Abstract
X-linked nephrogenic diabetes insipidus (NDI) is caused by mutations in the arginine vasopressin (AVP) receptor type 2 (AVPR2) gene. A 20-month old boy and his 8-year old brother both presented with polyuria, polydipsia, and failure to thrive. Both boys demonstrated partial response to DDAVP (1-desamino-8-D AVP or desmopressin) administration, thus NDI diagnosis was delayed; it was not until exome sequencing showed AVPR2 defects that their diagnosis was made and proper treatment was instituted. Both patients were hemizygous for two AVPR2 variants; one was predicted in silico to affect AVPR2 mRNA splicing. Both were functionally studied in vitro; a minigene assay revealed that the novel AVPR2 c.276 A>G mutation creates a cryptic splice acceptor site that leads to 5’ truncation of AVPR2 exon 2 when tested in HEK293 human kidney cell line. Both patients have been treated with high dose DDAVP with a remarkable improvement of their symptoms and accelerated linear growth and weight gain.
Conclusion: We present here a unique case of partial X-linked NDI due to an AVPR2 splice site mutation; patients with DI of unknown etiology may harbor splice site mutations that are initially underestimated in their pathogenicity on sequence analysis.
Keywords: X-linked nephrogenic diabetes insipidus, Arginine vasopressin receptor type 2, Partial nephrogenic diabetes insipidus, Pathogenic splice site mutation
Introduction
Diabetes insipidus (DI) is characterized by complete or partial loss of arginine vasopressin (AVP) function leading to polyuria, polydipsia, and hypernatremia. This is due to either posterior pituitary defects leading to low, absent, or erroneous AVP production (central DI), or due to a disruption in the signalling machinery mediating AVP effects in the kidney (nephrogenic DI, NDI).
AVP receptor type 2 (AVPR2) is a G-protein coupled receptor [6] that acts as the primary mediator of the water conserving action of AVP in the kidney. Activation of AVPR2 by AVP binding ultimately leads to the translocation of aquaporin 2 (AQP2) to the apical surface of the principal cells in the kidney collecting duct; this occurs via activation of the cAMP-dependent protein kinase (PKA) pathway, thereby increasing water reabsorption and urine osmolality [9].
Mutations in AVPR2 lead to X-linked NDI. The AVPR2 gene resides on chromosome Xq28 and consists of 3 exons [6]. More than 220 AVPR2 variants have been shown to cause X-linked NDI, and there is considerable phenotypic variability in the response to fluid deprivation and DDAVP (1-desamino-8-D AVP or desmopressin) administration [9, 1]. Missense mutations can lead to (partially) preserved receptor expression and function and hence a milder DI phenotype [8]. Three AVPR2 splice site mutations have been described in patients with X-linked NDI [9].
Here we present two male siblings with an identical, novel splice site mutation in AVPR2 leading to partial X-linked NDI. The defects were identified by exome sequencing after multiple attempts to identify the nature of disease in these two siblings had failed previously. We provide a report describing the patients’ clinical features as well as functional analysis of the effect of the mutation on RNA splicing.
Results
A 20-month old boy (II-4, Fig. 1A) presented with failure to thrive, nausea, recurrent fevers, polyuria, and polydipsia. He was enrolled in the NIH Undiagnosed Diseases program [3,4] as well as NHGRI clinical protocol 76-HG-0238, “Diagnosis and Treatment of Patients with Inborn Errors of Metabolism and Other Genetic Disorders”; his parents gave written informed consent. The boy was born at 39 weeks after a pregnancy that was complicated by polyhydramnios. Laboratory evaluation showed hypernatremia (146 mEq/L) with elevated serum osmolality (304 mOsm/kg) and inappropriately low urine osmolality (105 mOsm/kg, Table 1). AVP levels were elevated (38, range 0.7–3.8 pg/mL). Pituitary MRI showed the absence of a posterior pituitary bright spot (Suppl. Fig. 1); while this is a typical radiological finding in central DI, the posterior pituitary bright spot is also often absent in NDI [2]. A water deprivation test failed to adequately increase urine osmolality (Table 1). However, DDAVP administration (1 μg s.c.) increased urine osmolality slightly to 347 mOsm/kg, consistent with partial NDI.
Fig 1.
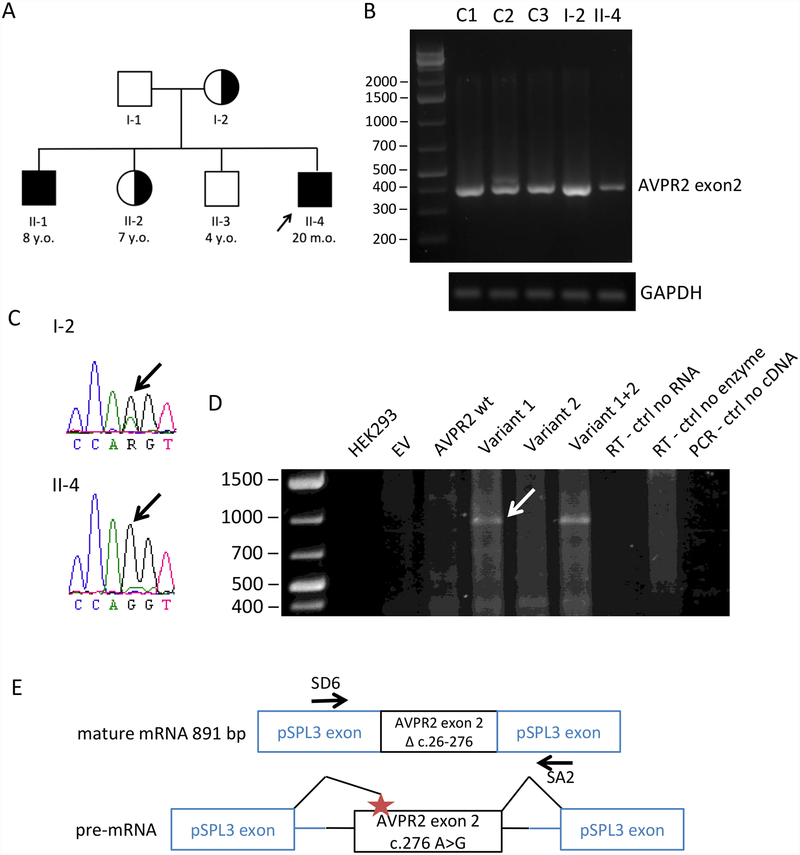
Family pedigree of the proband and analysis of splice site mutations in AVPR2. A. Family pedigree of the proband (arrow), his 3 siblings and his parents. Unaffected (empty symbols), affected (filled symbols), carrier (half symbols), male (square), female (circle). B. RT-PCR was performed on peripheral blood lymphocyte cDNA from three controls (C1–C3), the affected patient (II-4) and his mother (I-2), with primers covering most of exon 2 and both sides of AVPR2 c. 276 A>G. A control PCR on the same samples was performed using primers for GAPDH. C. Sequencing of the AVPR2 exon 2 RT-PCR products of the patient and his mother where the locus of the mutation is highlighted (arrows). D. Minigene assay. cDNA from untransfected HEK293, empty vector-transfected HEK293 (EV), the AVPR2 wt and three mutants in pSPL3, and negative controls (reverse transcription negative controls without reverse transcriptase or without RNA, and RT-PCR negative control without cDNA). The arrow points to the mutation-specific band that was sequenced. E. Schematic of the RT-PCR product from the c.276 A>G mutant within the minigene assay lacking the 5’ end of exon 2. The location of the SD6 and SA2 primers used in the minigene assay is indicated.
Table 1.
Laboratory findings of both patients during fluid deprivation/AVP stimulation tests.
| Parameter | II-4 (2013) | II-4 (2012) | II-1 (2013) | |
|---|---|---|---|---|
| Basal | Serum osmolality (207–295 mOsm/kg) |
304 | 313 | 291 |
| Serum Na+ (135–145 mEq/L) |
146 | 146 | 137 | |
| Urine osmolality (mOsm/L) |
105 | 234 | 60 | |
| AVP (0.7–3.8 pg/mL) |
38 | 20 | 7.5 | |
| After fluid deprivation | Serum osmolality (207–295 mOsm/kg) |
n.p. | 315 | n.p. |
| Serum Na+ (135–145 mEq/L) |
n.p. | 146 | n.p. | |
| Urine osmolality (mOsm/L) |
n.p. | 257 | n.p. | |
| After DDAVP | Serum osmolality (207–295 mOsm/kg) |
317 | 314 | 301 |
| Serum Na+ (135–145 mEq/L) |
147 | 149 | 147 | |
| Urine osmolality | 347 | 511 | 216 |
Normal ranges in parentheses, n.p. not performed.
The patient’s older brother (II-1, then 8 years old, Fig. 1A), also presented with polyuria, polydipsia, and failure to thrive and was subsequently diagnosed with DI (Table 1), while the two parents, a sister and a brother do not have DI (Fig. 1A).
Initial Sanger sequencing reported no strongly pathogenic variants in AVPR2 or AQP2, but mention was made of a possible splicing effect of a synonymous change, c.276A>G (variant 1). We performed exome sequencing and found the same variant and a second variant in AVPR2, c.26 −6 T>G (rs56689668; variant 2), both predicted in silico to affect mRNA splicing. The two affected children were hemizygous for these variants; the patients’ mother (I-2) and sister (II-2) were heterozygous for these variants (Fig. 1A).
The novel AVPR2 c.276 A>G variant was predicted in silico to create a cryptic splice acceptor site at c.277 that could lead to skipping of the first 251 bases of exon 2 and create a 5’ truncated exon 2. The second variant was identified in the 1000 Genomes Project with an allele frequency of 0.003 [5] and could reduce the efficiency of the natural splice acceptor site of intron 2 (c.26).
AVPR2 mRNA species in peripheral blood lymphocytes of the affected patient, his mother, and three healthy controls were tested by reverse transcription PCR (RT-PCR) for the predicted effect of variant 1. The forward primer binds within the predicted truncated region of AVPR2 exon 2. RT-PCR products were present in all samples at the sizes expected for exon 2 without truncation (Fig. 1B). Sequencing of the RT-PCR products confirmed the presence of AVPR2 exon 2 in both the proband and the mother; the patient’s mRNA contained only the c.276 A>G mutation whereas the mother’s mRNA contained both the mutation and the wild-type (Fig. 1C), so we did not find evidence of alternative splicing in the lymphocyte mRNA of the patient.
Both variants were functionally studied in a minigene assay. Exon 2 of wild-type AVPR2, and exon 2 containing one or both variants together with the flanking intronic sequences, were cloned into the pSPL3 vector. After transfection into HEK293 human kidney cells, mRNA synthesis from the plasmids using the cells’ own transcription and splicing machinery led to mRNA products containing exon 2 of AVPR2 flanked by two exons from the pSPL3 vector (Fig. 1E).
Variant 1 leads to an approximately 900 bp product, consistent with a 5’ truncated exon 2 lacking its first 251 bases (Fig. 1D), which was confirmed by sequencing of this band. The c.26 −6 T>G variant did not have an independent effect in this assay, and the construct containing both variants led to the same product as that seen with the c.276 A>G mutation alone (Fig. 1D).
Both the patient and his affected brother are currently being treated with DDAVP, which has led to improvement of DI symptoms as well as improved growth and weight gain (Suppl. Fig. 1B and 1C). Both patients were initially treated with thiazide diuretics and ibuprofen, which did not have a significant effect on polyuria and polydipsia; patient II-1 continued to have a fluid intake of more than 3 liters every day, with frequent urination (every 1–2 hours) and nocturia (at least 3–4 times each night). After establishment of the diagnosis of X-linked NDI due to the AVPR2 splice site mutation, treatment with DDAVP was initiated. Due to the high dosage required, both patients receive DDAVP nasal spray (0.90 mg/day II-1 and 0.45 mg/day II-4) in the highly concentrated formulation typically used to treat Von Willebrand disease (Stimate).
Discussion
We detected a novel AVPR2 splice site mutation as a cause of partial X-linked NDI in two male siblings. The novel AVPR2 c. 276 A>G mutation causes alternative splicing leading to the 5’ truncation of exon 2, as shown in a minigene assay. This truncation causes a frameshift followed by a premature stop codon, that would most likely target the mRNA for nonsense-mediated decay and lead to absence of AVPR2 protein; this is likely to underlie the pathogenic mechanism for NDI in these patients.
AVPR2 mutations causing NDI can vary in their functional severity; clinical symptoms and the response to DDAVP can be diverse. Three cases of X-linked NDI caused by splice site mutations [9] have been described, but to our knowledge no AVPR2 splice site mutation has been functionally studied. This is also the first time that exome sequencing has led to the identification of a genetic defect clarifying the nature of the disease in children with a confusing form of DI, and led to improvement of their clinical situation.
AVPR2 nonsense mutations generally lead to nonsense-mediated decay and hence complete absence of the receptor [10]. Although partial NDI phenotypes have been described, these are mostly found in patients with missense mutations in which the AVPR2 receptor is present at the cell membrane [8]. However, the newly created splice acceptor site in our case may not be sufficiently strong to abolish all correct splicing of AVPR2. The natural splice acceptor site of intron 1 could retain some of its function, which may allow the production of a small amount of correctly spliced AVPR2 mRNA. This would be consistent with the clinical phenotype of partial NDI with a response to unusually high dose DDAVP. To our knowledge, this is the first clinical and functional description of an AVPR2 splice site mutation leading to a partial NDI phenotype.
Standard management of X-linked NDI in children includes hydrochlorothiazide and indomethacin [10]. These treatments, however, only partially improve symptoms, generally decreasing urine volume by about 30–70 % [7]. Partial NDI can be treated with high doses of DDAVP, but conventional subcutaneous administration would require multiple injections per day and oral administration a large volume of tablets [2]. Consequently, our patients are treated with nasal DDAVP, which is available at a higher dosage.
In conclusion, patients with DI of unknown etiology can harbor splice site mutations whose pathogenicity may initially be underestimated on routine sequence analysis. The predicted amino acid sequence was not affected by this novel mutation, and functional studies were required to demonstrate an effect on mRNA splicing. Furthermore, AVPR2 splice site mutations may lead to a partial NDI phenotype that can be confusing in terms of its pathophysiology; this form of NDI can be managed by high dose DDAVP administration.
Supplementary Material
What is known:
X-linked nephrogenic diabetes insipidus is caused by mutations in the arginine vasopressin receptor type 2 (AVPR2), and disease severity can vary depending on the functional effect of the mutation.
What is new:
We demonstrate here that a splice site mutation in AVPR2 identified by WES leads to partial X-linked nephrogenic diabetes insipidus in two male siblings.
Treatment with high dose DDAVP led to improvement of polyuria and polydipsia, weight gain, and improved growth.
Abbreviations
- AQP2
aquaporin 2
- AVP
Arginine vasopressin
- AVPR2
Arginine vasopressin receptor type 2
- DDAVP
1-desamino-8-D AVP or desmopressin
- DI
Diabetes insipidus
- NDI
Nephrogenic diabetes insipidus
- RT-PCR
reverse transcription PCR
References
- 1.Babey M, Kopp P, Robertson GL (2011) Familial forms of diabetes insipidus: clinical and molecular characteristics. Nature reviews Endocrinology 7:701–714 [DOI] [PubMed] [Google Scholar]
- 2.Faerch M, Christensen JH, Corydon TJ, Kamperis K, de Zegher F, Gregersen N, Robertson GL, Rittig S (2008) Partial nephrogenic diabetes insipidus caused by a novel mutation in the AVPR2 gene. Clinical endocrinology 68:395–403 [DOI] [PubMed] [Google Scholar]
- 3.Gahl WA, Markello TC, Toro C, Fajardo KF, Sincan M, Gill F, Carlson-Donohoe H, Gropman A, Pierson TM, Golas G, Wolfe L, Groden C, Godfrey R, Nehrebecky M, Wahl C, Landis DM, Yang S, Madeo A, Mullikin JC, Boerkoel CF, Tifft CJ, Adams D (2012) The National Institutes of Health Undiagnosed Diseases Program: insights into rare diseases. Genet Med 14:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gahl WA, Tifft CJ (2011) The NIH Undiagnosed Diseases Program: lessons learned. JAMA 305:1904–1905 [DOI] [PubMed] [Google Scholar]
- 5.Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juul KV, Bichet DG, Nielsen S, Norgaard JP (2014) The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. American journal of physiology Renal physiology 306:F931–940 [DOI] [PubMed] [Google Scholar]
- 7.Moeller HB, Rittig S, Fenton RA (2013) Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocrine reviews 34:278–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neocleous V, Skordis N, Shammas C, Efstathiou E, Mastroyiannopoulos NP, Phylactou LA (2012) Identification and characterization of a novel X-linked AVPR2 mutation causing partial nephrogenic diabetes insipidus: a case report and review of the literature. Metabolism: clinical and experimental 61:922–930 [DOI] [PubMed] [Google Scholar]
- 9.Spanakis E, Milord E, Gragnoli C (2008) AVPR2 variants and mutations in nephrogenic diabetes insipidus: review and missense mutation significance. Journal of cellular physiology 217:605–617 [DOI] [PubMed] [Google Scholar]
- 10.Wesche D, Deen PM, Knoers NV (2012) Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatric nephrology [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.