Abstract
BACKGROUND
Population measures of sterility are traditionally constructed for women, despite fertility and sterility being conditions of the couple. Estimates of male sterility provide insight into population-level sterility, and complement estimates based solely on women.
OBJECTIVE
This study seeks to estimate male sterility for the Gwembe Tonga of Zambia using male birth histories collected by the Gwembe Tonga Research Project from 1957 to 1995, while providing context by estimating female sterility for the Gwembe Tonga, as well as female sterility in all of Zambia, from Zambian DHS data (1992, 1997, 2001–02, and 2007).
METHODS
Sterility is measured using the Larson-Menken subsequently infertile indicator. Estimates are produced using discrete time event history analysis.
RESULTS
The odds of sterility were higher for women than men, though women’s odds of sterility were only 1.5 times that of men’s in the middle reproductive years. The odds of sterility increased steadily with age for both men and women, and across all datasets. However, women’s sterility increased much more sharply with age than men’s did, and women’s odds of sterility were higher than men’s at all reproductive ages.
1. Introduction
Population measures of fertility and sterility are usually constructed from birth histories from women, and thus limited to the population of women, rather than the general population. However, fertility or its absence are conditions experienced by a couple, and the causes of sterility can be related to the male partner, the female partner, or both partners. Medical studies (Folkvord, Odegaard, and Sundby 2005) and anthropological studies (Gerrits 1997, Dryer et al 2004) provide direct evidence of male sterility in multiple locations in Africa. Determining whether sterility is due to male or female disorders is often difficult, and generally less is known about the prevalence of male sterility (McFalls and McFalls 1984). McFalls and McFalls (1984) estimate between 20 and 60% of couple infertility across populations is accounted for in whole or in part by male sterility. As sterility, primary or secondary, is potentially related to either the male or female member of the couple, estimating male sterility can provide a more complete picture of population-level sterility than female estimates alone.
This analysis aims to describe the sterility of Gwembe Tonga men by applying Larsen and Menken’s (1989, 1991) subsequently infertile measure, using incomplete birth histories. Data for the Gwembe Tonga provide a unique opportunity to estimate male sterility, because male birth histories were recorded. Juxtaposed with measurement of sterility for the women in the same population, this analysis seeks to describe sterility among the entire Gwembe Tonga population. Measures of female sterility from Zambia Demographic and Health Surveys (ZDHS) data from 1992, 1996, 2001–02, and 2007 are also presented to provide national context for the Gwembe Tonga analysis.
2. Background
2.1. Sterility in Africa
Fertility rates vary widely across and within countries in sub-Saharan Africa (for example, Bongaarts, Frank, and Lesthaeghe 1984), and evidence from demographic measurement of sterility has shown wide variation across the continent as well. Earlier studies have tried to measure the inability to produce a living child, and we use the word sterility for any measures of the inability to produce a living child. Larsen (2000) found relatively low rates of primary sterility but high rates of secondary sterility. Rates of secondary sterility ranged from less than 10% to 25% for women age 25–44 (Larsen 2000). Other researchers have found similar variation (Ericksen and Brunette 1996). Frank (1983) found great variance in rates of primary sterility by country and also by ethnic group in Africa. Bongaarts et al (1984) cite substantial variation in measured primary sterility across Africa, varying from 3% to 20% or higher, and noted substantial variation within countries. Similarly, Jensen (1995) found substantial differences in secondary sterility rates in two Kenyan communities.
2.2. Zambia and the Gwembe Tonga
Zambia is a landlocked country in southern Africa with an estimated mid-year 2013 population of over 14 million. Life expectancy remains among the lowest in the world (52 years) with maternal mortality and infant mortality rates among the highest (World Factbook 2013). Fertility rates in Zambia are high; total fertility was estimated at 6.2 in 2007. Contraceptive use has increased from 15% of women in 1992 to 41% in 2007, 33% using a modern method in 2007 (CSO et al 2009). Relatively little information about sterility in Zambia has been published. Using parity progression ratios to analyze Zambian censuses, Sunil and Pillai (2002) found that the proportion of women who were sterile increased from 0.12 in 1980 to 0.15 in 1990, with evidence of regional variation. The authors estimated that sterility rates in Southern Province, where the Gwembe Tonga live, increased from 0.11 to 0.14 between 1980 and 1990 (Sunil and Pillai 2002).
This analysis estimates sterility among the Gwembe Tonga, using a data set collected from 1956–1995 (Clark 2001). The Gwembe Tonga traditionally lived in the valley of the Zambezi River, but many were forced to relocate in the late 1950s to make way for the Kariba Dam and its reservoir. Gwembe Tonga women marry early (mean age of 16.5 years) and nearly universally (97% married by age 45). Gwembe Tonga fertility rates have remained high (total fertility of 6) through the 1980s (Clark et al 1995). In the late 1950s, as many as 40% of men practiced polygyny (Colson 1971). Polygyny is still practiced, though less common (Clark 2001). Marriage and fertility practices are similar for the Gwembe Tonga and ZDHS national samples (Table 1).
Table 1:
Population size, marital and birth history descriptive statistics for women and men in the Gwembe Tonga and Zambia DHS (1992, 1996, 2001–02, and 2007) datasets
| Women | Men | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gwembe Tonga |
1992 ZDHS* |
1996 ZDHS |
2001–02 ZDHS |
2007 ZDHS |
Gwembe Tonga |
1996 ZDHS |
2001–02 ZDHS |
2007 ZDHS |
|
| Individuals aged 20 and over | 2206 | 4800 | 5637 | 5487 | 5269 | 1900 | 1307 | 1591 | 4829 |
| Ever married | 1768 | 4516 | 5219 | 5063 | 4747 | 1258 | 1041 | 1341 | 3910 |
| Married individuals used in analysis** | 1405 | 3615 | 4071 | 3416 | 2264 | 1021 | Not included in analysis | ||
| Mean age first marriage (SE) | 19.9 (0.16) |
17.2 (0.05) |
17.5 (0.05) |
17.7 (0.05) |
18.1 (0.05) |
25.6 (0.24) |
22.8 (0.13) |
22.6 (0.11) |
22.9 (0.07) |
| Women married more than once (%) |
143 | 1186 | 1346 | 1240 | 1009 | 1,083 | 349 | 524 | 994 |
| (8%) | (26%) | (26%) | (25%) | (21%) | (84%) | (34%) | (39%) | (27%) | |
| Men with more than 1 wife (%) | Not applicable | 337 (27%) |
93 (10%) | 122 (10%) |
284 (8%) |
||||
| Percent of polygynists with exactly 2 wives |
68% | 94% | 84% | 91% | |||||
| Mean age in years at first birth (SE) |
20.4 | 18.1 | 18.3 | 18.3 | 18.5 | 25.0 | Not available | ||
| (0.07) | (0.05) | (0.04) | (0.04) | (0.04) | (0.18) | ||||
| Mean birth interval (SE) | 2.7 (0.02) |
2.8 (0.97) |
2.8 (1.04) |
2.9 (1.01) |
3.0 (1.02) |
2.4 (0.02) |
|||
| Proportion with last closed birth interval ≥ 5 years |
5.6% | 21.2% | 23.5% | 21.0% | 21.5% | 4.2% | |||
| Mean number of live births (SE) | 4.1 (0.08) |
4.6 (0.04) |
4.5 (0.04) |
4.4 (0.04) |
4.3 (0.04) |
4.5 (0.16) |
4.8 (0.12) |
5.0 (0.11) |
4.7 (0.05) |
| Mean number of living children (SE) |
3.1 | 3.8 | 3.6 | 3.7 | 3.6 | 3.3 | 3.9 | 4.1 | 4.0 |
| (0.06) | (0.4) | (0.03) | (0.03) | (0.03) | (0.11) | (0.10) | (0.09) | (0.05) | |
| Proportion childless§ | 8.10% | 2.41% | 2.73% | 2.74% | 2.53% | 17.10% | Not available | ||
The 1992 ZDHS did not contain a male sample.
For the Gwembe Tonga, these are people continuously married for 5 years preceding each observation included in analysis; for the ZDHS, these are women who married at least 5 years prior to the observation and were still married at the time of the survey.
Proportion childless was estimated for those married at least 7 years before last observation using Larsen’s method (Larsen, 2000).
3. Data
Data for the Gwembe Tonga come from the Gwembe Tonga Research Project, begun by Elizabeth Colson and Thayer Scudder in 1956, with yearly data on unions and births through 1995. Four villages are included in the dataset, and the sample includes all the inhabitants of these villages, as well as individuals born to or marrying members of the sample, or migrating into the villages. Individuals left the sample through death or by moving away (Clark 2001). These data are ideal for estimating sterility for men because birth histories are separately available for both men and women. To compare sterility estimates for the Gwembe Tonga with Zambia, Demographic and Health Survey data from 1992 (ZDHS 1992), 1996 (ZDHS 1996), 2001–02 (ZDHS 2001–02) and 2007 (ZDHS 2007) are used. Analysis was restricted to currently married individuals at least 20 years of age in the ZDHS, because divorce dates were not available, and periods of marriage and separation could not be identified in those data. All individuals who had ever been married, men and women, over 20 years old were included from the Gwembe Tonga data, with analysis limited to observations for which the individual had been married for five consecutive years prior to the observation. Years when an individual was separated after the first union or the first years of any union were excluded from analysis to ensure exposure to pregnancy, following Larsen and Menken (1989, 1991). Analysis was limited to individuals aged 20 years or over, following the recommendation of Larsen and Menken (1991), based on their sensitivity analysis, which found a substantial difference in true and assigned age at sterility for ages below 20 years.
The period covered by this data was tumultuous for the Gwembe Tonga, and evidence shows that social organization and behaviors changed over this period in ways that may impact fertility desires and practices and fecundity (Clark et al. 1995). For analysis, time periods were selected to capture key events for the Gwembe Tonga and Zambia, though consultation with the Gwembe Tonga Research Project (Thayer Scudder, personal communication). Period one, 1950 to 1963, covers a brief period before the Kariba dam project and relocation and resettlement. Period two, 1964 to 1972, covers nine years of relative stability and economic growth. 1973 to 1981, period three, saw dramatic deterioration of the Zambian political economy and the war for Zimbabwe Independence, which disrupted economic and social services for the Gwembe Tonga. In period four, 1982 to 1990, health conditions deteriorated and HIV/AIDS became a large problem in Zambia. During period five, 1991 to 1999, health problems and the burden of HIV/AIDS continued as the economy stagnated, and there were a series of floods and droughts. In the final period, 2000 to 2008, the economy improved and access to HIV care and treatment improved. No data used for the Gwembe Tonga fell in the last period, though some of this period is captured in the 2001–02 and 2007 ZDHS data. Later ZDHS datasets did not have observations for the earliest time periods. Over 50% of observations in the 2007 ZDHS were in the last time period, and for the 2007 ZDHS, time periods were collapsed to 1973–1999 and 2000–07 to accommodate sparseness in certain age and time period categories.
4. Methods
This analysis uses Larsen and Menken’s (1989, 1991) method for measuring population sterility, defined as the inability to have a live birth, to estimate sterility of Gwembe Tonga men and women. The measure is implemented in the same manner for both men and women. This analysis is the first of which the authors are aware that estimates male sterility at the population level using a measure that has been exclusively used for women, but can be applied here because of the availability of male birth history data. Larsen and Menken’s subsequently infertile indicator measures the proportion of individuals who are subsequently infertile after a certain age, using incomplete birth histories. For this measure, infertility is defined as an individual being observed for a specified time T without giving birth, despite being sexually active and not using contraception. This method estimates the proportion of individuals who become sterile between age a and age a + T, at some age a*. An indicator for sterility was assigned for each person-year. For person-years that meet the criteria for inclusion in the dataset (age 20 years or older, married at least five years earlier, followed for the following five years), an individual was sterile if, in the last, open birth interval, the individual did not give birth during any of the following five years.
T is generally five years, as birth intervals are usually no longer than five years. However, women with open birth intervals longer than five years will be categorized as infertile using this method, risking overestimation in populations with wider than average birth intervals. Larsen and Menken (1991) argue that women who had subsequent births outside of the interval were likely sub-fecund, and in both the male and female Gwembe Tonga data, as well as all ZDHS datasets, average birth intervals were below five years (Table 1). However, while few last, closed birth-intervals were longer than five years for the Gwembe Tonga women (5.6%) or men (4.2%), more that 20% of women in each ZDHS dataset had last, closed birth-intervals longer than five years (Table 1). It is possible the fact that the larger portion of ZDHS last, closed birthintervals are longer than five years is related to the tendency for births to be shifted to later than five years, prior to the survey, to avoid answering more questions (for example, see Pullum 2006). With these longer, later birth-intervals, estimates of secondary sterility may be inflated in the ZDHS datasets. However, we see that estimates of sterility from the ZDHS datasets are well below those of the Gwembe Tonga (Figure 1).
Figure 1:
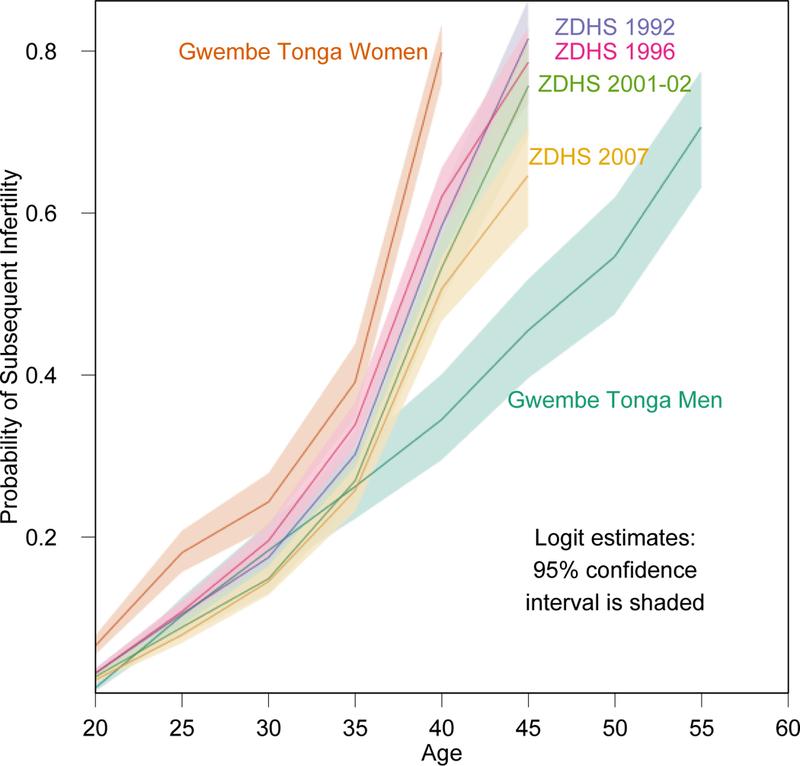
Predicted probability of being infertile by age with 95% confidence intervals
There are some limitations to this measure. Some sexually inactive individuals may be included, even when only including those who are married continuously. Excluding never-married and divorced individuals from the measure is likely to underestimate infertility; evidence suggests that subfecund women are more likely to be divorced than fecund women (Larsen and Menken 1994). Contraception further complicates the measure, as women who are practicing contraception could be counted as sterile, despite being fecund. Larsen (1994) outlined contraceptive use conditions in which the subsequently infertile measure could be estimated with negligible bias, but adequate detail about contraceptive use for determining whether these conditions are met is not available in the ZDHS (and often not available generally). In this analysis, two ways of dealing with contraceptive use were used for the ZDHS data, and are discussed briefly in the results.
This study uses discrete-time event history analysis (Allison 1984). Logistic regression is used to estimate the hazard of being subsequently infertile, by age and time. This approach incorporates covariates for age and time (calendar period). Sex and an interaction term between sex and age are included in the pooled Gwembe Tonga model to allow direct comparison of male and female sterility. A person-year file was created for the analysis. The time period (position on the calendar) corresponding to each person-year was taken from the calendar date when the person-year started. All five-year age groups were included in all analyses, with the last age category for women being 40 or 45 years and older, and men’s age categories continuing to 55 years and older. Being sterile was measured with the subsequently infertile measure (Larsen and Menken 1989, 1991). This indicator was defined for all observed person-years that were preceded by five consecutive years of marriage. An individual was subsequently infertile in a given year if they were exposed to risk of pregnancy during the subsequent five years and did not have a live birth. Probabilities of being sterile were predicted for five-year age categories spanning reproductive ages. Gwembe Tonga men, Gwembe Tonga women, and each of the four ZDHS survey datasets were analyzed separately. Gwembe Tonga men and women were also analyzed in a pooled dataset, to allow comparisons between male and female sterility. For the ZDHS models, including an indicator variable for Southern Province, where the Gwembe Tonga live, did not improve model fit. Rather, it resulted in an odds ratio close to 1.0, whose 95% confidence interval included 1.0. Consequently, we present ZDHS estimates for Zambia as a whole, rather than restricted to Southern Province. Results are presented for each of the seven models in Tables 2 and 4.
Table 2:
Odds ratios (with standard errors) for being subsequently infertile obtained from logistic regression for Gwembe Tonga women, men and women and men combined
| Women | Men | Pooled Women and Men | |
|---|---|---|---|
| Age Group | |||
| 20–24 | Reference group | ||
| 25–29 | 3.5 (0.3)* | 10.7 (2.4)* | 10.7 (2.4)* |
| 30–34 | 5.5 (0.6)* | 22.8 (5.4)* | 22.6 (5.4)* |
| 35–39 | 12.3 (1.4)* | 40.8 (10.0)* | 40.1 (9.8)* |
| 40–44** | 86.0 (10.5)* | 64.5 (16.2)* | 63.1 (15.8)* |
| 45–49§ | 106.4 (27.2)* | 154.3 (38.9)* | |
| 50–54 | 158.4 (42.8)* | ||
| 55+ | 338.5 (96.7)* | ||
| Time Period | |||
| 1950–1963 | Reference group | ||
| 1964–1972 | 2.0 (0.4)* | 2.1 (0.6)* | 2.1 (0.7)* |
| 1973–1981 | 2.3 (0.5)* | 3.2 (1.0)* | 2.6 (0.3)* |
| 1982–1990 | 3.5 (0.8)* | 5.6 (1.7)* | 4.2 (0.8)* |
| 1991–1999 | 12.5 (2.8)* | 18.5 (5.8)* | 14.5 (2.6)* |
| Sex | |||
| Male | Reference group | ||
| Female | 6.5 (1.6)* | ||
| Sex and Age Interaction | |||
| Female*25–29 | 0.3 (0.1)* | ||
| Female*30–34 | 0.2 (0.1)* | ||
| Female*35–39 | 0.3 (0.1)* | ||
| Female*40–44 | 0.8 (0.2) | ||
| Female*45+ | 2.3 (0.7) | ||
| Intercept | 0.01 (0.0)* | 0.002 (0.0)* | 0.002 (0.0)* |
| Pseudo R2 | 0.32 | 0.29 | 0.31 |
| Cases | 1,405 | 1,021 | 2,426 |
| Observations | 20,069 | 16,141 | 36,210 |
Significant at the p<0.01 level
For women only, the 40–44 age group includes all women age 40 and over.
For the pooled model, the 45–49 age group includes all individuals age 45 and over
Table 4.
Odds ratios (with standard errors) for being subsequently infertile obtained through logistic regression for women from the 1992, 1996, 2001–02 and 2007 ZDHS surveys, treating women using contraception as though they were fertile
| 1992 | 1996 | 2001–02 | 2007 | |
|---|---|---|---|---|
| Age group | ||||
| 20−24 | Reference group | |||
| 25−29 | 3.5 (0.3)* | 3.5 (0.2)* | 3.5 (0.3)* | 3.8 (0.3)* |
| 30−34 | 5.7 (0.5)* | 6.5 (0.5)* | 5.8 (0.5)* | 7.3 (0.7)* |
| 35−39 | 10.4 (1.1)* | 12.3 (1.1)* | 11.0(1.2)* | 14.3 (1.6)* |
| 40−44 | 31.0 (3.8)* | 33.2 (3.7)* | 31.2 (3.9)* | 37.9 (5.2)* |
| 45 and older | 83.0 (17.4)* | 65.1 (11.1)* | 70.6 (13.9)* | 66.7 (12.4)* |
| Time Period** | ||||
| 1964−1972 | Reference group | No observations | ||
| 1973−1981 | 1.8 (0.5) | 10.6 (7.4)* | Reference group | |
| 1982−1990 | 3.8 (1.2)* | 22.9 (16.5)* | 1.6 (0.4) |
Reference group (1973–1999) |
| 1991−1999 | 5.7 (1.8)* | 45.2 (32.9)* | 2.6 (0.8)* | |
| 2000−2007 | N/A | N/A | 4.2 (1.3)* | 1.5 (0.2)* |
| Intercept | 0.01 (0.0)* | 0.001 (0.0)* | 0.01 (0.0)* | 0.02 (0.0)* |
| Pseudo R2 | 0.19 | 0.22 | 0.19 | 0.18 |
| Cases | 3399 | 3813 | 3744 | 3636 |
| Observations | 40233 | 45708 | 44099 | 43040 |
Significant at the p<0.01 level
For the 2007 ZDHS most observations were in the latest time period and earlier time periods were combined to adjust for the relatively few observations.
5. Results
The primary objective of this analysis is to produce estimates of sterility for men: something that is not generally done, because birth-history data for men are very uncommon. Male sterility by age is presented in Figure 1, along with estimates of female sterility from the Gwembe Tonga and ZDHS datasets. These estimates are from the regressions in Table 2 and 4 for each dataset and include 95% confidence intervals. The predicted probabilities can be interpreted as the proportion of the population experiencing sterility in an age group. Fewer men are experiencing sterility at any age than women. For example approximately 40% of Gwembe Tonga women are sterile by age 35, while sterility among Gwembe Tonga men does not reach that level until age 45. These age-specific differences between men and women are made explicit in Table 3, which provides the odds of being sterile for a woman compared to a man from the pooled model. While much higher at the youngest and oldest ages, in the middle of reproductive years, aged 30 to 34, women are only 1.5 times more likely to be sterile than men, indicating that their increased risk of sterility is felt most in their early and late reproductive years.
Table 3:
Odds ratios of being subsequently infertile for women compared to men by age, computed from the logistic regression with interaction term for the pooled Gwembe Tonga men and women data
| Age | Odds |
|---|---|
| 20–24 | 7.4 |
| 25–29 | 2.2 |
| 30–34 | 1.5 |
| 35–39 | 2.0 |
| 40–44 | 5.8 |
| 45 and older | 15.6 |
Table 1 seeks to illustrate the similarities and differences in marital and fertility behaviors across the data sets as well as provide the initial numbers of individuals from which the final set of observations was drawn. Ages at first marriage, ages at first birth, birth intervals, number of births and number of living children were similar for women in all datasets and similar for men in all datasets. For men and women, across all models, the odds of being sterile increased steadily with age, with near certainty of sterility at the oldest ages (which was an open interval and included all the oldest respondents under study); nearly 80% of Gwembe Tonga women and women in the earlier ZDHS data were sterile in the last age groups, whereas about 70% of men were sterile in the last age group, which was similar to women in the later ZDHS data (see Table 2 and Figure 1). This steady increase was true in the pooled model for men and women as well. From Figure 1 we can see that the steady increase of the probability of being sterile with age is evident in all groups, but that the increase is much steeper for women than it is for men. Even though women in the later ZDHS surveys have probabilities of sterility at both young and old ages that are similar to Gwembe Tonga men, the slope of their increase in sterility with age is similar to that of the Gwembe Tonga women and earlier ZDHS samples.
Contraceptive use among the Gwembe Tonga was negligible during the period under analysis (Sam Clark and Thayer Scudder, personal communication), so this was ignored in their estimates. Modern contraceptive use was increasing throughout Zambia in the period covered by the ZDHS surveys. For the four ZDHS datasets, the results shown in Table 4 consider all current users of contraception to be fertile. This represents a minimum level of sterility in the populations, as presumably a non-negligible proportion of women using contraception may be sterile but considered fecund. The models were also run (not shown) excluding all contraceptive-users from the risk set entirely, which resulted in higher levels of sterility. In 1992, when only 16% of married women were using any method of contraception at the time of the survey, the estimated probability of sterility is similar for the two approaches, but by 2007, when 33% of married women were using contraception, the proportion of women who were sterile differed significantly between the two approaches, indicating a sizeable effect of contraceptive use on the measure (which was documented in Larsen 2000). For comparison and discussion, only the estimates treating all contraceptive-users as fertile are used, representing the minimum estimate of sterility.
Odds of sterility increased over time for all models (Tables 2 and 4). This analysis is not intended to investigate the effect of time-period on sterility, because the relationship between time and sterility is complex, and data on factors that may explain this relationship are not available. In this analysis, time is included only to control for overall secular changes in the level of sterility.
6. Discussion
The Gwembe Tonga Research Project dataset provides a unique opportunity to estimate male sterility, and to compare the sterility of men with that of women. Our findings demonstrate that the prevalence of male sterility increases steadily with age, though not as sharply as for women. While sterility is generally less common among men, the difference is not constant over the life course. In fact, women’s odds of being sterile are only moderately higher than men’s in the middle of the reproductive years.
We use Larsen and Menken’s subsequently infertile indicator to measure sterility. The reliability of paternity reporting is a potential limitation for any study of male sterility. In addition to the potential confusion of social and biological paternity, men may be unaware of children or choose to deny paternity. Data from both the Gwembe Tonga and ZDHS surveys indicate that most men reported at least one child. Data for the Gwembe Tonga were collected as part of annual censuses of the four villages; this limits recall bias that could undermine cross-sectional birth histories from men, especially in relation to births outside of stable, long-duration unions. The increasing prevalence of contraception in the more recent ZDHS survey data is another potential challenge for estimates of population sterility using this measure. Acknowledging this, we present results showing the lower bound of sterility. Obviously, high prevalence of female-controlled contraception would greatly complicate attempts to measure male sterility through birth histories, though for the period under study for the Gwembe Tonga, contraceptive use was negligible (Communication with the Gwembe Tonga Research Project). Sterility estimates for women in the ZDHS data were consistently lower than those for the Gwembe Tonga, this may be due in part to underestimation caused by including all contraceptive users in the risk set (assuming that they are all fertile when we know that a small fraction are not). The inclusion criteria used for the ZDHS samples was less strict with respect to marriage duration, because data on union histories was less detailed. This may result in overestimation of female sterility, because some of the women are not at risk of becoming pregnant. Additionally, substantially more women in the ZDHS samples reported last, closed birth intervals longer than five years than either Gwembe Tonga women or men. If ZDHS women do experience last birth intervals much longer than five years, the measure used here may overestimate sterility by classifying women as sterile after they have gone five years without giving birth, even though they may go on to give birth later. However even with so many women in the ZDHS samples having last, closed birth intervals longer than five years, the mean length of the last birth interval was less than 3.5 years for all ZDHS samples, compared to 2.3 for Gwembe Tonga men and 2.7 for Gwembe Tonga women. Evidence suggests that about 10% of births may be inaccurately reported outside the five years prior to the interview to avoid additional questions in Demographic and Health Surveys (Pullum, 2006). Even with these two potential sources of upward bias in the estimates for the ZDHS samples, sterility estimates for the ZDHS women were lower than those for the Gwembe Tonga women.
This study shows that men’s sterility increases with age in a manner similar to the age-related increase for women. However, men’s probability of sterility increases much more slowly than women’s, which is expected due to men’s longer reproductive period. In the final age group, men and women alike reached very high probabilities of sterility.
7. Acknowledgments
Supported in part by K01 HD057246, R01 HD054511 and R01 HD070936 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). For helpful comments and access to data, we thank Elizabeth Colson and Thayer Scudder, the Gwembe Tonga inhabitants, and Stewart Tolnay.
References
- Allison P (1984). Event History Analysis: Regression for longitudinal event data. Newbury Park, CA: Sage Publications. [Google Scholar]
- Bongaarts J, Frank O, and Lesthaeghe R (1984). The Proximate Determinants of Fertility in Sub-Saharan Africa. Population Development and Review 10(3): 511–537. doi: 10.2307/1973518. [DOI] [Google Scholar]
- Central Statistical Office (CSO), Ministry of Health (MOH), Tropical Diseases Research Centre (TDRC), University of Zambia, and Macro International Inc (2009). Zambia Demographic and Health Survey 2007. Calverton, Maryland, USA: CSO and Macro International Inc. [Google Scholar]
- Clark SJ (2001).An Investigation into the Impact of HIV on Population Dynamics in Africa. [Ph.D. dissertation]. Philadelphia: University of Pennsylvania, Department of Demography. [Google Scholar]
- Clark SJ, Colson E, Lee J, and Scudder T (1995). Ten Thousand Tonga: A Longitudinal Anthropological Study from Southern Zambia, 1956–1991. Population Studies 49(1): 91–109. doi: 10.1080/0032472031000148266. [DOI] [Google Scholar]
- Colson E (1971). The social consequences of resettlement: The impact of the Kariba resettlement upon the Gwembe Tonga. Manchester: University of Manchester for University of Zambia. [Google Scholar]
- Dryer SJ, Abrahams N, Mokoena NE, and van der Spuy ZM (2004). You are a man because you have children: experiences, reproductive health knowledge and treatment-seeking-behavior among men suffering from couple infertility in South Africa. Human Reproduction 19(4): 960–967. doi: 10.1093/humrep/deh195. [DOI] [PubMed] [Google Scholar]
- Ericksen K and Brunette T (1996). Patterns and predictions of infertility among African women: A cross-national survey of twenty-seven nations. Social Science and Medicine 42(2): 209–220. doi: 10.1016/0277-9536(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Folkvord S, Odegaard OA, and Sundby J (2005). Male infertility in Zimbabwe. Patient Education and Counseling 59(3): 239–243. doi: 10.1016/j.pec.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Frank O (1983). Infertility in Sub-Saharan Africa: Estimates and Implications. Population Development and Review 9(1): 137–144. doi: 10.2307/1972901. [DOI] [Google Scholar]
- Gerrits T (1997). Social and cultural aspects of infertility in Mozambique. Patient Education and Counseling 31(1): 39–48. doi: 10.1016/S0738-3991(97)01018-5. [DOI] [PubMed] [Google Scholar]
- Jensen A (1995). The status of women and the social context of reproduction. Journal of International Development 7(1): 61–79. doi: 10.1002/jid.3380070105. [DOI] [PubMed] [Google Scholar]
- Larsen U (2000). Primary and secondary infertility in sub-Sarharan Africa. International Journal of Epidemiology 29(2):285–291. doi: 10.1093/ije/29.2.285. [DOI] [PubMed] [Google Scholar]
- Larsen U and Menken J (1989). Measuring Sterility from Incomplete Birth Histories. Demography 26(2): 185–201. doi: 10.2307/2061519. [DOI] [PubMed] [Google Scholar]
- Larsen U and Menken J (1991). Individual-Level Sterility: A New Method of Estimation with Application to Sub-Saharan Africa. Demography 28(2): 229–247. doi: 10.2307/2061277. [DOI] [PubMed] [Google Scholar]
- McFalls JA and McFalls MA (1984). Disease and Fertility. Orlando, FL: Academic Press. [Google Scholar]
- Pullum TW (2006). An Assessment of Age and Date Reporting in the DHS Surveys, 1985–2003 Methodological Reports No. 5. Calverton, Maryland: Macro International. [Google Scholar]
- Sunil TS and Pillai VK (2002). Sterility in Zambia. Annals of Human Biology 29(4): 414–421. doi: 10.1080/03014460110100919. [DOI] [PubMed] [Google Scholar]
- The World Factbook 2013–2014 (2014). Washington, DC: Central Intelligence Agency [Google Scholar]
- Zambia Demographic and Health Survey, 1992 (1993). Columbia, MD: University of Zambia, Zambia Central Statistics Office and Macro International, Inc. [Google Scholar]
- Zambia Demographic and Health Survey, 1996 (1997). Calverton, MD: Zambia Central Statistics Office and Macro International, Inc. [Google Scholar]
- Zambia Demographic and Health Survey, 2001–2002 (2003). Calverton, MD: Zambia Central Statistics Office, Zambia Central Board of Health and ORC Macro. [Google Scholar]
- Zambia Demographic and Health Survey, 2007 (2009). Calverton, MD: Zambia Central Statistics Office and Macro International. [Google Scholar]


