Abstract
Introduction
Opioids prescribed after surgery accounted for 5% of the 191 million opioid prescriptions filled in 2017. Approximately 80% of the opioid pills prescribed by surgical care providers remain unused, leaving a substantial number of opioids available for non-medical use. We developed a multi-component intervention to address surgical providers’ role in the overprescribing of opioids. Our study will determine effective strategies for reducing post-surgical prescribing while ensuring adequate post-surgery patient-reported pain-related outcomes, and will assess implementation of the strategies.
Methods and analysis
The Minimising Opioid Prescribing in Surgery study will implement a multi-component intervention, in an Illinois network of six hospitals (one academical, two large community and three small community hospitals), to decrease opioid analgesics prescribed after surgery. The multi-component intervention involves four domains: (1) patient expectation setting, (2) baseline assessment of opioid use, (3) perioperative pain control optimisation and (4) post-surgical opioid minimisation. Four surgical specialities (general, orthopaedics, urology and gynaecology) at the six hospitals will implement the intervention. A mixed-methods approach will be used to assess the implementation and effectiveness of the intervention. Data from the network’s enterprise data warehouse will be used to evaluate the intervention’s effect on post-surgical prescriptions and a survey will collect pain-related patient-reported outcomes. Intervention effectiveness will be determined using a triangulation design, mixed-methods approach with staggered speciality-specific implementation for contemporaneous control of opioid prescribing changes over time. The Consolidated Framework for Implementation Research will be used to evaluate the site-specific contextual factors and adaptations to achieve implementation at each site.
Ethics and dissemination
The study aims to identify the most effective hospital-type and speciality-specific intervention bundles for rapid dissemination into our 56-hospital learning collaborative and in hospitals throughout the USA. All study activities have been approved by the Northwestern University Institutional Review Board (ID STU00205053).
Keywords: opioids, overprescribing, diversion, implementation, cfir, mixed methods
Strengths and limitations of this study.
Iterative modification of the evidence-based components of the intervention will allow us to study how hospitals and specialities adapt and improve the components to meet their site-specific contextual needs.
The study will provide a generalisable framework for tailored implementation of post-surgical discharge pain management prescribing best practices to reduce patient and societal harm due to excess opioids without negatively affecting patient reported pain outcomes.
Comparison to within site non-intervention controls allows us to control for secular decline in opioid prescriptions, driven by the salience of the ‘opioid epidemic’ in popular press and public policy.
The study duration (3 years) is a relatively short time to expect complete implementation and to observe improvement.
Introduction
Background and existing literature
Nearly all surgical procedures result in some level of postoperative pain, although with varying intensity and duration, for which surgical providers prescribe pain medications, often opioids. In our prior study, in a single health system, opioids were prescribed after 95% of surgical procedures.1 Furthermore, we found that the patients only used, on average, a small number of pills (~5 pills) after elective general surgical procedures,1 confirming prior studies’ finding that between 70% to 90% of prescribed opioids after surgery remain unused by the patient.2–5 It has also been established that only a small fraction of unused opioid pills are properly disposed of6–8 leaving a substantial number of pills available for potential non-medical use and diversion.5 9
Indeed, 50% to 75% of non-medical opioid users obtain the drugs from relatives or friends, a concept known as ‘diversion’.10 11 In 2016, 11.5 million individuals (>12 years) reported using prescription drugs for a non-medical purpose in the past year.12 Every day, more than 115 people die after overdosing on opioids and, despite recent efforts to curb the crisis, the numbers are still steadily increasing.13 Moreover, opioid related poisoning caused 78 840 hospitalisations and 140 077 emergency department visits in 2015 alone.12 Finally, more than 80% of people who use heroin have a history of non-medical use of pain relievers first.11 14 Much of the focus has fallen on opioid prescribing by primary care physicians, particularly for chronic opioid use, but surgical providers also have a role in this epidemic, given the overprescribing of opioids after surgery.
While opioid prescriptions written by surgical providers, in 2017, accounted for only 5% of the 191 million dispensed opioid prescriptions in the USA,15 they nevertheless accounted for 10.4 million filled prescriptions.16 Many factors contribute to opioid prescribing decisions for pain control following a surgical procedure, including patient factors (eg, pain tolerance, opioid tolerance), procedural factors (eg, open incision, laparoscopic incision), legal influences (eg, laws that restrict the ability to electronically prescribe or offer refills remotely) and social factors (eg, distance to provider to obtain a refill prescription).3 17–39 Research also shows that surgical providers often use a default number of pills (eg, 60 pills after hernia surgery) with little attention to patient factors, often prescribing more pills than needed for the majority of the patients.3 5 27 40 41
The role of surgical providers in the opioid epidemic unfolds in three ways1: (i) overprescribing of opioids following surgery, which leaves unused opioids available for non-medical use and diversion2 2–5, (ii) inadequately providing perioperative patient education around safe use and proper disposal of unused opioid pills and expectation setting about post-surgical pain and pain management17 42 43 and3 (iii) contributing to the development of chronic post-surgical pain.3 5 17 40 42–46
It is imperative that the numerous factors influencing surgical provider opioid prescribing be addressed while ensuring that the patients still receive adequate post-surgical pain control. Minimising Opioid Prescribing in Surgery (MOPiS) is a multi-component intervention that addresses1 opioid overprescribing, for example by promoting baseline assessment of a patient prior to writing any opioid prescription to identify and address duplicative opioid prescriptions (eg, provider use of state prescription monitoring programmes (PMP))2 34 47 48, optimising pain control (eg, use of non-opioid analgesics as first-line therapy with opioid supplementation, if needed)49 50 and3 minimising reliance on opioid prescriptions for pain control (eg, familiarisation of providers with evidence on alternatives to opioid analgesics and with the actual number of pills used by the patients for pain management).49 51 52
Study aims
The MOPiS study was developed, based on current evidence and national guidelines, to address four distinct domains1: expectation setting2, baseline assessment3, optimisation of perioperative non-opioid pain management and4 opioid minimisation at discharge.51–54
We will conduct a mixed-methods evaluation of the implementation and effectiveness of MOPiS by1 applying a concurrent, formative evaluation to identify barriers and enablers to implementation2, using the Consolidated Framework for Implementation Research (CFIR)3 55, performing a quantitative assessment of site-specific effectiveness and4 determining the necessary and sufficient conditions for improving post-surgical opioid prescribing without negatively affecting pain-related patient outcomes.
The study aims are
Aim 1: evaluate the implementation of a multi-component intervention to reduce post-surgical opioid prescribing, while ensuring optimal pain control across six hospitals
Hypothesis 1: Implementation requires site-specific contextual adaptations of the intervention by speciality-specific, multidisciplinary surgical teams.
Aim 2: assess changes in opioid prescribing and pain-related patient outcomes following implementation of the contextually specific, multi-component intervention
Hypothesis 2: Implementation of the intervention will lead to decreased post-surgical opioid prescriptions without a decline in patient-reported pain-related outcomes, compared with historical and contemporaneous controls.
Aim 3: assess the interaction between site-specific contextual factors and variation in the fidelity and intensity of implementation on post-surgical opioid discharge prescribing
Hypothesis 3: The context of the intervention, as well as, specific approaches to implementing the components of the intervention, will lead to greater improvement.
Theoretical frameworks
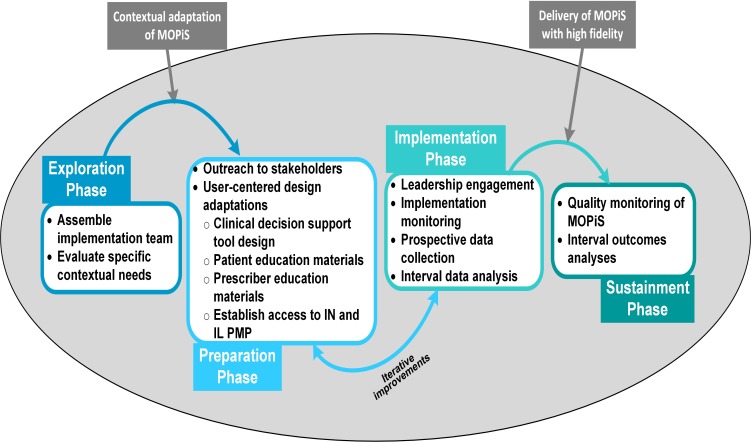
Implementation of the MOPiS intervention will be guided by the Exploration, Preparation, Implementation, Sustainment (EPIS) Framework.56 Figure 1 shows the adaptation of the EPIS Framework for MOPiS. In addition, the CFIR55 will be used to comprehensively explore the contextual needs and adaptations of the intervention in order to achieve robust and sustainable implementation at each site.
Figure 1.
Application of EPIS phases to MOPiS Implementation. EPIS, Exploration, Preparation, Implementation, Sustainment; IN, Indiana; IL, Illinois; MOPiS, Minimising Opioid Prescribing in Surgery; PMP, prescription monitoring programmes.
Study significance
The MOPiS study will provide a generalisable framework for tailored implementation of post-surgical prescribing best practices to reduce patient and societal harm due to excess opioids without negatively affecting pain-related patient-reported outcomes. The study promotes evidence-based practices to identify effective strategies for contextually specific, system-level change, and it will provide both conceptual and practical insights to inform implementation of a system-level, evidence-based practice intervention in diverse hospitals, across the USA.
Methods and analysis
Overview of study design
The intervention will be implemented in four surgical areas (general, orthopaedics, urology and gynaecology) at six hospitals within a single health system. We will conduct a mixed-methods evaluation using formative evaluation techniques including qualitative (eg, focus groups, interviews, observations) and quantitative (eg, process and outcome measure performance reports) data collection to identify barriers and facilitators to the implementation and effectiveness of the intervention.57
Data collection, analysis and interpretation will be continuous before, during and after implementation with identified barriers being fed back iteratively to the hospitals for site-specific contextual adaptations. In addition, we will perform a quantitative evaluation to determine the effectiveness of the intervention to decrease post-surgical discharge opioid prescribing without negatively affecting pain-related patient-reported outcomes. To determine effectiveness, we will also use cases from non-intervention surgical specialities (plastic surgery, vascular surgery and neurosurgery) as contemporaneous within-site controls. Once effective strategies are identified, they will be implemented in the non-intervention specialities for study equipoise. Finally, we will combine the qualitative and quantitative results to ascertain which components of the intervention, in combination with site-specific contextual factors, are most effective in reducing opioid prescribing. The goal is to identify the most effective hospital-type and speciality-specific intervention bundles.
Setting
The study will be conducted at six hospitals within a health system, in Illinois. The hospitals are diverse with significant differences in size, location, population served and level of academical or community focus (table 1) and have a single enterprise data warehouse (EDW) for data collection.
Table 1.
Hospitals and specialities participating in MOPiS
| Hospital 1 | Hospital 2 | Hospital 3 | Hospital 4 | Hospital 5 | Hospital 6 | |
| Category | Large academic | Large community | Large community | Small community | Small community | Small community |
| Teaching status | Teaching | Non-teaching | Non-teaching | Non-teaching | Non-teaching | Non-teaching |
| No of beds | 894 | 198 | 392 | 159 | 98 | 25 |
MOPiS, Minimising Opioid Prescribing in Surgery.
Intervention
For each of the four domains, the intervention includes one or more main components:
Patient expectation setting
Standardised Patient Education: Patient education materials will be developed with the aim of improving adherence to safe opioid use.29 34 51 52 54 58 Educational materials will include printed brochures, as well as, interactive patient modules to educate patients on how to safely use, store and dispose of opioids. All educational material will be user-informed and literacy-level testing will be performed by our patient advisory council.
Baseline assessment of opioid use
Automated IL-PMP Lookup: A key approach to reducing the number of opioids available for diversion is to ensure that the patients cannot access multiple opioid prescriptions simultaneously. The Prescription Monitoring Programme (PMP) is an electronic database that tracks the prescribing and dispensing of controlled substances (Schedule II-V), including opioids, with data on individual patients and individual prescribers.59 Clinicians can log into the PMP to view a patient’s prior filled prescriptions. As part of the intervention, the PMP database will be integrated into the electronic health record (EHR) at each hospital to allow for an automated connection and to overcome technical and nuisance barriers associated with manual PMP lookup. Moreover, each time a provider attempts to order opioids, we plan to design an automatic lookup in the Illinois PMP (IL-PMP), so that prior opioid prescriptions will automatically appear next to the new opioid order.
Perioperative pain control optimisation
Online Provider Education: Interactive, web-based, educational modules will be developed to educate providers on safe opioid prescribing, leveraging multimodal pain control strategies, setting pain management expectations with the patients and educating the patients on opioid safety. Content experts in perioperative pain management will help to develop the modules. The modules will be targeted toward all surgical care providers, including surgeons, advanced practice providers, nurses and pharmacists.
Postoperative opioid minimisation
Electronic Health Record-based CDS Tools: Computerised decision support (CDS) tools can change prescribing behaviour, prevent medical errors and improve evidence-based clinical practice.60–63 We will gather procedure-specific opioid use data and combine this with existing evidence for procedure-specific prescribing guidelines to develop guidelines for appropriate prescription opioid quantity by procedure. Discharge order sets will incorporate the recommended quantity as a default. Surgical providers will retain the option of increasing the quantity of opioid pills by providing a brief explanation. Additionally, the discharge order sets will also include pain management order sets with non-opioid pain management alternatives (eg, ibuprofen, ice packs).
Monthly Comparative Prescribing Reports: We will develop automated monthly comparative prescribing reports for individual surgical providers, detailing their prescribing data compared with blinded peer data and compared with prescribing recommendations by surgical procedure.
Adaptation of intervention components
We anticipate variation in the extent to which each hospital and each speciality adapts the intervention components, depending on hospital and speciality-specific contextual factors. The formative evaluation of MOPiS, conducted throughout the study timeframe, will enable the identification and documentation of hospital- and speciality-specific adaptations and improvements to the components to meet hospital- and speciality-specific contextual needs.
Characteristics of study participants
To study the systems and processes of care involved in post-surgical pain medication prescribing, including opioid prescribing, surgical providers and staff participating in the implementation will be research study participants. Surgical providers and staff, as participants in the qualitative data collection, will be identified using purposive sampling, based on subject matter expertise. Additionally, the patients will be interviewed. Potential patient participants will be identified by the surgical providers. English-speaking adults of any gender and ≥18 years of age who are willing to speak about their experience with the implementation of preoperative opioid education and expectation setting and post-surgical pain management will be invited to participate. Patients will be interviewed until saturation of themes is reached.
In addition, quantitative data about the patients who have undergone selected surgical procedures at each of the participating hospitals will be gathered for both intervention and control specialities, using a survey and electronic data abstraction from the EDW.
Patient and public involvement
To ensure that patient education materials are patient and family-centred, the health system’s patient advisory council will review materials. Additionally, advisory panel members, including a patient safety foundation representative, will provide feedback on study materials and findings, and will support dissemination efforts.
Data collection components
Readiness Assessment: Prior to implementation, we will conduct a survey to assess the readiness of each hospital and each speciality to implement the necessary process changes and to identify additional needed resources. The survey will be conducted among all surgical providers involved in the implementation of the intervention.
Interviews: We will conduct semi-structured confidential interviews with key stakeholders at each hospital and within each speciality to include, but not be limited to, clinic nurses, post-anaesthesia care unit (PACU) nurses, surgeons, advanced practice nurses, physician assistants, pharmacists and surgical residents (where applicable). Interview questions will focus on assessing the local adaptation and implementation of each intervention component, as well as, the individual’s perceptions of the utility of each component. We will specifically inquire about barriers to change, including questions about the culture and work environment, in order to gain input about any site-specific barriers and facilitators during implementation.
Focus Groups: During the implementation phase, we will conduct focus groups at each hospital, using a set of structured questions to guide the discussion. Focus group participants will include an attending physician, a PACU nurse, an outpatient clinic nurse, a resident or physician assistant where applicable and a representative from each speciality’s administrative leadership. Each focus group will consist of six to eight participants and last for approximately 1 hour. The data from the focus groups will provide important information about issues related to the division’s participation in the intervention.
Observations: We will conduct a series of ethnographical observations of preoperative clinical encounters at each hospital to help us understand hospital and speciality-specific contextual factors that affect the adaptation and implementation of the preoperative components (eg, observe the functioning of a preoperative clinic and patient encounter).64–66 The observer will assume the role of ‘peripheral-member researcher,’ which brings an ‘outsider’s perspective’ to the observation to allow for accurate appraisal of activities. The site visit team will collect data using a semi-structured observation tool.67–69 We will use observation to assess the patient exposure to the intervention components.
Patient Interviews: We will conduct interviews with a random selection of patients in each hospital to ask the patients about their experiences with, and exposure to, opioid education, expectation setting and post-surgical pain management to provide real-time patient satisfaction and exposure data to the implementation team.
Medical Chart Abstraction: Using data abstraction queries from the system-wide enterprise data warehouse (EDW), we will develop an automated report to identify prescribing patterns and process measure adherence by domain of optimal use, as detailed in table 2. In addition, the EDW will generate information on hospital characteristics and contextual factors. To validate the EDW data and any data that cannot be queried through the EDW, certified data abstractors will conduct manual chart reviews on a random sample of eligible patient charts.
Table 2.
Process measures for optimal postoperative opioid use
| Setting | Domain | Process measure | Measure | Implementation outcomes | Variable type |
| Preoperative | Expectation setting | Preoperative narcotic education | Preoperative education documented in preop note or preop clinic note | Fidelity | dichotomous |
| Opioid education tool distributed | Fidelity | dichotomous | |||
| Observation (5 to 10 clinic appointments) | Exposure | qualitative | |||
| Baseline assessment | Chronic opioid use investigated | PMP user look-up registry | Fidelity | dichotomous | |
| Chronic pain addressed | Chronic pain tool distributed | Fidelity | dichotomous | ||
| Referral to pain specialist | Fidelity | dichotomous | |||
| Addiction risk assessment | NIDA risk screen performed (preop documentation) | Fidelity | dichotomous | ||
| Perioperative | Optimising pain control (minimising opioid use) | Preoperative analgesic given | EMR MAR from OR or anaesthesia record | Exposure | dichotomous |
| Pre-incision local anaesthetic | Dictated in operative note | Exposure | dichotomous | ||
| Anaesthesia type | Anaesthesia Record | Exposure | categorical | ||
| Anaesthesia adjuncts (eg, regional block, epidural, intravenous lidocaine, etc) | Anaesthesia record | Exposure | categorical | ||
| Multimodal pain control | EMR MAR from inpatient stay | Exposure | dichotomous | ||
| Postoperative | Opioid minimisation | Consult PMP | PMP user look-up registry (monthly per 100 patients) | Fidelity | dichotomous |
| Communicate with PCP | Documentation in discharge record of coordination with PCP | Fidelity | dichotomous | ||
| Discharge education information provided | Post-surgical pain handout provided | Fidelity | dichotomous | ||
| Documentation of education in discharge record | Fidelity | dichotomous |
EMR, electronic medical record; MAR, medication administration record; NIDA, National Institute on Drug Abuse; OR, operating room; PCP, primary care physician; PMP, prescription monitoring programmes.
Patient Surveys: We will use online surveys to collect pain-related patient-reported outcomes among the patients who have undergone selected surgical procedures at each of the participating hospitals within the intervention and control specialities. Patients will be asked to rate their pain (0 to 10) daily for the first 7 days following discharge and, the morning of each day (postoperative day 2 to 8), they will be asked to report the number of opioid pills consumed in the prior 24 hours. The Patient-Reported Outcomes Measurement Information System (PROMIS) Pain Intensity Short Form will be administered using computerised adaptive testing preoperatively, and then at 2 weeks, 3 months, 6 months and 1 year post-surgically.
Table 3 provides a summary of the different data collection components.
Table 3.
Summary of data collection components and measures
| Data collection component | Implementation phase (EPIS framework) | Measures | |||
| Exploration | Preparation | Implementation | Sustainment | ||
| Readiness assessment | Readiness to implement, resources needed | ||||
| Observations | Patient exposure to education and expectation setting | ||||
| Provider interviews | Perceptions of implementation barriers, process, safety culture and intervention effectiveness | ||||
| Focus groups | Issues related to division’s participation in intervention | ||||
| Patient interviews | Patient exposure to education and expectation setting, patient satisfaction | ||||
| Medical chart abstraction | Implementation process measure adherence(table 2) | ||||
| Patient surveys | Patient-reported pain scores, opioid storage and disposal behaviours | ||||
| Secondary data sources | Hospital characteristics | ||||
EPIS, Exploration, Preparation, Implementation, Sustainment.
Secondary Data Sources: Data on hospital characteristics will be obtained from the American Hospital Association Annual Hospital Survey and the Centres for Medicare and Medicaid Services Payment Update Impact Files, including: bed size, discharge volume, surgical case volume, resident-to-bed ratio/teaching status, ownership, case mix index, core-based statistical area and nurse-to-bed ratio.
Data analyses
Measures of intervention effectiveness
The primary outcome will be the number of pills prescribed per surgical case (patient/case-level measure).
Secondary outcomes include the strength, quantity and formulation of each post-surgical opioid prescribed, converted to morphine milligram equivalents.
The electronical survey will use PROMIS Pain Intensity Short Form questions that capture how pain interferes with a patient’s quality of life over the preceding 7 days. This will function as a balancing measure to assess potential negative impacts of the intervention.
Quantitative data analysis
We will perform a quantitative evaluation to determine the effectiveness of the intervention to decrease post-surgical discharge opioid prescribing without negatively affecting patient-reported pain outcomes.
Change in Opioid Prescribing: A comparison of the mean number of pills prescribed per case post-intervention (Years 2 and 3) compared with pre-intervention (Year 1) will be performed, clustered within surgeons, specialities and hospital. Because the intervention is at the system-level and the cluster size may not support three or four levels of clustering, analyses will be designed to account for the 24 clusters at the site-speciality level (table 1). First, we will assess pre- and post-intervention differences in mean number of pills prescribed per case, using a one-sided paired Student’s t-test with clustered SE (or Wilcoxon rank-sum test for non-normally distributed data) to test the hypothesis that our intervention will reduce the mean number of pills prescribed per case. We will also use statistical models with controls for patient, speciality and hospital characteristics. We will explore several models to assess appropriateness and robustness of findings across specifications. Approaches may include a linear ordinary least squares fixed effects regression model of the form:
| (1) |
where is the number of pills prescribed for each case i, in speciality-site j, in year t; is an intercept, and are coefficients on dummy variables indicating cases from the post-intervention period, is a vector of patient covariates and is a set of 23 speciality-site indicators (“fixed effects”) which control for all time-invariant speciality-site characteristics, measured and unmeasured, that only vary across speciality-sites but are invariant within. In effect, this conservatively controls for all between-speciality-site differences and identifies treatment differences within sites. We hypothesise that the intervention will reduce the number of prescribed pills per case, thus we anticipate <0 and <0. A more stringent test of sustained improvement over time implies that . Because fixed effects linear models preclude the inclusion of any speciality-site covariates that may be of interest, we will also explore estimation of two-level hierarchical linear models with speciality-site random intercepts.
- Intervention Effect on Case-Level Patient-Reported Outcomes Measurement Information System Pain Intensity Short Form Pain Scores: Pre-post and difference-in-differences, as outlined above, will be calculated. However, we hypothesise that the coefficients on study year will not be statistically different from zero.
- Sensitivity Analyses: We will do several sensitivity analyses, including1: with and without inclusion of patient and speciality covariates in the models2; examining the association between hospital, surgical speciality, procedural characteristics and missing data and3 examining spillover effects by assessing operations within the hospital similar to those studied but performed by surgeons outside of the targeted specialities, serving as contemporaneous controls (eg, emergency general surgery or acute care surgery procedures).
- Power Calculations: The sample size is expected to be stable in the four specialities in each hospital (table 1). On average, we expect approximately 1888 cases per speciality by site. We conducted a baseline assessment of opioid prescribing and found a mean of 30 pills and SD of 10. Assuming a baseline prescription rate of 30 pills per case, a conservative hypothetical estimate of speciality by site intra-cluster correlations of 0.2, alpha = 0.05 and power = 80%, we anticipate being able to detect a minimal difference of 4.58 pills in mean rates before-and-after the intervention if the SD remains 10. This would increase to a minimum detectable decrease of 9.16 pills assuming SD = 20, or minimal detectable difference of 16.21 pills if the SD = 50.
Measures of implementation effectiveness
Feasibility: Conducting pre-implementation interviews with key stakeholders at each hospital will provide important information about perceived barriers to the intervention and whether interviewees think it will be possible to implement the intervention.
Fidelity: Throughout the implementation, we will use data from the EDW queries to measure the extent to which each component is actually implemented, using the process measures noted above. Fidelity of implementation will also be assessed through the preoperative clinic observations.
Exposure: The degree to which the intervention components were actually experienced by the patient will be measured through data abstracted from the EDW, observations of preoperative clinic encounters and patient surveys.
Intensity: We will estimate a summary score, based on clinician and patient surveys, observations and focus groups.
Qualitative comparative analysis
In the final phase of the study, we will assess which components of the intervention, in combination with site-specific contextual factors, are most effective in reducing opioid prescribing. Specifically, we will conduct Qualitative Comparative Analysis (QCA) by combining the results of the qualitative and quantitative data to identify the necessary and sufficient conditions for improved opioid prescribing while preventing negative pain-related patient outcomes.
Using QCA, we will be able to identify more than one causal pathway to the outcome of interest (eg, reducing opioid prescribing) and identify conjunctional causation or the conditions that may only display their effects in conjunction with other conditions. QCA is a case-oriented mathematical approach that examines relationships between conditions and an outcome.70 QCA answers the question: what conditions, alone or in combination with other conditions, are necessary or sufficient to produce the outcome of interest?
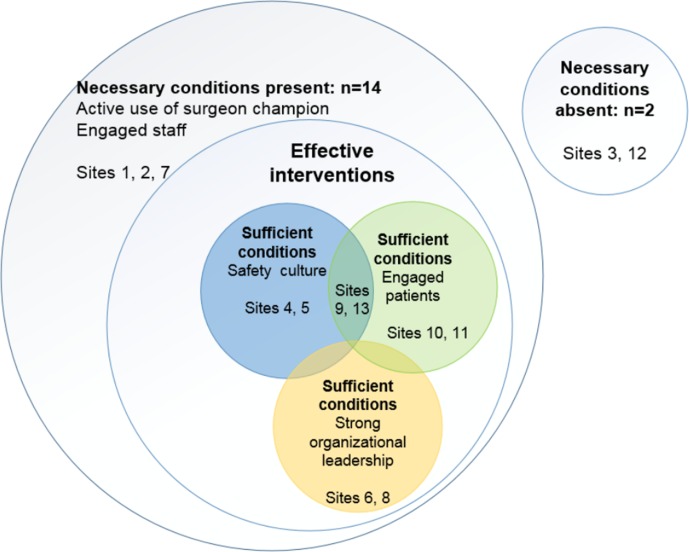
Data: The first step in QCA is to develop a conceptual model, based on input from the qualitative results. The transcripts from the surveys, interviews and observations will be coded and analysed to identify potential causal relationships. The research team will use a computer-assisted qualitative data analysis software (MAXQDA) to support the analysis. During analysis, categorical themes will be identified and applied to further transcripts. We will conduct a hybrid form of textual analysis which combines both inductive and deductive logics.71 72 The analytical strategy will be informed by the task at hand (assessment of the implementation of the intervention), as well as, the desire to allow unanticipated themes to emerge from the data and to allow participant understandings to be revealed, as in our prior work.73 74 Themes identified from the qualitative research will provide insight into site-specific implementation adaptations and how participants anticipate implementation of the intervention to reduce opioid prescribing while effectively controlling post-surgical pain. Results of the analysis will be used to build the QCA conceptual model. The conceptual model will emphasise the conditions that need to be in place, either individually or in combination, for the intervention to be effective at a specific hospital (ie, the necessary conditions) and what conditions, either individually or in combination, would produce the outcome in a specific hospital (ie, sufficient conditions). For example, using the results of the qualitative research, we might find the conditions most likely to influence implementation success include use of a surgeon champion, engaged staff, safety culture, engaged patients and strong organisational leadership. Figure 2 illustrates a hypothetical example that considers the necessary and sufficient set-theoretical relationships. As shown in the figure, necessary conditions must be present for an outcome to occur.
Figure 2.
Example of present and absent conditions for outcomes to occur.
Analysis: Crisp-set QCA analysis will be used to dichotomise the conditions for each hospital as either ‘having’ or ‘not having’ each condition. Data from the 24 sites within the six hospitals will be rank-ordered from highest to lowest on two outcomes (opioid prescriptions and pain-related patient measures). A ‘truth table’ will be constructed to assess whether all logically possible configurations have empirical cases. At the conclusion of the truth table analysis, we will return to the qualitative data to provide rich examples of the cases.
Sensitivity Analyses: Sensitivity analyses will examine relationships between change scores and numerous formulations of the domain-specific implementation scores to assess the robustness of the findings.
Ethics and dissemination
In this study, we describe the protocol for implementation and evaluation of a multifaceted opioid minimisation intervention. The study aims to identify the most effective hospital-type and speciality-specific intervention bundles for rapid dissemination into our 56-hospital learning collaborative and in hospitals throughout the USA. Dissemination activities will be further supported by our External Advisory Panel which includes public health leaders at the federal, state and city levels, as well as experts in medication diversion and addiction, public safety and health and drug policy and patient advocacy. Furthermore, findings will be disseminated through a combination of traditional approaches (peer-reviewed publications and conference presentations) and newer technology-driven approaches (social media accounts, websites and webinars). Dissemination materials will be prepared as soon as data are available and when analyses have been finalised.
Supplementary Material
Footnotes
Contributors: JJS conceptualised the study, developed the quantitative analysis plan and wrote significant portions of the manuscript. JJ also conceptualised the study, developed the qualitative analysis plan and wrote significant portions of the manuscript. WLAS, RH and MLS supported in writing and editing of the manuscript and detailing the study methodology. BL, JLH and KYB supported in the study conceptualisation and critically reviewed various drafts of the manuscript. All authors read and approved the final manuscript.
Funding: Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R34DA044752. Funding for this study runs from 1 September, 2017, through 31 August, 2020. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests: None declared.
Ethics approval: All study activities have been approved by the Northwestern UniversityInstitutional Review Board (ID STU00205053).
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Patient consent for publication: Not required.
References
- 1. Stulberg JJ BE, Nooromid MJ. Analysis of Northwestern Medicine’s Electronic Data Warehouse, 2016. [Google Scholar]
- 2. Ringwalt C, Gugelmann H, Garrettson M, et al. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res Manag 2014;19:179–85. 10.1155/2014/857952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bates C, Laciak R, Southwick A, et al. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol 2011;185:551–5. 10.1016/j.juro.2010.09.088 [DOI] [PubMed] [Google Scholar]
- 4. Morris BJ, Mir HR. The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg 2015;23:267–71. 10.5435/JAAOS-D-14-00163 [DOI] [PubMed] [Google Scholar]
- 5. Hill MV, McMahon ML, Stucke RS, et al. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg 2017;265:709–14. 10.1097/SLA.0000000000001993 [DOI] [PubMed] [Google Scholar]
- 6. Gray J, Hagemeier N, Brooks B, et al. Prescription Disposal Practices: A 2-Year Ecological Study of Drug Drop Box Donations in Appalachia. Am J Public Health 2015;105:e89–e94. 10.2105/AJPH.2015.302689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasak JM, Roth Bettlach CL, Santosa KB, et al. Empowering Post-Surgical Patients to Improve Opioid Disposal: A Before and After Quality Improvement Study. J Am Coll Surg 2018;226:235–40. 10.1016/j.jamcollsurg.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewis ET, Cucciare MA, Trafton JA. What do patients do with unused opioid medications? Clin J Pain 2014;30:654–62. 10.1097/01.ajp.0000435447.96642.f4 [DOI] [PubMed] [Google Scholar]
- 9. Levy B, Paulozzi L, Mack KA, et al. Trends in Opioid Analgesic-Prescribing Rates by Specialty, U.S., 2007-2012. Am J Prev Med 2015;49:409–13. 10.1016/j.amepre.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. (HHS Publication No. SMA 17-5044, NSDUH Series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 2017. [Google Scholar]
- 11. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use United States, 2008-2011. JAMA Intern Med 2014;174:802–3. 10.1001/jamainternmed.2013.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Diseases Control and Prevention (CDC). 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States: CDC National Center for Injury Prevention and Control, 2018. [Google Scholar]
- 13. Wide-ranging online data for epidemiologic research (WONDER. GA: Atlanta: Statistics CNCfH, ed, 2017. [Google Scholar]
- 14. Centers for Disease Control and Prevention. Annual Surveillance Report of Drug-Related Risks and Outcomes — United States, 2017. Surveillance Special Report 1: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 2017. [Google Scholar]
- 15. Centers for Diseases Control and Prevention (CDC). U.S. Opioid Prescribing Rate Maps 2018. 2018. https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html (Updated 3 Oct 2018).
- 16. Guy GP, Zhang K. Opioid Prescribing by Specialty and Volume in the U.S. Am J Prev Med 2018;55:e153–e155. 10.1016/j.amepre.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Volkow ND, McLellan TA, Cotto JH, et al. Characteristics of opioid prescriptions in 2009. JAMA 2011;305:1299–301. 10.1001/jama.2011.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 2012;172:425–30. 10.1001/archinternmed.2011.1827 [DOI] [PubMed] [Google Scholar]
- 19. Bartels K, Mayes LM, Dingmann C, et al. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One 2016;11:e0147972 10.1371/journal.pone.0147972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Breivik H. How to implement an acute pain service. Best Pract Res Clin Anaesthesiol 2002;16:527–47. 10.1053/bean.2002.0259 [DOI] [PubMed] [Google Scholar]
- 21. Calcaterra SL, Yamashita TE, Min SJ, et al. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med 2016;31:478–85. 10.1007/s11606-015-3539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carroll IR, Hah JM, Barelka PL, et al. Pain duration and resolution following surgery: an inception cohort study. Pain Med 2015;16:2386–96. 10.1111/pme.12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carroll I, Barelka P, Wang CK, et al. A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg 2012;115:1–702. 10.1213/ANE.0b013e31825c049f [DOI] [PubMed] [Google Scholar]
- 24. Clarke HA, Katz J, McCartney CJ, et al. Perioperative gabapentin reduces 24 h opioid consumption and improves in-hospital rehabilitation but not post-discharge outcomes after total knee arthroplasty with peripheral nerve block. Br J Anaesth 2014;113:855–64. 10.1093/bja/aeu202 [DOI] [PubMed] [Google Scholar]
- 25. Clarke H, Poon M, Weinrib A, et al. Preventive analgesia and novel strategies for the prevention of chronic post-surgical pain. Drugs 2015;75:339–51. 10.1007/s40265-015-0365-2 [DOI] [PubMed] [Google Scholar]
- 26. Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014;348:g1251 10.1136/bmj.g1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dowell D, Zhang K, Noonan RK, et al. Mandatory Provider Review And Pain Clinic Laws Reduce The Amounts Of Opioids Prescribed And Overdose Death Rates. Health Aff 2016;35:1876–83. 10.1377/hlthaff.2016.0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennedy-Hendricks A, Gielen A, McDonald E, et al. Medication Sharing, Storage, and Disposal Practices for Opioid Medications Among US Adults. JAMA Intern Med 2016;176:1027–9. 10.1001/jamainternmed.2016.2543 [DOI] [PubMed] [Google Scholar]
- 29. Dualé C. Prolonged use of opioids after surgery. BMJ 2014;348:g1280 10.1136/bmj.g1280 [DOI] [PubMed] [Google Scholar]
- 30. Gomes T, Juurlink DN, Dhalla IA, et al. Trends in opioid use and dosing among socio-economically disadvantaged patients. Open Med 2011;5:e13–22. [PMC free article] [PubMed] [Google Scholar]
- 31. Greer SM, Dalton JA, Carlson J, et al. Surgical patients' fear of addiction to pain medication: the effect of an educational program for clinicians. Clin J Pain 2001;17:157–64. 10.1097/00002508-200106000-00008 [DOI] [PubMed] [Google Scholar]
- 32. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag 2015;5:97–105. 10.2217/pmt.15.7 [DOI] [PubMed] [Google Scholar]
- 33. Chaparro LE, Smith SA, Moore RA, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013;7:CD008307 10.1002/14651858.CD008307.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Society ISM. Recommendations for Deterring Improper Use of Opioids, 2015. [Google Scholar]
- 35. Mason MJ, Golladay G, Jiranek W, et al. Depression Moderates the Relationship Between Pain and the Nonmedical Use of Opioid Medication Among Adult Outpatients. J Addict Med 2016;10:408–13. 10.1097/ADM.0000000000000253 [DOI] [PubMed] [Google Scholar]
- 36. McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain 2012;13:988–96. 10.1016/j.jpain.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menendez ME, Mellema JJ, Ring D. Attitudes and self-reported practices of hand surgeons regarding prescription opioid use. Hand 2015;10:789–95. 10.1007/s11552-015-9768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mixter CG, Meeker LD, Gavin TJ. Preemptive pain control in patients having laparoscopic hernia repair: a comparison of ketorolac and ibuprofen. Arch Surg 1998;133:432–7. [DOI] [PubMed] [Google Scholar]
- 39. Gordon DB, Dahl J, Phillips P, et al. The use of "as-needed" range orders for opioid analgesics in the management of acute pain: a consensus statement of the American Society for Pain Management Nursing and the American Pain Society. Pain Manag Nurs 2004;5:53–8. 10.1016/j.pmn.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 40. Rodgers J, Cunningham K, Fitzgerald K, et al. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am 2012;37:645–50. 10.1016/j.jhsa.2012.01.035 [DOI] [PubMed] [Google Scholar]
- 41. Sun EC, Darnall BD, Baker LC, et al. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med 2016;176:1286–93. 10.1001/jamainternmed.2016.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res 2015;8:695–702. 10.2147/JPR.S91924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hahn KL. Strategies to prevent opioid misuse, abuse, and diversion that may also reduce the associated costs. Am Health Drug Benefits 2011;4:107–14. [PMC free article] [PubMed] [Google Scholar]
- 44. Burcu M, Zito JM, Metcalfe L, et al. Trends in Stimulant Medication Use in Commercially Insured Youths and Adults, 2010-2014. JAMA Psychiatry 2016;73:992–3. 10.1001/jamapsychiatry.2016.1182 [DOI] [PubMed] [Google Scholar]
- 45. Zhou C, Florence CS, Dowell D. Payments For Opioids Shifted Substantially To Public And Private Insurers While Consumer Spending Declined, 1999-2012. Health Aff 2016;35:824–31. 10.1377/hlthaff.2015.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bao Y, Pan Y, Taylor A, et al. Prescription Drug Monitoring Programs Are Associated With Sustained Reductions In Opioid Prescribing By Physicians. Health Aff 2016;35:1045–51. 10.1377/hlthaff.2015.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Services DoHaH. Prescription Drug Monitoring Program Interoperability Standards: A Report to Congress. Washington, DC 2013. [Google Scholar]
- 48. Finklea KSL, Bagalman E. Prescription Drug Monitoring Programs. Congressional Research Service CRS Report Prepared for Members and Committees of Congress 2014. [Google Scholar]
- 49. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17:131–57. 10.1016/j.jpain.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 50. Renthal W. Seeking Balance Between Pain Relief and Safety: CDC Issues New Opioid-Prescribing Guidelines. JAMA Neurol 2016;73:513–4. 10.1001/jamaneurol.2016.0535 [DOI] [PubMed] [Google Scholar]
- 51. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep 2016;65:1–49. 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 52. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248–73. 10.1097/ALN.0b013e31823c1030 [DOI] [PubMed] [Google Scholar]
- 53. Council NS. Prescription Nation 2016, 2016. [Google Scholar]
- 54. Administration SAaMHS. SAMHSA Opioid overdose prevention toolkit. 2016. Report No: (SMA) 16-4742.
- 55. Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aarons GA, Hurlburt M, Horwitz SM. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health 2011;38:4–23. 10.1007/s10488-010-0327-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Creswell JW. A concise introduction to mixed methods research: SAGE publications, 2015. [Google Scholar]
- 58. Michie S, Johnston M, Abraham C, et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26–33. 10.1136/qshc.2004.011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yeh B. Legal Issues Relating to the Disposal of Dispensed Controlled Substances, 2010. [Google Scholar]
- 60. Slight SP, Berner ES, Galanter W, et al. Metadata Correction: Meaningful Use of Electronic Health Records: Experiences From the Field and Future Opportunities. JMIR Med Inform 2015;3:e32 10.2196/medinform.5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Galanter WL, Thambi M, Rosencranz H, et al. Effects of clinical decision support on venous thromboembolism risk assessment, prophylaxis, and prevention at a university teaching hospital. Am J Health Syst Pharm 2010;67:1265–73. 10.2146/ajhp090575 [DOI] [PubMed] [Google Scholar]
- 62. Galanter W, Falck S, Burns M, et al. Indication-based prescribing prevents wrong-patient medication errors in computerized provider order entry (CPOE). J Am Med Inform Assoc 2013;20:477–81. 10.1136/amiajnl-2012-001555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Galanter WL, Bryson ML, Falck S, et al. Indication alerts intercept drug name confusion errors during computerized entry of medication orders. PLoS One 2014;9:e101977 10.1371/journal.pone.0101977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Benning A, Ghaleb M, Suokas A, et al. Large scale organisational intervention to improve patient safety in four UK hospitals: mixed method evaluation. BMJ 2011;342:d195 10.1136/bmj.d195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reeves S, Kuper A, Hodges BD. Qualitative research methodologies: ethnography. BMJ 2008;337:a1020 10.1136/bmj.a1020 [DOI] [PubMed] [Google Scholar]
- 66. Taxis K, Barber N. Ethnographic study of incidence and severity of intravenous drug errors. BMJ 2003;326:684 10.1136/bmj.326.7391.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barach P, Johnson JK, Ahmad A, et al. A prospective observational study of human factors, adverse events, and patient outcomes in surgery for pediatric cardiac disease. J Thorac Cardiovasc Surg 2008;136:1422–8. 10.1016/j.jtcvs.2008.03.071 [DOI] [PubMed] [Google Scholar]
- 68. Bognár A, Barach P, Johnson JK, et al. Errors and the burden of errors: attitudes, perceptions, and the culture of safety in pediatric cardiac surgical teams. Ann Thorac Surg 2008;85:1374–81. 10.1016/j.athoracsur.2007.11.024 [DOI] [PubMed] [Google Scholar]
- 69. Johnson JK, Barach P, Vernooij-Dassen M, et al. Conducting a multicentre and multinational qualitative study on patient transitions. BMJ Qual Saf 2012;21 Suppl 1(Suppl 1):i22–i28. 10.1136/bmjqs-2012-001197 [DOI] [PubMed] [Google Scholar]
- 70. Ragin C. The comparative method: Moving beyond qualitative and quantitative strategies. Berkeley: University of California Press, 1987:85–118. [Google Scholar]
- 71. Lofland J, Lofland L. Analyzing social settings. Belmont, CA: Wadsworth Publishing Company, 2006. [Google Scholar]
- 72. Miles M, Huberman A, Saldana J. Qualitative Data Analysis: A Methods Sourcebook2014. Los Angeles, CA: SAGE Publications Inc, 2014. [Google Scholar]
- 73. Johnson JK, Woods DM, Stevens DP, et al. Joy and challenges in improving chronic illness care: capturing daily experiences of academic primary care teams. J Gen Intern Med 2010;25 Suppl 4(Suppl 4):581–5. 10.1007/s11606-010-1408-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stevens DP, Bowen JL, Johnson JK, et al. A multi-institutional quality improvement initiative to transform education for chronic illness care in resident continuity practices. J Gen Intern Med 2010;25 Suppl 4:574–80. 10.1007/s11606-010-1392-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.