Abstract
Background:
The goal of this randomized clinical trial was to examine the efficacy of a cognitive behavioral stress reduction treatment for reducing disability among veterans with chronic multisymptom illness (CMI).
Method:
Veterans (N=128) who endorsed symptoms of CMI were randomized to: usual care (n=43), in-person (n=42) or telephone-delivered cognitive behavioral stress management (n=43). Assessments were conducted at baseline, three months, and twelve months. The primary outcome was limitation in roles at work and home (i.e., ‘role physical’). Reductions in catastrophizing cognitions were evaluated as a mechanism of action.
Results:
Intent-to-treat analyses showed no statistically significant main effect (F(2, 164)=.58, p=.56) or interaction effect (F(4,164)=.94, p=.45) for role physical. Over time, veterans improved in their physical function (F(2,170)=5.34, p<.01; ὴ2partial=.06), PTSD symptoms (F(2,170)=9.39, p<.01; ὴ2partial=.10), depressive symptoms (F(2,170)=10.81, p<.01, ὴ2partial=.11), and physical symptoms (F(2, 172)=12.65, p<.01; ὴ2partial=.13), but these improvements did not differ across study arms over time. Completer analyses yielded similar results. There were no differences in catastrophizing between arms.
Conclusion:
Findings suggest stress reduction may not be the right target for improving disability among veterans with CMI. Veterans with CMI may need intervention that directly impacts medical self-management to improve disability.
Keywords: chronic multisymptom illness, disability, clinical trial, cognitive behavioral therapy, stress management, null results
Cognitive behavioral treatment produces small-to-moerate effects for reducing the disability and physical symptoms of Chronic Multisymptom Illness (CMI) and other symptom based conditions (e.g., chronic fatigue syndrome, fibromyalgia) (Glombiewski et al., 2010; Hoffman, Papas, Chatkoff, & Kerns, 2007; Malouff, Thorsteinsson, Rooke, Bhullar, & Schutte, 2008)). While effective, 50% or more of patient’s with chronic symptom conditions do not respond to cognitive behavioral treatment (Malouff, Thorsteinsson, Rooke, Bhullar, & Schutte, 2008). There is a need to understand for whom cognitive behavioral treatments work and to develop tailored treatments for sub-groups who do not currently benefit (Ehde, Dillworth, & Turner, 2014). Veterans comprise one sub-population who have elevated rates of CMI, yet initial evidence suggests low adherence and response to cognitive behavioral treatment compared to civilians (Donta et al., 2003; Malouff et al., 2008). The goal of this study was to test a tailored cognitive behavioral stress reduction treatment for veterans with CMI.
There have been increases in poorly understood physical symptoms and CMI (i.e., medically unexplained symptoms and syndrome) among US Veterans since at least the US Civil War and among European Veterans since the Boer War (Hyams, Wignall, & Roswell, 1996; Jones et al., 2002) McAndrew, Helmer, Phillips, Ray, & Quigley, In Press; Mohanty et al., 2015). This was particularly true for US Veterans returning from Operation Desert Shield/Desert Storm of whom an estimated 30% meet criteria for CMI, also called Gulf War Illness (Fukuda et al., 1998)).
The symptom burden experienced by veterans with CMI causes disability, defined as impairment in the ability to complete daily activities and participate in social engagement. McAndrew and colleagues (2015) showed that both Desert Shield/Desert Storm and OEF/OIF veterans with chronic fatigue syndrome (a condition closely related to CMI) reported disability levels more than one standard deviation below the population mean.
There have been few randomized controlled trials of cognitive behavioral treatment for veterans with CMI and to our knowledge there is only one published trial. The U.S. Veterans Affairs (VA) Cooperative Study #470 was a multicenter randomized clinical trial of cognitive behavioral treatment, aerobic exercise, or both among 1,092 Veterans with CMI who had been deployed to Desert Shield/Desert Storm (Donta et al., 2003). Treatments lasted 12 weeks and adherence was low with only 36% of those in the cognitive behavioral treatment arm attending at least two-thirds of the sessions. Improvements were modest with 18.5% of those in the cognitive behavioral treatment arm meeting the study’s primary outcome criterion for improvement in physical function (Donta et al., 2003).
In his editorial response to this study, Hotopf (2003) questioned the validity of the underlying theoretical model of the treatment for this population. The treatment was based on the fear-avoidance model which was developed for civilians with chronic fatigue syndrome. The fear-avoidance model assumes partients avoid physical and mental activity due to a belief that these can exacerbate the symptoms of CMI. Treatment addresses activity avoidance by teaching partients to slowly reengage in physical and/or mental activities. It is not known whether veterans with CMI avoid exertion and/or if this is a factor that maintains disability. Hotopf (2003) argued for smaller clinical trials tailored to veterans with CMI.
Research conducted since this trial suggests that increased psychological stress is a factor that maintains disability among veterans with CMI. The chronic physical symptoms of CMI lead to increased stress with 16% of veterans with CMI experiencing depression and 22% experiencing an anxiety disorder (M. S. Blanchard et al., 2006). While evidence does not support that psychological stress is the primary cause of CMI or related conditions (Henningsen, Zimmermann, & Sattel, 2003), stress does make managing CMI more difficult. As a result, veterans with CMI and higher levels of psychological stress have worse disability as compared to veterans with CMI and lower levels of psychological stress (Hotopf et al., 2004). Further, veterans with CMI and higher levels of psychological stress have worsening disability over time as compared to veterans with CMI and lower levels of psychological stress. Possible mechanisms for this association include stress interfering with health care decision-making, stress negatively impacting health behaviors such as poor nutrition and stress leading to avoidance of activities. Each of these may maintain or aggravate disability.
The current study tested a cognitive behavioral treatment for stress reduction that addressed patient’s cognitive and emotional responses to CMI, particularly catastrphizing. Catastrophizing is a cognitive response style of having exaggerated thoughts in response to pain and physical symptoms such as “this pain will kill me” or “I cannot stand it anymore” (Keefe et al., 2000; Minton, Richardson, Sharpe, Hotopf, & Stone, 2010; M. J. Sullivan et al., 2001; Theunissen, Peters, Bruce, Gramke, & Marcus, 2012; Turner, Jensen, & Romano, 2000). Reductions in catastrophizing are a strong predictor of reduced psychological stress for civilians with chronic pain (Spinhoven et al., 2004; Thorn et al., 2007; Turner, Holtzman, & Mancl, 2007). The current study compared usual care to telephone delivered cognitive behavioral treatment for stress reduction to in-person delivered cognitive behavioral treatment for stress reduction. The cognitive behavioral treatment was delivered both over the telephone and in person because chronic symptoms may make it difficult to access healthcare. The primary dependent variable for this analysis was the role-physical subscale of the SF-36 which captures limitations in the ability to engage in work and home roles. Secondary outcomes include the physical function subscale of the SF-36 which captures perceived ability to be physically active, PTSD symptoms, depressive symptoms and physical symptoms.
METHODS
Participants
Veterans who reported symptoms consistent with CMI and who frequently used U.S. Veterans Affairs (VA) healthcare services in Fiscal Year 2004 or 2005 were invited to participate in this study. Participants were enrolled January 2005 through February 2007. CMI was operationalized as reporting 5 or more symptoms on the Patient Health Questionnaire somatic symptom scale (PHQ-15). Frequent healthcare service use was operationalized as being at or higher than 88% of healthcare utilizers at a northeastern VA Medical Center. Participants had to report having a negative emotional response to their physical symptoms (e.g., anger, depression, anxiety) to ensure there was an appropriate target for the treatment. Participants also had to be English speaking, enrolled in the VA healthcare system for at least one year, report use of VA healthcare for at least 50% of their healthcare and able to use the a telephone. Exclusion criteria included serious comorbid psychiatric diagnosis (i.e., psychotic disorder, dementia, cognitive disorder, intellectual disability, anorexia or other eating disorder) or a serious comorbid medical disorder (i.e., cancer, chronic renal insufficiency, chronic severe hepatic disease).
Procedures
This study received Veterans Affairs New Jesey Healthcare System Institutional Review Board and Research and Development Committee approvals. Participants were recruited by an electronic medical review. Potentially eligible veterans were mailed a letter and follow-up telephone calls were made to recruit and screen. Finally, potential participants’ medical records were reviewed by a medical doctor to determine whether high utilization of medical services in the past year could be explained by a medical condition other than CMI (e.g., cancer) that was not identified in the initial selection process.
Interested and eligible veterans were consented into the study. They were administered part of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) to determine the presence of psychosis. Random allocation was conducted using a random numbers generator and block randomization, whereby enrolled participants were assigned to one of three arms: in-person cognitive behavioral stress reduction treatment, telephone-delivered cognitive behavioral stress reduction treatment, or usual care. Participants were randomized from early 2005 to early 2007. Recruitment stopped at the end of the funding period. Do to research oversight regulations the dataset is not available to the public. There were no significant adverse events related to the treatment.
Power analyses were conducted apriori setting the Type 1 error at 1.67% (5% rate Bonferroni-corrected for 3 comparisons) and the power equal to .80 and eta-squared of .09 (9% of the variance can be accounted for by the study arms) indicated a sample of 50 participants per arm would be sufficient for analyses.
Telephone and In-Person Cognitive Behavioral Treatment.
The cognitive behavioral stress reduction treatment consisted of 10 sessions and was based on two manuals that were used in civilian and veteran samples (Guarino et al., 2001; Hellman, Budd, Borysenko, McClelland, & Benson, 1990). The current treatment emphasized cognitive and emotional responses to stress using a Rational Emotional Behavior Therapy (REBT; (Ellis, 1997)) framework. REBT is a type of cognitive behavioral treatment that focuses on challenging maladaptive cognitive beliefs. Participants were taught how to assess and dispute stress-inducing beliefs about their symptoms and other stressors using the A (Activating event), B (Belief), C (emotional/behavioral Consequence), D (Disputation) and E (Effect of revised beliefs) model. The sessions also entailed: (a) how to distinguish physical sensations from symptoms; (b) how to re-assess the meaning of pain and other physical sensations; (c) how to reduce anxiety about medical uncertainty; (d) how to cope with chronic symptoms; and (e) how to combat fears of (re)injury and overcome inactivity. The cognitive behavioral treatment was delivered in person or over the telephone depending on to which arm the participant was randomized. No restrictions were placed on any veteran’s engagement in other medical or psychiatric care.
Usual Care.
Participants in the usual care group received their regular VA care plus assessments. The study did not provide additional treatment and there were no other study contacts other than the assessments.
Assessment
Assessments consisted of written questionnaires and were conducted at baseline, three month and 12 months. Baseline assessments were conducted following informed consent and included a demographics survey. Follow up assessments were conducted by mailing the participants a packet of questionnaires with a stamped self-addressed envelope with instructions for return. Assessors were blinded to randomized treatment arm. Participants were compensated $25 for completing assessments at baseline and 12-month follow-up.
Patient Health Questionnaire Somatic Symptom Scale (PHQ-15).
The PHQ-15 is a brief, well validated self-report measure of 15 physical symptoms that account for over 90% of physical complaints reported by outpatients in primary care (Kroenke, Spitzer, & Williams, 2002). Symptoms include: stomach pain, back pain, pain in joints or limbs, menstrual pain, headache, chest pain, dizziness, fainting, heart palpitations, shortness of breath, bowel complaints, pain during sexual intercourse, nausea/gas/indigestion, fatigue, and trouble sleeping. Each of these symptoms are rated as: 0 “not bothered at all”; 1 “bothered a little”; or 2 “bothered a lot” and higher scores represent greater symptom burden, with scores of 5, 10, 15 representing cutoff points for low, medium and high, respectively (Kroenke et al., 2002). A cut-off of five was chosen to capture the greatest number of veterans experiencing difficulties from physical symptoms.
Veterans RAND 36-Item Health Survey (VR-36).
Adapted from the RAND 36-item health survey developed through the Medical Outcomes Study, the VR-36 is a standardized, brief measure of health-related quality of life used to assess physical and emotional functioning in medically ill veterans (Kazis et al., 2006; Kazis et al., 1999; Ware, 1992). Like the SF-36, the VR-36 has eight subscales with transformed scores ranging from 0 to 100 where higher scores denote higher levels of function. It differs from the SF-36 in that it offers a 5-point response scale (“no, none of the time,” “yes, a little of the time,” “yes, some of the time,” “yes, most of the time” and “yes, all of the time”), rather than “yes’ or “no” responses offered in the SF-36 (Kazis, Miller, et al., 2004). Two of the subscales, role physical limitation (RP) and physical functioning (PF) were used as dependent variables (McHorney, Ware, & Raczek, 1993). The role physical scale assessed work-related impairment (e.g., accomplished less, limited in kind, had difficulty, or cut down time) and the physical function scale was used to assess routine physical functions such as lifting, walking, and bending. Validity and reliability have been established through numerous studies (Kazis, Lee, et al., 2004).
PTSD Checklist-Civilian Version (PCL-C).
This is a brief (17 items), standardized, self-report measure commonly used to assess the presence and severity of symptoms that correspond with PTSD criteria in the DSM-IV (Association, 1994; E. B. J.-A. Blanchard, J.; Buckley, T. C.; Forneris, C. A., 1996; Weathers, Litz, Herman, Huska, & Keane, 1993, October). Participants are asked to rate the degree, ranging from 0 (not at all) to 5 (extremely), to which they have been bothered by a symptom in response to a stressful experience (e.g., repeated, disturbing memories, thoughts, or images of a stressful experience from the past; avoid thinking about or talking about a stressful experience from the past or avoid having feelings related to it). Overall, the PCL is considered psychometrically valid and reliable (McDonald & Calhoun, 2010; Wilkins, Lang, & Norman, 2011).
Patient Health Questionnaire Depression Screener (PHQ-9).
This brief, self-report, psychometrically validated measure of depression was adapted from the Primary Care Evaluation of Mental Disorders (PRIME-MD) and has been widely used across various medical populations (Kroenke, Spitzer, Williams, & Lowe, 2010; Spitzer, Kroenke, Williams, & Group, 1999). Responses range from “not at all = 0” to “nearly every day = 3;” cut points of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe levels of depressive symptoms (Kroenke et al., 2010). The PHQ-9 has been found be valid and reliable in detecting and monitoring depression, and is sensitive to change with a five-point decline considered a clinically significant improvement (Kroenke et al., 2010).
Catastrophizing Scale.
To assess dysfunctional thinking patterns, the ‘Helplessness’ subscale of the Pain Catastrophizing Scale (PCS; (M. J. L. Sullivan, Bishop, & Pivik, 1995) was modified by broadening the instructions to include any health symptom, rather than only pain. The ‘Helplessness’ subscale of the PCS has demonstrated adequate internal reliability and has been associated with affective responses to pain as well as pain-related disability (Osman et al., 1997; M. J. L. Sullivan et al., 1995; M. J. L. Sullivan, Lynch, M. E., Clark, A. J., 2005). In questions that included the word “pain” the word “it” was substituted in reference to their physical symptoms (e.g., “I worry all the time about whether the pain will end” was changed to “…whether it will end”).
Analyses
Differences in baseline variables between treatment arms (in-person cognitive behavioral stress reduction treatment, telephone-delivered cognitive behavioral stress reduction treatment, or usual care) were examined using ANOVA and chi-square analysis. The potential differential effect of therapist on the dependent variables at each time point (baseline, 3 months, and 12 months) was also examined using repeated measures ANOVA. A series of repeated measures ANOVAs was used to determine if statistically significant differences existed between treatment arms on dependent variables over time.
The primary dependent variable for this analysis was the VR-36 subscale ‘role physical limitation’ and secondary dependent variables were physical functioning, PTSD symptoms, depressive symptoms and physical symptoms. For each repeated measure ANOVA, change over time was examined for the specified dependent variable, as well as the interaction of treatment arm by change in the dependent variable over time. These were conducted using both intent-to-treat and completers analysis approaches.
To better understand the results, a series of planned exploratory analyses was conducted First, the role of catastrophizing as a mechanism of improvement was tested using baseline and three month assessment (immediately post-treatment), as this is where changes in a mechanistic variable would most likely be found. The analyses was performed using completers only, in order to determine whether receipt of treatment led to changes in catastrophizing. A repeated measures ANOVA in which treatment arm served as the between-subject variable and catastrophizing over time as the within-subject variable, was used to examine the main effect of time, and the interaction of treatment arm and catastrophizing over time.
Regression analyses were then used to determine whether changes in catastrophizing over time (i.e., catastrophizing at three months while controlling it at baseline) were associated with changes in the dependent variables regardless of treatment arm (i.e., dependent variable at three months while controlling for the dependent variable at baseline).
Results
Randomization
No significant differences in age, ‘role physical,’ physical symptom severity, depression, PTSD and physical function were found across arms at baseline (see Table 1). There were also no differences in gender (female=5.5%, male=94.5%) or report of current mental health treatment (48%) across arms at baseline. There were also no differences in self report of education obtainment (some high school=10%, high school graduate=41%, some college=35%, college graduate=7%, graduate school=6%) race (Black or African American=50%, White=45%, Other=5%) or ethnicity (Latino/a=6%, non-Latino/a=94%) at baseline. Finally, there were no differences in reporting being deployed oversees (71%) or to the Gulf Region (3%). No participants reported receiving cognitive behavioral treatment within the past year.
Table 1:
Examination of baseline characteristics (mean (standard deviation)) shows no significant differences at baseline.
| Telephone CBT (n=42) | In-person CBT (n=43) | Usual Care (n=43) | |
|---|---|---|---|
| Age | 57.6 (6.6) | 55.4 (8.2) | 56.8 (7.3) |
| Role Physical (SF-36) | 32.4 (11.7) | 32.4 (12.2) | 30.9 (10.9) |
| Physical Function (SF-36) | 30.8 (8.2) | 29.8 (10.7) | 31.6 (11.5) |
| Depressive Symptoms (PHQ-9) | 14.9 (6.4) | 15.7 (5.6) | 14.7 (7.2) |
| PTSD Symptoms (PCL) | 56.6 (15.1) | 53.7 (14.4) | 55.0 (17.7) |
| Physical Symptom (PHQ-15) | 13.2 (4.5) | 12.7 (5.4) | 14.6 (5.3) |
*PQH=patient health questionnaire, PCL=post traumatic stress disorder checklist, SF-36=short-form 36, CBT=cognitive behavioral treatment
Therapist Effects
Study providers consisted of five doctoral level clinical psychologists. The time-by-therapist interaction was not significant for any dependent variables, suggesting that there were no significant differential effects of therapists on the dependent variables over time.
Intent to Treat
The impact of treatment on the primary dependent variable, ‘role physical,’ was examined using ANOVA (see Table 2). There was no main effect of time indicating that participants ‘role physical’ scores did not significantly improve over time (F(2, 164)=.58, p=.56). There were also no differences among the treatment arms (treatment × time: F(4,164)=.94, p=.45). The secondary outcomes were physical functioning, PTSD symptoms, depressive symptoms and physical symptoms. For physical function there was a significant main effect, such that participants’ physical function scores improved over time (F(2,170)=5.34, p<.01; ὴ2partial=.06). However, change in physical function over time did not differ across the treatment arms (treatment × time: F(4,170)=.15, p=.96). Similar to findings on physical function scores, there was a main effect of time such that PTSD symptoms (F(2,170)=9.39, p<.01; ὴ2partial=.10), depressive symptoms (F(2,170)=10.81, p<.01, ὴ2partial=.11) and physical symptoms (F(2, 172)=12.65, p<.01; ὴ2partial=.13) improved over time but this change was the same across treatment arms (treatment × time: F(4, 170)=.46, p=.77, F(4,170)=.26, p=.91, and (F(4,172)=2.0, p=.10; ὴ2partial=.04 for PTSD, depression and physical symptoms, respectively).
Table 2:
Intent-to-treat analysis: Mean (standard deviation) of outcome variables for each arm at baseline, 3 months and 12 months.
| Telephone CBT (n=32) | In-person CBT (n=32) | Usual Care (n=31) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 m | 12 m | Baseline | 3 m | 12 m | Baseline | 3 m | 12 m | |
| Role Phys | 33.8 (12.1) | 31.6 (11.4) | 35.6 (13.2) | 33.9 (11.5) | 36.6 (11.6) | 35.0 (11.0) | 33.6 (10.1) | 34.9 (11.8) | 34.9 (13.9) |
| Phys Fx | 31.3 (9.1) | 33.2 (11.5) | 34.5 (10.9) | 30.7 (9.8) | 32.5 (10.8) | 34.0 (10.6) | 33.2 (11.8) | 33.6 (11.7) | 35.4 (11.8) |
| PTSD | 57.2 (16.2) | 49.7 (15.6) | 53.2 (15.9) | 50.2 (13.0) | 46.3(18.3) | 47.2 (17.4) | 52.8 (17.8) | 47.0 (17.7) | 47.3 (15.9) |
| Depression | 15.1 (6.6) | 12.7 (8.0) | 12.0 (7.7) | 14.9 (5.5) | 11.7 (7.0) | 11.7 (7.0) | 13.4 (6.9) | 11.8 (6.5) | 11.1 (7.3) |
| Physical Sx | 13.2 (4.5) | 11.8 (5.8) | 11.4 (5.0) | 11.6 (5.5) | 10.1 (5.5) | 10.9 (5.8) | 14.5 (4.8) | 11.2 (5.9) | 10.7 (5.2) |
*Role Phys=role physical, Phys Fx=physical function, PTSD=post traumatic stress disorder symptoms, Sx= Symptoms, CBT=cognitive behavioral treatment
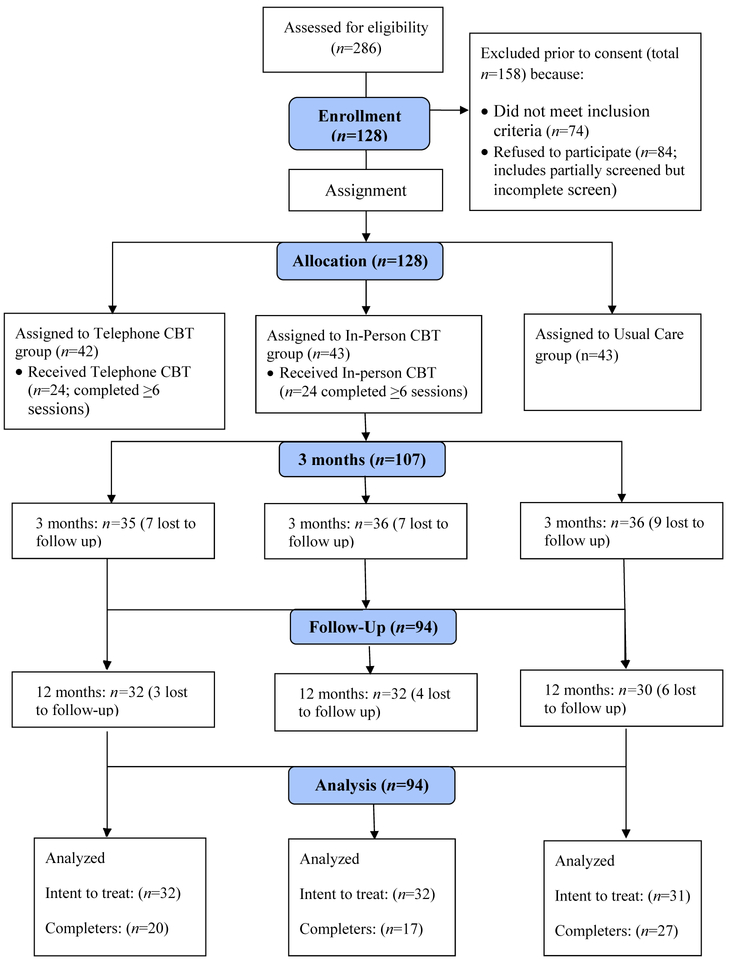
Attrition (see Figure 1) resulted in missing data, which was imputed using the last observation carried forward method. Only 0.6% of the measures were unanswered from participants who otherwise completed the assessment. With data imputed, the pattern of results remained the same; none of the time-by-treatment interaction terms were significant, suggesting that there were no differences between the arms on any of our dependent variables (results available upon request).
Figure 1:
Participant Flow Diagram
Completer Analysis
Completion was defined a priori as completing 6 or more sessions of the in-person or telephone delivered cognitive behavioral stress reduction treatment; the average number of completed sessions was six. Independent t-test showed that treatment completion did not differ between the telephone-delivered intervention (57% completed) or the in-person intervention (56%). The results of the completer analyses were qualitatively the same as the intent-to-treat analyses (see Table 3). There were no differences between the treatment arms over time for any of the dependent variables (i.e., treatment × time interactions were non-significant).
Table 3:
Completer analysis (completed 6 or more treatment sessions): Mean (standard deviation) for outcome variables for each arm at baseline, 3 months and 12 months.
| Telephone CBT (n=17) | In-person CBT (n=20) | Usual Care (n=27) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 month | 12 month | Baseline | 3 month | 12 month | Baseline | 3 month | 12 month | |
| Role Phys | 35.7 (11.9) | 31.5 (11.5) | 37.8 (12.9) | 34.1 (12.1) | 36.3 (12.8) | 35.6 (11.8) | 33.6 (10.1) | 34.9 (11.8) | 34.9 (13.9) |
| Physical Fx | 32.2 (9.4) | 34.0 (11.3) | 34.4 (11.4) | 31.5 (11.6) | 33.2 (11.7) | 34.9 (12.0) | 33.2 (11.8) | 33.6 (11.7) | 35.4 (11.8) |
| PTSD | 53.4 (15.7) | 46.7 (15.3) | 51.3 (12.7) | 49.8 (11.8) | 43.8 (17.3) | 46.0 (15.4) | 52.8 (17.8) | 47.0 (17.7) | 47.3 (15.9) |
| Depression | 14.3 (6.4) | 12.5 (9.2) | 11.9 (7.9) | 14.3 (5.9) | 10.4 (6.2) | 10.1 (6.7) | 13.4 (6.9) | 11.8 (6.5) | 11.1 (7.3) |
| Physical Sx | 13.4 (4.3) | 12.1 (5.7) | 11.2 (4.8) | 11.0 (4.8) | 9.8 (5.8) | 9.3 (5.2) | 14.5 (4.8) | 11.2 (5.9) | 10.7 (5.2) |
*Role Phys=role physical, Phys Fx=physical function, PTSD=post traumatic stress disorder symptoms, Sx= Symptoms, CBT=cognitive behavioral treatment
Specifically, the analyses showed that there was no main effect of time on ‘role physical’ (F(2, 122)=.94, p=.39) and no differences between the treatment arms on changes to ‘role physical’ (treatment × time: F(4,122)=1.29, p=.28). We also found that physical function (F(2,128)=3.55, p=.03; ὴ2partial=.05), PTSD (F(2, 128)=8.12, p<.01; ὴ2partial=.11), depression (F(2, 128)=8.03, p<.01; ὴ2partial=.11) and physical symptoms (F(2,130)=11.24, p<.01; ὴ2partial=.15) improved over time. These changes did not differ between the treatment arms (treatment × time) for physical function (F(4,128)=.21, p=.93), PTSD (F(4, 128)=.41, p=.80), depression (F(4,128)=.44, p=.78), nor physical symptoms (F(4,130)=1.10, p=.36).
Catastrophizing: Potential Mechanism of Change Analysis
We examined if our proposed mechanism of change (i.e., reduction in catastrophizing) was different across the treatment arms. Results showed a statistically significant effect of time, such that catastrophizing went down from baseline to three months (i.e., the end of treatment; F(1, 79)=9.36, p<.01; ὴ2partial=.11). However, there was not a statistically significant time-by-treatment interaction, suggesting that improvements in catastrophizing did not differ across treatment arms (mean (standard deviation): telephone-delivered cognitive behavioral treatment (baseline=11.04 (5.95), 3 month=8.57 (6.02)), in-person cognitive behavioral treatment (baseline=12.26 (6.5), 3 month=9.13 (6.29)), usual care (baseline=10.44 (7.23), 3 month=9.5 (7.34)). Cognitive behavioral stress reduction treatment, either telephone or in person, did not significantly improve catastrophizing (i.e., the proposed mechanism of action) as compared to usual care.
We next examined if changes in catastrophizing (operationalized as catastrophizing at three months while controlling for catastrophizing at baseline) was predictive of changes in our dependent variables (operationalized as the dependent variable at three months controlling for our dependent variable at baseline). Analyses showed reductions in catastrophizing were associated with improvements in functioning and mental health symptoms while controlling for baseline characteristics. Lower catastrophizing at three months was predictive of better role functioning at three months (β=−.43, p<.01), fewer PTSD symptoms at three months (β=.30, p<.01) and fewer depressive symptoms at three months (β=.33, p<.01). Lower catastrophizing at three months did not predict better physical function at three months (β=.17, NS) or fewer physical symptoms at three months (β=.08, NS).
Discussion
The current clinical trial compared a cognitive behavioral treatment for stress reduction delivered over the telephone to cognitive behavioral treatment for stress reduction delivered in person to usual care for veterans with CMI. Over the course of the study (baseline to 12 months), there was no improvement in ‘role physical,’ defined as the ability to physically engage in life roles at work or home. During the 12 months of the study, all treatments arms showed improvement in physical function, PTSD symptoms, depressive symptoms and physical symptoms. Contrary to our prediction, neither cognitive behavioral treatment arm showed greater improvement than usual care in primary or secondary outcomes (i.e., physical function, PTSD symptoms, depression symptoms or physical symptoms).
We examined whether there were changes in the proposed mediator, reductions in catastrophizing cognitions. All treatment arms showed a reduction in catastrophizing cognitions over time. This reduction in catastrophizing cognitions was associated with improvements in health function, PTSD symptoms and depressive symptoms. This is consistent with previous longitudinal studies that found reduced catastrophizing was associated with improvements in symptoms and functioning for civilians with chronic symptomatic conditions (e.g., chronic pain, fibromyalgia, chronic fatigue syndrome; (Jensen, Turner, & Romano, 2001; Michael & Burns, 2004; Spinhoven et al., 2004). We did not find that the cognitive behavioral treatment reduced catastrophizing cognitions more than usual care.
This study failed to find that cognitive behavioral stress reduction treatment led to improvement in any of our dependent variables compared to usual care. We propose the most likely reason for the null effect of the cognitive behavioral treatment is that stress reduction is the wrong treatment target. While cognitive behavioral stress reduction treatments improve disability for some medical conditions (Goldenberg et al., 1994), other studies point to the benefits of focusing on skills and behavior related to illness management.
For example, depression and diabetes are highly comorbid and depression is known to lead to worse adherence to diabetes treatments and worse diabetes outcomes (Gonzalez et al., 2008). Cognitive behavioral treatments that treat comorbid depression and diabetes by teaching skills that link and improve management of both (e.g., improve adherence to medication, increase physical activity) show improvements in both diabetes management and depression outcomes (Safren et al., 2014). These improvements are greater than interventions that focus on treating depression alone (Lin et al., 2006; Lustman, Freedland, Griffith, & Clouse, 2000; Lustman, Griffith, Freedland, Kissel, & Clouse, 1998). CMI and diabetes are similar in that the management of both conditions is time intensive and complicated. The management of CMI requires scheduling breaks and activities around symptoms, working closely with medical providers, using problem-solving to determine which self-management approaches improve disability. The current stress reduction intervention attempted to improve disability by changing thoughts and behaviors that maintain stress, rather than building skills and behavior to help manage the illness. This approach may not have been direct enough to impact outcomes. A better target may have been helping veterans to problem-solve how to better manage their CMI and increase activities of their choosing.
It is also possible that a research design consideration could have masked a true impact of treatment. The primary design consideration is if our cognitive behavioral treatment was delivered effectively. Although all therapists received weekly supervision with an expert in cognitive behavioral therapy for CMI, we did not have a measure of therapist adherence to the treatment or a measure of competency of the therapists. The cognitive behavioral stress reduction treatment was designed to target catastrophizing cognitions, yet it did not significantly improve catastrophizing cognitions more than usual care. Other cognitive behavioral treatments for patients with chronic symptomatic conditions (i.e., chronic pain) have shown improvements in catastrophizing with similar sample sizes. Further, these other trials have shown that changes in catastrophizing are associated with improvements in disability outcomes (Cassidy et al, 2011; Turner et al, 2000; Kristjansdottir et al, 2013). A stronger or more effective implementation could have led to improvements in outcomes in this study. However, it is likely that a more effective implementation of the treatment would be difficult to apply in real world clinics. Historically, the efficacy of cognitive behavioral treatments is lower in effectiveness as compared to efficacy trials because of difficulties implementing the treatments.
Another consideration is that we limited our inclusion criteria to include only veterans who used a high level of healthcare seeking in the past year. We may have enrolled these veterans at an extreme point in their condition and their improvement over time may reflect the natural influence of regression to the mean. Alternatively, because we only looked at the previous year, we do not know if these veterans were consistently high utilizers or had simply started a number of new treatments, some of which may have been taking effect during our data collection period.
An alternate research design consideration is whether we had sufficient power to find an effect. Our initial power analysis suggested the need for 50 participants per group which we did not achieve. However, the means, standard deviations, and mean changes over time are fairly consistent across the three arms (see Tables 1, 2 and 3) suggesting that there was little effect rather than insufficient power to find an effect on our primary or secondary outcomes of interest. Our results do not suggest that there was a clinically meaningful difference between the CBT and usual care arms that did not reach statistical significance.
Future work should exmine the generalizability of these findings. This is the second trial to find a cognitive behavioral treatment is less effective for veterans with symptom based conditions than civilians (Donta et al., 2003). Similar to veterans, civilians with a history of trauma are 2.7 times more likely to experience clinically significant medically unexplained symptoms (Afari et al., 2014). To our knowledge, no study has examined the efficacy of cognitive behavioral treatment for civilians with medically unexplained symptoms or a symptom based condition (e.g., fibromyalgia) and a history of trauma.
In conclusion, this clinical trial of cognitive behavioral stress reduction treatment delivered over the telephone or in person compared to usual care did not show a significant improvement of cognitive behavioral treatment over usual care. This is possibility because targeting stress management is too distal to achieve the desired disability outcome. Future trials should consider interventions that directly target illness self-management to improve the disability of people with CMI.
Declarations:
Telemedicine Treatment for Veterans with Gulf War Illness Clinical Trials #NCT00129454; This work, Telemedicine Treatment for Veterans with Gulf War Illness Clinical Trials #NCT00129454, is supported by the War Related Illness & Injury Study Center, VA Health Services Research & Development Grant Nos. GWI04–355 and CDA 13–017.
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the Department of Defense or the United States government.
References
- Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, & Cuneo JG (2014). Psychological Trauma and Functional Somatic Syndromes: A Systematic Review and Meta-Analysis. Psychosomatic Medicine, 76(1), 2–11. doi: 10.1097/psy.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP (1994). Diagnostic and statistical manual of mental disorders: DSM-IV (4th ed.): Washington, DC: American Psychiatric Association. [Google Scholar]
- Blanchard EB, J.-A. J; Buckley TC; Forneris CA (1996). Psychometric properties of the PTSD Checklist (PCL). Behaviour Research and Therapy, 34, 669–673. [DOI] [PubMed] [Google Scholar]
- Blanchard MS, Eisen SA, Alpern R, Karlinsky J, Toomey R, Reda DJ, … Kang HK (2006). Chronic Multisymptom Illness Complex in Gulf War I Veterans 10 Years Later. Am J Epidemiol, 163(1), 66–75. doi: 10.1093/aje/kwj008 [DOI] [PubMed] [Google Scholar]
- Donta ST, Clauw DJ, Engel CC Jr., Guarino P, Peduzzi P, Williams DA, … Feussner JR (2003). Cognitive behavioral therapy and aerobic exercise for Gulf War veterans’ illnesses: a randomized controlled trial. [Clinical Trial Randomized Controlled Trial Research Support, U.S. Gov’t, Non-P.H.S.]. JAMA, 289(11), 1396–1404. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Dillworth TM, & Turner JA (2014). Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. American Psychologist, 69(2), 153. [DOI] [PubMed] [Google Scholar]
- Ellis A, Harper A, Powers M (1997). A Guide to Rational Living: Wilshire Book Co. [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, … Reeves WC (1998). Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA, 280(11), 981–988. [DOI] [PubMed] [Google Scholar]
- Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, & Hofmann SG (2010). Psychological treatments for fibromyalgia: a meta-analysis. PAIN®, 151(2), 280–295. [DOI] [PubMed] [Google Scholar]
- Guarino P, Peduzzi P, Donta ST, Engel CC, Clauw DJ, Williams DA, … Feussner JR (2001). A multicenter two by two factorial trial of cognitive behavioral therapy and aerobic exercise for Gulf War veterans’ illnesses: design of a veterans affairs cooperative study (CSP# 470). Controlled clinical trials, 22(3), 310–332. [DOI] [PubMed] [Google Scholar]
- Hellman CJ, Budd M, Borysenko J, McClelland DC, & Benson H (1990). A study of the effectiveness of two group behavioral medicine interventions for patients with psychosomatic complaints. Behavioral Medicine, 16(4), 165–173. [DOI] [PubMed] [Google Scholar]
- Henningsen P, Zimmermann T, & Sattel H (2003). Medically Unexplained Physical Symptoms, Anxiety, and Depression: A Meta‐Analytic Review. Psychosomatic Medicine, 65(4), 528–533. [DOI] [PubMed] [Google Scholar]
- Hoffman BM, Papas RK, Chatkoff DK, & Kerns RD (2007). Meta-analysis of psychological interventions for chronic low back pain. Health psychology, 26(1), 1. [DOI] [PubMed] [Google Scholar]
- Hotopf M (2003). Treating Gulf War veterans’ illnesses--are more focused studies needed? [Comment Editorial]. JAMA, 289(11), 1436–1437. [DOI] [PubMed] [Google Scholar]
- Hotopf M, David A, Hull L, Nikalaou V, Unwin C, & Wessely S (2004). Risk factors for continued illness among Gulf War veterans: a cohort study. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Psychol Med, 34(4), 747–754. doi: 10.1017/S0033291703001016 [DOI] [PubMed] [Google Scholar]
- Hyams KC, Wignall FS, & Roswell R (1996). War syndromes and their evaluation: from the US Civil War to the Persian Gulf War. Annals of Internal Medicine, 125(5), 398–405. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, & Romano JM (2001). Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. [Research Support, U.S. Gov’t, P.H.S.]. J Consult Clin Psychol, 69(4), 655–662. [DOI] [PubMed] [Google Scholar]
- Jones E, Hodgins-Vermaas R, McCartney H, Everitt B, Beech C, Poynter D, … Wessely S (2002). Post-combat syndromes from the Boer war to the Gulf war: a cluster analysis of their nature and attribution. Bmj, 324(7333), 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazis LE, Lee A, Spiro III A, Rogers W, Ren XS, Miller DR, … Haffer SC (2004). Measurement comparisons of the medical outcomes study and veterans SF-36 health survey. Health Care Financ Rev, 25(4), 43–58. [PMC free article] [PubMed] [Google Scholar]
- Kazis LE, Miller DR, Clark JA, Skinner KM, Lee A, Ren XS, … Ware JE Jr. (2004). Improving the response choices on the veterans SF-36 health survey role functioning scales: results from the Veterans Health Study. J Ambul Care Manage, 27(3), 263–280. [DOI] [PubMed] [Google Scholar]
- Kazis LE, Miller DR, Skinner KM, Lee A, Ren XS, Clark JA, … Fincke BG (2006). Applications of Methodologies of the Veterans Health Study in the VA Healthcare System: Conclusions and Summary. Journal of Ambulatory Care Management, 29(2), 182–188. [DOI] [PubMed] [Google Scholar]
- Kazis LE, Ren XS, Lee A, Skinner K, Rogers W, Clark J, & Miller DR (1999). Health Status in VA Patients: Results from the Veterans Health Study. American Journal of Medical Quality, 14(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, & Caldwell DS (2000). The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain, 87(3), 325–334. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, & Lowe B (2010). The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. General Hospital Psychiatry, 32(4), 345–359. doi: doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JBW (2002). The PHQ-15: Validity of a New Measure for Evaluating the Severity of Somatic Symptoms. Psychosomatic Medicine(64), 258. [DOI] [PubMed] [Google Scholar]
- Malouff JM, Thorsteinsson EB, Rooke SE, Bhullar N, & Schutte NS (2008). Efficacy of cognitive behavioral therapy for chronic fatigue syndrome: a meta-analysis. Clin Psychol Rev, 28(5), 736–745. [DOI] [PubMed] [Google Scholar]
- McDonald SD, & Calhoun PS (2010). The diagnostic accuracy of the PTSD Checklist: A critical review. Clinical Psychology Review, 30(976–987). doi: doi: 10.1016/j.cpr.2010.06.012 [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, & Raczek AE (1993). The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and Clinical Tests of Validity in Measuring Physical and Mental Health Constructs. Medical Care, 31(3), 247–263. [DOI] [PubMed] [Google Scholar]
- Michael ES, & Burns JW (2004). Catastrophizing and pain sensitivity among chronic pain patients: moderating effects of sensory and affect focus. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. Ann Behav Med, 27(3), 185–194. doi: 10.1207/s15324796abm2703_6 [DOI] [PubMed] [Google Scholar]
- Minton O, Richardson A, Sharpe M, Hotopf M, & Stone P (2010). Drug therapy for the management of cancer-related fatigue. [Meta-Analysis Review]. Cochrane Database Syst Rev(7), CD006704. doi: 10.1002/14651858.CD006704.pub3 [DOI] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, & O’Neill E (1997). Factor structure, reliability, and validity of the Pain Catastrophizing Scale. Journal of Behavioral Medicine, 20(6), 589–605. [DOI] [PubMed] [Google Scholar]
- Spinhoven P, ter Kuile M, Kole-Snijders AMJ, Mansfeld MH, den Ouden D-J, & Vlaeyen JWS (2004). Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain. European Journal of Pain, 8(3), 211–219. doi: 10.1016/j.ejpain.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, & Group PHQPCS (1999). Validation and utility of a self-report version of PRIME-MD. Journal of the American Medical Association, 282(18), 1737–1744. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, & Lefebvre JC (2001). Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain, 17(1), 52–64. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop S, & Pivik J (1995). The Pain Catastrophizing Scale: Development and validation. Psychological Assessment, 7(4), 524–532. [Google Scholar]
- Sullivan MJL, Lynch ME, Clark AJ (2005). Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain, 113(3), 310–315. doi: doi: 10.1016/j.pain.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Theunissen M, Peters ML, Bruce J, Gramke H-F, & Marcus MA (2012). Preoperative Anxiety and Catastrophizing: A Systematic Review and Meta-analysis of the Association With Chronic Postsurgical Pain. Clin J Pain, 28(9), 819–841. doi: 10.1097/AJP.0b013e31824549d6 [DOI] [PubMed] [Google Scholar]
- Thorn BE, Pence LB, Ward LC, Kilgo G, Clements KL, Cross TH, … Tsui PW (2007). A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. The Journal of Pain, 8(12), 938–949. [DOI] [PubMed] [Google Scholar]
- Turner JA, Holtzman S, & Mancl L (2007). Mediators, moderators, and predictors of therapeutic change in cognitive–behavioral therapy for chronic pain. Pain, 127(3), 276–286. [DOI] [PubMed] [Google Scholar]
- Turner JA, Jensen MP, & Romano JM (2000). Do beliefs, coping, and catastrophizing independently predict functioning in patients with chronic pain? Pain, 85(1), 115–125. [DOI] [PubMed] [Google Scholar]
- Ware JE, S. CD (1992). The MOS 36-Item Short-Form Health Survey (SF-36). Medical Care, 30(6), 473–483. [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, & Keane TM (1993, October). The PTSD checklist: Reliability, validity and diagnostic utility. Paper presented at the Annual Meeting of the International Society for Traumatic Stress Studies, San Antonio, TX. [Google Scholar]
- Wilkins KC, Lang AJ, & Norman SB (2011). Synthesis of the psychometric properties of the PTSD Checklist (PCL) Military, Civilian and Specific versions. Depression and Anxiety, 28(7), 596–606. doi: doi: 10.1002/da.20837 [DOI] [PMC free article] [PubMed] [Google Scholar]