Abstract
Background:
Distress intolerance is linked to the maintenance of panic disorder and cigarette smoking, and may underlie both problems.
Method:
Smokers (n = 54; 40.7% panic disorder) were recruited for an experimental study; half were randomly assigned to 12-hour nicotine deprivation and half smoked as usual. The current investigation consisted of secondary, exploratory analyses from this larger experimental study. Four distress intolerance indices were examined as predictors of anxious responding to an emotional elicitation task (10% carbon dioxide (CO2)-enriched air challenge); anxious responding was in turn examined as a predictor of post-challenge panic and nicotine withdrawal symptoms.
Results:
The Distress Tolerance Scale (DTS) was significantly negatively associated with anxious responding to the challenge (β = −0.41, p = 0.017). The DTS was negatively associated with post-challenge increases nicotine withdrawal symptoms indirectly through the effect of anxious responding to the challenge (b = −0.485, CI95% (–1.095, –0.033)). This same indirect effect was found for post-challenge severity of panic symptoms (b = −0.515, CI95% (–0.888, –0.208)). The DTS was directly predictive of post-challenge increases nicotine withdrawal symptoms, in the Opposite direction (β = 0.37, p = 0.009), but not panic symptom severity.
Conclusions:
Anxious responding in response to stressful experiences may explain the impact of perceived distress intolerance on panic and nicotine withdrawal symptom expression.
Keywords: Distress intolerance, biological challenge, CO2, withdrawal, panic disorder
Introduction
There are established bidirectional relations between panic psychopathology and cigarette smoking (Abrams et al., 2008a; Cosci et al., 2010; Zvolensky and Bernstein, 2005). For example, cigarette smoking is prospectively associated with an increased risk of panic attacks and panic disorder (Breslau and Klein 1999; Breslau et al., 2004; Johnson et al., 2000) and, conversely, panic psychopathology is associated with smoking maintenance (e.g., Farris et al., 2014b) and poorer cessation outcomes (Piper et al., 2011). These interrelations may, in part, be understood by a shared diathesis. Indeed, growing evidence suggests that one’s perceived or objective ability (or inability) to withstand aversive emotional or physiological states (i.e. distress intolerance) is linked to problematic substance use as well as affective symptoms and disorders (Leventhal and Zvolensky, 2015; Leyro et al., 2010). Indeed, existing literature on distress intolerance suggests it is a key vulnerability factor associated with cigarette smoking maintenance (e.g. Brown et al., 2005) and panic psychopathology (e.g. Keough et al., 2010). Specifically, as compared with nonsmokers, smokers are less able to tolerate distress (e.g. pain via a Cold Pressor task, Pulvers et al., 2012; Mirror-Tracing Task, Quinn et al., 1996), and high distress intolerant smokers, in particular, are more likely to drop out of cessation programs (MacPherson et al., 2008), and are more likely to lapse during a self-guided quit attempt (Abrantes et al., 2008; Brown et al., 2005) and in experimental relapse analogue tasks (Kahler et al., 2013). In addition, distress intolerance is related to cognitive processes that may perpetuate smoking behavior. For example, distress intolerance is linked to greater expectancies about the negative reinforcement properties of smoking and smoking to reduce negative affective states (Leyro et al., 2008), and in the context of acute abstinence, distress intolerance may enhance smoking reinforcement (Perkins et al., 2010).
In regard to anxiety and panic symptoms, distress intolerance appears to importantly mark risk for more severe psychopathology (Schmidt et al., 2006). Theoretically, it is posited that higher levels of distress intolerance may be associated with greater tendencies to react with fear to aversive interoceptive provocation (Schmidt et al., 2011). Indeed, perceived distress intolerance is directly (Keough et al., 2010) and indirectly associated with self-reported panic symptoms (Kraemer et al., 2013). Lastly, bio-behaviorally indexed distress intolerance is associated with greater physical anxiety concerns (a domain-specific effect; Johnson et al., 2012) and greater traumatic stress hyperarousal symptoms (Berenz et al., 2012).
Initial cross-sectional investigations examining smoking/panic interplay have found that among smokers, physical distress intolerance is directly and indirectly associated with more severe arousal-based anxiety symptoms (Brandt et al., 2012; Farris et al., 2014a). Experimental work has found that, among a sample of smokers who underwent two voluntary hyperventilation challenges, greater panic-reactivity during the first challenge predicted shorter persistence during the second voluntary challenge (i.e. shorter latency to discontinuing interoceptive provocation task; Marshall et al., 2008). Together, these findings suggest that the in ability to tolerate distress may place smokers at greater risk for panic-relevant symptoms and in vivo responding to a physiological stressor.
Parallel lines of research have independently, and to a lesser extent simultaneously, examined the role of distress intolerance in terms of smoking- and panic-relevant clinical outcomes. However, to our knowledge, work to date has (a) not utilized a multi-method assessment of distress intolerance to examine panic–cigarette smoking associations, and (b) been limited to examining panicrelevant outcomes. In addition, this work has not consistently examined these panic–smoking relations in the context of a stressor task or negative affective states. This is particularly relevant to smoking given smoking deprivation experienced in the early stages of cessation (e.g. 24 hours after quitting) can be conceptualized as critical ‘window’ for experiencing acute distress (Shiffman et al., 2004). Second, examination of the relation between distress intolerance and nicotine withdrawal in response to a physiological stressor may clarify mechanisms at play in the relation between stress and nicotine withdrawal severity.
To fill these gaps, the current investigation consisted of exploratory secondary data analyses from an experimental study (Leyro and Zvolensky, 2013) to test the role of distress intolerance in terms of panic symptom severity and changes in nicotine withdrawal symptoms experienced after a biological challenge (10% carbon dioxide (CO2) challenge). Biological challenge paradigms have been utilized in experimental psychopathology research to produce physiological sensations associated with anxiety/panic-like symptoms (e.g. chest discomfort, dizziness, sweating, shortness of breath; Abrams et al., 2008a; 2011a, 2011b). In addition, distress intolerance is most frequently conceptualized as a domain-general trait (Trafton and Gifford, 2011), that an individual’s ability to remain in contact with distress is theoretically Situationally indiscriminant. Based on this conceptualization of distress intolerance, we expected that among the pre-challenge measures of distress intolerance, the domain- general index (per the Distress Tolerance Scale; DTS) would be the most robust predictor (relative to domain-specific measurements; Bernstein et al., 2011) of (a) anxious responding to the CO2 challenge, and (b) post-challenge clinical processes (panic symptoms severity and increases in withdrawal symptom relative to pre-challenge). Anxious responding to the CO2 challenge was expected to be directly predictive of post-challenge severity of panic symptoms and changes nicotine withdrawal symptoms, and indirectly account for the relations between distress intolerance on post-challenge outcomes.
Method and materials
Participants and procedures
Participants were non-treatment-seeking smokers, approximately half of whom met criteria for panic disorder (as defined by DSMIV-TR; APA, 2000), who were recruited to participate in a 2-day experimental study (the parent study) examining aspects of panic disorder and nicotine withdrawal (Leyro and Zvolensky, 2013). Inclusion criteria included: (1) being a daily smoker for at least the past year (cigarettes per day ⩾ 7); (2) being 18–65 years old; and (3) reporting a willingness to abstain from smoking for a 12-hour period. Exclusion criteria included: (1) a current medical condition that contraindicated CO2 administration (cardiovascular, endocrine, pulmonary, respiratory (including severe asthma), or gastrointestinal illness); (2) having decreased the number of cigarettes smoked per day by more than half in the past 6 months; (3) limited mental competency (not oriented to person, place, or time) and the inability to give informed, voluntary, written consent to participate; (4) pregnancy or the possibility of being pregnant (by self-report); (5) current use of nicotine replacement therapy; (6) current or past history of psychotic-spectrum symptoms or disorders; (7) current substance dependence; (8) prior experience with CO 2 challenge; (9) suicidality (assessed using the SCID-I/NP; suicidality/depression section); and (10) any current use of psychotropic medication which could impact the effectiveness of the laboratory challenge.
Baseline visit.
At the first visit, participants were assessed for current (12 months) Axis I psychopathology using the Structured Clinical Interview-Non-Patient Version for DSM-IV Disorders (SCID-I/NP; First et al., 2007), to assess study eligibly. Smoking rate and history were assessed with the Smoking History Questionnaire (Brown et al., 2002), and nicotine dependence via the Fagerström Test of Cigarette Dependence (FTCD; Fagerström, 2012). As part of the baseline assessment, four distress intolerance tasks were completed (described in Measures section), in addition to a larger self-report battery (not utilized in the current secondary analyses). As a function of the parent study design, participants were randomly assigned to either smoking deprivation for 12 hours prior to their laboratory visit, or to smoke as usual. This manipulation was primarily utilized to ensure variability in nicotine withdrawal symptom severity prior to the biological challenge.
Experimental visit.
During the laboratory visit, smoking deprivation was verified via carbon monoxide analysis of breath sample (<10 ppm, Cocores, 1993); all higher scores were considered as smoking as usual. Following this, participants were fitted with a breathing mask and physiological monitoring equipment to assess continuous pCO2, respiration, heart rate, and skin conductance. During a 10-minute baseline period, participants were asked to sit still and relax, and were given regular room air through the mask. During the 4-minute challenge portion of the procedure, participants breathed 10% CO2-enriched air, a validated method of inducing panic-relevant responding. Following this, participants completed a 10-minute recovery period during which they again were given regular room air through the mask. Participants were not notified when the CO2 air was turned on or off; however, they were told that that baseline and recovery periods would last 10 minutes, while the challenge portion would last 4 minutes. Following the recovery period, participants were unhooked from the mask and monitoring equipment, debriefed, compensated $40, and sent home (for additional details regarding the sample and study procedures, please see Leyro and Zvolensky, 2013).
In the present secondary analyses, participants were included on the basis of having all available data on all study-specific variables (n = 54; Mage = 30.0, SD = 12.3; 46.3% female). Participants were primarily white (96.3%) and the majority completed at least part of college (63.0%). The average daily smoking rate of this sample was 18.8 cigarettes (SD = 6.8), and severity of cigarette dependence was reported as low to moderate (FTCD; Fagerström, 2012: M = 3.7, SD = 1.82). The mean latency to smoking initiation after waking was 33.5 minutes (SD = 39.9 minutes); 70.4% (n = 38) of participants reported smoking within the first 30 minutes after waking.1 The average number of current (past year) Axis I psychological disorders was 1.2 (SD = 1.4), and 40.7% (n = 22) met criteria for panic disorder (as defined by the DSM-IV-TR). For complete descriptive information on psychopathology in the sample, please see Leyro and Zvolensky (2013). Half of the sample (n = 27; 50.0%) were randomized to 12-hour smoking deprivation and the other half smoked as usual.
Measures
Indices of distress intolerance.
The DTS (Simons and Gaher, 2005) was used to index participants’ perceptions of their ability to tolerate mental distress. The DTS is composed of 15 items answered on 5-point Likert-type scales ranging from (1) strongly agree to (5) strongly disagree that evaluate participants’ ability to experience and endure negative emotional states. Items are summed and a mean score is computed; the possible range is 1–5, with higher scores reflecting greater tolerance (lower intolerance) for distress. This scale has good psychometric properties, including high internal consistency and appropriate convergence with other self-report ratings of affective distress and regulation (Simons and Gaher, 2005), including among smokers (Leyro et al., 2011). The scale incorporates items that assess appraisal, tolerance, absorption, and regulation.
The Discomfort Intolerance Scale (DIS; Schmidt et al., 2006) is a 5-item self-report measure that assesses the degree to which a respondent agrees with statements related to their perceived intolerance of physical distress or discomfort (e.g. “I take extreme measures to avoid feeling physically uncomfortable”) on a 7-point Likert-type scale (0 = not at all like me to 6 = extremely like me). Possible scores range from 0–30, with higher scores reflecting greater intolerance for physical discomfort. The DIS has demonstrated good psychometric properties in past work (Mitchell et al., 2013; Schmidt et al., 2006).
The Breath-Holding Task (Asmundson and Stein, 1994) is a behavioral assessment of distress intolerance. During the task, participants are read a standardized script instructing them how to complete the task. The script prompts participants to inhale as deeply as possible and then exhale once a full breath is achieved. At the completion of the exhalation, the participants, again, breathe in as deeply as possible and, this time, are prompted to hold their breath as long as they can (Asmundson and Stein, 1994). The length of time the participants are able to hold their breath is recorded via a stopwatch. No encouragement is given by the experimenter to promote duration. This task was then completed again. Here, the first and second breath-holding trials were highly correlated (r = 0.91, p < 0.001). The second trial was used in the current study. This task has been frequently used as measure of physical distress intolerance (Brown et al., 2009; Hajek et al., 1987), with shorter durations of breath-holding indicating greater intolerance of physical distress.
The Computerized Mirror-Tracing Persistence Task (MTPT-C; Lejuez et al., 2003) is a behaviorally oriented assessment that measures an individual’s ability to complete a difficult and frustrating computer task (e.g. latency to end the mirror-tracing task). The MTPT-C directs participants to trace a red dot along the shape of a star using a computer mouse; the computer mouse is programmed such that the mouse and cursor move in opposite directions of one another (e.g. when the mouse is moved left, the cursor moves right). If participants fail to move the mouse or move the mouse outside the lines of the shape, a loud buzzer sounds from the computer, and the cursor moves back to the beginning position. Although not aware of a time limit, participants were given up to 300 seconds to complete the task, with shorter latency to end the task being used as an index of distress intolerance. No monetary reward was provided for MTPT-C performance in the current study.
Anxious responding to CO2 challenge.
Participants were asked to rate their Subjective Unit of Distress (SUDS; Wolpe, 1958) from 0 (no anxiety) to 100 (extreme anxiety) on a visual analogue scale before the challenge procedure, after each minute of the challenge procedure, immediately after the challenge, and following each recovery minute. For the current analysis, each participant’s rating of SUDS following minute three of the 10% CO2 challenge was used to reflect their maximum distress (i.e. conceptualized as anxious responding to the CO2 stressor). We chose this rating rather than the rating following minute 4 because the air was switched back to room air directly before participants were asked to give this rating. Therefore this rating may have been confounded by a sense of relief that the challenge had been terminated.
Post-challenge outcomes.
Upon termination of the 4-minute 10% CO2 challenge portion of the procedure, participants completed ratings of panic and withdrawal symptoms. The Diagnostic Symptoms Questionnaire (DSQ; Sanderson et al., 1988, 1989) is a rating scale of 15 DSM-IV-TR panic symptoms that are rated on a severity scale of 0 (not at all) to 8 (very strongly felt). A mean score was computed, such that higher scores reflected more severe panic attack symptoms. The Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1986) was completed to index withdrawal symptom severity. The MNWS is an 8-item self-report of nicotine withdrawal symptoms, with items rated on a 6-point scale; higher scores reflect greater subjective withdrawal symptoms. A total score was derived from the mean response per item. The MNWS was completed pre-challenge to document pre-challenge subjective withdrawal. The MNWS was also completed post-challenge to examine changes in withdrawal post-CO2 provocation. A difference score was computed to account for differences in pre- challenge acute nicotine withdrawal (experienced as a result of the design of the parent study). Higher values from the change score reflected greater changes (increases) in withdrawal symptoms post-challenge.
Data analysis strategy
Analyses were conducted in AMOS 21.0 (Arbuckle, 2012) and path modeling using Maximum Likelihood imputation and estimation was employed. A path model was constructed to test the effects of the four distress intolerance indices in terms of predicting anxious responding to CO2 provocation. The significant distress intolerance indices were also examined in terms of post-challenge panic symptoms and changes in nicotine withdrawal, directly and indirectly occurring through anxious responding to the CO2 challenge. Gender and smoking rate (cigarettes per day) were tested as possible model covariates. Despite randomization of smokers to nicotine deprivation, the parent study did not observe a main effect for pre-challenge withdrawal in the pre-diction of challenge responding (Leyro and Zvolensky, 2013). However, deprivation condition and panic disorder status were entered as predictors of anxious responding to the CO2 challenge, to control for these effects beyond the distress intolerance indices. With regard to post-challenge withdrawal, the utilization of a MNWS change score accounted for pre-challenge variability by equating groups.2
Model fit was assessed using the Comparative Fit Index (CFI) and the Root Mean Square Error of Approximation (RMSEA). CFI is a goodness-of-fit measure of the amount of variance and covariance in the data set accounted for by the implied model, with values 0.90 or above considered an acceptable fit (Kline, 2011). A RMSEA of 0.05 or less indicates good model fit, and RMSEA under 0.10 is considered in the acceptable range (Kline, 2011). Iterative model improvement was conducted in consultation of modification indices and conceptual meaningfulness.
Results
Descriptively, distress intolerance indices were as follows: DTS: M = 3.5, SD = 1.01 (observed range 1.1–5.0); DIS: M = 11.9, SD = 6.60 (observed range: 0–28); MTPT-C: M = 149.8 seconds, SD = 101.15 (observed range: 15.1–300.0 s); Breath-Holding: M = 61.7 seconds, SD = 27.56 (observed range: 15.0–136.0 s). CO2 reactivity to the biological challenge was indicated by SUDS rating at minute 3 of the task: M = 63.9, SD = 34.99 (observed range: 0–100). Table 1 presents zero-order correlations between study variables. To assess current smoking status prior to the biological challenge, expired carbon monoxide breath sample analysis was conducted: average values at baseline for full sample were 17.7 ppm (SD = 7.29); and at the experimental visit was 4.7 ppm (SD = 3.74) for deprivation group and 20.3 ppm (SD = 8.18) for smoking as usual group (t(51) = 9.01, p < 0.001).
Table 1.
Descriptive characteristics and bivariate correlations.
| Variable | Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender (% F) | 25 (46.3) | – | ||||||||
| 2. Panic Dx (% Y) | 22 (40.7) | −.014 | – | |||||||
| 3. Cigarettes per day | 18.8 (6.84) | .033 | −.220 | – | ||||||
| 4. DTS-total | 3.5 (1.01) | −.207 | −.419** | .050 | – | |||||
| 5. DIS-total | 11.9 (6.60) | .124 | .280* | .053 | −.638** | – | ||||
| 6. MTPT-C | 149.8 (101.15) | −.004 | .128 | −.101 | .087 | −.128 | – | |||
| 7. BH duration | 61.7 (27.56) | −.410** | .062 | −.203 | .229 | −.205 | .301* | – | ||
| 8. Anxious responding | 63.9 (34.99) | .217 | .065 | .129 | −.436** | .311* | .023 | −.199 | – | |
| 9. Post- DSQ | 2.9 (1.85) | .247 | .244 | −.154 | −.422** | .287* | .189 | −.112 | .692** | – |
| 10. Post- MNWSa | 7.5 (5.68) | .059 | −.277* | .152 | .235 | −.133 | .211 | −.031 | .148 | .080 |
p < 0.05
p < 0.01 Note: Gender (coded 0 = male, 1 = female); Panic disorder (0 = no, 1 = yes); DTS-total: Distress Tolerance Scale; DIS-total: Discomfort Intolerance Scale; MTPT-C: Mirror-Tracing Persistence, seconds of persistence; BH Duration: duration in seconds on the Breath-Holding Persistence Task; Anxious Responding: Subjective Units of Distress at minute 3 of CO2 challenge; Post-DSQ: Post-challenge panic symptom severity per the Diagnostic Symptom Questionnaire; Post-MNWS: Nicotine withdrawal per the Minnesota Nicotine Withdrawal Scale from pre- to post-challenge. Grey boxes with underlined correlation coefficients represent errors terms that were allows to correlate in path model (see Figure 1).
A change score from pre-challenge to post-challenge was used in analyses, but post-challenge raw scores were reported in table for meaningful interpretation. An average increase in withdrawal was reported post-challenge (increase M = 0.50, SD = 3.5).
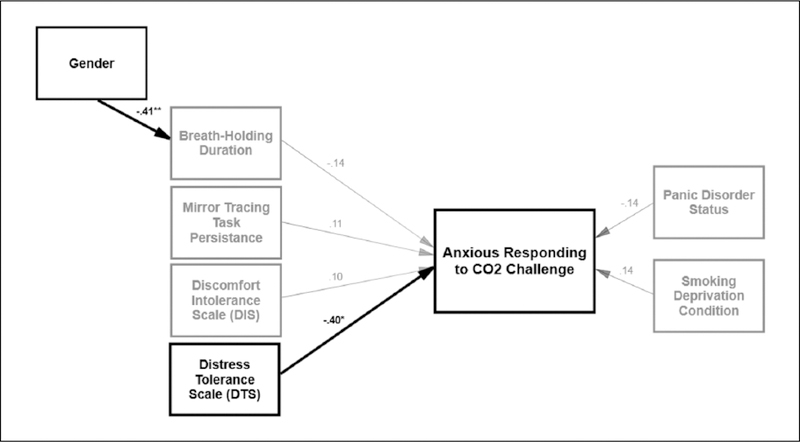
Next, a path model was constructed and examined in terms of its consistency with the theoretical model (see Figure 1). In the proposed path model, model fit was acceptable (χ2(19) = 24.23, p = 0.188; CFI = 0.91, RMSEA = 0.07). All four distress intolerance indices were examined in terms of predicting anxious responding to the CO2 challenge. Error terms for distress intolerance measures (by measurement method; self-report versus behavioral task) were allowed to correlate with each other based on an a priori decision (McHugh et al., 2011). Both behavioral measures of distress intolerance (Breath-Holding Task and MTPT-C) were inter-correlated (r = 0.33, p = 0.023), and both self-report measures of distress intolerance (DTS and DIS) were inter-correlated (r = −0.64, p < 0.001). No statistically significant cross-method bivariate correlations were observed (Table 1). Gender (coded 0 = male, 1 = female) was added as predictor Breath-Holding duration (r = −0.41, p = 0.004), based on modification indices and theoretical relevance suggesting gender differences in this physical distress intolerance index (Hogan et al., 2015; MacPherson et al., 2008). Smoking rate (cigarettes per day) was removed from the model based on modification indices. Only the DTS was significantly predictive of anxious responding during the CO2 challenge (β = −0.40, p = 0.017), such that higher levels of distress intolerance were associated with greater SUDS ratings at minute 3 of CO2 challenge. These effects were significant after adjusting for the non-significant effects of panic disorder status and deprivation condition on anxious responding to the CO2 challenge. No other distress intolerance indices were predictive of anxious responding to the CO2 challenge.
Figure 1.
Model of distress tolerance indices in predicting anxious responding to CO2 challenge. Note. Grey lines are non-significant effects; thick black regression lines indicate significant effects. * p < 0.05, **p < 0.01 Error terms are not displayed for ease in viewing.
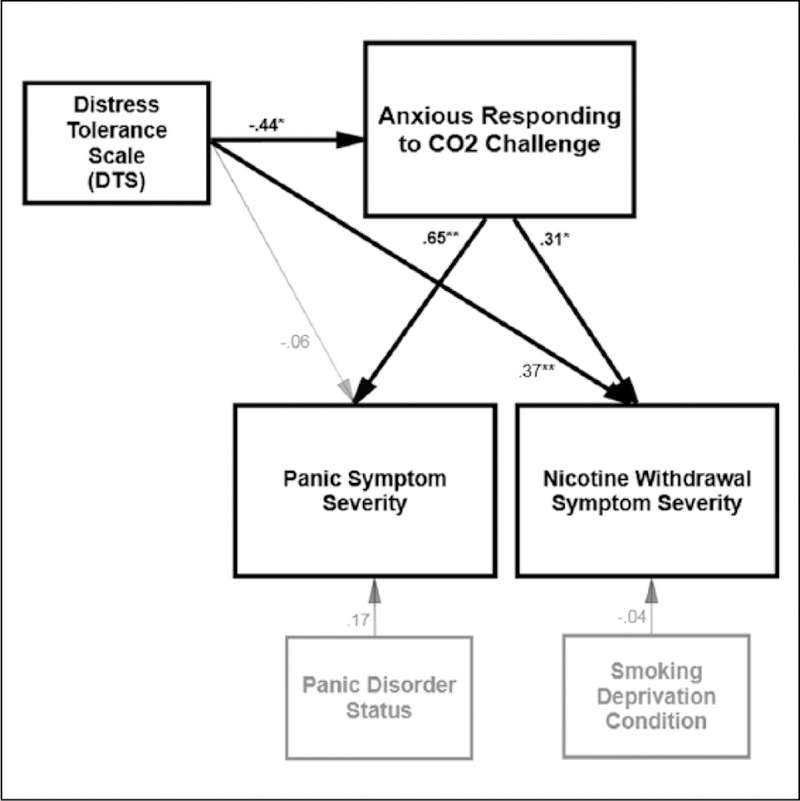
Next, a second model was constructed to test the effect of the DTS in predicting post-challenge panic symptom severity and changes in nicotine withdrawal symptoms directly and indirectly through the effect of anxious responding to the CO2 challenge (Figure 2). Model fit was excellent (χ2(7) = 3.73, p = 0.811; CFI = 1.00, RMSEA = 0.01). Panic disorder status was entered as a predictor of post-challenge panic symptom severity, and deprivation status was entered as a predictor of post-challenge changes in nicotine withdrawal symptom severity. Both of these covariates had non-significant effects. The DTS was allowed to correlate with baseline panic disorder status, based on modification indices (r = −0.42, p = 0.005) and theoretical relevance (Schmidt et al., 2011). Results revealed that anxious responding to the CO2 challenge was positively predictive of post-challenge panic symptoms (β = 0.65, p < 0.001) and increases in subjective withdrawal symptoms, relative to pre-challenge withdrawal (β = 0.32, p = 0.028). There was a counterintuitive direct effect of DTS on post-challenge outcomes, such that the DTS was positively predictive of post-challenge increases in subjective nicotine withdrawal (β = 0.37, p = 0.009). The DTS was not associated with post-challenge panic symptom severity. Next, the effect of the DTS on panic and nicotine withdrawal outcomes was examined through the indirect effect anxious responding to the CO2 challenge, which was tested using RMediation (Tofighi and Mackinnon, 2011). Results indicated that the DTS significantly and negatively predicted changes in nicotine withdrawal which was accounted for by anxious responding to the CO2 challenge, a*b = −0.485, SE = 0.273 (95% CI: –1.095, –0.033). Moreover, despite a non-significant direct effect, there was a significant negative association for the DTS on post-challenge panic symptom severity which occurred through the effect of anxious responding to the CO2 challenge, a*b = −0.515, SE = 0.174, (95% CI: –0.888, –0.208).
Figure 2.
Path model of DTS in predicting post-challenge changes in nicotine withdrawal and panic symptom severity through the effect of anxious responding to CO 2 challenge. Note. Grey lines are non-significant effects; thick black regression lines indicate significant effects. * p < 0.05, **p < 0.01 Error terms and correlations are not displayed for ease in viewing.
Discussion
Literature supports the role of distress intolerance in the prediction of poor smoking cessation outcomes (Brandon et al., 2003; Brown et al., 2009), maintenance of anxiety psychopathology (Schmidt et al., 2011), and as a mechanism linking smoking and anxiety (Marshall et al., 2008). The current experimental study utilized a multi-methodological approach to further explicate the associations between distress intolerance in smokers in terms responding to a biological challenge stressor task. Here, only a self-report domain-general measure of distress intolerance (per the DTS) was (negatively) predictive of anxious responding to the CO2 challenge. Interestingly, despite the bivariate association between the DTS and DIS, perceived emotional distress intolerance (per the DTS; e.g. “I can’t handle feeling distress or upset”) and perceived physical discomfort intolerance (per the DIS; e.g. “When I begin to feel physically uncomfortable, I quickly take steps to relieve the discomfort”), while related, appear to be distinct constructs with divergent predictive effects (Bernstein et al., 2009, 2011). It is also worth noting that, consistent with prior work (Bernstein et al., 2011; McHugh et al., 2011), behaviorally indexed distress intolerance measures were inter-correlated, as were self-report measures, but no significant cross-method associations were observed.
In addition, as expected, anxious responding to the CO2 challenge directly predicted greater severity of panic symptoms post-challenge. Covarying for panic disorder status suggests that this effect may not be unique to panic-disordered individuals. This finding is consistent with other work that has found that CO2 provocation reliably leads to increases panic arousal post- challenge, which is intensified among smokers relative to non- smokers (Abrams et al., 2008b), and complements the few prior experimental investigations that have specifically examined the role of reactivity to interoceptive challenges in the elicitation of panic-relevant arousal among smokers (Abrams et al., 2011a; Marshall et al., 2008; Vujanovic and Zvolensky, 2009). Moreover, the current study uniquely found that anxious responding to the CO2 challenge was significantly associated with post-challenges increases in nicotine withdrawal symptoms, relative to withdrawal symptoms prior to the task, and after covarying for the effects of the primary study manipulation of deprivation status.
Interestingly, the DTS was negatively associated with increases in nicotine withdrawal severity post-challenge and panic symptoms severity post-challenge, due to the indirect effect of anxious responding to the CO2 task. That is, as scores on the DTS decreased (indicating higher distress intolerance), greater anxious responding to the CO2 task was observed, which in turn accounted for increases in the experience of subjective nicotine withdrawal post-challenge and panic symptoms. The significant indirect effect findings are consistent with theoretical conceptualizations of distress intolerance, which suggest that this cognitive vulnerability factor is only ‘activated’ in the context of an emotionally stressful experience, giving rise to poorer emotional outcomes (for an example in trauma literature, see Vujanovic et al., 2013). Prior work has found that smokers with high distress intolerance, in the context of negative affect, are at greater risk for smoking lapse (Abrantes et al., 2008). Together, these findings underscore the importance of emotional context, suggesting that perceived distress intolerance may uniquely mark risk for panic psychopathology and smoking-relevant processes, when experienced in the context anxious responding/distress due to a stressor (e.g. CO2 challenge), which is consistent with prior related work (Marshall et al., 2008) and theory (Zvolensky et al., 2010). Importantly, without considering the context of the CO2 challenge, the association between the DTS and the experience of changes in nicotine withdrawal post-challenge (but not panic symptom severity) was reversed. Potentially, this positive association of distress tolerance and nicotine withdrawal experiences can be understood by the tendency to (mis)attribute sensations experienced subsequent to the CO2 challenge as nicotine withdrawal (versus CO2-related sensations), or is a spurious finding. Certainly, it would be important to replicate this direct effect finding.
These findings also offer unique clarification of mechanisms by which distress/stress exacerbates nicotine withdrawal. Despite a large body of research suggesting proximal changes in stress impact nicotine withdrawal severity and vice versa (Kassel et al., 2003), placing these individuals at greater risk for continued use (Childs and De Wit, 2010; McKee et al., 2011; Todd, 2004), little work has investigated mechanisms that may explain how stress relates to perceptions of nicotine withdrawal. Two clear possibilities exist: First, it is possible that stress leads to an actual increase in nicotine withdrawal symptoms. For example, stress-precipitated changes in physiology result in faster elimination of nicotine from the bloodstream (Schachter et al., 1977). A second hypothesis is that intense and acute stress, such as that experienced during a biological challenge, results in physiological sensations that are indistinguishable from those experienced during nicotine withdrawal (Hatsukami et al., 1985), thereby conflating symptom reports (Parrott, 1995; Todd, 2004). This may be particularly relevant for smokers who are sensitive to, or unable to tolerate, such distress. In the present investigation, the observed relation between anxious responding to the CO2 challenge (subjective distress to task) and change in nicotine withdrawal, as well as the indirect effect of anxious responding on the distress intolerance– withdrawal interplay, support the latter. This novel finding is consistent with theory suggesting that certain smokers with heightened risk for anxiety pathology (e.g. anxiety sensitivity; tendency to catastrophically interpret the meaning of anxiety and internal bodily sensations; Zvolensky et al., 2014) may subjectively report greater nicotine withdrawal symptoms, particularly in the context of stress (e.g. acute nicotine deprivation), making them more susceptible to greater reliance on smoking to manage discomfort/distress, greater nicotine dependence, and increased risk of lapse in the context of cessation.
There are several limitations that warrant mention. First, the current sample was small in size, thus these exploratory findings should be replicated in larger samples to increase confidence in the generalizability of the findings. Relatedly, the current sample consisted of community-recruited smokers who were primarily white, well-educated, and low–moderately nicotine-dependent smokers (e.g. 29.6% of sample reported smoking their first cigarette of the day after more than 30 minutes of waking). It is important to examine this model in a racially and socioeconomiccally diverse groups of smokers, and among those with higher levels of nicotine dependence. It is possible that in a more nicotine-dependent sample, distress tolerance may differentially impact withdrawal and panic effects. It is also worth noting, however, the changing characteristics of smokers in the United States (Center for Disease Control and Prevention, 2014), which documents the declining overall smoking rate in the United States. Thus, while examination of these processes in a more nicotine-dependent sample may provide additional insight into these important relations, it is also possible that this current sample is representative to the current demographic of smokers, at least in terms of smoking behavior/rate. Also, our sample met for a wide range of co-occurring psychopathology and substance use, perhaps increasing generalizability, with 40.7% meeting criteria for current panic disorder. Despite covarying for panic disorder status in our models, we cannot rule out the possibility that unmeasured affective or cognitive vulnerabilities within this group, or other risk factors associated with high rates of co- morbid substance use and psychopathology in our sample, may have driven some of the observed effects. However, our sample was underpowered to examine additional mediators and moderators, and future work seeking to replicate these findings should be open to this possibility. In addition, anxious responding to the challenge was indexed by subjective anxiety rating (SUDS) during the biological challenge. Future work would benefit from examining behavioral responding during the challenge to index intolerance, for example self-initiated termination of exposure to CO2-enriched air (Bernstein et al., 2011; Brown et al., 2002), wherein participants are instructed that they can discontinue CO2 delivery at any point during the task (e.g. pull mask). Such experimental design permits the experimenter to compute a task- persistence variable—the amount of time participant was able to withstand the physiological discomfort of breathing CO2enriched air. In the interest of further explicating the difference of emotional reactivity and intolerance, it would be useful for future work to explore how the anxious responding, behavioral intolerance, and perceived distress intolerance (per the DTS) differentially relate to panic-relevant and smoking-relevant responding.
Broadly, these experimental findings add to the growing evidence that, when in the context of an emotional/physiological stressor (in this case CO2 challenge), distress intolerance is an important individual difference factor relevant to the expression of panic symptoms and experience of withdrawal symptoms among smokers. Clinically speaking, smokers who perceive themselves as being unable to tolerate negative emotional states may be particularly vulnerable to reacting with distress to interoceptive changes (e.g. somatic changes experienced during a cessation attempt due to acute nicotine withdrawal symptoms). Such heightened reactivity may, in turn, increase susceptibility to experience greater panic-like symptoms (even among those smokers with no history of panic psychopathology) and more intense nicotine withdrawal symptoms during acute quitting stages. This process may contribute to increased risk of early termination of a cessation attempt (i.e. shorter time to re-initiating smoking). For these high-risk smokers, it may be particularly important to integrate distress tolerance skill-building into standard cognitive-behavioral smoking cessation programs in order to (a) encourage acceptance of intense, acute, and unpredictable experiences of negative emotional states and willingness to experience such states, (b) provide exposure to panic-like experiences and acute nicotine withdrawal symptoms to practice acceptance based responding to negative emotional responses (prior to the actual quit attempt), and (c) increase self-efficacy for ability to successfully persist through the cessation processes (Brown et al., 2008, 2013; Gifford et al., 2004).
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a National Institute of Drug Abuse grant awarded to Dr. Teresa Leyro (F31-DA024919–03). Ms. Farris is supported by a pre-doctoral National Research Service Award from the National Institute of Drug Abuse (F31-DA035564–02).
Footnotes
Declaration of Conflicting Interests
The authors Ms. Farris, Dr. Zvolensky, and Dr. Leyro declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: In the past 2 years, Dr. Otto has served as a paid consultant for MicroTransponder Inc., Concert Pharmaceuticals, and ProPhase; provided expert consensus opinion for Otsuka Pharmaceuticals, received royalty support for use of the SIGH-A from ProPhase, and received book royalties from Oxford University Press, Routledge, and Springer. The content presented does not necessarily represent the official views of the National Institutes of Health, and that the funding sources had no other role other than financial support.
Notes
To examine the extent to which latency to smoking initiation upon waking (within 30 minutes versus longer than 30 minutes) may have impacted nicotine dependence/withdrawal effects, a series of independent sample t-tests were conducted. Results indicated that these groups did not statistically differ in terms of cigarettes per day, expired CO at baseline or at the experimental visit, or level of nicotine withdrawal at visit 2 (all p values > 0.05). In addition, participants who reported smoking after 30 minutes of waking were equally represented across experimental manipulation conditions (n = 9 in smoking as usual; n = 7 in deprivation; χ2 test non-significant).
A total of 9 and 8 parameters were estimated were estimated in Models 1 and 2, thus the sample size was sufficiently powered per Kline’s (2011) recommendation of between 5–20 times cases to estimated parameters (albeit at the lower end of the recommendation, of a ratio of approximately 6:1).
References
- Abrams K, Leger K, Schlosser L, et al. (2011a) Nicotine withdrawal exacerbates fear reactivity to CO(2)-induced bodily sensations among smokers. Nicotine Tob Res 13: 1052–1058. [DOI] [PubMed] [Google Scholar]
- Abrams K, Schlosser L, Leger K, et al. (2011b) Panic-relevant cognitive processes among smokers. J Cogn Psychother 25: 71–81. [Google Scholar]
- Abrams K, Schruers K, Cosci F, et al. (2008a) Biological challenge procedures used to study co-occurring nicotine dependence and panic disorder. Addict Behav 33: 1463–1469. [DOI] [PubMed] [Google Scholar]
- Abrams K, Zvolensky MJ, Dorflinger L, et al. (2008b) Fear reactivity to bodily sensations among heavy smokers and nonsmokers. Exp Clin Psychopharmacol 16: 230–239. [DOI] [PubMed] [Google Scholar]
- Abrantes AM, Strong DR, Lejuez CW, et al. (2008) The role of negative affect in risk for early lapse among low distress tolerance smokers. Addict Behav 33: 1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA (2000) Diagnostic and Statistical Manual of Mental Disorders – (4th Ed. Text Revision). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Arbuckle JL (2012) IBM® SPSS® AMOS™ 21.0 User’s Guide Chicago, IL: IBM Corporation. [Google Scholar]
- Asmundson GJG and Stein MB (1994) Triggering the false suffocation alarm in panic disorder patients by using a voluntary breath-holding procedure. Am J Psychiatry 151: 264–266. [DOI] [PubMed] [Google Scholar]
- Berenz EC, Vujanovic AA, Coffey SF, et al. (2012) Anxiety sensitivity and breath-holding duration in relation to ptsd symptom severity among trauma exposed adults. J Anxiety Disord 26: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A, Marshall EC and Zvolensky MJ (2011) Multi-method evaluation of distress tolerance measures and construct(s): Concurrent relations to mood and anxiety psychopathology and quality of life. J Exp Psychopathol 2: 386–399. [Google Scholar]
- Bernstein A, Zvolensky MJ, Vujanovic AA, et al. (2009) Integrating anxiety sensitivity, distress tolerance, and discomfort intolerance: A hierarchical model of affect sensitivity and tolerance. Behav Ther 40: 291–301. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, et al. (2003) Pretreatment task persistence predicts smoking cessation outcome. J Abnorm Psychol 112: 448–456. [DOI] [PubMed] [Google Scholar]
- Brandt CP, Johnson KA, Schmidt NB, et al. (2012) Main and interactive effects of emotion dysregulation and breath-holding duration in relation to panic-relevant fear and expectancies about anxiety-related sensations among adult daily smokers. J Anxiety Disord 26: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N and Klein DF (1999) Smoking and panic attacks: An epidemiologic investigation. Arch Gen Psychiatry 56: 1141. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP and Kessler RC (2004) Psychiatric disorders and stages of smoking. Biol Psychiatry 55: 69–76. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, et al. (2002) Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol 111: 180. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, et al. (2005) Distress tolerance and early smoking lapse. Clin Psychol Rev 25: 713–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, et al. (2009) A prospective examination of distress tolerance and early smoking lapse in adult selfquitters. Nicotine Tob Res 11: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, et al. (2008) Distress tolerance treatment for early-lapse smokers: Rationale, program description, and preliminary findings. Behav Mod 32: 302–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Reed KM, Bloom EL, et al. (2013) Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine Tob Res 15: 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (2014) Current cigarette smoking among adults — United States, 2005–2012. MMWR 63: 29–34. [PMC free article] [PubMed] [Google Scholar]
- Childs E and De Wit H (2010) Effects of acute psychosocial stress on cigarette craving and smoking. Nicotine Tob Res 12: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocores J (1993) Nicotine dependence: Diagnosis and treatment. Psychiatr Clin N Am 16: 49–60. [PubMed] [Google Scholar]
- Cosci F, Knuts IJE, Abrams K, et al. (2010) Cigarette smoking and panic: A critical review of the literature. J Clin Psychiatry 71: 606–615. [DOI] [PubMed] [Google Scholar]
- Fagerström K (2012) Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine Tob Res 14: 75–78. [DOI] [PubMed] [Google Scholar]
- Farris SG, Vujanovic AA, Hogan J, et al. (2014a) Main and interactive effects of anxiety sensitivity and physical distress intolerance with regard to PTSD symptoms among trauma-exposed smokers. J Trauma Dissoc 15: 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, Blalock JA, et al. (2014b) Negative affect and smoking motives sequentially mediate the effect of panic attacks on tobacco-relevant processes. Am J Drug Alcohol Abuse 40: 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. (2007) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, NonPatient Edition (SCIDI/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, et al. (2004) Acceptance-based treatment for smoking cessation. Behav Ther 35: 689–705. [Google Scholar]
- Hajek P, Belcher M and Stapleton J (1987) Breath-holding endurance as a predictor of success in smoking cessation. Addict Behav 12: 285–288. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Hughes JR and Pickens R (1985) Characterization of tobacco withdrawal: Physiological and subjective effects. NIDA Res Monogr 53: 56–67. [PubMed] [Google Scholar]
- Hogan J, Farris SG, Brandt C, et al. (2014) Predictors of breath-holding duration among treatment-seeking tobacco users. J Subst Use E-pub ahead of print [Google Scholar]
- Hughes JR and Hatsukami D (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43: 289–294. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, et al. (2000) Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA 284: 2348–2351. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Berenz EC and Zvolensky MJ (2012) Nonclinical panic attack history and anxiety sensitivity: Testing the differential moderating role of self-report and behavioral indices of distress tolerance. Cogn Ther Res 36: 603–611. [Google Scholar]
- Kahler CW, Mchugh RK, Metrik J, et al. (2013) Breath holding duration and self-reported smoking abstinence intolerance as predictors of smoking lapse behavior in a laboratory analog task. Nicotine Tob Res 15: 1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR and Paronis CA (2003) Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull 129: 270–304. [DOI] [PubMed] [Google Scholar]
- Keough ME, Riccardi CJ, Timpano KR, et al. (2010) Anxiety symptomatology: The association with distress tolerance and anxiety sensitivity. Behav Ther 41: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB (2011) Principles and Practice of Structural Equation Modeling Third edition. New York, NY: The Guilford Press. [Google Scholar]
- Kraemer KM, Luberto CM and Mcleish AC (2013) The moderating role of distress tolerance in the association between anxiety sensitivity physical concerns and panic and PTSD-related re-experiencing symptoms. Anxiety Stress Coping 26: 330–342. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW and Brown RA (2003) A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor. Behav Therapist 26: 290–293. [Google Scholar]
- Leventhal AM and Zvolensky MJ (2015) Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychol Bull 141: 176–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM and Zvolensky MJ (2013) The interaction of nicotine withdrawal and panic disorder in the prediction of panic-relevant responding to a biological challenge. Psychol Addict Behav 27: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Bernstein A, Vujanovic AA, et al. (2011) Distress Tolerance Scale: A confirmatory factor analysis among daily cigarette smokers. J Psychopathol Behav Assess 33: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Zvolensky MJ and Bernstein A (2010) Distress tolerance and psychopathological symptoms and disorders: A review of the empirical literature among adults. Psychol Bull 136: 576–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Zvolensky MJ, Vujanovic AA, et al. (2008) Anxiety sensitivity and smoking motives and outcome expectancies among adult daily smokers: Replication and extension. Nicotine Tob Res 10: 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Stipelman BA, Duplinsky M, et al. (2008) Distress tolerance and pre-smoking treatment attrition: Examination of moderating relationships. Addict Behav 33: 1385–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EC, Zvolensky MJ, Vujanovic AA, et al. (2008) Panic reactivity to voluntary hyperventilation challenge predicts distress tolerance to bodily sensations among daily cigarette smokers. Exp Clin Psychopharmacol 16: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Daughters SB, Lejuez CW, et al. (2011) Shared variance among self-report and behavioral measures of distress intolerance. Cogn Ther Res 35: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, et al. (2011) Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol 25: 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MA, Riccardi CJ, Keough ME, et al. (2013) Understanding the associations among anxiety sensitivity, distress tolerance, and discomfort intolerance: A comparison of three models. J Anxiety Disord 27: 147–154. [DOI] [PubMed] [Google Scholar]
- Parrott AC (1995) Smoking cessation leads to reduced stress, but why? Int J Addict 30: 1509–1516. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, et al. (2010) Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology 210: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, et al. (2011) Anxiety diagnoses in smokers seeking cessation treatment: Relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction 106: 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvers K, Hood A, Limas EF, et al. (2012) Female smokers show lower pain tolerance in a physical distress task. Addict Behav 37: 1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn EP, Brandon TH and Copeland AL (1996) Is task persistence related to smoking and substance abuse? The application of learned industriousness theory to addictive behaviors. Exp Clin Psychopharmacol 4: 186–190. [Google Scholar]
- Sanderson WC, Rapee RM and Barlow DH (1988) Panic induction via inhalation of 5.5% CO2 enriched air: A single subject analysis of psychological and physiological effects. Behav Res Ther 26: 333–335. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Rapee RM and Barlow DH (1989) The influence of an illusion of control on panic attacks induced via inhalation of 5.5% carbon dioxide-enriched air. Arch Gen Psychiatry 46: 157–162. [DOI] [PubMed] [Google Scholar]
- Schachter S, Silverstein B, Kozlowski LT, et al. (1977) Studies of the interaction of psychological and pharmacological determinants of smoking. J Exp Psychology 106: 3–12. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Mitchell M, Keough M, et al. (2011) Anxiety and its disorders. In: Zvolensky MJ, Bernstein A and Vujanovic AA (eds) Distress Tolerance: Theory, Research, and Clinical Applications New York, NY: Guilford Press, pp.105–125. [Google Scholar]
- Schmidt NB, Richey JA and Fitzpatrick KK (2006) Discomfort intolerance: Development of a construct and measure relevant to panic disorder. J Anxiety Disord 20: 263–280. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ and Gilbert DG (2004) Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res 6: 599–614. [DOI] [PubMed] [Google Scholar]
- Simons JS and Gaher RM (2005) The Distress Tolerance Scale: Development and validation of a self-report measure. Motiv Emot 29: 83–102. [Google Scholar]
- Todd M (2004) Daily processes in stress and smoking: effects of negative events, nicotine dependence, and gender. Psychol Addict Behav 18: 31–39. [DOI] [PubMed] [Google Scholar]
- Tofighi D and Mackinnon DP (2011) Rmediation: An R Package for mediation analyses confidence intervals. Behav Res Methods 43: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA and Gifford EV (2011) Biological bases of distress tolerance. In: Zvolensky MJ, Bernstein A and Vujanovic AA (eds) Distress Tolerance: Theory, Research, and Clinical Applications New York, NY: Guilford Press, pp.80–102. [Google Scholar]
- Vujanovic AA and Zvolensky MJ (2009) Anxiety sensitivity, acute nicotine withdrawal symptoms, and anxious and fearful responding to bodily sensations: A laboratory test. Exp Clin Psychopharmacol 17: 181–190. [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Hart AS, Potter CM, et al. (2013) Main and interactive effects of distress tolerance and negative affect intensity in relation to PTSD symptoms among trauma-exposed adults. J Psychopathol Behav Assess 35: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J (1958) Psychotherapy by Reciprocal Inhibition Palo Alto, CA: [Google Scholar]
- Stanford University Press. Zvolensky MJ and Bernstein A (2005) Cigarette smoking and panic psychopathology. Curr Direct Psychol Sci 14: 301–305. [Google Scholar]
- Zvolensky MJ, Farris SG, Guillot CR, et al. (2014) Anxiety sensitivity as an amplifier of the subjective and behavioral tobacco abstinence effects. Drug Alcohol Depend 142: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Vujanovic AA, Bernstein A, et al. (2010) Distress tolerance: Theory, measurement, and relations to psychopathology. Curr Direct Psychol Sci 19: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]