Abstract
Purpose
This pilot study was done to determine the feasibility and accuracy of UF/NCI phantoms and Monte Carlo retrospective dosimetry and had 2 aims: 1) To determine the anatomic accuracy of UF/NCI Phantoms by comparing 3D organ doses in National Wilms Tumor Study (NWTS) patient-matched UF/NCI phantoms to organ doses in corresponding patient-matched CT scans and 2). To compare in-field and out-of-field organ dosimetry using two dosimetry methods - standard RT treatment planning systems (TPS) and Monte Carlo (MC) dosimetry in these two anatomic models.
Methods
Twenty NWTS patient-matched DICOM files of UF/NCI phantoms and CT scans were imported into the Pinnacle RT treatment planning system. The NWTS RT fields (whole abdomen, flank, whole lung or a combination) and RT doses (10–45Gy) were reconstructed in both models. Both TPS and MC dose calculations were performed. For Aim 1, the mean doses to the heart, kidney, thyroid gland, testes and ovaries using TPS and MC in both models were statistically compared. For Aim 2, the TPS and MC dosimetry for these organs in both models were statistically compared.
Results
For Aim 1, there was no significant difference between phantom and CT scan dosimetry for any of the organs using either TPS or MC dosimetry. For Aim 2, there was a significant difference between TPS and MC dosimetry for both CT scan and phantoms for all organs. While the doses for in-field organs were similar for both TPS and MC, the doses for near-field and out-of-field organs were consistently higher for 90–100% of MC doses, however the absolute dose difference was small (<1Gy).
Conclusions
This pilot study has demonstrated that the patient-matched UF/NCI phantoms together with Monte Carlo dosimetry is an accurate model for performing retrospective 3D dosimetry in large scale epidemiology studies such as the NWTS.
Keywords: Radiation, dosimetry, phantom model, Monte Carlo dosimetry, Epidemiology
INTRODUCTION
The National Wilms Tumor Study (NWTS) conducted 5 clinical trials that accrued 9236 Wilms tumor (WT) patients during 1969–2002 (1–7). In the earlier NWTS protocols, radiation therapy (RT) fields were designed using 2D methods, and later in the era of 3D dose planning, investigators were given the option of using either 2D or 3D treatment planning as most fields were anteroposterior (AP-PA) fields to the whole lung, flank or whole abdomen. For RT quality assurance (QA) review of patients enrolled on NWTS protocols, RT records including paper diagrams of RT portals indicating field extent with respect to skeletal anatomy and x-ray simulator and RT portal films from a sample of patients were reviewed by study investigators. The NWTS did not have the capability of digital data acquisition and storage and thus there was no information available on 3D organ dosimetry. Thus for the NWTS Late Effects Study (LES), RT correlation with specific late effects was analyzed by using prescribed doses to standard Wilms tumor RT fields rather than by 3D organ dosimetry.
Computational human phantoms such as the University of Florida and National Cancer Institute Phantoms (UF/NCI) represent the newest generation of computational surrogates of patient anatomy that permit preservation of anatomic realism and anthropometric modeling that was not previously available (8, 9). The UF/NCI library contains 158 pediatric phantoms with heights ranging from 85 to 185 cm (10 cm increments) and weights ranging from 10 to 125 kg (5 kg increments). All phantoms are available in Digital Imaging and Communications in Medicine (DICOM) format with pre-contoured organs that can be readily used for treatment planning and dosimetry. This computational human phantom series is freely available for research purpose (Please contact Dr. Choonsik Lee (choonsik.lee@nih.gov) (10). Many reports have shown that Monte Carlo (MC) dosimetry is more accurate than standard Treatment Planning System (TPS) dosimetry, especially for out-of-field organs and heterogeneous regions (11–16). This pilot study was done to determine the feasibility and accuracy of UF/NCI phantoms and Monte Carlo retrospective dosimetry and had 2 aims: 1) To determine the anatomic accuracy of UF/NCI Phantoms by comparing 3D organ doses in National Wilms Tumor Study (NWTS) patient-matched UF/NCI phantoms to organ doses in corresponding patient-matched CT scans and 2). To compare in-field and out-of-field organ dosimetry using two dosimetry methods - standard RT treatment planning systems (TPS) and Monte Carlo (MC) dosimetry in these two anatomic models.
MATERIALS AND METHODS
A representative sample of 20 NWTS patients, ten boys and ten girls were selected from the NWTS database (Data and Statistical Center, Seattle WA). Their mean age was 50.5 months (15–88 months), mean height was 103.65 cm. (81–129 cm.) and mean weight was 16.7 kg. (9–21 kg. As the original CT scans of these patients was not available, we obtained patient-matched (by height, weight and sex) DICOM files of CT scans from the imaging archive of the Quality Assurance Review Center, Lincoln RI and UF/NCI Phantoms from the phantom library (Dr. Choonsik Lee). These NWTS patients received the following RT fields: Flank (2 patients), Whole Abdomen (WA) (6 patients), Whole Lungs (1 patient) and combination of fields (11 patients). The RT doses were as follows: Flank (10–30Gy), Whole Abdomen (10–40Gy) and Whole lung (12–15Gy) (Tables 1 and 2). These patient-matched CT scans and Phantom DICOM files were imported into the Pinnacle RT treatment planning system v9.10 (TPS) at Northwestern University. The NWTS patient-specific RT fields (whole abdomen, flank, whole lung or a combination) and RT doses (10–45Gy) were reconstructed using TPS for these 20 patients in both patient-matched CT and phantoms. The RT field reconstruction and TPS dose planning was supervised by an experienced radiation oncologist (JAK) and senior physicist (MGK) based on review of patient records from the NWTS. The normal organ contours (heart, thyroid gland, kidney, testes, ovaries and uterus) on the 20 CT scans and phantoms were reviewed by a pediatric radiologist (CR). While there was good anatomic correlation for all other organs, the position of the ovaries were inaccurate in the phantoms. Thus the ovaries were re-contoured based on published MRI-based guidelines (17).
Table 1.
Comparison of TPS and MC Organ doses in 10 male NWTS patient-matched CT scans and UF/NCI Phantoms.
| NWTS RT Prescription | Mean Left kidney Gy | Mean Heart Gy | % heart >= 5Gy (cc) | Mean Thyroid Gy | Mean Testes Gy RIGHT +LEFT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | TPS | MC | Δ | TPS | MC | Δ | TPS | MC | Δ | TPS | MC | Δ | TPS | MC | Δ | ||
| 1 | Lt Flank 18Gy/WA20Gy | Phantom | 39.66 | 39.40 | 0.26 | 9.40 | 9.69 | 0.29 | 39.67 | 42.63 | 2.96 | 0.25 | 0.46 | 0.21 | 1.99 | 2.74 | |
| CT Scan | 38.21 | 38.02 | 0.19 | 9.89 | 10.18 | 0.29 | 43.78 | 46.40 | 2.62 | 0.29 | 0.56 | 0.27 | NA | NA | |||
| Δ | 1.45 | 1.38 | 0.49 | 0.50 | 4.11 | 3.77 | 0.04 | 0.10 | |||||||||
| 2 | Lt flank 40 Gy | Phantom | 39.27 | 40.04 | 0.77 | 5.66 | 6.08 | 0.42 | 21.45 | 23.02 | 1.57 | 0.29 | 0.37 | 0.08 | 0.38 | 0.43 | 0.05 |
| CT Scan | 40.21 | 40.61 | 0.40 | 5.17 | 5.48 | 0.31 | 17.25 | 18.83 | 1.58 | 0.30 | 0.34 | 0.04 | 0.29 | 0.31 | 0.02 | ||
| Δ | 0.94 | 0.57 | 0.49 | 0.60 | 4.20 | 4.18 | 0.01 | 0.03 | 0.09 | 0.12 | |||||||
| 3 | Rt flank 30 Gy | Phantom | 2.70 | 2.81 | 0.11 | 0.97 | 1.06 | 0.09 | 0.00 | 0.00 | 0.00 | 0.13 | 0.17 | 0.04 | 0.25 | 0.38 | 0.13 |
| CT Scan | 1.90 | 1.83 | 0.07 | 0.86 | 0.96 | 0.10 | 0.00 | 0.00 | 0.00 | 0.15 | 0.12 | 0.03 | 0.27 | 0.40 | 0.13 | ||
| Δ | 0.80 | 0.98 | 0.11 | 0.11 | 0.00 | 0.00 | 0.02 | 0.05 | 0.02 | 0.02 | |||||||
| 4 | WA 30 Gy | Phantom | 31.08 | 30.86 | 0.22 | 9.75 | 10.13 | 0.38 | 43.05 | 49.69 | 6.64 | 0.62 | 0.77 | 0.15 | 2.63 | 3.31 | |
| CT Scan | 29.62 | 29.31 | 0.31 | 10.21 | 10.50 | 0.29 | 44.33 | 47.88 | 3.55 | 0.20 | 0.65 | 0.45 | NA | NA | |||
| Δ | 1.46 | 1.55 | 0.46 | 0.37 | 1.28 | 1.81 | 0.42 | 0.13 | |||||||||
| 5 | WA 34.5 Gy | Phantom | 35.76 | 35.27 | 0.49 | 10.56 | 10.95 | 0.39 | 43.03 | 48.33 | 5.30 | 0.69 | 0.87 | 0.18 | 3.05 | 3.97 | 0.92 |
| CT Scan | 35.06 | 34.77 | 0.29 | 10.66 | 11.01 | 0.35 | 37.84 | 42.82 | 4.98 | 0.27 | 0.80 | 0.53 | 4.06 | 5.71 | 1.65 | ||
| Δ | 0.70 | 0.51 | 0.10 | 0.06 | 5.19 | 5.51 | 0.42 | 0.07 | 1.01 | 1.74 | |||||||
| 6 | WA 10.5Gy/WholeLung 1.5Gy | Phantom | 11.05 | 11.02 | 0.03 | 12.32 | 12.19 | 0.13 | 100.00 | 100.00 | 0.00 | 6.86 | 7.33 | 0.47 | 0.96 | 1.40 | 0.44 |
| CT Scan | 11.16 | 11.20 | 0.04 | 12.38 | 12.30 | 0.08 | 100.00 | 100.00 | 0.00 | 6.65 | 7.19 | 0.54 | 1.80 | 2.59 | 0.79 | ||
| Δ | 0.11 | 0.19 | 0.06 | 0.11 | 0.00 | 0.00 | 0.21 | 0.14 | 0.84 | 1.20 | |||||||
| 7 | WA 34.5 Gy | Phantom | 35.37 | 35.26 | 0.11 | 11.65 | 12.07 | 0.42 | 45.37 | 48.91 | 3.54 | 0.65 | 0.85 | 0.20 | 4.33 | 6.24 | 1.91 |
| CT Scan | 35.07 | 34.73 | 0.34 | 10.68 | 11.01 | 0.33 | 37.94 | 42.81 | 4.87 | 0.24 | 0.80 | 0.56 | 3.98 | 5.70 | 1.72 | ||
| Δ | 0.30 | 0.53 | 0.97 | 1.06 | 7.43 | 6.10 | 0.41 | 0.05 | 0.35 | 0.54 | |||||||
| 8 | Whole Lung+WA 11.5 Gy | Phantom | 12.05 | 12.10 | 0.05 | 11.99 | 11.97 | 0.02 | 100.00 | 100.00 | 0.00 | 7.90 | 8.25 | 0.35 | 1.45 | 2.34 | 0.89 |
| CT Scan | 11.77 | 11.71 | 0.06 | 11.87 | 11.68 | 0.19 | 100.00 | 100.00 | 0.00 | 6.42 | 6.89 | 0.47 | 2.32 | 2.98 | 0.66 | ||
| Δ | 0.28 | 0.39 | 0.12 | 0.29 | 0.00 | 0.00 | 1.48 | 1.36 | 0.87 | 0.64 | |||||||
| 9 | Whole Lung 15Gy/Lt flank 25Gy | Phantom | 41.97 | 41.76 | 0.21 | 16.33 | 16.20 | 0.13 | 100.00 | 100.00 | 0.00 | 9.63 | 9.98 | 0.35 | 0.37 | 0.92 | 0.55 |
| CT Scan | 40.13 | 40.00 | 0.13 | 16.08 | 16.04 | 0.04 | 100.00 | 100.00 | 0.00 | 8.56 | 8.94 | 0.38 | 0.42 | 0.81 | 0.39 | ||
| Δ | 1.84 | 1.76 | 0.25 | 0.16 | 0.00 | 0.00 | 1.07 | 1.05 | 0.05 | 0.10 | |||||||
| 10 | WA 20Gy/ Whole Lung 13.5Gy | Phantom | 19.94 | 19.88 | 0.06 | 14.41 | 14.43 | 0.02 | 100.00 | 99.91 | 0.09 | 6.77 | 7.22 | 0.45 | 0.34 | 0.72 | 0.38 |
| CT Scan | 18.75 | 18.81 | 0.06 | 14.46 | 14.54 | 0.08 | 100.00 | 100.00 | 0.00 | 6.00 | 6.29 | 0.29 | 0.46 | 0.79 | 0.33 | ||
| Δ | 1.19 | 1.08 | 0.05 | 0.11 | 0.00 | 0.09 | 0.77 | 0.93 | 0.12 | 0.06 | |||||||
Note: Please see P values in the text under “Results”. ‘Δ’ Is the absolute difference in dose (Gy). NA – not applicable as the CT scans did not include testicles. WA – Whole Abdomen. When multiple sites were treated, these sites may be treated in a single field (+) or as separate fields (/).
Table 2.
Comparison of TPS and MC Organ doses in 10 female NWTS patient-matched CT scans and UF/NCI Phantoms.
| NWTS RT Prescription | Mean Left kidney Gy | Mean Heart Gy | % heart >= 5Gy (cc) | Mean Thyroid Gy | Mean Ovary Gy RIGHT + LEFT |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | TPS | MC | Δ | TPS | MC | Δ | TPS | MC | Δ | TPS | MC | Δ | TPS | MC | Δ | ||
| 11 | WA 10.5Gy/Whole Lung 12Gy | Phantom | 10.48 | 10.46 | 0.02 | 12.63 | 12.58 | 0.05 | 100.00 | 100.00 | 0.00 | 5.61 | 5.95 | 0.34 | 10.99 | 10.85 | 0.14 |
| CT Scan | 11.19 | 11.25 | 0.06 | 12.73 | 12.73 | 0.00 | 100.00 | 100.00 | 0.00 | 5.26 | 5.40 | 0.14 | 11.31 | 11.24 | 0.07 | ||
| Δ | 0.71 | 0.80 | 0.10 | 0.15 | 0.00 | 0.00 | 0.35 | 0.55 | 0.32 | 0.39 | |||||||
| 12 | Whole Lung 14 Gy | Phantom | 0.78 | 0.88 | 0.10 | 14.29 | 14.25 | 0.04 | 100.00 | 100.00 | 0.00 | 6.52 | 6.89 | 0.37 | 0.06 | 0.08 | 0.02 |
| CT Scan | 1.02 | 1.15 | 0.13 | 14.47 | 14.52 | 0.05 | 100.00 | 100.00 | 0.00 | 6.33 | 6.80 | 0.47 | 0.12 | 0.09 | 0.03 | ||
| Δ | 0.24 | 0.27 | 0.18 | 0.27 | 0.00 | 0.00 | 0.19 | 0.10 | 0.06 | 0.01 | |||||||
| 13 | WA 40 Gy | Phantom | 41.03 | 40.96 | 0.07 | 14.49 | 14.89 | 0.40 | 49.08 | 53.68 | 4.60 | 0.68 | 0.82 | 0.14 | 41.89 | 41.33 | 0.56 |
| CT Scan | 42.27 | 42.33 | 0.06 | 11.84 | 12.27 | 0.43 | 40.20 | 46.42 | 6.22 | 0.81 | 1.06 | 0.25 | 41.37 | 42.31 | 0.94 | ||
| Δ | 1.24 | 1.37 | 2.65 | 2.62 | 8.88 | 7.25 | 0.13 | 0.25 | 0.52 | 0.98 | |||||||
| 14 | Whole Lung 15Gy/Lt flank 22.5Gy | Phantom | 42.20 | 42.07 | 0.13 | 16.26 | 16.31 | 0.05 | 100.00 | 100.00 | 0.00 | 6.88 | 7.61 | 0.73 | 18.07 | 17.88 | 0.19 |
| CT Scan | 39.33 | 39.29 | 0.04 | 16.09 | 15.72 | 0.37 | 100.00 | 100.00 | 0.00 | 4.51 | 5.19 | 0.68 | 17.22 | 16.81 | 0.41 | ||
| Δ | 2.87 | 2.78 | 0.17 | 0.59 | 0.00 | 0.00 | 2.37 | 2.42 | 0.85 | 1.07 | |||||||
| 15 | WA 20 Gy | Phantom | 20.44 | 20.38 | 0.06 | 7.08 | 7.29 | 0.21 | 37.54 | 41.97 | 4.43 | 0.22 | 0.36 | 0.14 | 20.32 | 19.92 | 0.40 |
| CT Scan | 21.09 | 21.18 | 0.09 | 5.92 | 6.14 | 0.22 | 32.16 | 34.11 | 1.95 | 0.41 | 0.53 | 0.12 | 21.18 | 21.16 | 0.02 | ||
| Δ | 0.65 | 0.80 | 1.16 | 1.15 | 5.38 | 7.86 | 0.19 | 0.17 | 0.86 | 1.23 | |||||||
| 16 | Whole Lung 12Gy/ WA 10.5Gy | Phantom | 11.13 | 11.09 | 0.04 | 12.54 | 12.45 | 0.09 | 100.00 | 99.96 | 0.04 | 9.35 | 9.34 | 0.01 | NA | NA | |
| CT Scan | 11.42 | 11.35 | 0.07 | 12.64 | 12.69 | 0.05 | 100.00 | 99.96 | 0.04 | 8.60 | 8.82 | 0.22 | 11.19 | 11.13 | 0.06 | ||
| Δ | 0.29 | 0.26 | 0.10 | 0.23 | 0.00 | 0.00 | 0.75 | 0.52 | |||||||||
| 17 | WA 10.5 Gy | Phantom | 10.82 | 10.84 | 0.02 | 1.97 | 2.11 | 0.14 | 14.94 | 14.95 | 0.01 | 0.21 | 0.25 | 0.04 | 11.06 | 10.93 | 0.13 |
| CT Scan | 10.90 | 10.91 | 0.01 | 2.96 | 3.11 | 0.15 | 23.06 | 23.99 | 0.93 | 0.27 | 0.32 | 0.05 | 11.15 | 11.02 | 0.13 | ||
| Δ | 0.08 | 0.07 | 0.99 | 1.01 | 8.12 | 9.03 | 0.06 | 0.06 | 0.09 | 0.09 | |||||||
| 18 | Whole Lung 13.5Gy/WA 21Gy | Phantom | 21.50 | 21.64 | 0.14 | 14.45 | 14.58 | 0.13 | 100.00 | 100.00 | 0.00 | 6.27 | 6.61 | 0.34 | 22.19 | 22.04 | 0.15 |
| CT Scan | 21.92 | 21.85 | 0.07 | 14.29 | 14.20 | 0.09 | 100.00 | 100.00 | 0.00 | 6.99 | 7.20 | 0.21 | 22.68 | 21.95 | 0.73 | ||
| Δ | 0.42 | 0.21 | 0.16 | 0.37 | 0.00 | 0.00 | 0.72 | 0.59 | 0.49 | 0.10 | |||||||
| 19 | WA15G/W Lung 14G/Lt flank 3Gy | Phantom | 18.60 | 18.69 | 0.09 | 14.58 | 14.56 | 0.02 | 100.00 | 100.00 | 0.00 | 6.19 | 6.49 | 0.30 | 15.58 | 15.48 | 0.10 |
| CT Scan | 19.12 | 19.00 | 0.12 | 14.90 | 15.00 | 0.10 | 100.00 | 100.00 | 0.00 | 5.73 | 6.11 | 0.38 | 16.01 | 16.06 | 0.05 | ||
| Δ | 0.52 | 0.31 | 0.32 | 0.44 | 0.00 | 0.00 | 0.46 | 0.38 | 0.43 | 0.58 | |||||||
| 20 | WA 30Gy/ Rt Lung+Flank13.5Gy | Phantom | 32.21 | 31.81 | 0.40 | 19.58 | 20.03 | 0.45 | 91.37 | 93.79 | 2.42 | 3.40 | 4.28 | 0.88 | 30.86 | 30.30 | 0.56 |
| CT Scan | 32.17 | 32.31 | 0.14 | 20.32 | 20.62 | 0.30 | 86.07 | 87.91 | 1.84 | 2.98 | 3.63 | 0.65 | 32.57 | 32.25 | 0.32 | ||
| Δ | 0.04 | 0.50 | 0.74 | 0.58 | 5.30 | 5.88 | 0.42 | 0.65 | 1.71 | 1.94 | |||||||
Note: Please see P values in the text under “Results”. ‘Δ’ Is the absolute difference in dose (Gy). NA – not applicable as the phantoms did not have ovaries. WA – Whole Abdomen. When multiple sites were treated, these sites may be treated in a single field (+) or as separate fields (/).
For Aim 1, evaluating the anatomic accuracy of the phantoms, the mean TPS doses of the heart, kidney, thyroid gland, testes and ovaries were obtained for all patient-matched CT scans and phantoms. The percent (%) heart volume receiving >=5Gy (threshold dose for cardiac mortality in the French Study) was also determined (18). For Aim 2, comparing TPS and MC dosimetry, the DICOM files of both CT scan and phantom anatomic models with RT planning and dosimetry data (TPS) were sent to the NCI for MC radiation transport simulation to provide more accurate out-of-field doses. The MC calculations were performed using the X-ray Voxel Monte Carlo (XVMC) code (CL, MM, and JWJ) (19–21). The methodology for converting the DICOM data into an input file for the XVMC simulation was described in a previous publication by Lee et al. (10). Extensive benchmarking of the XVMC dosimetry method was performed by comparing against ion chamber measurements in a water phantom for different field sizes and depths as described in a recent publication by Mille et al. (22). All simulations were run on NCI Biowulf high-performance Linux computing cluster which has 95,000+ cores.
Statistical analysis was performed using Wilcoxon rank-sum test (comparison of two anatomic models - UF/NCI phantoms vs. CT scans) and Signed rank test (comparison of two dosimetric methods - TPS vs. MC dosimetry). These data were summarized by means and ranges and analyses were conducted in SAS v9.4.
RESULTS
Comparison of Organ Dosimetry in NWTS patient-matched phantoms and CT scans
Tables 1 and 2 show the mean TPS and MC doses of the heart, kidney, thyroid gland, testes, ovary and the % heart receiving >=5Gy for 10 male and 10 female NWTS patient-matched phantoms and CT scans (Columns in Table 1, 2) (Aim 1) (Fig. 1, 2). The NWTS protocol RT fields and doses for these patients are also shown. There was good dosimetric correlation between these two models as demonstrated by the small absolute difference in RT doses (Table 1, 2). For the entire patient cohort the overall mean cumulative dose difference (TPS and MC) (range) for the various organs between CT scans and phantoms was 0.61 Gy (0.01–2.87 Gy). The mean difference between % heart volume receiving >=5Gy between these 2 models was 2.54% (0–9.03%). There was no significant difference between phantom and CT dosimetry for any of the organs with both TPS and MC dosimetric methods. The P-values for the entire patient cohort for phantom doses vs. CT dose comparisons for the two dosimetric methods (TPS and MC) were as follows: Mean heart dose (phantom vs. CT models): TPS (0.92) and MC (0.89); Mean kidney dose (phantom vs. CT): TPS (0.98) and MC (0.99); Mean thyroid gland dose (phantom vs. CT): TPS (0.60) and MC (0.62); Mean testes dose (phantom vs. CT): TPS (0.67) and MC (0.95), Mean ovary dose (phantom vs. CT): TPS (0.76) and MC (0.76); % heart volume receiving >=5Gy (phantom vs. CT): TPS (0.87) and MC (0.96).
Figure 1.
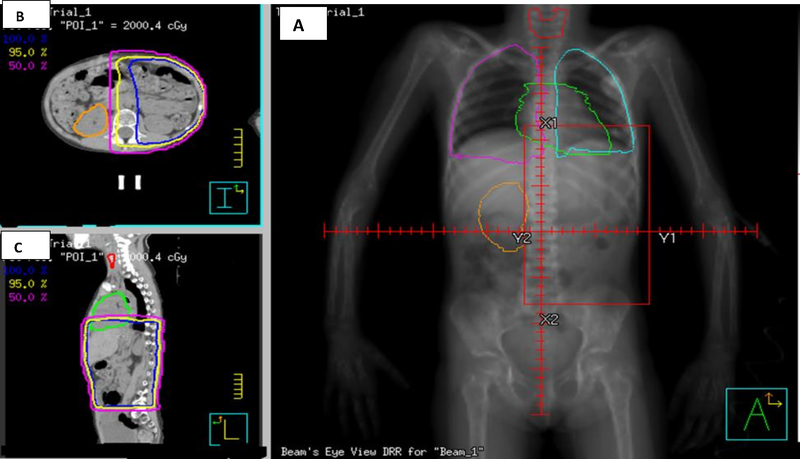
A) Digitally Reconstructed Radiograph (DRR) of a left flank RT field (20Gy) for an 85 month old female NWTS patient-matched CT scan; B) Axial CT scan image showing TPS planned isodose curves (95%, 5%, 0.5%); C) Sagittal CT scan image showing similar isodose lines. The heart, thyroid gland, lungs and ovaries are shown.
Figure 2.
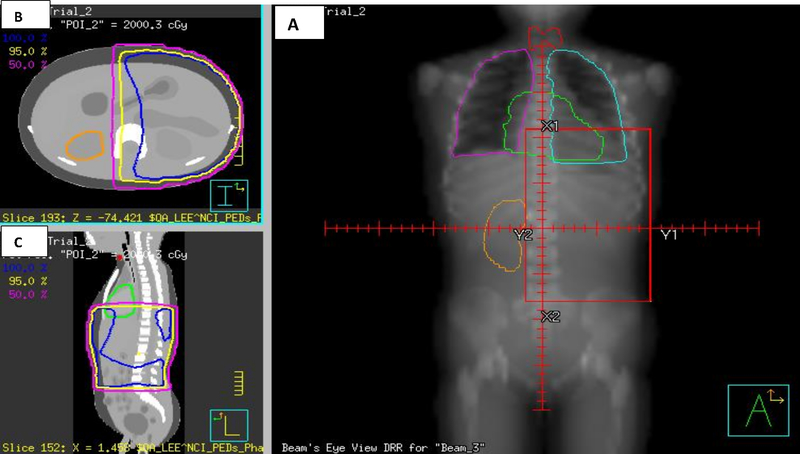
A) Digitally Reconstructed Radiograph (DRR) of a left flank RT field (20Gy) for an 85 month old female NWTS patient-matched NCI Phantom; B) Axial image showing TPS planned isodose curves (95%, 5%, 0.5%); C) Sagittal image showing similar isodose lines. The heart, thyroid gland, lungs and ovaries are shown.
Comparison of Organ Dosimetry using TPS and MC dosimetry in both Patient-matched Phantoms and CT scans
Tables 1 and 2 show the TPS and MC doses for 10 male and 10 female NWTS patient-matched phantoms and CT scans (Rows in Table 1, 2) (Aim 2) (Figure 1, 2). For the entire patient cohort, the mean cumulative difference in dose (range) between TPS vs. MC for various organs in both CT and phantom models were as follows: Left kidney 0.81 Gy (0.04–2.87 Gy) vs. 0.81 Gy (0.02–2.78 Gy); Heart 0.48 Gy (0.05–2.65 Gy) vs. 0.54 Gy (0.06–2.62 Gy); Thyroid 0.52 Gy (0.01–2.37 Gy) vs. 0.48 Gy (0.03–2.42 Gy); Testes 0.42 Gy (0.02–1.01 Gy) vs. 0.55 Gy (0.02–1.74 Gy) and Ovaries 0.59 Gy (0.06–1.71 Gy) vs. 0.71 Gy (0.01–1.94 Gy). There was a significant difference between TPS and MC organ doses in both CT and phantom models and the P values were as follows: mean Left kidney dose (TPS vs. MC): Phantom model (<0.0001) and CT model (<0.0001); mean heart dose (TPS vs. MC): Phantom model (<0.0001) and CT model (<0.0001); % heart volume >=5Gy (TPS vs. MC): Phantom model (0.001) and CT (0.005); Mean thyroid gland dose (TPS vs. MC): Phantom model (<0.0001) and CT model (<0.0001); Mean testes dose (TPS vs. MC): Phantom model (0.002) and CT model (0.02); Mean ovary dose (TPS vs. MC): Phantom model (0.004) and CT model (0.002).
The organs were then classified as either in-field (<5 cm from field edge), near-field (>5 but <10 cm from field edge) and out-of-field (>10 cm from field edge) based on their anatomic relationship to the different RT fields prescribed for each patient. The overall mean dose difference (range) between TPS and MC dosimetry for all in-field, near-field and out-of-field organs in both models were: 0.17 Gy (0.00–0.73 Gy); 0.32 Gy (0.01–0.49 Gy) and 0.58 Gy (0.02–1.91 Gy), respectively. While the dose for in-field organs was similar when using the TPS and MC methods, the doses for near-field and out-of-field organs were consistently higher for the MC compared to the TPS dosimetry approach for the majority of dose calculations performed (90–100%). On average, the MC dose to distant organs such as testes and thyroid gland was larger than that calculated by the TPS by 31.6% (6.8–59.6%) and 18.25% (0.07–70.1%), respectively. However, the absolute dose difference between MC and TPS for testes and thyroid was small (< 1 Gy).
DISCUSSION
The seminal NWTS publications from the NWTS late effects study (LES) were for 5 late effects targeted for data collection from patients (Second Malignant Neoplasms, Congestive heart failure, Restrictive Pulmonary disease, Stage Renal Disease, and Adverse Pregnancy Outcomes (23–32). All these reports have consistently implicated higher RT doses, larger RT fields and doxorubicin as important causative factors for these late effects. However, as described earlier, these studies only considered RT fields and prescribed RT doses without organ dosimetry.
While modern RT treatments have the capability of providing accurate 3D organ dosimetry, many of the late effects discussed above will require decades of follow-up before any dose-volume correlations can be made. PENTEC (Pediatric Normal Tissue Effects in the Clinic) is a collaborative effort that is currently analyzing past published literature on 3D organ-RT tolerance in children (33). The main limitation for PENTEC is that the majority of past publications did not provide meaningful 3D dosimetry correlation with late effects. This retrospective dosimetry study, will make available 3D organ dosimetry that can be correlated with these NWTS late effects after decades of follow up. This knowledge will in turn help the current generation of children receive safer RT treatments that may result in a lower incidence of these late effects using modern technology such as IMRT or protons. The NWTS LES data linking congestive heart failure and lung RT has led to the development of a cardiac sparing IMRT protocol that will be adopted in the next generation of COG renal tumor protocols (34).This phantom dosimetry study will also provide physicians, parents and survivors evidence that can be used to develop more robust follow-up guidelines aimed at risk reduction and mitigation of the impact of treatment-induced late toxicity in childhood cancer survivors.
Accurate dose estimation for in-field and out-of-field organs is crucial for evaluating dose-response relationship for both tumor and organs at risk (OAR). While the modern RT TPS is fairly accurate for estimating doses both in-field and near-field locations, it is not accurate for out-of-field dose calculations (11–16). Howell et al. showed that commercial RT TPS (Eclipse) underestimated out-of-field doses by an average of 40% compared to water phantom measurements and this error was greater (up to 55%) at distances >11 cm from edge of RT fields (15). Others have also reported that MC dose calculation algorithm measures out-of-field doses accurately (12–16). The main dose contributors for out-of-field doses include machine head leakage, scatter through machine components, and internal tissue scatter which are not fully accounted for in TPS calculations. The XVMC Monte Carlo code that was used in this report was validated with water phantom measurements out to 30 cm beyond the field edge (22). In this report extensive benchmarking of the XVMC dosimetry method was performed by comparing against ion chamber measurements in a water phantom for different field sizes and depths. Errors in the in-plane dose-profiles are generally smaller than 25%, except at 20 cm beyond the field-edge where they can be as large as 50%. This accuracy is acceptable because the dose received by tissues at these distances is typically quite small (<1% of prescribed dose).
In this report we have demonstrated the feasibility and accuracy of retrospective dosimetry using this UF/NCI phantom model by demonstrating the lack of any statistical difference between 3D organ doses (both TPS and MC) between 20 NWTS patient-matched phantoms and CT scans. Further, we have also shown that while the in-field doses were similar between TPS and MC dosimetry approaches, the out-of-field target organ doses were consistently higher with MC dosimetry compared to standard TPS measurements in these two models. This UF/NCI phantom model would be superior to other older phantom models used by late effects studies such as the Childhood Cancer Survivor Study (CCSS) (35). The older phantom models use dose measurements that are limited to points in a water or anthropomorphic phantom which may differ according to the size of the patient. On the other hand, the image-based 3D dosimetry approach as used in this study uses height- and weight-matched phantoms and can provide dose at every voxel element in the body. While CT scans represents the gold standard for modern RT dosimetry, for the conduct of retrospective dosimetry in large scale epidemiology studies, patient-matched phantoms have the advantage over patient-matched CT scans in that they come with all organs pre-contoured; thus, avoiding the labor-intensive step of organ segmentation in a large number of CT scans. We propose to use this NCI Phantom Monte Carlo dosimetry model as part of a NIH funded R01 grant to perform retrospective dosimetry on nearly 5000 NWTS patients to study the correlation between 3D organ doses and specific late effects such as congestive heart failure, restrictive pulmonary disease, second malignant neoplasms, end stage renal disease, infertility and adverse pregnancy outcomes.
CONCLUSIONS
This pilot study has demonstrated that the patient-matched UF/NCI phantoms together with Monte Carlo dosimetry is an accurate model for performing retrospective 3D dosimetry. This model is a valuable tool for performing retrospective dosimetry in large scale epidemiology studies such as the NWTS and will permit a more accurate correlation compared to existing methods between 3D organ RT doses and late toxicities observed in childhood cancer survivors. This information will in turn guide the safer delivery of modern RT treatments and also help develop more accurate follow-up guidelines to mitigate the impact of RT on high-risk cancer survivors.
ACKNOWLEDGEMENTS
R01CA219013. The Monte Carlo calculations used in this study were funded by the intramural research program of the National Institutes of Health (NIH), National Cancer Institute, Division of Cancer Epidemiology and Genetics and utilized the computational resources of the NIH High-Performance Computing Biowulf cluster (https://hpc.nih.gov).
ABBREVIATIONS
- UF/NCI
UNIVERSITY OF FLORIDA/NATIONAL CANCER INSTITUTE
- NWTS
NATIONAL WILMS TUMOR STUDY
- RT
RADIATION THERAPY
- Gy
GRAY
- CT
COMPUTERIZED TOMOGRAPHY SCANS
- TPS
TREATMENT PLANNING SYSTEM
- DICOM
DIGITAL IMAGING AND COMMUNICATIONS IN MEDICINE
- 2D
TWO DIMENSIONAL
- 3D
THREE DIMENSIONAL
- WT
WILMS TUMOR
- AP-PA FIELDS
ANTEROPOSTERIOR – POSTEROANTERIOR FIELDS
- D50
DOSE TO 50% OF ORGAN VOLUME (MEAN DOSE)
- QA
QUALITY ASSURANCE
- LES
LATE EFFECTS STUDY
- ICRP
INTERNATIONAL COMMISSION ON RADIATION PROTECTION
- WA
WHOLE ABDOMEN
- XVMC
X-RAY VOXEL MONTE CARLO
- PENTEC
PEDIATRIC NORMAL TISSUE EFFECTS IN THE CLINIC
- COG
CHILDREN’S ONCOLOGY GROUP
- CCSS
CHILDHOOD CANCER SURVIVOR STUDY
- OAR
ORGAN AT RISK
- Rt
RIGHT
- Lt
LEFT
Footnotes
Conflict of Interest: No
REFERENCES
- 1.D’Angio GJ, Evans AE, Breslow N, et al. : The treatment of Wilms’ tumor. Results of the National Wilms’ Tumor Study, Cancer 1976; 38:633–646. [DOI] [PubMed] [Google Scholar]
- 2.D’Angio GJ, Evans A, Breslow N, et al. : The treatment of Wilms tumor. Results of the Second National Wilms’ Tumor Study, Cancer 1981; 47:2302–2311. [DOI] [PubMed] [Google Scholar]
- 3.Thomas PRM, Tefft M, Farewell VT, et al. : Abdominal relapses in irradiated Second National Wilms’ Tumor Study patients, J Clin Oncol 1984; 2:1098–1101. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PRM, Tefft M, Compaan PJ, et al. : Results of two radiotherapy randomizations in the Third National Wilms’ Tumor Study (NWTS-3). Cancer 1991; 68:1703–1707. [DOI] [PubMed] [Google Scholar]
- 5.D’Angio GJ, Breslow N, Beckwith JB, et al. : Treatment of Wilms’ tumor. Results of the Third National Wilms’ Tumor Study, Cancer 1989; 64:349–360. [DOI] [PubMed] [Google Scholar]
- 6.Green DM, Breslow NE, Beckwith JB, et al. : A comparison between single dose and divided dose administration of dactinomycin and doxorubicin, J Clin Oncol 1998; 16:237–245. [DOI] [PubMed] [Google Scholar]
- 7.Grundy PE, Breslow NE, Li S, et al. : Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable histology Wilms’ tumor. A report from the National Wilms’ Tumor Study Group, J Clin Oncol 2005; 23:7312–7321. [DOI] [PubMed] [Google Scholar]
- 8.Lee C, Lodwick D, Hurtado J et al. The UF family of reference hybrid phantoms for computational radiation dosimetry. Phys Med Biol 2010; 55: 339–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geyer AM, O’Reilly SO, Lee C et al. The UF/NCI family of hybrid computational phantoms representing the current US population of male and female children, adolescents and adults – application to CT dosimetry. Phys Med Biol 2014; 59: 5225–5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C, Jung JW, Pelletier C et al. Reconstruction of organ dose for external radiotherapy patients in retrospective epidemiologic studies. Phys Med Biol 2015; 60: 2309–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chetty IJ, Curran B, Cygler JE et al. Report of the AAPM Task Group No.105: Issues associated with clinical implementation of Monte Carlo-based photon and electron external beam treatment planning. Med Phys 2007; 34: 4818–4853. [DOI] [PubMed] [Google Scholar]
- 12.Kry SF, Titt W, Followill D et al. A Monte Carlo model for out-of-field dose calculation from high-energy photon therapy. Med Phys 2007; 34: 3489–3499. [DOI] [PubMed] [Google Scholar]
- 13.Song JH, Shin JK, Kay CS et al. Comparison of dose calculations between pencil-beam and Monte Carlo algorithms of the iPlan RT in arc therapy using homogenous phantom with 3DVH software. Radiat Oncol 2013; 8: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bednarz B, Xu XG. Monte Carlo modeling of a 6 and 18MV Varian Clinac medical accelerator for in-field and out-of-field dose calculations: development and validation. Phys Med Biol 2009; 54: 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell RM, Scarboro SB, Kry SF et al. Accuracy of out-of-field dose calculations by a commercial treatment planning system. Phys Med Biol 2010; 55: 6999–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger T, Sauer OA. Monte Carlo versus pencil beam/collapsed cone dose calculation in a heterogeneous multi-layer phantom. Phys Med Biol 2005; 50: 859–868. [DOI] [PubMed] [Google Scholar]
- 17.Bardo DM, Black M, Schenk K et al. Location of the ovaries in girls from newborn to 18 years of age: reconsidering ovarian shielding. Pediatr Radiol 2009; 39: 253–259. [DOI] [PubMed] [Google Scholar]
- 18.Pein F, Sakiroglu O, Dahan M et al. Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumor at the Institut Gustave Roussy. Br J Cancer 2004; 91: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawrakow I, Fippel M. Investigation of variance reduction techniques for Monte Carlo photon dose calculation using XVMC. Phys Med Biol 2000; 45: 2163–2183. [DOI] [PubMed] [Google Scholar]
- 20.Kawrakow I, Fippel M. Investigation of variance reduction techniques for Monte Carlo photon dose calculation using XVMC. Phys Med Biol 2000; 45: 2163–2183. [DOI] [PubMed] [Google Scholar]
- 21.Kawrakow I, Fippel M, Friedrich K. 3D electron dose calculation using a voxel based Monte Carlo algorithm (VMC). Med Phys 1996; 23: 445–457. [DOI] [PubMed] [Google Scholar]
- 22.Mille MM, Jung JW, Lee C, Kuzmin GA, Lee C. Comparison of normal tissue dose calculation methods for epidemiological studies of radiotherapy patients. J Radiol Prot 2018; 10.1088/1361-6498/aabd4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green DM, Grigoriev YA, Nan B et al. Congestive heart failure after treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol 2001; 19: 1926–1934. [DOI] [PubMed] [Google Scholar]
- 24.Lange JM, Takashima JR, Peterson SM et al. Breast cancer in female survivors of Wilms tumor: A report from the National Wilms Tumor Late Effects Study. Cancer 2014; 120: 3722–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalapurakal JA, Peterson S, Peabody EM et al. Pregnancy outcomes after abdominal irradiation that included or excluded the pelvis in childhood Wilms tumor survivors: A report from the National Wilms Tumor Study. Int J Radiat Oncol Biol Phys 2004; 58: 1364–1368. [DOI] [PubMed] [Google Scholar]
- 26.Green DM, Peabody EM, Nan B et al. Pregnancy outcome after treatment for Wilms tumor: A report from the National Wilms tumor Study Group. J Clin Oncol 2002; 20: 2506–2513. [DOI] [PubMed] [Google Scholar]
- 27.Green DM, Lange JM, Peabody EM et al. Pregnancy outcome after treatment for Wilms tumor: A report from the National Wilms tumor Long-term Follow-up Study. J Clin Oncol 2010; 28: 2824–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DM, Lange JM, Qu A et al. Pulmonary disease after treatment for Wilms tumor: A report from the National Wilms Tumor Long-term Follow-up Study. Pediatr Blood Cancer 2013; 60: 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange J, Peterson SM, Takashima JR et al. Risk factors for end stage renal disease in non-WT1 syndromic Wilms tumor. J Urol 2011; 186: 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breslow NE, Collins AJ, Ritchey ML et al. , End stage renal disease in patients with Wilms tumor: Results from the National Wilms tumor Study Group and the United States Renal Data System. J Urol 2005; 174: 1972–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cotton CA, Peterson S, Norkool PA et al. Early and Late mortality after diagnosis of Wilms tumor. J Clin Oncol 2009; 27: 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breslow NE, Takashima JR, Whitton JA et al. Second Malignant Neoplasms following treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol 1995; 13: 1851–1859. [DOI] [PubMed] [Google Scholar]
- 33.Constine L, Hodgson D, Bentzen S. Pediatric treatment planning II: The PENTEC report on normal tissue complications. www.aapm.org/meetings/2014am/PRSessions.
- 34.Kalapurakal JA, Gopalakrishnan M, Walterhouse D. Final report of a prospective clinical trial of cardiac sparing whole-lung intensity modulated radiation therapy in patients with metastatic pediatric tumors. Int J Radiat Oncol Biol Phys 2016; 96 suppl:118–9. [Google Scholar]
- 35.Stovall M, Weathers R, Kasper C et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Rad Res 2006; 166: 141–157. [DOI] [PubMed] [Google Scholar]