Abstract
The increased use of self-luminous displays, especially in the evening prior to bedtime, has been associated with melatonin suppression, delayed sleep and sleep curtailment. The present study set out to investigate whether the Night Shift application provided by Apple Inc. for use on its portable electronic devices is effective for reducing acute melatonin suppression, a well-established marker of circadian phase. Participants experienced four experimental conditions: a dim light control, a high circadian stimulus true positive intervention and two Night Shift interventions delivering low and high correlated colour temperature light from the devices. Melatonin suppression did not significantly differ between the two Night Shift interventions, which indicates that changing the spectral composition of self-luminous displays without changing their brightness settings may be insufficient for preventing impacts on melatonin suppression.
1. Introduction
The human circadian system, as measured by acute melatonin suppression and phase shifting of the dim light melatonin onset (DLMO), a well-established marker of the biological clock, is maximally sensitive to short-wavelength (blue) light. Recent technological developments and the advent of light-emitting diodes (LEDs) have led to larger and brighter devices (e.g. televisions, computer displays, cell phones and tablets) that, in some cases, can emit more short-wavelength light. Exposure to short-wavelength light in the evening and at night carries risks for adverse effects in humans, including acute melatonin suppression, delayed sleep and, with frequently occurring exposures, circadian disruption. Circadian disruption has been associated with poor sleep quality and has been linked to mood disorders, such as depression, and increased risks of chronic diseases such as diabetes, obesity, cardiovascular disease and cancer.1
Night-time exposure to short-wavelength light from electronic devices has been shown to suppress secretion of the hormone melatonin.2–4 Examining the effects of evening exposure to LED-backlit computer displays, Cajochen et al. showed that a five-hour exposure to displays with a high short-wavelength content significantly suppressed melatonin compared to non-LED backlit displays of equal luminance.5 Figueiro et al. demonstrated that a two-hour exposure to cathode ray tube computer displays induced a slight, but not statistically significant reduction in melatonin concentrations in college students.6 Wood et al. showed that melatonin levels were significantly suppressed after a two-hour exposure, but not after a one-hour exposure, to iPads at full brightness.7 A more recent within-subjects study conducted by Chang et al. showed that light received at the cornea of 12 young adults from the nighttime use of e-readers (≅32 lux, four-hour exposure) significantly decreased melatonin secretion and subjective sleepiness while also reducing electroencephalogram (EEG) theta activity compared to reading a conventional printed book in ambient lighting conditions (≅1 lux).8 (An increase in theta activity has been associated with an increase in sleepiness. 9) Moreover, assessment of circadian phase, as measured by melatonin suppression, after five consecutive nights in both conditions revealed a 1.5-hour delay for participants in the e-reader condition. Examining the effects of a shorter duration of exposure, Grønli et al. showed that compared to reading a printed book in ambient light (≅26.7 lux), a half-hour exposure to iPads operating at full brightness (≅58 lux) decreased subjective sleepiness and delayed slow-wave brain activity during sleep by about 30 minutes, as measured by EEG.10
Considered together, these studies suggest that commercial self-luminous electronic devices can adversely affect sleep and circadian physiology. In response, several device manufacturers and third-party software developers have introduced applications that adjust the spectral composition of self-luminous displays to reduce their short-wavelength light emissions in an attempt to avoid decrements to sleep and circadian health. The goal of the present study was to test the effectiveness of one such application, Night Shift, which was released in 2016 by Apple Inc. with the aim of improving sleep quality among users of its self-luminous portable electronic devices. The iPad was selected for the study because 8.9 million of the devices were sold worldwide in the first quarter of 2017 alone, outselling its nearest two competitors combined and accounting for nearly 25% of all tablet sales.11 Moreover, the newly released Night Shift application had never been tested before.
2. Method
2.1. Participant selection
A total of 12 participants (five female) with a median±standard deviation (SD) age of 22.5±3.7 years took part in this two-phase study involving six participants in each phase, the first occurring in June 2016 and the second in June 2017. Using suppression data and variances from similar past studies,3,7 an a priori power calculation (SD=0.15) revealed that 12 subjects were needed to observe a large effect size at 99.9% statistical power to significantly detect 25% melatonin suppression and a power of 93.4% to significantly detect 15% melatonin suppression. All participants were pre-screened for major health problems, such as bipolar disorder, seasonal depression, cardiovascular disease, diabetes and hypertension. Participants were excluded from the experiment if they were taking over-the-counter melatonin or prescription hypertension medications, antidepressants, sleep medicine or beta-blockers. Participants reporting eye diseases such as cataracts and glaucoma were also excluded. All participants were required to maintain a consistent sleep–wake schedule, with bedtimes no later than 23:00 and wake times no later than 07:30, during the week leading up to each night-time study session to maintain their melatonin circadian rhythm. Participants were also required to refrain from caffeine consumption 12 hours prior to the start of each session. All human studies conducted by the research team (Lighting Research Center, Rensselaer Polytechnic Institute) conform to 45 CFR 46 and international ethical standards,12 and are reviewed, approved and monitored by the Rensselaer Institutional Review Board. Informed consent was obtained from all study participants.
2.2. Experimental conditions and apparatus
The participants reported to the laboratory on four nights, each separated by at least one week to allow for a wash-out period between the experimental conditions. Participants in each phase of the study were randomly divided into two equal groups and exposed to four experimental conditions that were scheduled in a pre-decided, counterbalanced order to avoid effects of order, learning or adaptation on the study’s outcome measure. A single condition was experienced by the participants for the duration of each experimental session. For all conditions, participants viewed identically configured iPads for the duration of the experiment, and display brightness levels for all conditions were maintained at their maximum setting for the duration of the exposure. From a fixed distance of 30.5 cm (12 inches) for a white background at full brightness, the iPads in the Night Shift Low CCT and the Night Shift High CCT interventions delivered photopic illuminances at the cornea of 54.3 lux and 97.8 lux, respectively. It should be noted, however, and as reported below, measurements from the Dimesimeter worn by the participants showed that actual average exposure amounts varied depending on what participants were watching during each session, as the screen background was not always white.
Over the course of the study, all participants were exposed to a high circadian stimulus (CS) ‘true positive’ intervention, Blue Light Goggles, which was calibrated to deliver 40 lux of ‘blue’ light (λmax=470 nm) at the cornea. The device used for this intervention was composed of two LEDs mounted above the eyes and directed at the participants’ line of sight through the centre of each eye opening on lensless commercial eyeglasses frames that were modified for this purpose. Polycarbonate translucent tape covered each LED to diffuse the emitted light, minimise glare and avoid blue-light hazard.13,14 Before each experimental session, the goggles were calibrated using an optical fibre with a Lambertian diffuser on one end. The researchers independently measured the irradiances of the left and right LEDs and made any necessary adjustments. The device’s light level (40 lux) was selected to provide a CS of 0.45 that was predicted to be above threshold (CS=0.1) and below saturation (CS=0.7), following the metric employed by the Rea et al. model, 15,16 which assesses the circadian effectiveness of a light source for acute suppression of melatonin after a one-hour exposure.
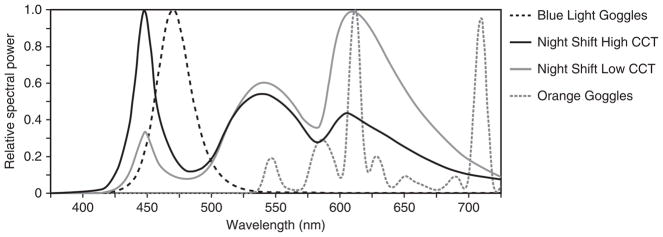
The study also used two spectrally distinct lighting interventions for the iPad (iPad Air 2, IOS 9.3, Apple Inc., Cupertino, CA, USA) that were generated by adjusting the ‘colour temperature’ slide control of the device’s Night Shift application to either extreme of its ‘less-warm’ (5997 K) or ‘more-warm’ (2837 K) range. The correlated colour temperatures (CCTs) of both Night Shift interventions (i.e. Night Shift Low CCT and Night Shift High CCT) were measured using a spectrometer (Model USB650 Red Tide Spectrometer, Ocean Optics, Winter Park, FL, USA). The relative spectral power distributions (SPDs) for the lighting conditions are shown in Figure 1. Finally, all of the participants were exposed to a dim light condition, Orange Goggles, which served as the control for the baseline melatonin suppression calculations. On the control night, participants wore orange-tinted glasses (SAF-T-CURE Orange UV Filter, Chicago, IL, USA) that filtered out radiation <525nm to ensure minimal impact on the circadian system.17 For the Blue Light Goggles and Orange Goggles conditions, the iPad displays were covered with orange-tinted media that similarly filtered out radiation <525nm (Roscolux #21 golden amber, Rosco Laboratories, Stamford, CT) to eliminate any CS from the screens.
Figure 1.
The relative spectral power distributions for the lighting interventions used in this study.
Participants exposed to the Night Shift Low CCT and Night Shift High CCT interventions wore lensless eyeglasses frames fitted with a circadian light meter called a Dimesimeter,18 which measured corneal light exposures by recording the light stimulus at 30-second intervals during the two-hour exposure. The circadian-effective light (CLA) levels were calculated from the resulting photometric data using post-processing algorithms following the Rea et al. model,15,16 and the CLA values were transformed into CS values using a logistic function based on the human circadian system’s response.19 During each intervention, light levels at the cornea were also spot-checked every 30 minutes using the spectrometer. The same devices and protocol were used in both phases (i.e. June 2016 and June 2017) of the study.
2.3. Protocol
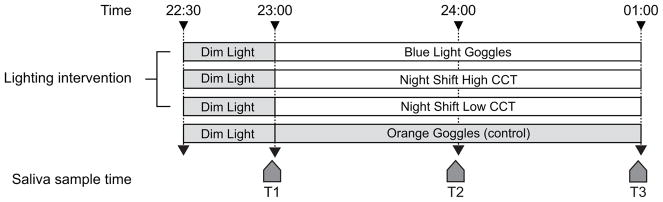
For each night-time experimental session, each participant arrived at the laboratory at 22:30 and remained in dim light (<5 lux at eye level) for 30 minutes, followed by a two-hour exposure to one of the four experimental conditions. The dim light stimulus remained on for the duration of all experimental sessions in all four conditions. Over the course of each 2.5-hour session, three saliva samples were collected from each participant; the first sample was taken immediately before the beginning of the experimental condition after a 0.5-hour dim light exposure and the two remaining samples were taken thereafter at one-hour intervals while experiencing the condition (Figure 2). At 01:00, after the final saliva sample was collected, participants left the laboratory. During exposure to the four experimental conditions, participants were free to choose whatever viewing content (e.g. games, on-line shopping, reading, etc.) they preferred in order to simulate typical use of the devices.
Figure 2.
Protocol used in this study, showing the relationship between experimental conditions and saliva sample times (T1–T3).
Saliva samples were collected using the Salivette system (Sarstedt AG, Nümbrecht, DE), wherein the participant chews on a plain cotton cylinder, which is then placed in a test tube and centrifuged for 10 minutes at 3000g. Each saliva sample was frozen (−20°C) immediately after it was taken, and the samples were assayed in a single batch using melatonin radioimmunoassay kits. The reported sensitivity of the saliva sample assay was 2.9 pg/mL, and the intra- and inter-assay coefficients of variability were 6.9% and 14%, respectively.
2.4. Data analysis
Using the spectral irradiance distributions from the Dimesimeter measurements, the α-opic irradiances were calculated (Table 1) for each experimental condition with the International Commission on Illumination’s (CIE)20,21 SI-compliant version of the Lucas et al. toolbox.22 The α-opic irradiance metric refers to how each of the human photoreceptors responds to light stimulus. The α-opic irradiance, Ee,α, was determined by convolving the spectral irradiance of the light source, Ee,λ (λ), for each wavelength, with the desired action spectrum, sα(λ), where sα(λ) is normalised to one at its peak
Table 1.
Calculations of the five α-opic irradiances for all experimental conditions, following the SI-compliant approach recommended by the CIE.
| Experimental condition | Cyanopic irradiancea (μWcm−2) | Melanopic irradiancea (μWcm−2) | Rhodopic irradiancea (μWcm−2) | Chloropic irradiancea (μWcm−2) | Erythropic irradiancea (μWcm−2) |
|---|---|---|---|---|---|
| iPad with Blue Light Goggles | 21.0 | 35.6 | 30.4 | 18.2 | 10.5 |
| iPad Night Shift High CCT (5997 K) | 6.0 | 7.7 | 9.6 | 11.1 | 11.5 |
| iPad Night Shift Low CCT (2837 K) | 1.3 | 3.8 | 5.9 | 9.3 | 12.1 |
| iPad with Orange Goggles (control) | 0 | 0 | <0.1 | <0.5 | <1.0 |
| (1) |
Given that the α-opic irradiance values provided in Table 1 do not refer to the predicted response by the circadian system (i.e. melatonin suppression), CLA and CS values were calculated using data from the Dimesimeter (Table 2). The spectral irradiance at the cornea was converted into CLA, reflecting the spectral sensitivity of the circadian system, and then, second, transformed into the CS, reflecting the absolute sensitivity of the circadian system. CS is a measure of the effectiveness of the retinal light stimulus for the human circadian system from threshold (CS=0.1) to saturation (CS=0.7).15,16 The following equations were used to determine CLA and CS
Table 2.
Photopic illuminance (lux), circadian light (CLA), circadian stimulus (CS) and melatonin suppression at T2 (after one-hour exposure) and at T3 (after two-hour exposure) for each of the experimental conditions.
| Experimental conditiona | Photopic illuminance (lux) | Circadian light CLAb | Circadian stimulus mean CSb | Mean±SEM melatonin suppression at T2 (%) | Mean±SEM melatonin suppression at T3 (%) |
|---|---|---|---|---|---|
| Blue Light Goggles | 40 | 619 | 0.45 | 32±0.05 | 51±0.04 |
| Night Shift High CCT (5997 K) | 69.6±31.4 | 93.5±29.4 | 0.13±0.04 | 15±0.05 | 19±0.05 |
| Night Shift Low CCT (2837 K) | 71.4±52.9 | 56.9±23.9 | 0.08±0.03 | 8±0.03 | 12±0.03 |
| Orange Goggles (control) | <5 | <1.0 | 0.00 | NA | NA |
Stimulus reported for Blue Light Goggles intervention was calibrated and measured prior to each experimental session. Photopic illuminances reported for the Night Shift interventions are the mean±SEM values recorded by the Dimesimeter over the duration of exposure. The CLA and CS values reported for both Night Shift interventions are mean±SEM. Photopic illuminance for the Orange Goggles condition was measured using an illuminance meter during the experiment.
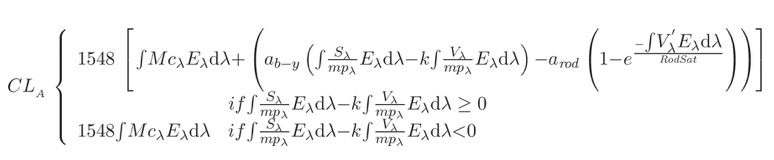 |
(2) |
where:
CLA: circadian light; the constant, 1548, sets the normalisation of CLA so that 2856K blackbody radiation at 1000 lux has a CLA value of 1000
Eλ: light source spectral irradiance distribution
Mcλ: melanopsin (corrected for crystalline lens transmittance) sensitivity
Sλ: S-cone fundamental
mpλ: macular pigment transmittance
Vλ: photopic luminous efficiency function
V′λ: scotopic luminous efficiency function
RodSat: half-saturation constant for bleaching rods=6.5W/m2
k=0.2616
ab-y=0.700
arod=3.300
| (3) |
Melatonin suppression for each lighting intervention was determined by comparing melatonin levels collected during the control night (i.e. Orange Goggles) to those collected at the same saliva sample times (T2 and T3) on the lighting intervention nights. For each night, specifically, the melatonin concentrations at T2 (after a one-hour exposure) and T3 (after a two-hour exposure) were first normalised to T1, and the melatonin suppression at T2 and T3 was then calculated using the following formula
| (4) |
where:
Mn=the normalised melatonin concentration at each saliva sample time during the intervention
Md=the normalised melatonin concentration at each saliva sample time during the control
Statistical analyses were performed using repeated measures analysis of variance (ANOVA) with ‘saliva sample time’ and ‘lighting intervention’ as within-subjects factors and independent variables. Further evaluation for the main effects and interactions was performed using post-hoc, 2-tailed Student’s t-tests. Results were considered to be statistically significant if the p value was <0.05.
3. Results
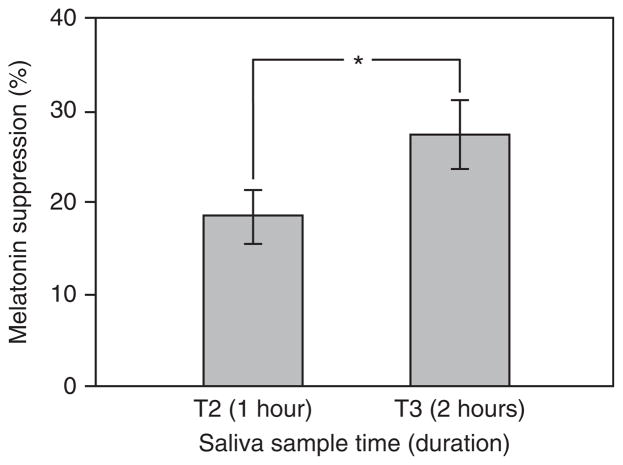
The repeated measures ANOVA revealed a significant main effect of saliva sample time (F1,11=14.83, p <0.05), indicating that longer duration exposures suppress melatonin to a greater degree during participants’ biological night (Figure 3).
Figure 3.
The main effect of saliva sample time on melatonin suppression across all lighting interventions. The error bars represent standard error of the mean; * represents statistical significance (p <0.05).
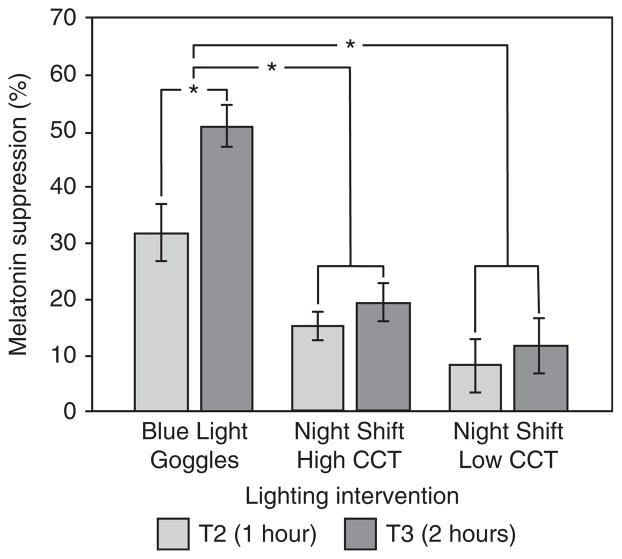
The ANOVA also revealed a significant main effect of lighting intervention (F2,22= 28.07, p <0.05) (Figure 4). Post-hoc analysis, using a paired sample 2-tailed Student’s t-test with a Bonferroni correction, revealed that, over the two-hour exposure, the mean±standard error of the mean (SEM) melatonin suppression from exposure to the Blue Light Goggles intervention (41±4.1%) was significantly greater than that observed for both the Night Shift Low CCT (10±2.7%) (t23=5.77, p <0.05) and Night Shift High CCT (17±4.6%) (t23=8.09, p <0.05) lighting interventions. Mean melatonin suppression after exposure to the Night Shift Low CCT and the Night Shift High CCT interventions was not significantly different (t23=−3.16, p > 0.05).
Figure 4.
The main effect of lighting intervention on melatonin suppression, as well as the significant interaction between lighting intervention and saliva sample time (T2, T3) on melatonin suppression. The error bars represent standard error of the mean; * represents statistical significance (p <0.05).
One-sample t-tests showed that suppression after one-hour and two-hour exposures to all of the lighting interventions was significantly different from zero. The significant interaction between lighting intervention and saliva sample time (F2,22=11.33, p <0.05) (see Figure 4) showed that melatonin suppression was significantly greater after a two-hour exposure than after a one-hour exposure only in the Blue Light Goggles intervention.
For the Blue Light Goggles intervention, suppression values were significantly different from zero at T2 (24:00) (t11=6.20, p <0.05) and at T3 (01:00) (t11=13.42, p <0.05). For the Night Shift High CCT intervention, suppression values were significantly different from zero at T2 (24:00) (t11=3.30, p <0.05) and at T3 (01:00) (t11=3.83, p <0.05). For the Night Shift Low CCT intervention, suppression values were also significantly different from zero at T2 (24:00) (t11=3.14, p <0.05) and at T3 (01:00) (t11=3.50, p <0.05). Table 2 shows the mean±SEM melatonin suppression at T2 and T3.
4. Discussion
The results of the present study indicate that all three lighting interventions significantly suppressed melatonin over two durations of exposure (one-hour and two-hour) during the participants’ biological night. As hypothesised, the greatest suppression was obtained when participants were exposed to the Blue Light Goggles intervention, which was used as the ‘true positive’ intervention. These findings are consistent with previous studies examining the effect of night-time exposures to self-luminous electronic devices on melatonin suppression. One study involving 13 young adult participants showed that the night-time (23:00 to 01:00) use of self-luminous devices (iPads) suppressed melatonin by 7% and 23% following one-hour and two-hour exposures, respectively.7 A follow-up study involving 20 adolescent participants who viewed a variety of self-luminous devices (computers, tablets, e-readers, televisions and/or cell phones) for three hours prior to their normal bedtimes demonstrated that one-hour and two-hour exposures suppressed melatonin by 23% and 38%, respectively.3 Together, these studies suggest an increased sensitivity to light at night among adolescents and highlight the importance of considering the duration of exposure to such devices when specifying technological or operational design recommendations. It should be noted that although the amount of melatonin suppression, especially from the Night Shift Low CCT intervention, was close to threshold, it is not known how this amount of suppression induces circadian disruption, delays sleep or affects health. Larger, more comprehensive epidemiological studies should investigate how the long-term use of these self-luminous displays affects people, especially adolescents and children.
The results of the present study indicate that the Night Shift Low CCT and Night Shift High CCT interventions both provided stimuli that resulted in some suppression of nocturnal melatonin after one-hour and two-hour exposures, but post-hoc analysis revealed that melatonin suppression did not significantly differ between these two spectrally distinct modes. These results therefore suggest that adjusting a self-luminous display’s spectral composition without adjusting its brightness setting may be insufficient for avoiding adverse impacts on melatonin secretion and circadian system function and further emphasise the importance of considering both the spectral and absolute sensitivities of the human circadian system with respect to photic stimulus.
Another interesting observation from the present study is that after the first hour of exposure, melatonin suppression appeared to saturate for both Night Shift interventions but not for the Blue Light Goggles intervention, suggesting that, while the stimulus was effective at inhibiting melatonin secretion initially, it was not strong enough to counter the natural rise in melatonin levels over the course of the participants’ biological night.
Finally, the significantly higher melatonin suppression observed after exposure to the Blue Light Goggles intervention compared to both Night Shift interventions is consistent with the predictions shown in Table 2. Perhaps more importantly, the percentage melatonin suppression observed for both Night Shift interventions after a one-hour exposure closely corresponded to the respective CS values for each intervention provided by the Rea at al. model.15,16 Specifically, the CS value of 0.08 for the Night Shift Low CCT intervention corresponded to the observed 8% melatonin suppression, and the CS value of 0.13 for the Night Shift High CCT intervention was quite close to the observed 15% melatonin suppression (see Table 2). Broadly speaking, the ability to predict light’s effectiveness for influencing acute melatonin suppression could be a very useful tool for researchers, manufacturers, specifiers and lighting designers.
One of the limitations of this study is the degree of freedom granted to the participants in selecting the viewing distance and media content when using their respective iPads. Any potential variability within the individual spectral and absolute characteristics of the stimulus, however, was accounted for by the data collected from the Dimesimeters and cross-checked with spot measurements using a spectrometer every 30minutes. The content viewed by the participants during the interventions was also noted. Furthermore, the study’s within-subjects experimental design ensured that any random effects were minimised.
Another limitation worth noting is that we did not monitor and control for participants’ photic history leading up to the laboratory session. Given that all participants were fulltime employees or students with regular schedules, however, it was assumed that they would receive consistent light exposures during all four weeks of the study. Lastly, one must be very cautious in making far-reaching deductions from the data presented, as the study considered only one model of self-luminous electronic tablet and a single application.
Overall, the results of the present study may be useful for developers, manufacturers and users of self-luminous electronic devices by emphasising considerations other than spectrum. Perhaps limiting the daily duration of exposure from two hours to one hour and reducing the light level by dimming such devices can be more effective in preventing circadian disruption. Lastly, since the use of self-luminous devices in the hours before bedtime is most prevalent in children and adolescents, it is paramount to consider their increased sensitivity to light at night while developing new applications such as Night Shift.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the study was provided by the Lighting Research Center’s Light and Health Alliance (Acuity Brands, CREE, Current by GE, Ketra, OSRAM, Philips and USAI Lighting).
The authors would like to thank Mark S. Rea, Andrew Bierman, Kassandra Gonzalez, Sharon Lesage, Greg Ward, Savanna Wemette and David Pedler for their technical and editorial assistance.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. Journal of Circadian Rhythms. 2010;8:2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood AW, Loughran SP, Stough C. Does evening exposure to mobile phone radiation affect subsequent melatonin production? International Journal of Radiation Biology. 2006;82:69–76. doi: 10.1080/09553000600599775. [DOI] [PubMed] [Google Scholar]
- 3.Figueiro MG, Overington D. Self-luminous devices and melatonin suppression in adolescents. Lighting Research and Technology. 2016;48:966–975. [Google Scholar]
- 4.Arendt J. Melatonin and human rhythms. Chronobiology International. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 5.Cajochen C, Frey S, Anders D, Späti J, Bues M, Pross A, Mager R, Wirz-Justice A, Stefani O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. Journal of Applied Physiology. 2011;110:1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- 6.Figueiro MG, Wood B, Plitnick B, Rea MS. The impact of light from computer monitors on melatonin levels in college students. Neuro Endocrinology Letters. 2011;32:158–163. [PubMed] [Google Scholar]
- 7.Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Applied Ergonomics. 2013;44:237–240. doi: 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strijkstra AM, Beersma DG, Drayer B, Halbesma N, Daan S. Subjective sleepiness correlates negatively with global alpha (8–12 Hz) and positively with central frontal theta (4– 8 Hz) frequencies in the human resting awake electroencephalogram. Neuroscience Letters. 2003;340:17–20. doi: 10.1016/s0304-3940(03)00033-8. [DOI] [PubMed] [Google Scholar]
- 10.Grønli J, Byrkjedal IK, Bjorvatn B, Nødtvedt Ø, Hamre B, Pallesen S. Reading from an iPad or from a book in bed: the impact on human sleep. A randomized controlled crossover trial. Sleep Medicine. 2016;21:86–92. doi: 10.1016/j.sleep.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Nations D. Is the iPad still popular? Retrieved 21 November 2017, from https://www.lifewire.com/is-the-ipad-still-popular-4066926.
- 12.World Medical Association. World Medical Association Declaration of Helsinki. The Journal of the American Medical Association. 2000;284:3043–3045. [PubMed] [Google Scholar]
- 13.Bullough JD. The blue-light hazard: a review. Journal of the Illuminating Engineering Society. 2000;29:6–14. [Google Scholar]
- 14.Bullough JD, Bierman A, Rea MS. Evaluating the blue-light hazard from solid state lighting. International Journal of Occupational Safety and Ergonomics. doi: 10.1080/10803548.2017.1375172. First published 6 October 2017. [DOI] [PubMed] [Google Scholar]
- 15.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Research Reviews. 2005;50:213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Rea MS, Figueiro MG, Bierman A, Hamner R. Modelling the spectral sensitivity of the human circadian system. Lighting Research and Technology. 2012;44:386–396. [Google Scholar]
- 17.Figueiro MG, Brons JA, Plitnick B, Donlan B, Leslie RP, Rea MS. Measuring circadian light and its impact on adolescents. Lighting Research and Technology. 2011;43:201–215. doi: 10.1177/1477153510382853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Lighting Research and Technology. 2013;45:421–434. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeitzer J, Dijk D, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. The Journal of Physiology. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Commission Internationale de l’Éclairage. Report on the First International Workshop on Circadian and Neurophysiological Photometry, 2013. Vienna: CIE; 2015. CIE TN 003:2015. [Google Scholar]
- 21.Commission Internationale de l’Éclairage. Report on the First International Workshop on Circadian and Neurophysiological Photometry; 2013; Vienna: CIE; 2015. [Last accessed on 21 November, 2017]. Open access downloadable Microsoft Excel version of the CIE’s SI-compatible irradiance toolbox. [Google Scholar]
- 22.Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, Price LL, Provencio I, Skene DJ, Brainard GC. Measuring and using light in the melanopsin age. Trends in Neurosciences. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]