Abstract
Giant unilamellar protein vesicles (GUPs) were formed with the adenosine A2A receptor (A2AR) incorporated in the lipid bilayer and observed protein partitioning into liquid ordered and liquid disordered phases. When no ligand is bound, A2AR partitions preferentially into the liquid disordered phase of GUPs, while ligand-bound A2AR partitions into the liquid ordered phase.
Lipid rafts are hypothesized areas on the cell plasma membrane where cholesterol, sphingolipids, and proteins may aggregate in a manner that modulates cellular signaling.[1–4] In recent decades, lipid rafts have been modeled extensively using phase-separating giant unilamellar vesicles (GUVs).[5, 6] GUVs allow for stringent control of membrane composition and direct observation of micron-scale lipid phase separation that mimics the hypothetical rafts.[4, 6, 7] GUVs of certain ternary lipid compositions can undergo liquid-liquid (l-l) phase separation where the liquid ordered (lo) phase (analogous to lipid rafts) is rich in cholesterol, saturated lipids and sphingolipids, and the liquid disordered phase (ld) is enriched in unsaturated lipids.[8, 9] Protein incorporated GUVs, known as giant unilamellar protein-vesicles (GUPs), offer a means to directly observe protein partitioning in phase-separating GUP compositions. Our group has previously reported on the partitioning of spinach aquaporin SoPIP2;1 and the human serotonin receptor 5-HT1AR in phase-separating GUPs.[10, 11] Here we report on the phase-separating behavior of the G protein-coupled receptor (GPCR) adenosine A2A receptor (A2AR) in GUPs and observe changes in its partitioning due to ligand binding.
GPCRs are the largest family of proteins in the human genome and are the target of over half of medical therapies on the market.[12] They participate in intracellular signaling cascades and are implicated in behavioral and psychiatric disorders. In particular, A2AR is implicated in addiction, in respiratory diseases such as chronic obstructive pulmonary disease (COPD) and is a target for Parkinson’s disease therapies.[13, 14] Furthermore since A2AR participates in immunological feedback loops, this receptor is being targeted for anti-tumor immunotherapies.[15] Assisted by the growing number of structural studies on ligand-bound A2AR, novel drug candidates are being developed and it has been shown that A2AR has a cholesterol consensus motif.[16, 17] Biochemical analysis and crystal structures of A2AR and other GPCRs have revealed significant changes in helix conformation depending on binding partners including ligands, G proteins, ions, and lipids. [18–23] Despite this, studies regarding the biophysical interactions of A2AR and the lipid bilayer of the plasma membrane remain scant.[24, 25] To better understand the biophysics of A2AR phase partitioning, we incorporated A2AR in phase separating vesicles made of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and cholesterol (chol) using a hydrogel-assisted method.[10, 11] This work focuses on a single membrane protein, A2AR, and uniquely addresses lipid-protein interactions. We investigated ligand-bound and ligand-unbound A2aR in purified and crude form and observed that A2aR not bound to ligand partitioned to the ld phase while agonist- or antagonist-bound A2aR partitioned into the lo phase. Further we observed that ligand-bound A2aR protein concentration in the lo phase was unaffected by the area of lo domains (Ao) in GUPS.
Two forms of A2AR were used in our investigations: purified protein (pA2AR) expressed in Sf9 insect cells and protein from crude membranes fragments (cA2AR) expressed in HEK-293 cells and purchased from Perkin Elmer (see SI).[18] Throughout this work, agonist-bound protein will be identified with (Ag) and antagonist-bound protein will be identified with (An). Protein purified from Sf9 cells (as described in the SI) was solubilized in a 0.01%/0.002% dodecyl-β-D-maltoside (DDM)/ cholesterol hemisuccinate (CHS), 25 mM HEPES buffer at pH 7.5 and then subsequently bound to 100μM ZM241385 antagonist (pA2AR(An)). pA2AR purity were determined using analytical aSEC, and proper protein incorporation in GUPs was confirmed by radioligand binding (Figure S1). Unbound apo cA2AR, agonist bound (cA2AR(Ag), final concentration N-ethyl-carboxyadenosine (NECA) 100μM) and antagonist bound (cA2AR(An), final concentration ZM241385 100μM) were used to identify ligand effects on protein partitioning. Both forms of A2AR were tagged using a rhodamine-labeled monoclonal antibody prior to incorporation in unilamellar GUPs for observations (fluorescence intensity controls, phase partitioning, cantibody binding controls and apo and ligand controls and other controls are shown in Figure S2, Figure S3, Figure S4, Figure S5, Figure S6, Figure S7 and Table S1 and Table S2). Antibody-labeled A2AR was incorporated in GUPs made from lipid compositions at various DOPC:DPPC:chol ratios (Table S2). All compositions are known to phase separate into lo and ld phases. [8] In all cases, the protein:lipid molar ratio was ~1:155 (See SI).The concentration of chol was increased over several GUP trials to identify lo and ld areas; since lo regions are chol-rich, the total lo area will increase with increasing chol concentration. GUPs were formed using the agarose hydration method for protein incorporation as described in the SI. Upon formation, GUPs were harvested by gentle pipetting, settled in an isosmotic glucose buffer and then transferred to observation chambers for viewing using confocal microscopy and subsequently analyzed (Figure S8). Vesicles were anchored to glass microscope slides via biotinylation for ease of imaging (see SI).
In all cases, the antibody-labeled protein segregated preferentially to certain regions of the GUP, indicating that it was participating in phase separation (See Figure 1). The only dye in this system was conjugated to the A2AR antibody. To identify the lo and ld phases of GUPs with pA2AR(An), apo cA2AR, cA2AR(An), and cA2AR(Ag), we quantified the ratio of bright area versus dark area in Z-stack projections of GUP confocal images while varying chol concentration (see SI Figure S3 for analysis). DOPC:DPPC:chol GUPs with concentrations of chol from 20 to 40 mol% were prepared. In GUPs containing A2aR bound to ligand, the bright areas increase with increasing chol content while in those containing apo cA2aR the bright areas decrease with increasing chol (Figure 1). The micrographs in Figure 1 show phase separating GUPs with varying amounts of chol. Since chol concentration positively correlates with percent area of the lo phase (Ao) we concluded that ligand-bound pA2AR(An), cA2AR(An), and cA2AR(Ag) partitions to the lo phase while ligand-unbound cA2AR partitions to the ld phase. Furthermore, protein partitioning is independent of CHS or DDM because cA2AR solubilized in either CHS or CHS/DDM (similar conditions to pA2AR) consistently partitions to the ld phase (Figure S9 and Figure S10).
Figure 1.
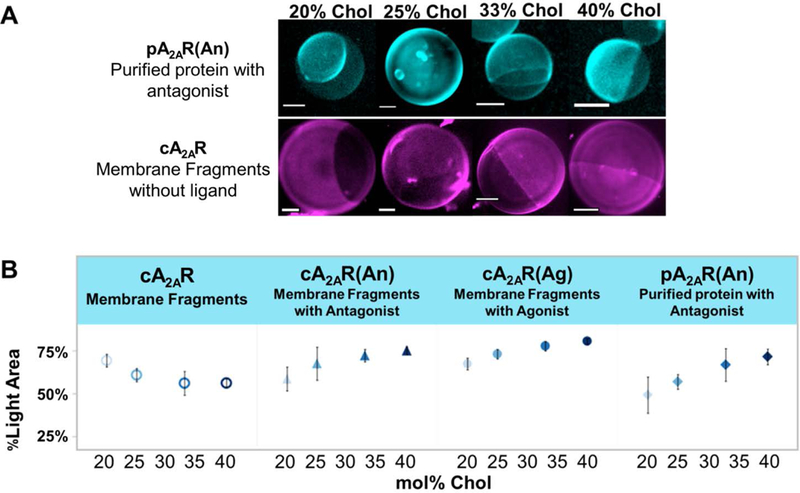
A) GUPs with incorporated A2AR show protein segregation to certain regions of vesicles. The top set of micrographs shows pA2AR(An) tagged with rhodamine-labeled antibody. The bottom set of micrographs shows apo cA2AR tagged with rhodamine-labeled antibody. For pA2AR(An), as mol% chol increases, the bright area of the GUP increases, while for cA2AR, as mol% chol increase, the dark area of the GUP increases. All scale bars are 5 μm. B) %Light area of GUP versus mol% of chol. On average 8 GUPs were analyzed per sample with each experiment being repeated 4 times. The error bars indicate standard error of the mean. In rhodamine-labeled antibody-tagged GUP samples with ligand-bound A2AR, %light area increases with increasing mole percent chol. However, in unbound protein, cA2AR, %light area decreases with increasing mole percent chol. This shows a protein partitioning dependence on ligand binding state.
Ligand-bound A2AR partitioning to the lo phase of phase-separating mixtures of GUPs could be due to changes in protein conformation or dynamics when ligand is bound to the GPCR. While previous reports on GPCR protein partitioning have prominently shown partitioning into the ld phase of phase separating lipid bilayers,[10, 11] some results have suggested that ligand binding with GPCRs causes co-segregation into lipid rafts.[26, 27] For example, in investigations isolating caveolae (which tend to associate with raft-like lipid compositions) from plasma membranes, subfractionation of caveolae and immunofluorescence have shown the G protein subunits Gαi and Gαs in these lipid rafts,[28] and the adenosine A1A GPCR has been shown to move into and out of these lipid rafts.[29] The lipid raft hypothesis suggests that proteins and signaling components aggregate into dynamic lipid rafts to strengthen signaling pathways and to avoid signaling protein cross-talk and degradation.[27, 30] While protein behavior in a cell plasma membrane will depend on protein-protein and protein-cytoskeleton interactions, GPCRs are known to undergo conformational changes during ligand binding and activation that change the receptor’s microenvironment in the plasma membrane.[31–33] Our results suggest and support theories on the dynamic aggregation of protein signaling components into cellular lipid rafts, which may be triggered by agonist binding.
GPCR functionality and membrane elasticity also support liquid ordered phase partitioning of A2AR. Most of this research has been done on rhodopsin and Garwisch et al. showed that rhodopsin could induce lipid order by hydrophilic matching phenomenon,[34] and this was further supported by MD simulations performed by Grossfield and coworkers.[35] Further research by Garwisch and coworkers has shown that elastic curvature stress of the lipid bilayer favors the active meta-II state of rhodopsin.[36, 37] A similar effect was observed by Brown and coworkers, who argued that the conformation energetics of the protein are linked to the continuum mechanical properties of the lipid bilayer.[38]
We have also observed that 5-HT1AR, is more active in ordered membranes.[39] Therefore it is not surprising that ligand-bound A2AR is observed here to partition into ordered domains in phase separating membranes.
To further assess protein partitioning, a partition coefficient K was calculated: K=io/id where io is the fluorescence intensity (a.u.) of the lo area and id is the fluorescence intensity (a.u.) of the ld area. As shown in Figure 2, K plotted against Ao has a negative slope for all samples. As Ao increased, the ratio of io/id decreased, indicating a decrease in the fluorescence intensity of the lo area (Figure 2). Furthermore for all ligand-bound samples, K values were greater than 1, whereas for ligand-unbound apo cA2AR, the K values were less than 1. The observed K values for ligand-unbound cA2AR is due to its opposite partitioning behavior. Without ligand bound, the protein partitions into the liquid disordered phase as described above and in the SI (SI control images). K values for ligand-unbound crude protein controls in 0.01% CHS or 0.01%/0.002% CHS/DDM are shown in Figure S10 and have the same trends as cA2AR in Fig 2. These results show that ligand-bound protein preference for the lo phase decreases as chol concentration increases in GUPs and because the trends in Figure 2 do not cross y=1 suggests that GPCR partitioning is independent of the size of the lo phase, Ao. In serotonin, rhodopsin, and β-adrenergic receptors, chol modulation and binding affects the orientation and population of receptor oligomers.[40–42] Thus spatial arrangement of GPCRs, including dimerization and oligomerization, in the plasma membrane may be mediated by lipid interactions. The decrease observed in K with increasing chol concentration may be related to such spatial organizational effects. Note that this effect is least pronounced (to the point of being nearly unobservable) for the cA2AR(An) sample. This could be due to interactions with endogenous lipids introduced in the crude membrane fragments.
Figure 2.
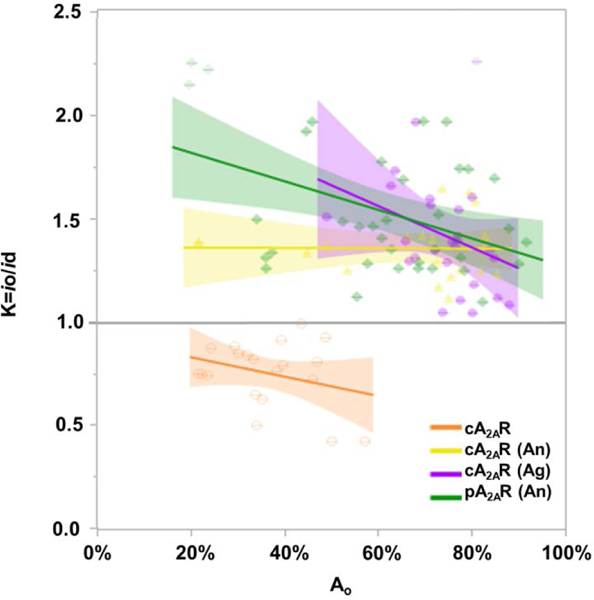
Partition coefficient (K) versus area of liquid ordered domain. All proteins investigated, bound and unbound, have a negative slope. The shaded areas around the lines show the goodness of fit.
To demonstrate that membrane-incorporated A2AR is biochemically active, we measured the activity of A2AR incorporated from crude membrane fragments into GUPs. The activity assay was performed as described in Gutierrez et al 2016,[39] where GUPs were formed to encapsulate BODIPY-GTPγS. Upon formation and settling, GUPs were incubated with the agonist NECA and fluorescent intensity increase due to receptor-catalyzed BODIPY-GTPγS binding to the Gα subunit of the G protein was tracked over time via microplate reader (Figure 3). This experiment was performed in GUPs made from two ratios of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) to chol. The protein is active at both chol concentrations, but receptor-catalyzed nucleotide exchange is faster at the higher chol concentration. The fact that the receptor is capable of catalyzing GDP-GTP exchange subsequent to reconstitution in GUPs demonstrates that the phase segregation phenomenon reported here reflects the behavior of a correctly folded, biologically active protein.
Figure 3.
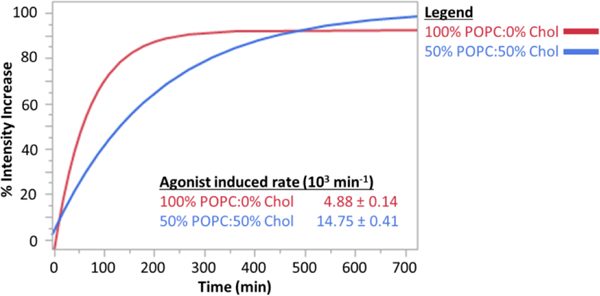
Fluorescence intensity change as a function of time for A2AR-incorporating GUPs incubated with a fluorescent GTP analog and stimulated by agonist. The A2AR was reconstituted from crude membrane fragments which also include the relevant G protein. The experiment was performed at two chol concentrations; higher activity was observed at higher chol concentration. These data show single exponential rates and the standard error mean of 6 replicates at each chol concentration.
The results here show that the behavior of the GPCR A2AR depends on lipid composition. Effects of lipid composition on GPCR function have been reported for rhodopsin, β2-adrenergic receptors, and serotonin receptors.[1, 22, 34, 36, 39, 43] For example, increases in chol concentration in lipid bilayers have been reported to increase the functional activity of the serotonin receptor 5-HT1AR and initial work on the A2AR receptor shows similar behavior (Figure S11).[39] Differences in lipid bilayer thickness, ordering, geometry, and pressure differences between lo and ld phases have also been shown to alter the activity of rhodopsin.[34, 36, 37] Differential partitioning into lipid regions with different bulk properties may therefore modulate the functional activity of A2AR. This is in addition to the potential of phase segregation to promote the aggregation of signaling components and regulate GPCR degradation and activity.[1, 43] Apart from signaling mechanisms linked to putative raft localization, the results here support the idea that lipid ordering preferentially supports ligand-bound configurations of A2AR.
Phase separating compositions of GUPs incorporated with purified and crude GPCR A2AR display lo partitioning when ligand is bound and ld partitioning when unbound. Preferential partitioning of ligand-bound receptor supports the hypotheses on transient dynamic lipid raft assemblies in the plasma membrane that aggregate for protein signaling and regulation. The interplay between ligand-mediated alterations in protein geometry and preferential location to membrane subcompartments may represent an important signal control mechanism for GPCRs and membrane proteins in general.
Supplementary Material
Acknowledgments
MGG was funded by a Viterbi Fellowship, Oakley Fellowship, and ARCS Scholarship. JD was funded by the USC SURE program. LCDO was funded by a Viterbi Fellowship. This work was supported by the Office of Naval Research (N0001-16-1-2382).
Footnotes
Conflicts of interest
There are no conflicts to declare
Supporting information for this article is given via a link at the end of the document. See DOI:10.1039/x0xx00000x
Notes and references
- 1.Levental I and Veatch SL, J Mol Biol 2016, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons K and Sampaio JL, Cold Spring Harbor perspectives in biology 2011, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike LJ, Journal of lipid research 2009, 50 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sezgin E, et al. , Biochimica et biophysica acta 2012, 1818. [DOI] [PubMed] [Google Scholar]
- 5.Wesołowska O, et al. , Acta Biochimica Polonica 2009, 56. [PubMed] [Google Scholar]
- 6.Dietrich C, et al. , Biophysical journal 2001, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sezgin E, et al. , Nature protocols 2012, 7. [DOI] [PubMed] [Google Scholar]
- 8.Veatch SLKSL, Biophysical journal 2003, 85, 3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veatch SL and Keller SL, Physical Review Letters 2005, 94. [DOI] [PubMed] [Google Scholar]
- 10.Hansen JS, et al. , Journal of the American Chemical Society 2013, 135, 17294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez MG and Malmstadt N, Journal of the American Chemical Society 2014, 136, 13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang R and Xie X, Acta pharmacologica Sinica 2012, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonneau O, et al. , Am J Physiol Lung Cell Mol Physiol 2006, 290. [DOI] [PubMed] [Google Scholar]
- 14.Cieslak M, Komoszynski M, and Wojtczak A, Purinergic Signal 2008, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leone RD, Lo YC, and Powell JD, Comput Struct Biotechnol J 2015, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katritch V, Cherezov V, and Stevens RC, Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.[Jafurulla M, Tiwari S, and Chattopadhyay A, Biochemical and biophysical research communications 2011, 404. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, et al. , Science 2012, 337. [Google Scholar]
- 19.Xu F, et al. , Science 2011, 332. [Google Scholar]
- 20.Liu W, et al. , Science 2012, 337. [Google Scholar]
- 21.Carpenter B, et al. , Nature 2016, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zocher M, et al. , Proceedings of the National Academy of Sciences of the United States of America 2012, 109. [Google Scholar]
- 23.Ye L, et al. , Nature 2016, 533. [Google Scholar]
- 24.Jazayeri A, Andrews SP, and Marshall FH, Chem Rev 2017, 117. [DOI] [PubMed] [Google Scholar]
- 25.Lebon G, et al. , Nature 2011, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fallahi-Sichani M and Linderman JJ, PLoS One 2009, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chini B and Parenti M, Journal of molecular endocrinology 2004, 32. [DOI] [PubMed] [Google Scholar]
- 28.Escriba PV, et al. , Biochimica et biophysica acta 2007, 1768. [DOI] [PubMed] [Google Scholar]
- 29.Lasley RD and Smart EJ, Trends in Cardiovascular Medicine 2001, 11. [DOI] [PubMed] [Google Scholar]
- 30.Chini B and Parenti M, Journal of molecular endocrinology 2009, 42. [DOI] [PubMed] [Google Scholar]
- 31.Barnett-Norris J, Lynch D, and Reggio PH, Life Sci 2005, 77. [DOI] [PubMed] [Google Scholar]
- 32.Jaakola VP, et al. , Science 2008, 322. [Google Scholar]
- 33.Xu F, et al. , Science 2011, 332. [Google Scholar]
- 34.Teague WE Jr., et al. , Faraday Discussions 2013, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salas-Estrada LA, et al. , Biophysical journal 2018, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soubias O, et al. , Biophysical journal 2010, 99. [Google Scholar]
- 37.Soubias O, et al. , Biophysical journal 2015, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botelho AV, et al. , Biochemistry 2002, 41. [Google Scholar]
- 39.Gutierrez MG, Mansfield K, and Malmstadt N, Biophysical journal 2016, 110, 2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobe F, et al. , Biochimica et biophysica acta 2008, 1783. [DOI] [PubMed] [Google Scholar]
- 41.Renner U, et al. , Molecular pharmacology 2007, 72. [DOI] [PubMed] [Google Scholar]
- 42.Cherezov V, et al. , Science 2007, 318. [Google Scholar]
- 43.Lorent JH and Levental I, Chemistry and physics of lipids 2015, 192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


