Abstract
Despite extensive research, the neurobiological risk factors that convey vulnerability to opioid abuse are still unknown. Recent studies suggest that the dopamine D3 receptor (D3R) is involved in opioid self-administration, but it remains unclear whether altered D3R availability is a risk factor for the development of opioid abuse and addiction. Here we used dopamine D3 receptor-knockout (D3-KO) mice to investigate the role of this receptor in the different phases of opioid addiction. D3-KO mice learned to self-administer heroin faster and took more heroin than wild-type mice during acquisition and maintenance of self-administration. D3R-KO mice also displayed higher motivation to work to obtain heroin reward during self-administration under progressive-ratio reinforcement, as well as elevated heroin-seeking during extinction and reinstatement testing. In addition, deletion of the D3R induced higher baseline levels of extracellular dopamine (DA) in the nucleus accumbens (NAc), higher basal levels of locomotion, and reduced NAc DA and locomotor responses to lower doses of heroin. These findings suggest that the D3R is critically involved in regulatory processes that normally limit opioid intake via DA-related mechanisms. Deletion of D3R augments opioid-taking and opioid-seeking behaviors. Therefore, low D3R availability in the brain may represent a risk factor for the development of opioid abuse and addiction.
Keywords: Heroin, dopamine, D3 receptors, reward, addiction, self-administration, reinstatement
Introduction
Opioid addiction is a chronic reoccurring disorder characterized by high rates of relapse (Smyth et al, 2010) and risk for overdose (CDC, 2017). Heroin, a synthetic opioid, is among the most addictive drugs of abuse (Chartoff and Connery, 2014; Reed et al, 2014), and is associated with massive personal and public health costs. In 2015 alone, heroin use disorder was estimated to cost the U.S. $51.2 billion, or $50,799 per user (Jiang et al, 2017). Rates of heroin abuse and dependence are rising, with diagnoses more than doubling from 2002 and 2013 and fatal overdoses increasing by 533% (Cicero et al, 2017; Kounang, 2017; Lipari and Hughes, 2013). Results from the 2015 National Survey on Drug Use and Health indicate that approximately 5.1 million individuals report using heroin in their lifetime (CBHSQ, 2016). These statistics and others have led many to declare the U.S. in the midst of an “opioid epidemic” (Kolodny et al, 2015; HHS, 2017).
Despite the rising prevalence of opioid addiction, treatments are limited and the neurobiological risk factors that convey vulnerability to opioid abuse remain unknown (Chartoff and Connery, 2014). The mesolimbic dopamine (DA) pathway, which originates in the ventral tegmental area (VTA) and projects to the nucleus accumbens (NAc) and prefrontal cortex, is a major neural substrate for drugs of abuse (Pierce and Kumaresan, 2006). Like many other drugs of abuse, opioids such as heroin increase extracellular DA levels in the NAc, where DA binds to post-synaptic receptors to trigger many molecular, physiological and behavioral changes (Pierce and Kumaresan, 2006). There are five DA receptor subtypes, which are classified into D1-like (D1, D5) and D2-like (D2, D3, D4) receptors based on their action on intracellular adenylate cyclase (Beaulieu and Gainetdinov, 2011). The D1 and D2 receptor subtypes have received substantial attention for their roles in substance abuse and addictive disorders (Volkow and Morales, 2015). However, the potential of both receptor subtypes as therapeutic targets is low due to their wide distributions throughout the brain and peripheral tissues and the concern for side effects (Childress and O’Brien, 2000; Desai et al, 1999; Xi and Gardner, 2007). In contrast, D3 receptors are enriched primarily in the mesolimbic DA system, particularly in the VTA and NAc, and therefore represent more attractive pharmacotherapeutic targets for the treatment of drug abuse (Heidbreder, 2008; Heidbreder and Newman, 2010; Sokoloff and Le Foll, 2017; Xi and Gardner, 2007).
Converging evidence in both humans and rodent models supports the involvement of the D3R in drug use disorders. For example, cocaine- and methamphetamine-dependent subjects show increased D3R availability in midbrain regions including the substantia nigra, hypothalamus and amygdala compared to healthy controls (Aleph Prieto, 2017; Boileau et al, 2016; Matuskey et al, 2014). The increase in D3R expression in the substantia nigra and ventral pallidum correlates positively with years of drug use (Matuskey et al, 2014). Genetic deletion of D3R causes escalation in cocaine-taking and cocaine-seeking behavior in mice D3R (Song et al, 2012). In addition, variations in the D3R gene are also associated with opiate dependence in humans (Duaux et al, 1998), and chronic opioid exposure is associated with a reduction in D3 expression in the amygdala in rats (Rosen et al, 2017). We have recently reported that acute pharmacological blockade of D3R inhibits heroin or oxycodone self-administration in rats and mice in a dose-dependent manner (Boateng et al, 2015; You et al, 2017). However, it is unknown whether altered D3R expression is associated with the development of opioid abuse and addiction. In the present study, we used transgenic D3R gene-knockout mice to study whether D3R loss is a susceptibility factor in the development of opioid abuse and addiction by examining animal drug-taking and drug-seeking behaviors during the acquisition of intravenous (i.v.) heroin self-administration, extinction, and reinstatement of drug-seeking. We also examined heroin self-administration maintained by different doses of heroin under fixed-ratio and progressive-ratio reinforcement schedules. Finally, we used in vivo microdialysis and a locomotor sensitization paradigm to study whether D3R deletion alters mesolimbic DA response to heroin, and thereby alters heroin-taking and heroin-seeking behaviors.
Materials and methods
Animals
Wild-type (WT) and DA D3R knockout (D3-KO) mice were generated and backcrossed to a C57BL/6J background at the National Institute on Drug Abuse (Baltimore, MD, USA) from three D3+/− breeding pairs purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were genotyped in our laboratory according to the mouse tail D3R-DNA-PCR protocol used by Charles River Laboratories (Wilmington, MA, USA). Experimental WT and D3-KO mice were matched for age (8–14 weeks) and weight (25–35 grams). During experimental testing, mice were housed individually in a climate-controlled animal colony room on a reversed light-dark cycle (lights on at 1900 h, lights off at 700 h). Food and water were available ad libitum. Mice were acclimated to housing conditions and handled once/day for 7 days before the start of experiments. All testing was conducted during the dark phase. All experimental procedures were carried out in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health.
Surgery
To enable heroin self-administration, a catheter was implanted into the right external jugular vein under ketamine anesthesia in each experimental subject, using aseptic surgical technique. Briefly, a 6.0-cm length of MicroRenathane tubing (ID 0.012″, OD 0.025″; Braintree Scientific Inc., Braintree, MA, USA) was inserted 1.2 cm into the right jugular vein and anchored to a 24-gauge steel cannula (Plastics One, Roanoke, VA, USA) that was bent at a 100° angle and mounted to the skull with cyanoacrylate glue and dental acrylic. A 2.5-cm extension of flexible tubing was connected to the distal end of the cannula. The mice were allowed 5–7 days for recovery, during which time 0.05 ml of 0.9% saline solution containing 20 IU/ml heparin and 0.33 mg/ml gentamycin was infused daily through the catheter to forestall clotting and infection. Thereafter, 0.05 ml of 0.9% saline solution containing 20 IU/ml heparin was infused immediately prior to and immediately following each daily heroin self-administration session. When needed, i.v. brevital (a barbiturate) was used to test catheter patency between the self-administration sessions. During heroin self-administration sessions, the flexible tubing extension was connected to a perfusion pump (Razel Scientific Instruments, Stamford, CT, USA) via a PE50 tubing connector. Outside self-administration sessions, the free end of the cannula guide was kept sealed at all times.
Apparatus
Operant test chambers (Model ENV-307A, Medical Associates, Georgia, VT, USA) were used to conduct i.v. heroin self-administration experiments. Briefly, each test chamber contained two levers located 2.5 cm above the floor (one active and one inactive), as well as a speaker and a yellow cue light located ~5 cm above the active lever. A house light mounted on the opposite wall signaled the start of each 3-hour test session and remained illuminated until the session ended. For heroin self-administration sessions, a liquid swivel mounted on a balance arm above the chamber allowed for i.v. drug delivery in freely-moving animals. Depression of the active lever resulted in the activation of an infusion pump; depression of the inactive lever was recorded but had no scheduled consequences. Each heroin infusion was paired with two discrete cues: illumination of the cue light above the active lever, and a cue tone that lasted for the duration of the infusion. Experimental events were controlled by a PC programmed in Medstate Notation and connected to a Medical Associates interface.
Heroin self-administration under FR1 reinforcement
To determine the effects of D3R deletion on heroin intake, after recovery from surgery mice were placed into operant chambers and allowed to lever-press for i.v. heroin delivered at a rate of 3.57μl/sec under a fixed-ratio (FR)1 reinforcement schedule (each lever press lead to one heroin infusion) for 3 h daily. During the infusion period (4.2 sec), additional responses were recorded but had no consequences. For the first 5 training sessions a 0.1 mg/kg/infusion heroin dose was available for self-administration, for sessions 6 through 10 a 0.05 mg/kg/infusion dose was available, and for sessions 11 through 15 a 0.025 mg/kg/infusion dose was available. Stable self-administration was defined as (i) earning at least 20 infusions per 3-h session, (ii) less than 20% variability in heroin infusions across 2 consecutive sessions, and (iii) an active/inactive lever press ratio exceeding 2:1. Mice that did not reach stability criteria were excluded from further experimentation (2 WT and 3 D3-KO mice, see Results). To prevent heroin overdose, a maximum of 50 infusions could be earned during each 3-h session. The number of heroin infusions earned, and active and inactive lever responses were recorded for each session in WT and D3-KO mice.
Heroin dose-response functions
To determine whether D3R deletion increases or decreases the pharmacological action of heroin in mouse brain, we compared the heroin self-administration dose-response curves between WT and D3-KO mice under the same experimental conditions. This is based on a well-accepted view that a leftward or upward shift of a drug dose-response curve may indicate an increase in its pharmacological action, and vice versa (Song et al., 2012). For this purpose, additional separate groups of mice (9 WT and 10 KO) were implanted with i.v. jugular catheters and were thereafter allowed to self-administer different unit doses (0.00625, 0.0125, 0.025, 0.05 or 0.1 mg/kg/infusion) of heroin under a FR1 schedule of reinforcement. Heroin self-administration began from the highest dose (0.1 mg/kg/infusion) and then continued for other doses of heroin in a counterbalanced order. The self-administration maintained by each dose of heroin lasted for 5–7 consecutive sessions until the stable self-administration criteria were met for at least 3 days. The averaged numbers of heroin infusions over the last 3 sessions of self-administration maintained by each dose of heroin were used for data comparisons between WT and D3-KO mice. After 3–5 days of stable responding, the heroin dose available for self-administration was switched for additional 5–7 days. Doses were made available in a randomized counterbalanced order, and infusion volume and duration remained identical for each dose. The number of heroin infusions earned, and active and inactive lever responses were recorded for each session in D3-KO and WT mice.
Heroin self-administration under progressive-ratio reinforcement
Additional groups of WT (n=12) and D3-KO (n=14) mice were used in this experiment. The methods for heroin self-administration under progressive-ratio (PR) reinforcement were as reported previously (Song et al, 2012). After establishing stable heroin self-administration under FR1 reinforcement, subjects were switched to heroin self-administration under PR reinforcement. During PR conditions, the work requirement (lever presses) needed to receive a heroin infusion was raised progressively within each test session according to the following PR series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, and 603 until the break point was reached (Richardson and Roberts, 1996). The break point was defined as the maximal work load (i.e., number of lever presses) completed for the last heroin infusion prior to a 1-h period during which no infusions were obtained by the animal. Animals were initially trained for heroin (0.025 mg/kg) self-administration under FR1 reinforcement schedule for 2 weeks, and then switched to heroin (0.025mg/kg/infusion) self-administration under progressive-ratio reinforcement schedule for 4 days, followed by 0.05 mg/kg/infusion for 3 additional days. The number of heroin infusions and break points under PR reinforcement were compared between WT and D3-KO mice.
Extinction and reinstatement testing
After the completion of the 3-dose heroin self-administration experiment under FR1 and FR2 reinforcement conditions (Fig. 1-A,B), the same groups of animals continued for heroin self-administration (0.025 mg/kg/infusion) under the FR2 schedule for additional 3–5 days until stable self-administration was achieved. Two WT mice and one KO mouse failed to meet the stable self-administration criteria, and therefore, were removed from this experiment. The remaining 10 WT and 11 D3-KO mice then underwent extinction and reinstatement test. During extinction, syringe pumps were turned off and the heroin-associated cue light and tone were unavailable. Thus, lever pressing was recorded but had no scheduled consequences. Extinction training continued for 20 days until the extinction criteria were met (active lever responding <20% of the self-administration baseline). Two WT mice and three D3-KO mice failed to meet the above extinction criteria, and therefore, were removed from the data analysis and the following reinstatement test. The remaining 8 WT mice and 7 D3-KO mice then received 0.25 mg/kg of heroin (i.p.) to evoke reinstatement of drug-seeking behavior. The priming dose of heroin was selected based on previously published reports of significant reinstatement to drug seeking to this dose after extinction training (e.g., de Vries et al, 1998; Leri and Stewart, 2001). During reinstatement testing, active lever presses lead to re-exposure to the cue light and tone previously paired with heroin infusions, but not to heroin infusions. Active and inactive lever responses were recorded for each extinction and reinstatement session.
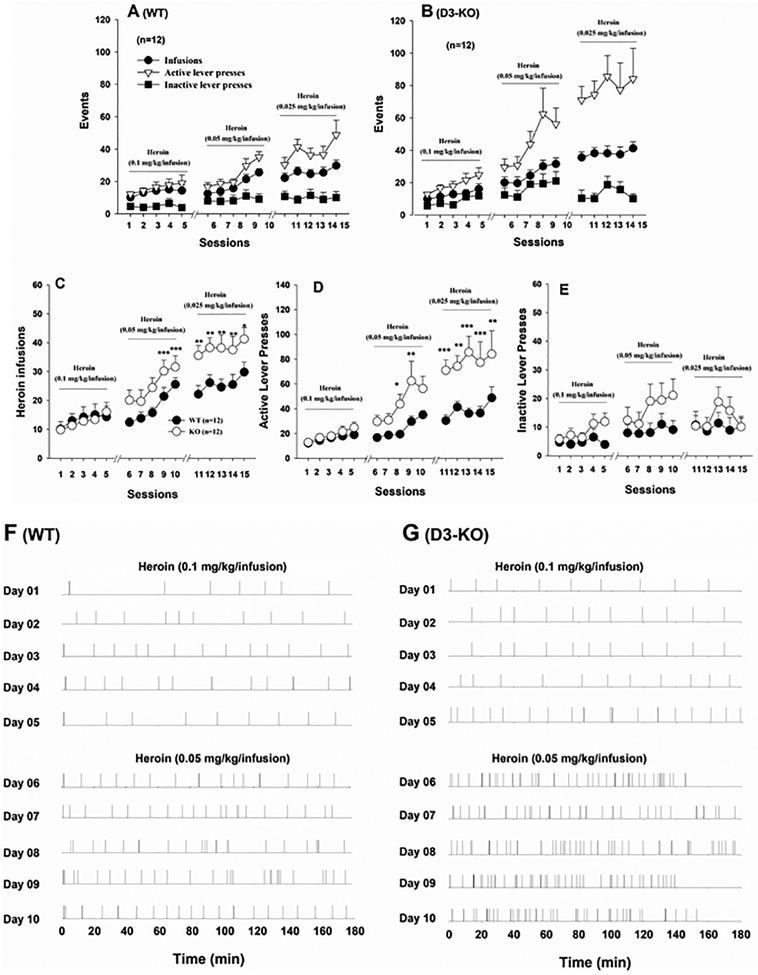
Figure 1.
Time courses of heroin self-administration during the acquisition and maintenance phases maintained by the different doses of heroin in WT and D3-KO mice. (A, B) Total numbers of heroin infusions, active lever presses and inactive lever presses in WT and D3-KO mice, respectively, illustrating the acquisition of heroin self-administration over time (sessions). (C,D,E) Group comparisons of heroin infusions, active lever responses, and inactive lever responses between WT and D3-KO during acquisition of heroin self-administration over time. (F,G) Representative heroin self-administration (infusions) within-session responding for heroin during the initial 10 days of self-administration training by WT and D3-KO mice. *p < 0.05; **p < 0.01; ***p < 0.001 compared with WT mice.
In vivo microdialysis
In vivo microdialysis was used to evaluate the impact of D3R deletion on baseline DA levels and heroin-induced DA release in the NAc. Anesthetized mice (ketamine; 400 mg/kg, i.p.) were implanted with a guide cannula (CMA-Carnegie Medicin-Sweden) in the striatum. Damage to the NAc was minimized by implanting the tips of the guide cannula 2 mm above the target site. Coordinates used for placement of the probe tips were AP +1.4 mm, ML ±1.5, and DV −3.5 mm, with an angle of 8° from vertical. Dialysis experiments were conducted in freely moving mice. Seven days after surgery, a probe (MAB 4.15.2.PES; SciPro) was inserted into the NAc at least 12 h before experiments began, to minimize manipulation-induced DA release during experimentation. After probe insertion, animals (n=6–8 per genotype) were transferred to microdialysis test cages (Phymep, Paris, France). Probes were connected to a syringe pump (Bioanalytical Systems) and perfused with a modified artificial cerebrospinal fluid (NaCl, 140 mM; KCl, 2.5 mM; CaCl2, 1.4 mM; MgCl2, 1.2 mM; Na2HPO4/NaH2PO4 (buffer), D-glutcose 5 mM, pH 7.4) at a rate of 0.1μl/min. Starting at least 2 h before sample collection, dialysis buffer was perfused through the probe (2.0 μl/min). Dialysis samples were collected every 20 min into 10 μL of 0.5 M perchloric acid, to prevent DA degradation. After 1 h of baseline collection, animals received one of 2 doses of heroin (1 or 2 mg/kg, s.c.) and collection continued for an additional 2 h. All samples were frozen at −80 °C until analysis. Dialysate DA concentrations were determined by high-performance liquid chromatography (HPLC) with electrochemical detection (ESA), following our previously published methods (Song et al, 2012). DA responses to heroin are expressed as percentage of baseline (defined as the average of the last three pre-injection values).
Locomotor activity
Locomotor activity was next examined in naïve groups of mice to determine the impact of D3R deletion on basal activity levels as well as activity following acute heroin administration. During the week before locomotor testing, mice were habituated to locomotor detection chambers (Accuscan Instruments) during 3 h daily sessions. On the test day, mice were placed in the chamber for 1 h of habituation, after which the effects of heroin on locomotion were measured over a 2 h period. Each heroin dose (0, 0.5, 1.0, 2.0 mg/kg, i.p.) was tested in 4 separate groups of mice (n=8 per group). The distance traveled before and after injections was collected in 10-min intervals using the VersaMax data analysis system (Accuscan Instruments).
Data analysis
All data are presented as mean (±SEM). Two-way ANOVAs with repeated measures for time were used to analyze heroin intake and seeking during self-administration, extinction and reinstatement testing, as well as locomotor activity and extracellular DA responses to heroin in D3-KO and WT mice. Post-hoc comparisons were carried out using the Newman-Keuls test. Student t-tests were used to compare mean heroin infusions or break points under PR reinforcement and basal levels of locomotion and extracellular NAc DA levels between D3-KO and WT mice.
Results
D3-KO mice earned more heroin during self-administration
To determine whether D3R deletion alters vulnerability to heroin use, one group of WT mice (n=14) and one group of D3-KO mice (n=15) were allowed to self-administer heroin, beginning with a dose of 0.1 mg/kg/infusion for 5 days under a fixed-ratio 1 (FR1) reinforcement schedule, followed by 0.05 mg/kg/infusion and 0.025 mg/kg/infusion under the FR1 reinforcement schedule. After 2 weeks of heroin self-administration, 2 WT and 3 D3-KO mice did not reach stable heroin self-administration criteria as stated above (i.e., <20 heroin infusions during daily 3 h session), and therefore were excluded from the study. Figures 1A and 1B show the time courses of the acquisition of heroin self-administration in WT and D3-KO mice, respectively, illustrating rapid discrimination of the heroin-paired active lever over the non-drug-paired inactive lever during the acquisition and maintenance phases of self-administration. Figures 1C–1E show that D3-KO mice earned more heroin infusions and made more active lever responses to obtain heroin reward than WT mice during the daily 3 h sessions. Two-way ANOVA with repeated measures over time revealed a significant genotype main effect (Fig. 1C -infusions, F1, 22=4.98, p<0.05; Fig. 1D – active lever responses, F1, 22=11.2, p<0.01; Fig. 1E – inactive responses, F1, 22=1.82, p>0.05), a time main effect (Fig. 1C, F14,308=18.30, p<0.001; Fig. 1D, F14,308=17.18, p<0.001; Fig. 1E, F14, 308=3.11, p<0.01), and a genotype × time interaction (Fig. 1C, F14,308=4.83, p<0.001; Fig. 1D, F14, 308=3.37, p<0.001; Fig. 1E, F14, 308=0.99, p>0.05). Individual group comparisons revealed significantly higher number of heroin infusions or active lever presses in D3-KO mice than in WT mice at many time points during acquisition, particularly at those time points when the self-administration was maintained by the lower doses of heroin (0.05 or 0.025 mg/kg/infusion). Figures 1F–1G show representative within-session heroin infusions during the first 10 days of self-administration training in a WT mouse and a D3-KO mouse. Specifically, a lower dose of heroin (0.05 mg/kg/infusion) maintained higher rates of self-administration with shorter inter-infusion intervals than a higher dose of heroin (0.1 mg/kg/infusion) in D3-KO mice compared to WT controls.
Deletion of D3 receptors upward-shifted heroin self-administration dose-response curves
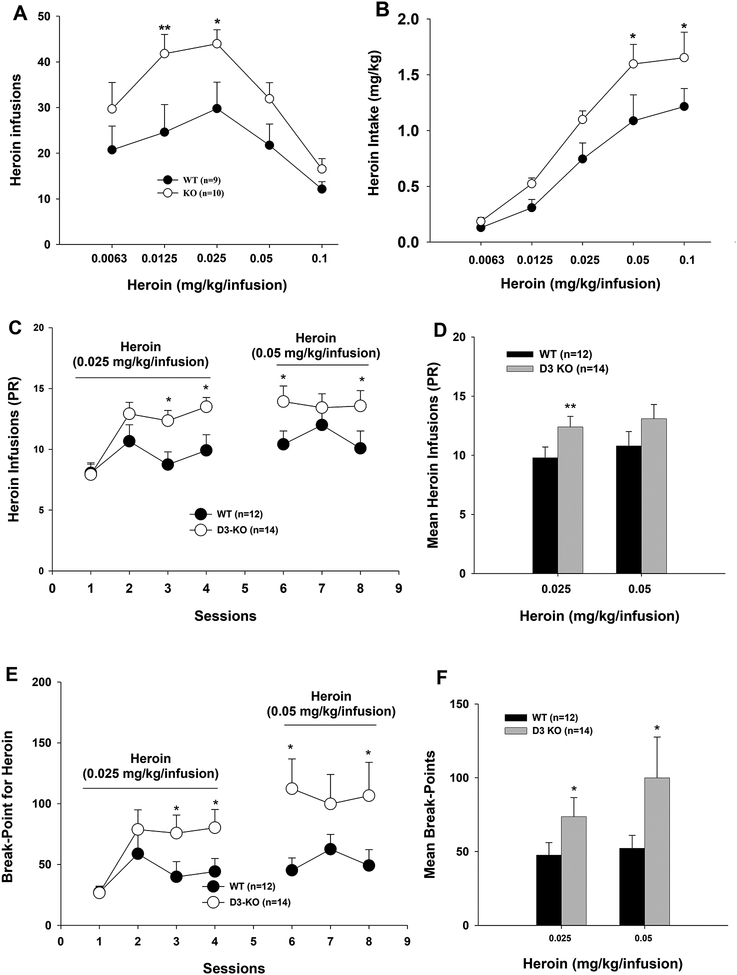
We next compared heroin self-administration dose-response curves between D3-KO and WT mice. As shown in Figure 2A, both D3-KO and WT mice exhibited inverted ‘U-shaped’ dose-response functions. However, D3R deletion significantly shifted the heroin dose-response curve upward compared to WT mice. Two-way ANOVA with repeated measures for heroin dose revealed a significant genotype main effect (Fig. 2A, F1, 17= 9.12, p<0.01), a dose main effect (F4,68= 9.16, p<0.001), and a genotype × dose interaction (F4,68= 2.75, p<0.05). Paired comparisons between KO and wild-type mice revealed significant increases in heroin self-administration in D3-KO mice at several unit doses of heroin (Fig. 2A). Figure 2B shows cumulative heroin intake (e.g., infusion number × unit heroin dose, mg/kg), illustrating a significant upward shift in the heroin dose-response intake function in D3-KO mice compared to WT mice (F1, 17= 10.38, p<0.01).
Figure 2.
Heroin self-administration maintained by different unit doses of heroin under fixed-ratio (A, B) or progressive-ratio (C-F) reinforcement schedules. Deletion of D3Rs in D3-KO mice shifted heroin self-administration dose-response curves upward (A) and heroin intake dose-response curves upward (B), as well as increased heroin infusions (C, D) and break-points for heroin reward (E, F) under PR reinforcement. *p < 0.05; **p < 0.01, compared with WT group.
Deletion of D3 receptors increased PR break-points during heroin self-administration
We then examined the effects of D3R deletion on motivation to respond for heroin under progressive-ratio (PR) reinforcement conditions (Richardson and Roberts, 1996). Again, D3-KO mice earned more heroin than WT mice (Figs. 2C–2D) and exhibited higher break-point levels for heroin reward under PR reinforcement (Figs. 2E–2F). Two-way ANOVA with repeated measures for time revealed a significant genotype main effect (Fig. 2C – heroin infusions, F1, 24=5.56, p<0.05; Fig. 2E – break-points, F1, 24=4.78, p<0.05), a time main effect (Fig. 2C, F6,144=4.31, p<0.01; Fig. 2E, F6,144=2.92, p=0.01), and a genotype × time interaction (Fig. 2C, F6,144=2.71, p<0.05; Fig. 2E, F6, 144=2.39, p<0.05). Figures 2D and 2F show the mean heroin infusions and break-point levels over the last 3 days of heroin self-administration, illustrating significantly higher motivation to work for heroin reward in D3-KO mice compared to WT mice.
D3-KO mice displayed higher heroin-seeking behavior during extinction
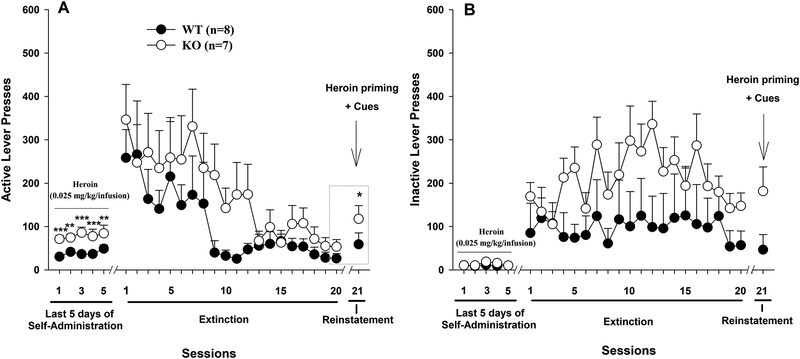
We next examined the effects of D3R deletion on combined heroin- and cue-induced relapse to drug-seeking after response extinction. Figure 3 shows extinction responding over 3 weeks, during which time active or inactive lever pressing did not produce heroin infusions or presentation of the heroin-associated cue light and tone. D3-KO mice displayed an overall higher heroin-seeking behavior than WT mice. However, this increase was not statistically significant. Two-way ANOVA with repeated measures revealed phenotype main effect (F1, 13 = 1.55, p>0.05), time main effect (F19,247 = 7.31, p<0.001), and phenotype × time interaction (F19,247 = 0.799, p>0.05). We then assessed heroin + cue-induced reinstatement of drug seeking on day 21 (Fig. 3A). We found that heroin priming (0.25 mg/kg, i.p.) plus heroin-associated cues produced reinstatement primarily in D3-KO mice. However, two-way ANOVA with repeated measures (for the data highlighted in the gray box in Fig. 3A) revealed a non-significant genotype main effect (F1, 13 = 2.44, p>0.05), a significant time main effect (F1,13 = 12.63, p<0.001), and a non-significant genotype × time interaction (F1.13 = 1.35, p>0.05). Post-hoc multiple-group comparisons revealed significant reinstatement response to heroin (q=5.03, p<0.05, Student-Newman-Keuls test). There were no significant differences in reinstatement responding between WT and KO mice (q=2.21, p>0.05). In addition, D3-KO displayed overall higher inactive lever responding than WT mice during 3 weeks of extinction, although this increase was not statistically significant between the two groups of mice (Fig. 3B – phenotype main effect, F1, 13=1.03, p>0.05).
Figure 3.
Heroin-seeking behavior in WT and D3-KO mice during extinction and (heroin + cue)-induced reinstatement. (A) Active and (B) Inactive lever responding in each phase of the experiment. The data in the gray box were used for statistical analysis of extinction (but see text for more details). *p < 0.05, compared to the last day of extinction.
D3-KO mice displayed higher baseline extracellular DA and lower DA response to heroin
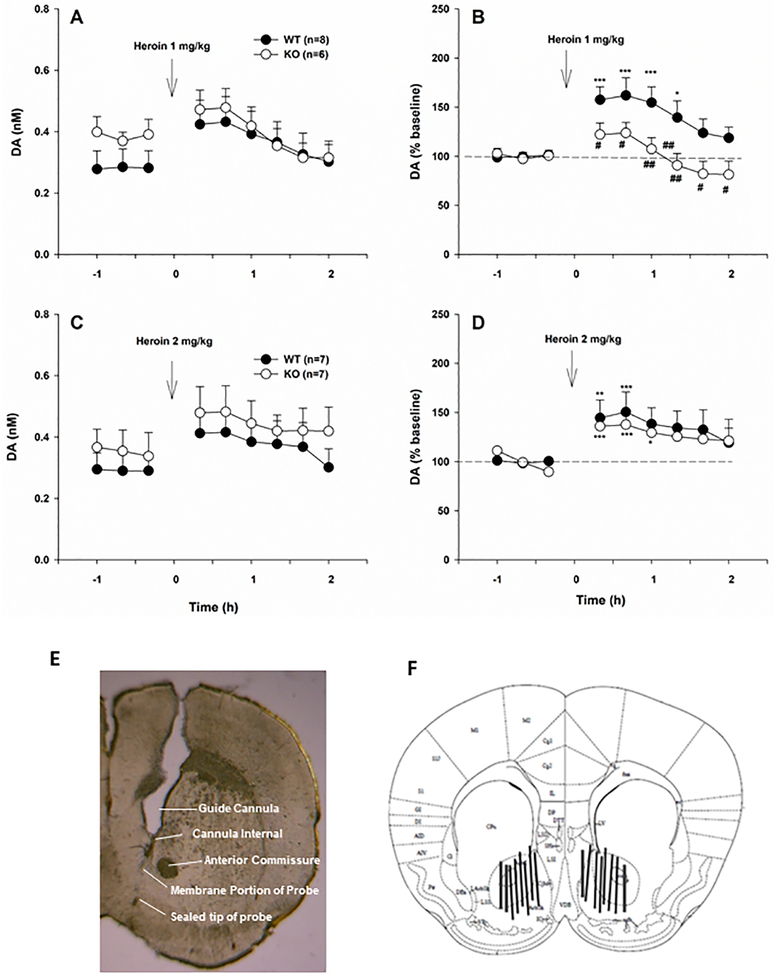
To determine whether altered heroin-seeking and heroin-taking behaviors observed in D3-KO mice correlates with altered DA responses to heroin, we investigated the effects of D3R deletion on baseline and heroin-enhanced extracellular DA levels in the NAc. Figures 4A and 4C show extracellular NAc DA levels (nM) before and after each dose of heroin (1 and 2 mg/kg, s.c.). The averaged baseline level of extracellular DA (over 3 baseline samples in each animal) showed significant differences between the two genotypes of mice (WT, n=15, 0.273 ± 0.033 nM vs. D3-KO, n=13, 0.384 ± 0.030 nM; t=−2.49, p<0.05, two-tailed test). Because of this difference in baseline DA levels, we normalized heroin-induced changes in DA to the percent change over baseline in each animal, to compare the DA-elevating effects of heroin in WT vs. D3-KO mice. We found that 1.0 mg/kg heroin significantly increased extracellular DA in the NAc of WT, but not in D3-KO mice (Fig. 4B; genotype main effect, F1, 12=7.26, p<0.05; time main effect, F8, 96=7.77, p<0.001; interaction, F8, 96=2.64, p<0.05). In contrast, 2.0 mg/kg heroin increased extracellular DA in both WT and D3-KO mice (Fig. 4D; time main effect, F8, 96=7.94, p<0.001), and there was no significant genotypic difference at this dose (genotype main effect, F1, 12=0.204, p>0.05; interaction, F8, 96=0.26, p>0.05). Figures 4E and 4F show the placement of dialysis probes, indicating that dialysis probes (membrane portion) were located in the NAc, including both the shell and core compartments.
Figure 4.
Extracellular DA levels in the NAc before and after heroin injection in WT and D3-KO mice. (A, C) Extracellular DA levels (nM) before and after different doses of heroin, illustrating a higher baseline level of extracellular DA in D3-KO compared to WT mice and an increased DA response to heroin in both phenotypes of mice. (B, D) DA responses to heroin expressed as percent of pre-injection baseline, illustrating blunted DA responses to heroin in D3-KO mice. (E, F) Representative histological image (E) and a diagram (F) illustrating the locations of the microdialysis probes in the NAc. *p <0.05; **p < 0.01; ***p < 0.001 compared with pre-injection baseline. #p < 0.05, ##p<0.01, compared with WT mice.
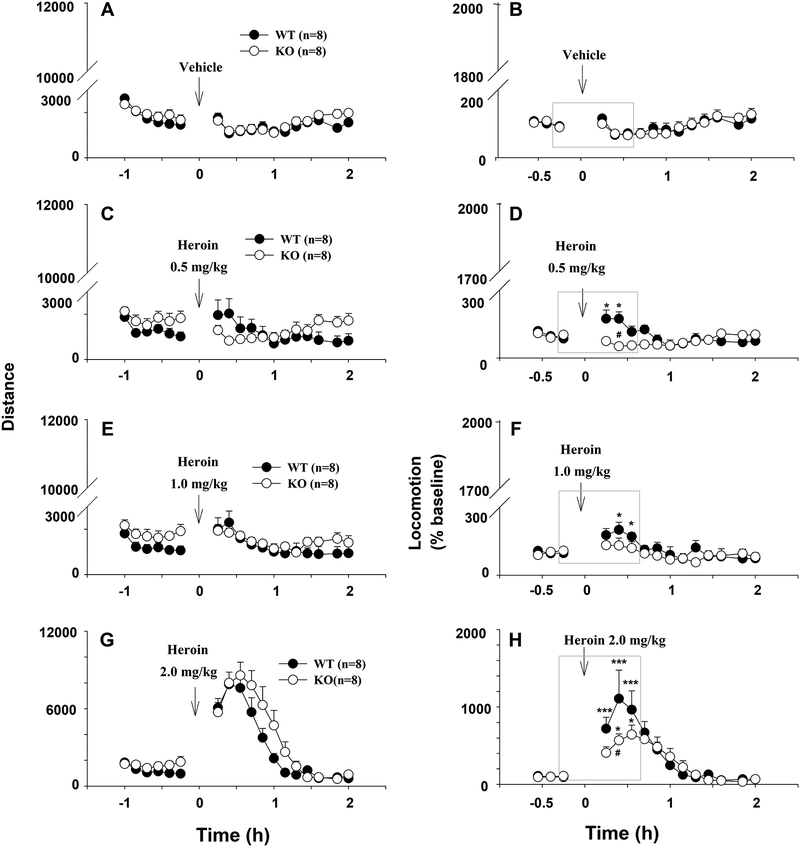
D3-KO mice displayed higher basal locomotion and reduced locomotor response to heroin
Finally, we determined whether the altered DA response to heroin generalizes to heroin’s effects on locomotion. Figure 5 shows that heroin produced dose-dependent increases in distance traveled by WT mice (left panels). In contrast, heroin increased distance traveled by D3-KO mice only at the highest dose tested (2.0 mg/kg); lower doses of heroin (0.5 and 1.0 mg/kg) did not significantly alter locomotor activity in D3-KO mice. In addition, D3-KO mice displayed higher basal locomotion than WT mice (averaged baseline over 30-min before heroin injection, D3-KO, n=8, 1912.6 ± 303.2 vs. WT, n=8, 1060.5 ± 201.9; t=−4.55, p<0.05). Because of this difference, we normalized heroin-enhanced locomotion to baseline (i.e., percent change over baseline). We found significantly lower locomotion responses to heroin in D3-KO mice (Fig. 5, right panels). Since the different locomotor responses were seen mainly within the 30 min after heroin injection, we further analyzed this component of the data separately (highlighted in the gray boxes, Fig. 5, right panels). Two-way ANOVA with repeated measures for time revealed significantly lower locomotor responses to heroin in D3-KO mice after 0.5 mg/kg heroin (Fig. 5D, genotype effect, F1, 14=0.67, p>0.05; time effect, F3, 42=4.22, p<0.05; genotype × time interaction, F3, 42=4.80, p<0.01), 2.0 mg/kg heroin (Fig. 5H, genotype, F1, 14=2.15, p>0.05; time, F3, 42=15.34, p<0.001; genotype × time interaction, F3, 42=2.75, p<0.05), but not after 1.0 mg/kg heroin (Fig. 5F, genotype, F1, 14=0.77, p>0.05; time, F3, 42=3.05, p<0.05; genotype × time interaction, F3, 42=1.07, p>0.05).
Figure 5.
Effects of heroin on locomotor activity in WT and D3-KO mice. (A, C, E, G) Distance travelled (cm) before and after saline or different doses of heroin; (B, D, F, H) Heroin-enhanced locomotion (expressed as percent of pre-injection baseline). The data highlighted in gray boxes (shown in B, D, F, H) were used for statistical analyses (but see text for more details). *p < 0.05; ***p < 0.001, compared with pre-injection baseline. #p < 0.05 compared with WT mice.
Discussion
In the present study, we found that genetic deletion of the D3R significantly escalated heroin-taking and heroin-seeking behavior, suggesting that D3R may play a role in regulatory processes that normally limit heroin intake. Compared to wild-type controls, mice lacking D3Rs took more heroin during the self-administration, showed elevated heroin dose-response functions, and displayed increased motivation to obtain heroin reward under a PR reinforcement schedule and higher heroin-seeking during extinction and reinstatement testing. Converging findings therefore suggest that deletion of D3R increases vulnerability to heroin in multiple behavioral measures related to opioid addiction. These results not only extend our previous report that deletion of D3R increases cocaine-taking and cocaine-seeking behaviors (Song et al, 2012), but also suggest that low D3R expression in the brain may constitute a risk factor for the development of opioid abuse and addiction. We note that the present findings that genetic deletion of D3R increases heroin self-administration appear to conflict with previous reports that acute pharmacological blockade of D3R significantly decreases heroin or oxycodone self-administration in a dose-dependent manner (Boateng et al, 2015; You et al, 2017). One possible explanation is that neuroadaptations may occur in the D3-KO mice, which compensate for the loss of D3R expression during the embryonic stage, resulting in a reduction in D3R function in mediating drug reinforcement (see more discussion below).
The molecular mechanisms underlying increased vulnerability to heroin in D3-KO mice are unknown. Classically, opioid reward is attributed to activation of mu opioid receptors located in GABAergic interneurons or GABAergic afferents in the VTA, which suppress GABAergic neuron activity and consequently disinhibit DA neurons projecting to the NAc (Fields and Margolis, 2015; Shabat-Simon et al, 2008). Prior studies indicate that D3-KO mice do not show alterations in mu opioid receptor expression and function compared to WT controls (Narita et al, 2003), suggesting that a non-opioid receptor mechanism may underlie increased heroin intake following D3R deletion. One possibility is that D3R deletion may augment the salience of heroin or heroin-associated cues, since higher levels of drug-taking and drug-seeking behavior is often interpreted as increased drug craving or salience of drug reward and drug-associated cues (Piazza et al, 2000). This interpretation is supported by the finding that D3-KO mice exhibited a vertical upward shift in heroin dose-response curves compared to WT controls. In addition, increases in PR breakpoint also reflect increased motivation for reward-seeking behavior (Richardson and Roberts 1996, Xi et al, 2005). Furthermore, in the absence of heroin, D3-KO mice exhibited overall higher drug-seeking behaviors during extinction and reinstatement, as assessed by higher active and inactive lever responses (although the increase in extinction responding was not statistically significant between the two genotypes).
How D3R deletion causes increased incentive or motivation for heroin-taking and heroin-seeking remains unknown. D3R is an inhibitory G-protein coupled receptor (Chen et al, 2009; Diaz et al, 2000) and is expressed in multiple phenotypes of neurons, including dopaminergic neurons in the VTA, dopaminergic terminals in the NAc, GABAergic neurons in the striatum, and glutamatergic neurons in the amygdala, hippocampus, and prefrontal cortex (Clarkson et al, 2017; Diaz et al, 2000; Li and Kuzhikandathil, 2012). Thus, deletion of D3 receptors in these neurons may cause a compensatory increase in presynaptic neurotransmitter release and/or neuronal excitability via a disinhibition mechanism, which may, in part, contribute to increased vulnerability to heroin or heroin-associated cues. This interpretation is supported by the finding that D3-KO mice displayed increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors (Xu et al, 1997) and increased genetic responses to cocaine (Kong et al, 2011). In addition, D3R regulates DA transporter (DAT) expression and DA reuptake by directly interacting with DAT or regulating DAT trafficking to and from the plasma membrane (Castro-Hernandez et al, 2015; McGinnis et al, 2016; Zapata et al, 2007). Our previous work indicates that D3-KO mice exhibit increased DAT expression in the ventral striatum and VTA (Song et al, 2012). This increased DAT expression may contribute to elevated vulnerability to drugs of abuse via faster clearance of extracellular DA and/or increased DA response rate to psychostimulants (Briegleb et al, 2004; Grace, 2001; Somkuwar et al, 2013; Zahniser and Sorkin, 2004).
An alternative explanation is that D3R deletion may blunt heroin’s rewarding efficacy, leading to a compensatory increase in heroin intake. This interpretation is supported by our findings that D3-KO mice displayed increased baseline levels of extracellular DA in the NAc and attenuated DA responses to heroin, mirroring observations of altered baseline locomotor activity and locomotor responses to heroin in the present study. These findings are consistent with our previous report that significant increases in baseline extracellular DA in the NAc was observed in D3-KO mice, which caused a decrease in NAc DA responses to cocaine (Song et al, 2012). Increased baseline extracellular DA is most likely due to a loss of presynaptic D3R that normally controls DA release. Increased extracellular DA may also lead to a reduction in DA response to heroin (defined as % change in extracellular DA over baseline) and postsynaptic DA receptor desensitization. Thus, higher heroin intake may be interpreted as a compensatory response to reduced DA responses to heroin or reduced drug reward after D3R deletion (Song et al, 2012;Yokel and Wise, 1975). Since the loss of drug reward may potentiate motivated behavior to take or seek more drug to compensate for the loss of rewarding effects, this interpretation may also explain why D3-KO mice displayed higher levels of heroin-seeking behavior during extinction and reinstatement testing. This interpretation is further supported by the finding that D3-KO mice displayed higher rates of heroin self-administration with shorter inter-infusion intervals similar to that maintained by a lower dose of heroin (Figs. 1F–1G). This heroin reward-blunting hypothesis may also well explain our recent finding that D3R antagonists significantly reduce heroin or oxycodone self-administration (Boutang et al., 2015; You et al, 2017). Paradoxically, findings in conditioned place preference models are mixed – D3R deletion produced a significant increase (Narita et al, 2003) or no significant alteration in morphine-induced place preferences (Frances et al, 2004; Wang et al, 2015). Thus, more studies are required to address this issue.
In addition to increases in heroin intake, D3-KO mice exhibited increased intake of a non-drug reinforcer relative to WT controls, as shown in our previous report that D3R deletion significantly escalated oral sucrose self-administration (Song et al, 2012) and observations in humans that striatal D2/D3R availability is reduced in obese subjects compared to normal-weight controls (van de Giessen et al, 2014). Moreover, in rodents D3R antagonists reduce food intake both in models of obesity as well as lean comparators (Thanos et al, 2008). Given the shared neural substrates of obesity and drug abuse (Volkow et al, 2013), these observations suggest that low D3R availability may represent a risk factor for addictive disorders more broadly.
We note that WT mice, and particularly D3-KO mice, showed consistently high responding on both active and inactive levers, which lasted 2–3 weeks or longer in some subjects during extinction (Song et al., 2012). This is significantly different from the observations in rats in which extinction responding is extinguished usually within 1–2 weeks from the last drug self-administration session (Xi et al., 2007; You et al., 2017). The underlying mechanisms for this extinction resistance in mice are unclear. Such high extinction responses may reflect higher motivation for reward-seeking or deficits in learning to extinguish drug-seeking behavior. In addition, we note that the reinstatement responses to heroin priming and reintroduction of the heroin-associated cues was not statistically significant in WT mice. This may be related to a species difference (between rats and mice) in extinction resistance or heroin responsivity, relatively small group sizes, or development of opioid tolerance after D3R deletion. Together, these findings suggest that D3-KO mice are more sensitive to heroin and thus more susceptible to heroin-associated cues observed during extinction.
In conclusion, findings in the present study indicate that genetic deletion of D3 receptors augments heroin-taking and heroin-seeking behaviors, suggesting that low D3R availability increases vulnerability to heroin use, possibly via both DA and non-DA mechanisms. The D3R may therefore represent a valuable biomarker to identify individuals at elevated risk for developing addictions, providing an opportunity for targeted interventions and treatments to minimize the prevalence of these costly disorders.
Highlights:
Deletion of D3Rs increased heroin self-administration and heroin intake
Deletion of D3Rs increased motivation for heroin during PR self-administration
Deletion of D3Rs increased basal levels of NAc extracellular DA and decreased DA responses to heroin
Deletion of D3Rs increased basal levels of locomotion and decreased locomotor responses to heroin
Reduced D3R availability in the brain may facilitate opioid abuse and addiction
Funding and Disclosure
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse (Z1A DA000389), National Institutes of Health, USA. The authors declare that there is no financial conflict of interest.
References
- Aleph Prieto G (2017). Abnormalities of Dopamine D3 Receptor Signaling in the Diseased Brain. J Cent Nerv Syst Dis 9: 1179573517726335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. (2002). Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32: 435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. (2011). The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63: 182–217. [DOI] [PubMed] [Google Scholar]
- Boateng CA, Bakare OM, Zhan J, Banala AK, Burzynski C, et al. (2015). High Affinity Dopamine D3 Receptor (D3R)-Selective Antagonists Attenuate Heroin Self-Administration in Wild-Type but not D3R Knockout Mice. J Med Chem 58: 6195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Payer D, Rusjan PM, Houle S, Tong J, et al. (2016). Heightened Dopaminergic Response to Amphetamine at the D3 Dopamine Receptor in Methamphetamine Users. Neuropsychopharmacology 41: 2994–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. (2004). Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: contribution of the dopamine transporter. Neuropsychopharmacology 29: 2168–79. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, et al. (2012). Cocaine self-administration in dopamine D(3) receptor knockout mice. Exp Clin Psychopharmacol 20: 352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hernandez J, Afonso-Oramas D, Cruz-Muros I, Salas-Hernandez J, Barroso-Chinea P, et al. (2015). Prolonged treatment with pramipexole promotes physical interaction of striatal dopamine D3 autoreceptors with dopamine transporters to reduce dopamine uptake. Neurobiol Dis 74: 325–35. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2016). 2015 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; Rockville, MD. [Google Scholar]
- Center for Disease Control. (2017). Opioid Overdose. (Sept. 18 2017; https://www.cdc.gov/drugoverdose/index.html).
- Chartoff EH, Connery HS. (2014). It’s MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol 5: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xu M. (2010). Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. J Neurochem 114: 530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Lao CL, Chen JC. (2009). The D(3) dopamine receptor inhibits dopamine release in PC-12/hD3 cells by autoreceptor signaling via PP-2B, CK1, and Cdk-5. J Neurochem 110: 1180–90. [DOI] [PubMed] [Google Scholar]
- Childress AR, O’Brien CP. (2000). Dopamine receptor partial agonists could address the duality of cocaine craving. Trends Pharmacol Sci 21: 6–9. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Kasper ZA. (2017). Increased use of heroin as an initiating opioid of abuse. Addict Behav 74: 63–66. [DOI] [PubMed] [Google Scholar]
- Clarkson RL, Liptak AT, Gee SM, Sohal VS, Bender KJ. (2017). D3 Receptors Regulate Excitability in a Unique Class of Prefrontal Pyramidal Cells. J Neurosci 37: 5846–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai JK, Goyal RK, Parmar NS. (1999). Characterization of dopamine receptor subtypes involved in experimentally induced gastric and duodenal ulcers in rats. J Pharm Pharmacol 51: 187–92. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. (1998). Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 10: 3565–3571. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, et al. (2000). Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20: 8677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duaux E, Gorwood P, Griffon N, Bourdel MC, Sautel F, et al. (1998). Homozygosity at the dopamine D3 receptor gene is associated with opiate dependence. Mol Psychiatry 3: 333–6. [DOI] [PubMed] [Google Scholar]
- Fields HL, Margolis EB. (2015). Understanding opioid reward. Trends Neurosci 38: 217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances H, Le Foll B, Diaz J, Smirnova M, Sokoloff P. (2004). Role of DRD3 in morphine-induced conditioned place preference using drd3-knockout mice. Neuroreport 15: 2245–9. [DOI] [PubMed] [Google Scholar]
- Grace A(2001). Psychostimulant actions on dopamine and limbic system function: relevance to the pathophysiology and treatment of ADHD. pp. 134–157. London, United Kingdom: Oxford University Press. [Google Scholar]
- Heidbreder C (2008). Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets 7: 410–21. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. (2010). Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci 1187: 4–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Lee I, Lee TA, Pickard AS. (2017). The societal cost of heroin use disorder in the United States. PLoS One 12: e0177323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, et al. (2015). The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health 36: 559–74. [DOI] [PubMed] [Google Scholar]
- Kong H, Kuang W, Li S, Xu M. (2011). Activation of dopamine D3 receptors inhibits reward-related learning induced by cocaine. Neuroscience 176: 152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounang N (2017). US heroin deaths jump 533% since 2002, report says.
- Leri F, Stewart J. (2001). Drug-induced reinstatement to heroin and cocaine seeking: a rodent model of relapse in polydrug use. Exp Clin Psycopharmacol 9: 297–306. [DOI] [PubMed] [Google Scholar]
- Li Y, Kuzhikandathil EV. (2012). Molecular characterization of individual D3 dopamine receptor-expressing cells isolated from multiple brain regions of a novel mouse model. Brain Struct Funct 217: 809–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari RN, Hughes A. 2013. Trends in Heroin Use in the United States: 2002 to 2013 In The CBHSQ Report. Rockville, MD. [PubMed] [Google Scholar]
- Matuskey D, Gallezot JD, Pittman B, Williams W, Wanyiri J, et al. (2014). Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug Alcohol Depend 139: 100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MM, Siciliano CA, Jones SR. (2016). Dopamine D3 autoreceptor inhibition enhances cocaine potency at the dopamine transporter. J Neurochem 138: 821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Mizuo K, Mizoguchi H, Sakata M, Narita M, et al. (2003). Molecular evidence for the functional role of dopamine D3 receptor in the morphine-induced rewarding effect and hyperlocomotion. J Neurosci 23: 1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. (2000). Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20: 4226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. (2006). The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30: 215–38. [DOI] [PubMed] [Google Scholar]
- Reed B, Butelman ER, Yuferov V, Randesi M, Kreek MJ. (2014). Genetics of opiate addiction. Curr Psychiatry Rep 16: 504. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Rosen LG, Rushlow WJ, Laviolette SR. (2017). Opiate exposure state controls dopamine D3 receptor and cdk5/calcineurin signaling in the basolateral amygdala during reward and withdrawal aversion memory formation. Prog Neuropsychopharmacol Biol Psychiatry 79: 59–66. [DOI] [PubMed] [Google Scholar]
- Shabat-Simon M, Levy D, Amir A, Rehavi M, Zangen A. (2008). Dissociation between rewarding and psychomotor effects of opiates: differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area. J Neurosci 28: 8406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth BP, Barry J, Keenan E, Ducray K. (2010). Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J 103: 176–9. [PubMed] [Google Scholar]
- Sokoloff P, Le Foll B. (2017). The dopamine D3 receptor, a quarter century later. Eur J Neurosci 45: 2–19. [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Darna M, Kantak KM, Dwoskin LP. (2013). Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: dopamine transporter function and cellular distribution in adulthood. Biochem Pharmacol 86: 309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. (2012). Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci U S A 109: 17675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, et al. (2008). The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav 89: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2017). The U.S. Opioid Epidemic. (Sept. 18 2017; https://www.hhs.gov/opioids/about-the-epidemic/index.html).
- van de Giessen E, Celik F, Schweitzer DH, van den Brink W, Booij J. (2014). Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J Psychopharmacol 28: 866–73. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M. (2015). The Brain on Drugs: From Reward to Addiction. Cell 162: 712–25. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. (2013). Obesity and addiction: neurobiological overlaps. Obes Rev 14: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YP, Wei SG, Zhu YS, Zhao B, Xun X, Lai JH. (2015). Dopamine receptor D1 but not D3 essential for morphine-induced conditioned responses. Genet Mol Res 14: 180–9. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. (2007). Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev 13: 240–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, et al. (1997). Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19: 837–48. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. (1975). Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science 187: 547–9. [DOI] [PubMed] [Google Scholar]
- You ZB, Gao JT, Bi GH, He Y, Boateng C, et al. (2017). The novel dopamine D3 receptor antagonists/partial agonists CAB2–015 and BAK4–54 inhibit oxycodone-taking and oxycodone-seeking behavior in rats. Neuropharmacology 126: 190–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. (2004). Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology 47 Suppl 1: 80–91. [DOI] [PubMed] [Google Scholar]
- Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, et al. (2007). Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem 282: 35842–54. [DOI] [PubMed] [Google Scholar]