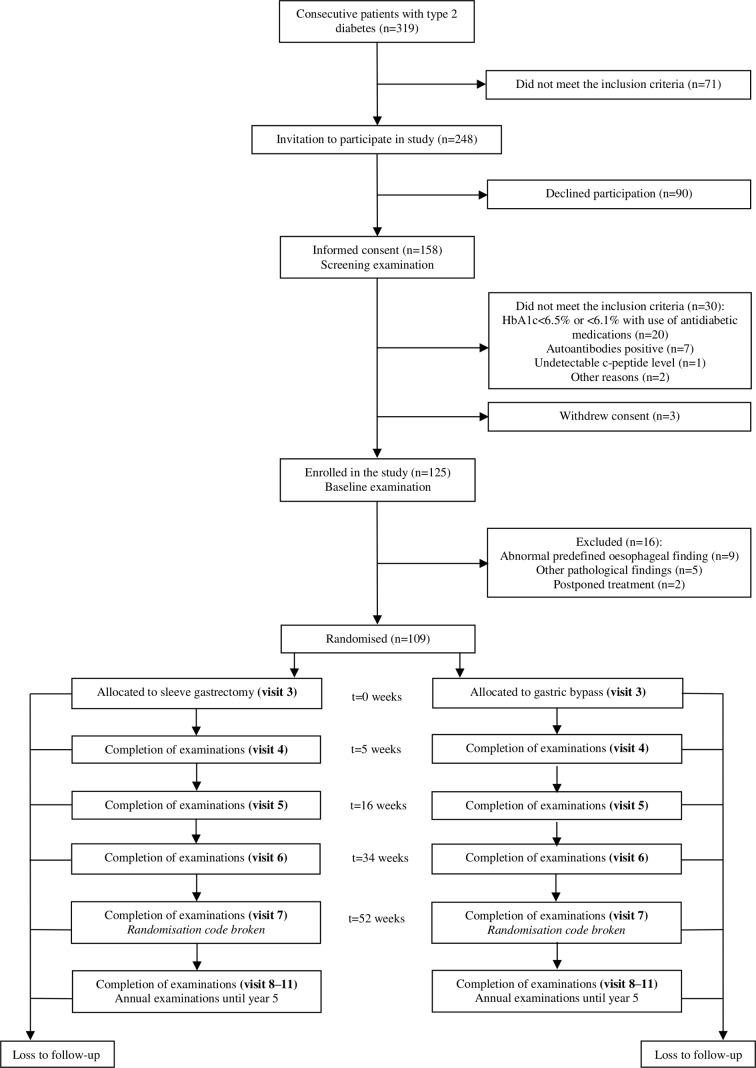
A total of 319 out of 1471 patients on the waiting list for bariatric surgery had type two diabetes. Among these, 248 patients fulfilled the inclusion criteria and were invited to participate in the study. Ninety patients declined participation and the remaining 158 patients gave informed consent and underwent the screening examination. A total of 30 patients did not meet the inclusion criteria at screening and three withdrew their consent. One hundred and twenty-five patients underwent baseline examination out of whom 16 were excluded due to abnormal gastrointestinal findings or other exclusion criteria, leaving 109 patients to be randomised and subsequently allocated to sleeve gastrectomy or gastric bypass. The first patient was included in January 2013 and the last patient was included in February 2018. Study flow chart and timeline are illustrated in figures 1 and 2, respectively.
Figure 1.
Flowchart.
Figure 2.
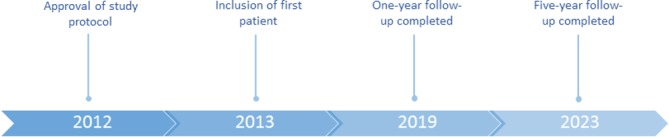
Timeline.
The Oseberg study includes several test and examinations very shortly after surgery when weight loss is expected to be low (5 weeks), after expected maximal weight loss (1 year) and during possible weight regain (one to 5 years). This comprehensive follow-up enables us to evaluate very early, medium and long-term effects of gastric bypass and sleeve gastrectomy with varying degrees of weight loss. Clinical examinations and tests with scheduled time points for assessment are listed in table 1, and a list of the blood, urine and faeces samples that have been and will be collected, including time points for collection, is presented in table 2. One-year follow-up will be completed in March 2019 and the end of the study period is in December 2023.
Table 2.
Method principles, sample matrix, units and analytical precision of laboratory measurements
| Analyte | Method principle | Sample matrix | Unit | Precision (CV, analytical) |
Time point for collection (visit number)* |
| Ferritin | ECLIA | Serum | µg/L | 7% | 1–11 |
| Iron | Photometry | Serum | µmol/L | 4% | 1–11 |
| Transferrin | Photometry | Serum | 1–11 | ||
| Vitamin B12 | ECLIA | Serum | pmol/L | 12% | 1–11 |
| Folic acid | ECLIA | Serum | nmol/L | 12% | 1–11 |
| C reactive protein | Photometry | Serum | mg/L | 5% | 1–11 |
| Creatinine | Photometry | Serum | µmol/L | 2.5% | 1–11 |
| Sodium | ISE | Serum | mmol/L | 1.0% | 1–11 |
| Potassium | ISE | Serum | mmol/L | 1.2% | 1–11 |
| Calcium | Photometry | Serum | mmol/L | 1.5% | 1–11 |
| Magnesium | Photometry | Serum | mmol/L | 3.0% | 1–11 |
| Phosphate | Photometry | Serum | mmol/L | 2.0% | 1–11 |
| Albumin | Photometry | Serum Urine |
g/L | 3.0% | 1–11 2, 4, 7 and 11 |
| Total protein | Photometry | Serum | g/L | 2.5% | 1–11 |
| Uric acid | Photometry | Serum | µmol/L | 4.0% | 1–11 |
| Glucose | Photometry | Serum/plasma | mmol/L | 2.0% | 1–4, 7 and 11 |
| Alanine aminotransferase | Photometry | Serum | U/L | 5% | 1–11 |
| Aspartate transaminase | Photometry | Serum | U/L | 9.0% | 1–11 |
| Alkaline phosphatase | Photometry | Serum | U/L | 3.0% | 1–11 |
| Gamma-glutamyl transpeptidase | Photometry | Serum | U/L | 3.0% | 1–11 |
| Lactate dehydrogenase | Photometry | Serum | U/L | 5.5% | 1–11 |
| Creatine kinase | Photometry | Serum Urine |
U/L | 5.0% | 1–11 2, 4, 7 and 11 |
| Bilirubin | Photometry | Serum | µmol/L | 5.0% | 1–11 |
| Amylase | Photometry | Serum | 1–11 | ||
| Total cholesterol | Photometry | Serum | mmol/L | 2.5% | 1–11 |
| HDL cholesterol | Photometry | Serum | mmol/L | 3.0% | 1–11 |
| LDL cholesterol | Photometry | Serum | mmol/L | 3.0% | 1–11 |
| Triglycerides | Photometry | Serum | mmol/L | 3.0% | 1–4, 7 and 11 |
| Thyroid-stimulating hormone | ECLIA | Serum | mIE/L | 5.0% | 1–11 |
| Unbound triiodothyronine | ECLIA | Serum | pmol/L | 5.0% | 1–11 |
| Unbound thyroxine | ECLIA | Serum | pmol/L | 5% | 1–11 |
| Parathyroid hormone | ECLIA | Plasma | pmol/L | 6.0% | 1–11 |
| 25-OH-vitamin D | ECLIA | Serum | nmol/L | 6.5% | 1–11 |
| Β-human chorionic gonadotropin† | ECLIA | Serum | IE/L | 5.0% | 1 |
| Paracetamol | Photometry | Serum | µmol/L | 3.0% | 2, 4, 7 and 11 |
| HbA1c | HPLC | Blood | % | 1.4% | 1–11 |
| Complete blood count | Photometry Impedance Flow cytometry |
Blood | g/dL % cells/L |
1.0%–10.0% | 1–11 |
| Thiamin | HPLC | Serum | nmol/L | 4.5% | 2 and 4–11 |
| Bone alkaline phosphatase | CLIA | Serum | U/L | 9.5 U/L 10% 45 U/L 13% |
2 and 4–11 |
| C-telopeptide of type I collagen | ECLIA | Serum | µg/L | 0.12 µg/L 13% 0.32 µg/L 8% |
2 and 4–11 |
| Procollagen type I N-terminal propeptide | ECLIA | Serum | µg/L | 5% | 2 and 4–11 |
| Insulin | ECLIA | Serum | pmol/L | 4% | 2, 4, 7 and 11 |
| C peptide | ECLIA | Serum | pmol/L | 4% | 2, 4, 7 and 11 |
| Anti-GAD | IP | Serum | ai | 0.25 ai 25% 1.45 ai 8% |
1 |
| Anti-IA2 | IP | Serum | ai | 0.32 ai 18% 1.66 ai 12% |
1 |
| Samples for storage | Serum, plasma, blood, urine and faeces | 1–4, 7 and 11 |
*Fasting blood samples visit 2, 4, 7 and 11.
†Women only.
ai, antibody index; CLIA, chemiluminescent immunoassay; CV, coefficient of variation; ECLIA, electro-CLIA; HPLC, high-performance liquid chromatography; IP, immunoprecipitation; ISE, ion selective electrode.
Sample size
This study has two primary endpoints and was powered thereafter. On the basis of previous research addressing glycaemic response of gastric bypass and sleeve gastrectomy in type 2 diabetic subjects9 19 33 34 remission rates of 75% and 50%, respectively, were assumed.
Data on disposition index after sleeve gastrectomy derived from a frequently sampled intravenous glucose tolerance test (FSIGT) was not available before study start, and data from a study addressing β-cell function after gastric bypass and a low-calorie diet was, therefore, used for sample size determination.35 Mean (SD) disposition index was 268 (232) after gastric bypass and 94 (92) after a low-calorie diet. On the basis of these figures mean (SD) disposition index after gastric bypass and sleeve gastrectomy was estimated to be 270 (160) and 180 (160) 1 year after surgery, respectively.
Given a 5% significance level and 80% power and an equal distribution to the two groups, a total study sample of 110 (remission) or 100 (disposition index) subjects was required to reveal a difference between groups. In order to accommodate possible dropouts (5% in previous study at our centre),9 we planned to include 120 subjects in the study. Due to a higher than expected number of excluded patients after baseline examinations, the baseline study population was increased to 125.
Recruitment
The electronic medical records of all patients on the waiting list for bariatric surgery at the Morbid Obesity Centre were reviewed by one of the principal investigators (DH). Patients eligible for inclusion were contacted by phone (DH) and invited to an information meeting a few months prior to surgery. Patients who then approved participation signed the informed consent and underwent a screening procedure.
Allocation—sequence generation and concealment
The allocation sequence was created by one of the bariatric surgeons (MS), not involved in the recruitment or follow-up of the patients, using a computerised random number generator (randomization.com) with a 1:1 allocation using blocks sizes of 10. Opaque envelopes were sequentially numbered, and a note with the procedure to be conducted, according to the randomisation list, was placed inside the envelope. The envelopes were then sealed and stored inside locked cabinets. Only authorised staff members, including the person who uploaded the sequence to the web-based randomisation service, have access to the allocation sequence. The investigators responsible for patient recruitment, or clinicians who are in contact with the patients, do not have access to the allocation sequence. The allocation for each specific patient was revealed, to the surgical staff only, in the operating room on the day of surgery. Afterwards, the envelope with the randomisation code was resealed and stored in the coordinating office in locked cabinets. The surgical staff were not involved with patient follow-up after the surgical procedure.
Blinding
The triple blinding refers to blinding of patients, study personnel engaged in all visits until the 1-year follow-up and the person(s) analysing the primary outcomes. Surgical incisions from the two procedures are identical, and postsurgical follow-up and treatment are similar for all patients. The randomisation code will remain concealed until data for the primary outcomes have been collected at 1 year. The research physician is then be responsible for including the actual type of surgery in the patient’s electronic medical record and for informing the patient. The code will only be broken during the first year if there is an unexpected complication or a need for reoperation. A list linking name and study identification number with study procedure is available at the study office and in the emergency department—all patients are aware of this. The research physician must report all code breaks (with reason) in the case report form.
Data collection methods
Primary outcomes
Remission of type 2 diabetes
Whole blood HbA1c will be analysed on a Tosoh high-performance liquid chromatography G8 analyser (Tosoh Corporation, Tokyo, Japan) with reagents from the supplier. The analytical variation (CVat) is estimated to be 1.4%.
Disposition index
The disposition index, a valid surrogate measure of β-cell function, is the product of insulin sensitivity and the insulin secretory response to a glucose challenge (insulin sensitivity index (Si)×acute insulin response (AIRg)). Si and AIRg will be estimated with the Bergman minimal model (MINMOD Millennium software),36 which uses glucose and insulin data obtained from a FSIGT.30–32 The laboratory analyses used for quantifying insulin and glucose are shown in table 2.
Execution of the FSIGT: The patients are asked to avoid vigorous physical activity 1 week prior to the test. The patients must also terminate treatment with long-acting GLP-1 analogues and other antidiabetic medications 6 weeks and 48 hours prior to the test, respectively. The participants are not allowed to drink (up to 2 dL of water is allowed), eat or smoke 8 hours prior to the test. Other morning medications are delayed until after the tests. A cannula is inserted into a cubital vein and the cannulated arm wrapped in a heat pad throughout the experiment for the collection of arterialised blood samples. A cannula is then inserted in the contralateral cubital vein for glucose and insulin infusion. Due to patient safety, the upper limit of fasting blood glucose prior to FSIGT was set to <20 mmol/L. Blood samples are drawn two times before (−5 and 0 min) and after 2, 4, 8, 19, 22, 30, 40, 50, 70, 90 and 180 min after the intravenous glucose load (300 mg/kg body weight). After 20 min, a bolus of insulin is administrated (0.03 U/kg body weight). Blood will, at all time points, be collected in (1) one tube, which will be centrifuged after 30 min, serum will then be put on ice and stored at −80°C until the analysis of insulin and (2) one tube containing lithium heparin, which will be centrifuged immediately before the analysis of plasma glucose the same day. See table 2 for method principles, sample matrix, units and analytical precision of glucose and insulin analysis.
Secondary outcomes
See online supplementary file 2 for data collection methods of the secondary outcomes. Routine laboratory measurements will be performed at the Central Laboratory, Vestfold Hospital Trust. The laboratory is accredited according to NS-EN ISO 15189 and serves as the main analytical facility in the hospital. Complete blood count will be analysed on Sysmex XN-9000 with reagents from the supplier (Sysmex Corporation, Kobe, Japan). General clinical chemistry and immunochemistry were analysed on Cobas 8000 with modules ISE, c702 and e801 (Roche Diagnostics, Basel, Switzerland). Thiamin, bone markers, insulin, C peptide, Anti-GAD and Anti-IA2 will be analysed at Oslo University Hospital using established methods. A complete list of method principles, sample matrix, units and analytical precision are given in table 2.
bmjopen-2018-024573supp002.pdf (238.1KB, pdf)
Retention
Once a participant is included, every reasonable effort is made to prevent attrition through the entire study period. In addition to the planned visits, all participants have, during the last 2 years, received two letters in connection with milestones and holidays. Distribution of letters will continue throughout the entire study period. Loss to follow-up is estimated to be 5% or less.9
Data management
Authorised individuals enter all data electronically and into the original study forms. Data integrity is enforced through referential data rules, valid values and range checks. Data are stored on a secure and password-protected electronic research server. The original study forms are stored in locked cabinets at the study location. Participant files will be kept in storage for a period of at least 10 years after completion of the study.
Statistical methods
Data will be analysed according to both the intervention into which patients were randomised (intention-to-treat analysis) and per-protocol. Descriptive data will be presented as mean (SD), median (range) or number (%). Between-group comparisons will be analysed using independent samples t-test, repeated measures analysis of variance, mixed model analysis and Mann-Whitney U test for continuous variables and χ2, Fisher’s exact test and binary logistic regressions for repeated measures for categorical variables as appropriate. Regression analysis will be used for the exploration of the independent effects of weight reduction and other variables on primary and secondary outcomes.
Data monitoring
The steering committee consists of a team of healthcare professionals, researchers and a patient representative. Members of the steering committee meet every sixth month and safeguard the interests of trial participants and monitor the overall conduct of the clinical trial. Adverse events are consecutively reported. Harms are specified as secondary outcomes in the section secondary outcome measures.
Ethics and dissemination
Research ethics approval
The study protocol was registered in an international trial register (ClinicalTrials.gov) on 03 December 2012. See online supplementary file 3 for a brief structured summary of the study (WHO Trial Registration Data Set). The study is conducted according to the Declaration of Helsinki.
bmjopen-2018-024573supp003.pdf (176.3KB, pdf)
Protocol amendments
Significant amendments to the protocol have been/will only be made after ethical approval by the regional ethics committee. For previous amendments, see online supplementary file 4.
bmjopen-2018-024573supp004.pdf (151.3KB, pdf)
Informed consent
After a thorough evaluation of the existing literature prior to the approval of the study protocol in 2012, and after balancing clinical effects and side-effects, no evidence was found suggesting gastric bypass or sleeve gastrectomy to be a better choice for patients with type 2 diabetes. Research assistants and patients were informed about the lack of evidence before the start of the study, and one of the principal investigators (DH) obtained informed consents in face-to-face meetings.
Ancillary studies
Additional biological samples (urine, blood, serum, plasma and faeces) will be obtained and stored for use in future studies. Information about storage and analyses of biological samples was covered in the original informed consent for the Oseberg study.
Confidentiality
All participants are given a study ID, which will be used during data analyses. The key linking name and study ID is kept on a password-protected server. Participants’ study information will not be released outside of the study without the written permission of the participant.
Access to data
Selected study personnel, authorised to enter data electronically, and investigators within the Oseberg study have access to the final trial data set. Researchers within the Oseberg study must seek approval from the principal investigators prior to initiating data analyses.
Ancillary and post-trial care
The patients will receive post-trial follow-up according to national guidelines.37
Dissemination
The protocol and the results of the study (primary and secondary endpoints at 1 and 5 years) will be published in international peer-reviewed journals in accordance with the ICMJE criteria for authorship (http://www.icmje.org/ethical_1author.html). The executive and steering committees will actively contribute to the involvement and inclusion of authors and the order of authorships in the planning of publications. The results will also be disseminated through networks of scientists, conferences, professionals, and policymakers and commissioners of weight management.
Supplementary Material
Acknowledgments
We would like to express our gratitude to all the participants, the patient representative and the study personnel involved in the Oseberg study.
Footnotes
Contributors: JH, DH, RS and NN conceived of the study. JH, DH, NN, RS, BS, LKJ, KIB, HLG and T-IK initiated the study design and DH, NN, LKJ, TOWH, BS and JL helped with implementation. HB, JH and DH wrote the manuscript. HB, JH, KIB, FF, JOG, HLG, EH, JKH, TOWH, LKJ, T-IK, RLK, NPK, ML, JL, NN, RS, KAS, BS, MS, TGV and DH contributed to the refinement of the study protocol and approved the final manuscript.
Funding: The study is organised and financed by the Vestfold Hospital Trust and the Morbid Obesity Centre. All employees receive a salary from their respective departments. In addition, applications for external funding will be submitted for future postdoctoral and PhD fellows, and for specific laboratory analyses. FF and KAS have received grants from the South-Eastern Norway Regional Health Authority.
Competing interests: None declared.
Ethics approval: The study protocol was approved by the regional ethics committee on 12 September 2012 (ref: 2012/1427/REK sør-øst B).
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: Investigator, committee member and co-worker affiliations: MH Knudsen (Patients’ Committee, Vestfold Hospital Trust, Tønsberg, Norway); F Bäckhed (The Wallenberg Laboratory and Sahlgrenska Center for Cardiovascular and Metabolic Research, Department of Molecular and Clinical Medicine, Institute of Medicine, University of Gothenburg, Sweden); JM Fredheim (Department of Otolaryngology, Head and Neck Surgery, Vestfold Hospital Trust, Tønsberg, Norway); N Grude (Department of Microbiology, Vestfold Hospital Trust, Tønsberg, Norway); E Hornes Halvorsen (Department of Radiology, Vestfold Hospital Trust, Tønsberg, Norway); J Juul Holst (Department of Biomedical Sciences, Endocrinology Research Section, Copenhagen, Denmark); AW Medhus (Department of Gastroenterology, Oslo University Hospital, Ullevål, Norway); L Olsson (The Wallenberg Laboratory and Sahlgrenska Center for Cardiovascular and Metabolic Research, Department of Molecular and Clinical Medicine, Institute of Medicine, University of Gothenburg, Sweden); D Sifrim (Barts and the London School of Medicine and Dentistry, UK); M Småstuen (Department of Biostatistics, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway) and E Svendsen Gjevestad (Department of Physical Medicine and Rehabilitation, Vestfold Hospital Trust, Stavern, Norway). Principal investigators: JH, DH and NN. Executive committee: JH, DH, NN and RS. Steering committee: JH, DH, NN, RS, KIB, HLG, TGV, JKH, LKJ, MHK and BS. Co-workers: FB, HB, FF, JMF, JOG, NG, EHH, EH, JJH, T-IK, RLK, NPK, ML, JL, AWM, LO, KAS, DS, MSn, ESG, MSk and TOWH. Organisational responsibilities: Executive committee: design and conduct of the Oseberg study; recruitment of patients; preparation of protocol, CRFs (case report forms) and revisions; organising steering committee meetings; maintenance of trial IT system and data entry; and data verification. Steering committee: agreement of final protocol; reviewing the progress of the study and if necessary agreeing changes to the protocol to facilitate the smooth running of the study; meetings twice a year and safety monitoring.
Patient consent for publication: Not required.
References
- 1. Puzziferri N, Roshek TB, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA 2014;312:934–42. 10.1001/jama.2014.10706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of Bariatric Surgery vs Medical Obesity Treatment With Long-term Medical Complications and Obesity-Related Comorbidities. JAMA 2018;319:291–301. 10.1001/jama.2017.21055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Society for MaBS. Estimate of Bariatric Surgery Numbers, 2011-2016. 2016. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
- 4. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–51. 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015;150:931–40. 10.1001/jamasurg.2015.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014;149:716–26. 10.1001/jamasurg.2014.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9. 10.1001/jama.2013.5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA 2018;319:291–301. 10.1001/jama.2017.21055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofsø D, Nordstrand N, Johnson LK, et al. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol 2010;163:735–45. 10.1530/EJE-10-0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramón JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg 2012;16:1116–22. 10.1007/s11605-012-1855-0 [DOI] [PubMed] [Google Scholar]
- 11. Peterli R, Wölnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 2009;250:234–41. 10.1097/SLA.0b013e3181ae32e3 [DOI] [PubMed] [Google Scholar]
- 12. Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 2012;22:740–8. 10.1007/s11695-012-0622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc 2012;26:2231–9. 10.1007/s00464-012-2166-y [DOI] [PubMed] [Google Scholar]
- 14. Morínigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg 2006;16:1594–601. 10.1381/096089206779319338 [DOI] [PubMed] [Google Scholar]
- 15. Hofsø D, Jenssen T, Bollerslev J, et al. Beta cell function after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol 2011;164:231–8. 10.1530/EJE-10-0804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin E, Liang Z, Frediani J, et al. Improvement in ß-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab 2010;299:E706–E712. 10.1152/ajpendo.00405.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basso N, Capoccia D, Rizzello M, et al. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg Endosc 2011;25:3540–50. 10.1007/s00464-011-1755-5 [DOI] [PubMed] [Google Scholar]
- 18. Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016;77:28–37. 10.1016/j.peptides.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 19. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76. 10.1056/NEJMoa1200225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS Randomized Clinical Trial. JAMA 2018;319:255–65. 10.1001/jama.2017.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salminen P, Helmiö M, Ovaska J, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA 2018;319:241–54. 10.1001/jama.2017.20313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keidar A, Hershkop KJ, Marko L, et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia 2013;56:1914–8. 10.1007/s00125-013-2965-2 [DOI] [PubMed] [Google Scholar]
- 23. Yang J, Wang C, Cao G, et al. Long-term effects of laparoscopic sleeve gastrectomy versus roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28-35 kg/m(2). BMC Surg 2015;15:88 10.1186/s12893-015-0074-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–13. 10.1056/NEJMoa1401329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lönroth H, Dalenbäck J, Haglind E, et al. Laparoscopic gastric bypass. Another option in bariatric surgery. Surg Endosc 1996;10:636–8. [PubMed] [Google Scholar]
- 26. American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care 2012;35(Suppl 1):S11–S63. 10.2337/dc12-s011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes 2012;35:1364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mancia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens 2007;25:1751–62. 10.1097/HJH.0b013e3282f0580f [DOI] [PubMed] [Google Scholar]
- 29. Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care 2009;32:2133–5. 10.2337/dc09-9036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–67. 10.1172/JCI110398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steil GM, Volund A, Kahn SE, et al. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes 1993;42:250–6. 10.2337/diab.42.2.250 [DOI] [PubMed] [Google Scholar]
- 32. Mari A. Assessment of insulin sensitivity and secretion with the labelled intravenous glucose tolerance test: improved modelling analysis. Diabetologia 1998;41:1029–39. 10.1007/s001250051027 [DOI] [PubMed] [Google Scholar]
- 33. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg 2011;146:143–8. 10.1001/archsurg.2010.326 [DOI] [PubMed] [Google Scholar]
- 34. Pournaras DJ, Aasheim ET, Søvik TT, et al. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg 2012;99:100–3. 10.1002/bjs.7704 [DOI] [PubMed] [Google Scholar]
- 35. Plum L, Ahmed L, Febres G, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity 2011;19:2149–57. 10.1038/oby.2011.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986;23:113–22. 10.1016/0169-2607(86)90106-9 [DOI] [PubMed] [Google Scholar]
- 37. Utredning og behandling av sykelig overvekt i spesialisthelsetjenesten Voksne. 2007. https://www.helse-sorost.no/Documents/Styret/Styrem%C3%B8ter/2008/vedlegg-sak-086-2008-Rapport%20-%20utredning%20og%20behandling%20av%20sykelig%20overvekt%20i%20spes%20helsetjenesten%20-%20voksne%20pdf%20211102.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024573supp001.pdf (219.1KB, pdf)
bmjopen-2018-024573supp002.pdf (238.1KB, pdf)
bmjopen-2018-024573supp003.pdf (176.3KB, pdf)
bmjopen-2018-024573supp004.pdf (151.3KB, pdf)