Abstract
This study utilized a novel robotic device, the ACT-4D, to investigate the relationship between the flexion synergy and stretch reflexes in individuals with chronic hemiparetic stroke. Because the flexion synergy influences the amount of elbow flexor muscle activation present in the paretic limb during tasks requiring shoulder abduction loading, it was hypothesized that stretch reflexes may be modulated by expression of this abnormal muscle coactivation pattern. To test this hypothesis, the ACT-4D was used to enable 10 individuals with chronic hemiparetic stroke to generate varying amounts of shoulder abduction torque while concurrently receiving elbow extension position perturbations. It was found that increased expression of the flexion synergy led to greater reflex amplitudes as well as lower reflex velocity thresholds. The physiological basis of the flexion synergy is briefly discussed, as are the implications of the flexion synergy and stretch reflexes for purposeful movement.
Keywords: flexion synergy, stretch reflex, chronic hemiparetic stroke, robotics
I. INTRODUCTION
Chronic hemiparetic stroke is frequently associated with a compromised ability to reach with the paretic upper limb especially when supporting the weight of the limb [1]. While it has been suggested that this impairment may be due in part to changes in muscle properties following stroke [2], it is likely that the predominant contributors to the overall functional deficit are abnormal muscle coactivation patterns and/or altered reflexes. Therefore, of particular relevance to the work to be presented herein are the so-called ‘flexion synergy’ and the stretch reflex.
Originally described clinically [3, 4], the flexion synergy has subsequently been quantified in individuals with chronic hemiparetic stroke [1, 5, 6] and is defined as the involuntary neural coupling of shoulder abductor activity with activation of elbow flexors in the paretic upper limb. This coupling is particularly limiting because it leads to the loss of independent joint control, hampering volitional elbow extension when shoulder abduction is concurrently required. Importantly, it should be noted that the expression of the flexion synergy is progressive; that is, as the amount of shoulder abductor activity is increased, so too is activity in the elbow flexors. This graded expression pattern therefore makes it more difficult, for example, to extend at the elbow while lifting a book than while supporting the weight of the limb alone.
In addition to the flexion synergy, stretch reflexes may also play a role in movement disorders associated with chronic hemiparetic stroke. Stretch reflexes are typically defined by three characteristics: velocity sensitivity, position sensitivity and a dependence upon the level of muscle activation present prior to the stretch [7–10]. Reflex amplitude is directly proportional to all three of these parameters, such that greater stretch velocities, joint angles or higher levels of muscular preactivation result in greater reflex amplitudes. Importantly, these reflex parameters are also inherently linked: an increase in muscular preactivation allows a lower velocity stretch or a stretch beginning at a more acute joint angle (i.e. shorter muscle length) to elicit a reflex, while a reflex can be elicited at a lower level of preactivation if the stretch velocity or muscle length is increased. Following stroke, it has generally become accepted that the velocity, joint angle and preactivation thresholds necessary for reflex evocation decrease [9, 11–15](although it should be noted that the physiological mechanisms responsible for these shifts remain unclear). Due to these reductions, there is an increased likelihood that the combination of a slower speed, shorter muscle length, and lower preactivation level stretch may elicit a reflex. It should be noted that despite the aforementioned dependence of the stretch reflex on position, velocity, and preactivation, the work to be presented here will focus exclusively on the latter two components.
Although the interplay between the flexion synergy and stretch reflexes remains largely uninvestigated (however see [16] for a recent study on this subject), clear hypotheses can be drawn. Specifically, expression of the flexion synergy leads to abnormally increased elbow flexor activity during reaching (i.e. elbow extension) motions that necessitate shoulder abduction loading. Increases in muscle preactivation are known to impact the stretch reflex by increasing its amplitude as well as decreasing the velocity and joint angle at which a reflex is evoked. Given the graded nature of flexion synergy and stretch reflex expression, it is hypothesized that progressive increases in shoulder abduction loading will lead to incremental increases in the electromyographic activity of the elbow flexors (e.g. biceps brachii), which will in turn lead to larger stretch reflex amplitudes and lower velocity thresholds as preactivation increases for a given starting angle. In other words, it is hypothesized that the expression of the flexion synergy in the paretic upper limb will modify the expression of stretch reflexes in the same limb. To test this hypothesis, a novel robotic device, the ACT-4D, was developed and utilized that allowed quantification of stretch reflexes during simultaneous expression of varying levels of the flexion synergy.
II. METHODS
A. The robotic device
The robotic device used in this experiment is the ACT-4D, which is a modified version of the ACT-3D [17, 18]. Briefly, the ACT-3D consists of an admittance-controlled Haptic-Master robotic device retrofitted with a parallelogram mechanism that fixes the angular orientation of the robot endpoint in space, a 6 DOF loadcell (JR3, Woodland, CA, USA), and a custom graphical user interface. The ACT-3D allows unrestrained movement in three dimensions, and can impose force profiles upon its user in each of these three dimensions. The ACT-4D retains these capabilities while additionally including a custom elbow perturbation system. The elbow perturbation system – mounted around the force/torque sensor of the ACT-3D – consists of the following: a FHA-17C-DC24 combined motor and 1:100 gearbox (Harmonic Drive, Peabody, MA, USA), two cable drums, and a forearm beam. The gearbox is connected to the motor drum, while the sensor drum (located over the force/torque sensor), serves as the attachment point for the forearm beam. The drums are connected via pretensioned cables, capable of transmitting torque from the motor drum to the sensor drum. While the sensor drum can rotate about the vertical axis of the loadcell, the forearm beam is fixed in place relative to the sensor drum. The system can generate a peak torque of approximately 75 Nm, an acceleration of 4,500 °/s2, and peak velocity of 450 °/s. Elbow rotations are measured via an encoder located in the motor, a magnetic potentiometer located on the sensor drum, and two accelerometers located on the forearm beam. The user’s arm is tethered to the forearm beam through a custom orthosis or a forearm-wrist-hand fiberglass cast.
B. Participants
Ten individuals with chronic hemiparetic stroke (> 1 year post-stroke) participated in this investigation. Fugl-Meyer Motor Assessment scores ranged from 16–41 of a possible 66, representing moderate to severe impairment. For inclusion in the study, potential participants were required to posses full passive range of motion of the elbow, and ≥ 90° of shoulder abduction and shoulder flexion. Participants were additionally required to exhibit some volitional control of elbow flexion/extension for obtaining maximum voluntary torques. Current use of antispastic medications was grounds for exclusion from the study.
All participants provided informed consent to participate in the study, which was approved by the Institutional Review Board of Northwestern University.
C. Experimental setup
For the purposes of collecting maximum voluntary torques (MVT), participants were seated in a Biodex experimental chair (Biodex Medical Systems, Shirley, NY), with their trunk secured by over the shoulder and lap belt restraints. A participant-specific fiberglass forearm-wrist-hand cast was fitted to the paretic limb, followed by placement of active differential surface electrodes (Delsys, Boston, MA) on the paretic biceps brachii. Participants were subsequently connected rigidly at the level of the wrist to a 6 DOF loadcell (JR3, Woodland, CA). The paretic limb was positioned such that it retained 85° shoulder abduction, 45° shoulder flexion, and 105° elbow flexion.
For the flexion synergy and stretch reflex phase of the protocol, participants were removed from the isometric testing rig and interfaced with the ACT-4D (Fig. 1). The medial epicondyle of the humerus was aligned with the center of rotation of the sensor drum described previously, with the casted forearm tethered to the forearm beam. Participants were again restrained by seat belts, and positioned such that they retained 85° of shoulder abduction and approximately 60° of shoulder flexion. Elbow angle was not fixed, but was free to move from approximately 60–150°.
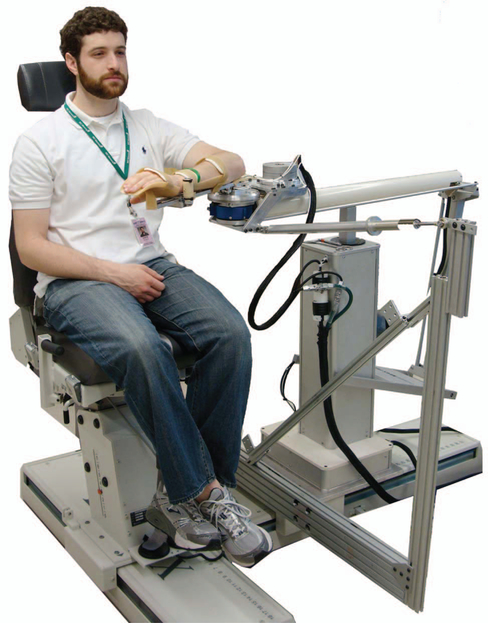
Fig. 1.
The ACT-4D system. The ACT-4D consists of an ACT-3D robotic system coupled with a dedicated elbow perturbation device and damper. The ACT-3D includes a modified Haptic-Master robot, a Biodex System 4 base station, and a custom graphical user interface (not shown). The ACT-4D is configured to allow movement in the vertical direction, impose forces along the vertical axis, and provide position perturbations in either elbow extension or flexion.
D. Experimental protocol
After obtaining shoulder abduction (SABD) MVTs, participants were transitioned to the flexion synergy/stretch reflex setup and protocol. Following placement of the paretic upper limb in the ACT-4D device (described above), participants were instructed to rest the paretic limb on a haptic surface, and relax completely. The ACT-4D was subsequently used to deliver a series of 30 stretches over the full range of motion (approximately 90° total range, from ~60–150° of elbow flexion), at a velocity of 6 °/s.
Individual flexion synergy/stretch reflex trials were subsequently acquired, each consisting of the following sequence of events (depicted in Fig. 2):
Fig. 2.
Trial sequence. Each trial began with a rest phase, followed by a lifting phase wherein participants lifted the limb above a haptic table while producing varying amounts of shoulder abduction torque. Subsequently, a position perturbation was applied to the limb by the ACT-4D during maintenance of shoulder abduction torque. After holding the limb in the extended position for 2 seconds, the ACT-4D returned the limb to the home position. The lower three panels are example data from one perturbation trial. Red trace: motor angle; blue trace: perturbation angular velocity profile; black trace: BIC EMG.
a mandatory resting phase of 2 s
a pre-stretch baseline phase that included lifting the limb from the haptic surface (i.e. producing SABD torque) and holding it in the elevated position for 2 s
a stretch perturbation phase, which was applied during the maintenance of the elevated limb posture/production of SABD torque
a holding phase requiring the participant to maintain the elevated posture/production of SABD torque for 2 s at the extended elbow position
a rest and return phase where the limb was lowered to the haptic surface and returned to the starting angle
Shoulder abduction torque was modulated by requiring participants to lift the paretic limb above the haptic surface, achieving a shoulder abduction angle of 85°, while the ACT-4D applied forces in the vertical direction. Shoulder abduction torque levels included 0, 12.5, 25, 37.5 and 50 % of MVT. For reference, 0% MVT gave participants the perception that their arm was weightless; that is, the weight of the arm was fully supported by the robot. Additionally, one level, designated ‘Table’, was used as a baseline condition where participants rested the paretic limb on a haptic surface without lifting or producing SABD torque. Position perturbations applied to the limb had peak velocities of 6, 15, 30, 45, 60, 90, 180 and 270°/s. Again for reference, a 90° perturbation at 6°/s took approximately 15 s to complete, whereas a 90° perturbation at 270°/s required approximately 0.333 s to complete. A set of synergy/reflex trials consisted of all 8 stretch velocities at each of the 6 SABD load levels. Three sets were completed per participant, with load level and stretch velocity presented in a random order.
E. Data analysis
Custom Matlab software (The MathWorks, Natick, MA) was used to analyze all data. EMG of the biceps brachii (BIC) was bandstop filtered from 55–65 Hz, then bandpass filtered between 10–100 Hz. Data were then rectified and smoothed using a 10ms 0-phase moving average filter and subsequently normalized by the peak EMG associated with its corresponding MVT. For each participant, the BIC EMG was ensemble averaged at each speed and SABD load combination. Baseline correction of EMG data was performed by removing from each trial the mean of 500ms of data prior to perturbation onset, so that reflex amplitudes subsequently computed reflected only the amount of EMG produced in addition to any preactivation. EMG data were parsed into three reflexive components: short latency reflexes (SLR), which spanned from 20–50ms post perturbation onset, long latency reflexes (LLR), which spanned the interval from 50–100ms post perturbation onset, and full reflexes (FULL), which were computed from 20–150ms post perturbation onset. Window averages of baseline corrected ensemble averaged BIC EMG data were taken at each reflexive component, and averaged across participants.
III. RESULTS
The response of the biceps brachii muscle to ramp and hold position perturbations provided during maintenance of varying levels of shoulder abduction (SABD) torque was investigated in the paretic upper limbs of 10 individuals with chronic hemiparetic stroke.
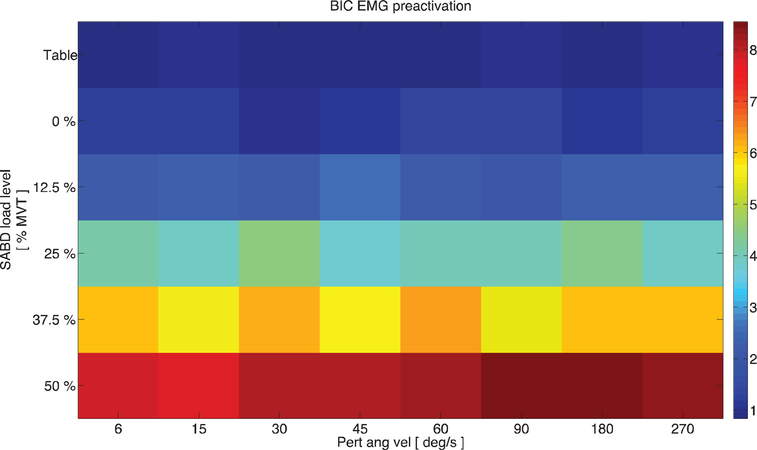
Fig. 3 demonstrates graphically the flexion synergy, as expressed across 10 participants. The ordinate represents the amount of shoulder abduction torque (% MVT) being produced by the group of participants. The abscissa represents the velocity of the impending stretch perturbation, and the colormap represents the amount of BIC preactivation present prior to application of stretches at the indicated velocities. It can be seen that as the requirement of SABD torque is increased, so too is the amount of spontaneously generated BIC activity, consistent with the definition of the flexion synergy.
Fig. 3.
The flexion synergy. Across-participant data representing expression of the flexion synergy. Ordinate: SABD load (%MVT) being produced by participants; abscissa: impending stretch velocity; colormap: amount of BIC EMG, expressed as % max, generated spontaneously in response to SABD loading prior to application of a stretch perturbation.
An example of a single representative participant’s synergy and reflex data can be seen in Fig. 4. Here, each column represents a given perturbation angular velocity, and each row a given SABD load. Each red trace is the ensemble average BIC activation of three trials at that velocity/load combination. It can be seen qualitatively from this figure that as velocity is increased (across a row), or as SABD load is increased (down a column), reflex magnitude is increased, both consistent with the hypotheses of this study.
Fig. 4.
Single-participant synergy + reflex data. This figure depicts BIC EMG data at each SABD load level and perturbation velocity for a single representative participant. Each red trace is the ensemble average of 3 perturbations at that load/velocity combination. It can be seen that as perturbation velocity or SABD loading is increased, reflex amplitude is also increased.
The trends shown in Fig. 4 were mirrored across participants, with significant effects of SABD load and perturbation velocity on reflex amplitude (P<0.05). In Fig. 5, across-participant data has been separated into the SLR, LLR and FULL reflex components. As with Fig. 4, each column represents a given perturbation velocity, while each row represents a given SABD load level. The colormap indicates the amplitude of the across-participant BIC reflex. It can be seen from Fig. 5 that although the short (SLR) and long (LLR) latency reflexes both increase with increasing velocity of stretch or increasing SABD load, the LLR component is larger than that of the SLR. Additionally, it should be reiterated that in both the ‘Table’ and ‘0%’ SABD loading conditions, the participants were not required to produce shoulder abduction torque. Therefore, despite the mechanical distinction between the two conditions (resting on a haptic surface vs floating above the haptic surface), the reflexes evoked were approximately equivalent.
Fig. 5.
Across-participant data. From left to right, the panels represent (1) short latency reflexes (20–50ms post perturbation onset), (2) long latency reflexes (50–100ms post perturbation onset), and full reflexes (20–150ms post perturbation onset). Ordinate: shoulder abduction load level; abscissa: perturbation angular velocity; colormap: BIC reflex amplitude, expressed as % max. It can be seen that across participants, reflex amplitude increases with SABD load or perturbation velocity. Note also that long latency reflexes have larger amplitudes than do short latency reflexes.
IV. DISCUSSION
This study utilized a novel robotic device, the ACT-4D, to investigate the potential effects of the flexion synergy on stretch reflexes in the paretic upper limb of individuals with chronic hemiparetic stroke. The ACT-4D allowed simultaneous manipulation of the shoulder abduction torque the user was required to generate while applying single joint elbow extension perturbations at varying velocities. This combination of synergy and stretch reflex manipulation provided unique insight into the relationship between these two motor abnormalities. It was hypothesized that increased expression of the flexion synergy would modify the stretch reflex by (1) increasing the reflex amplitude at a given perturbation velocity, and by (2) lowering the velocity at which a reflex could be elicited. Indeed, this hypothesis was confirmed in 10 individuals with moderate to severe chronic hemiparetic stroke
A. Physiological Relationship Between the Flexion Synergy and Stretch Reflexes
Although this study was not intended to investigate the neural substrates underlying the expression of the flexion synergy and altered stretch reflexes, it is nevertheless interesting to note that the two abnormalities share a putative physiological relationship in addition to the relationship discussed in previously. In particular, it is becoming increasingly accepted that the stereotypical pattern of muscle coactivation that emerges in the paretic upper limb following stroke may reflect an increased influence of residual motor resources following a stroke-induced decrease in ipsilesional corticofugal fiber density [19–22]. Potential sources of residual motor outlets include those motor pathways originating in the dorsal reticular formation, specifically the raphe nucleus and locus coeruleus. These pathways have been shown in animal models to project diffusely across vertical spinal cord segments, terminating in ventromedial portions of the cord where they innervate primarily the proximal flexor musculature [23]. Given this diffuse innervation pattern, an increased influence of these pathways would be likely to cause grouped movements and a loss of independent joint control, such as is seen with the flexion synergy.
While this notion levies a neuroanatomical argument to explain the coupling of multiple muscle groups, it is important to consider also the neurochemical implications of this argument. Although not exclusively monoaminergic, the reticular formation is the primary site of descending serotonergic (5-HT) and noradrenergic (NE) motor pathways in the brainstem [24]. At the level of the spinal cord, the monoamines 5-HT and NE have profound excitatory actions on motoneurons [25–29]. In the context of post-stroke stretch reflexes, the neuromodulatory actions of monoamines are thus well suited to amplify the motoneuronal responses to spindle-mediated ionotropic 1a afferent feedback, either by altering the recruitment threshold such that additional motor units are recruited for a given input or by changing intrinsic motoneuronal properties.
Additionally, stretch reflex alterations may be explained in part by an inability to relax the paretic muscle completely. This inability to achieve electromyographic quiescence may be predicated by the influence of a tonic depolarizing drive to the motoneuron pools innervating the muscle groups of interest. Although not unequivocal, recent evidence in support of such a tonic depolarizing drive has been provided by Mottram, et al, who argued that the comodulation of spontaneously firing motor units in ‘spastic’ muscle is mediated by tonic descending supraspinal input, potentially originating in brainstem nuclei [30].
It should be noted however that it is currently unknown whether these pathways may become upregulated following stroke or whether they are simply relied upon more heavily in the absence of primary motor outlets.
B. Synergies, Reflexes, and Movement Impairment
Using precise, impairment-based robotic approaches, Sukal and colleagues found that progressive increases in shoulder abduction loading led to commensurate decreases in horizontal reaching work area [31]. This finding has clear implications for functional movements, as a compromised ability to extend at the elbow directly affects purposeful interaction with the environment. The relationship between stretch reflexes and post-stroke reaching deficits is less straightforward. Many of the early investigations of stretch reflexes – as well as pervasive clinical assessments – attempted to characterize stretch reflexes under passive conditions [11–13, 15] (also, e.g., the Ashworth Scale). The results of these studies suggested that stretch reflexes routinely became ‘hyperactive’ in the paretic upper limb following stroke, notably exhibiting an enhanced velocity sensitivity that was absent in the non-paretic limb or the limbs of individuals without neurological injury [13, 14]. More recent work investigating stretch reflexes elicited during maintenance of preactivation has suggested that once active, the paretic and non-paretic musculature may respond similarly to a given stretch perturbation however [9]. This assertion raises the question of whether the hyperactivity noted passively plays a significant role in the overall post-stroke reaching deficit.
In light of this question, additional work is warranted that investigates the potential for reflexes to impact volitional movements in individuals with chronic hemiparetic stroke. In addition to considering the effect of preactivation on reflex expression, such would work also need to include assessments of volitional movement velocity, as this parameter is inextricably linked to both the flexion synergy and reflexes. For example, as expression of the flexion synergy increases – thereby increasing activity of the elbow flexors (i.e., the antagonistic muscle group during reaching) – reaching velocity is likely to decrease. While this decrease in velocity may make it less likely that the threshold for reflex evocation will be met, the increase in elbow flexor preactivation due to synergy expression will concurrently have the opposite effect. Alternatively, as the limb is unloaded and synergy expression becomes minimal, movement velocity may increase, but it will do so with an associated decrease in preactivation. Therefore, although it remains unclear precisely how stretch reflexes contribute to the overall movement disorder, the findings presented here suggest nominally that reflexes and the flexion synergy should not be considered in isolation from one another.
C. Clinical Implications
The results presented herein have clear implications for clinical rehabilitation interventions. Specifically, it is suggested that targeting the expression of the flexion synergy may be a particularly viable clinical strategy for individuals with all impairment levels given that such abnormal muscle coactivation patterns cannot be easily decoupled from the manifestation of stretch reflexes in the paretic upper limb. In other words, precisely because expression of the flexion synergy can facilitate the manifestation of stretch reflexes by abnormally elevating elbow flexor activity prior to and during a reaching movement, clinical interventions seeking to reduce the expression of the flexion synergy may also confer the added benefit of reducing the possible appearance of self-evoked stretch reflexes during reaching tasks.
Impairment-based robotic devices such as the ACT-4D have not only the capacity to quantify existing movement disorders, but can also be used to provide precise, quantitative outcome measures for myriad investigations. For example, many clinical assessments of movement disorders, such as the Ashworth/Modified Ashworth scale, are regarded as too crude and methodologically limited to quantify stretch reflexes adequately [32]. Given the recent surge of investigations that seek to test the effects of pharmacological agents such as baclofen, botulinum toxin, and others on muscle tone and reflexes, impairment-based robotics such as the ACT-4D provide an appealing means of quantifying the potential changes affected by the intervention.
ACKNOWLEDGMENT
The authors wish to thank Carolina Carmona, DPT, for assistance with participant recruitment and data collection.
Supporting grants: AHA: 10POST2790017 (PI: AHA Stienen), NIH: R01-HD039343 (PI: JPA Dewald), NIH: R01-NS054269 (PI: JPA Dewald).
REFERENCES
- [1].Dewald J and Beer R, “Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis,” Muscle and Nerve, vol. 24, pp. 273–283, 2001. [DOI] [PubMed] [Google Scholar]
- [2].Mirbagheri MM, et al. , “Natural history of neuromuscular properties after stroke: a longitudinal study,” J Neurol Neurosurg Psychiatry, vol. 80, pp. 1212–7, November 2009. [DOI] [PubMed] [Google Scholar]
- [3].Twitchell T, “The restoration of motor functino following hemiplegia in man,” Brain, vol. 74, pp. 443–480, 1951. [DOI] [PubMed] [Google Scholar]
- [4].Brunnstrom S, Movement Therapy in Hemiplegia New York: Harper and Row, 1970. [Google Scholar]
- [5].Dewald J, et al. , “Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. ,” Brain, vol. 118, pp. 495–510, 1995. [DOI] [PubMed] [Google Scholar]
- [6].Dewald J, et al. , “Upper-limb discoordination in hemiparetic stroke: implications for neurorehabilitation,” Topics in Stroke Rehabilitation, vol. 8, pp. 1–12, 2001. [DOI] [PubMed] [Google Scholar]
- [7].Smeets J and Erkelens C, “Dependence of autogenic and heterogenic stretch reflexes on pre-load activity in the human arm,” Journal of Physiology, vol. 440, pp. 455–465, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matthews P, “Observations on teh automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man,” Journal of Physiology, vol. 374, pp. 73–90, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burne J, et al. , “The spasticity paradox: movement disorder or disorder of resting limbs?,” Journal of Neurology, Neurosurgery and Psychiatry, vol. 76, pp. 47–54, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stein R, et al. , “Analysis of short-latency reflexes in human elbow flexor muscles,” Journal of Neurophysiology, vol. 73, pp. 1900–1911, 1995. [DOI] [PubMed] [Google Scholar]
- [11].Powers R, et al. , “Stretch reflex dynamics in spastic elbow flexor muscles,” Annals of Neurology, vol. 25, pp. 32–42, 1989. [DOI] [PubMed] [Google Scholar]
- [12].Ibrahim I, et al. , “Stretch-induced electromypgraphic activity and torque in spastic elbow muscles,” Brain, vol. 116, 1993. [DOI] [PubMed] [Google Scholar]
- [13].Thilmann A, et al. , “The mechanism of spastic muscle hypertonus. Variatino in reflex gain over the time course of spasticity. ,” Brain, vol. 114, pp. 233–244, 1991. [PubMed] [Google Scholar]
- [14].Thilmann A, et al. , “Pathological stretch reflexes on the “good” side of hemiparetic patients,” Journal of Neurology, Neurosurgery and Psychiatry, vol. 53, pp. 208–214, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Powers R, et al. , “Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis,” Annals of Neurology, vol. 23, 1988. [DOI] [PubMed] [Google Scholar]
- [16].Trumbower RD, et al. , “Contributions of altered stretch reflex coordination to arm impairments following stroke,” J Neurophysiol, vol. 104, pp. 3612–24, December 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sukal TM, et al. , “Source of work area reduction following hemiparetic stroke and preliminary intervention using the ACT3D system,” Conf Proc IEEE Eng Med Biol Soc, vol. 1, pp. 177–80, 2006. [DOI] [PubMed] [Google Scholar]
- [18].Sukal TM, et al. , “Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications,” Exp Brain Res, vol. 183, pp. 215–23, November 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lindenberg R, et al. , “Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke,” Neurology, vol. 74, pp. 280–287, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yao J, et al. , “Cortical overlap of joint representations contributes to the loss of independent joint control following stroke,” Neuroimage, vol. 45, pp. 490–499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schwerin S, et al. , “Ipsilateral versus contralateral cortical motor projectinos to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies,” Experimental Brain Research, vol. 185, pp. 509–519, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Perez M and Cohen L, “The corticospinal system and transcranial magnetic stimulation in stroke,” Topics in Stroke Rehabilitation, vol. 16, pp. 254–269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Davidson A and Buford J, “Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging,” Journal of Neurophysiology, vol. 92, pp. 83–95, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Holstege JC and Kuypers HG, “Brainstem projections to spinal motoneurons: an update,” Neuroscience, vol. 23, pp. 809–21, December 1987. [DOI] [PubMed] [Google Scholar]
- [25].Fedirchuk B and Dai Y, “Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production,” Journal of Physiology, vol. 557, pp. 355–361, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heckman C, et al. , “Active properties of motoneuron dendrites: diffuse descending neuromodulation, focused local inhibition,” Journal of Physiology, vol. 586, pp. 1225–1231, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heckman C, et al. , “Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior,” Trends in Neurosciences, vol. 26, pp. 688–695, 2003. [DOI] [PubMed] [Google Scholar]
- [28].Hultborn H, et al. , “Key mechanisms for setting the input-output gain across the motoneuron pool,” Progress in Brain Research, vol. 143, pp. 77–95, 2004. [DOI] [PubMed] [Google Scholar]
- [29].Powers R and Binder M, “Input-output functions of mammalian motoneurons,” Reviews of Physiology, Biochemistry and Pharmacology, vol. 143, pp. 137–263, 2001. [DOI] [PubMed] [Google Scholar]
- [30].Mottram C, et al. , “Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors,” Journal of Neurophysiology, vol. 102, pp. 2026–2038, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sukal T, et al. , “Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications,” Experimental Brain Research, vol. 183, pp. 215–223, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fleuren JF, et al. , “Stop using the Ashworth Scale for the assessment of spasticity,” J Neurol Neurosurg Psychiatry, vol. 81, pp. 46–52, January 2010. [DOI] [PubMed] [Google Scholar]