Abstract
Background.
Human fetal exposures to polybrominated diphenyl ethers (PBDEs) and their metabolites (OH-PBDEs) are unique from adults, and in combination with a different metabolic profile, may make fetal development more sensitive to adverse health outcomes from these exposures. However, we lack data to characterize human fetal PBDE exposures and the metabolic factors that can influence these exposures.
Objective.
We examined differences between 2nd trimester maternal and fetal exposures to PBDEs and OH-PBDEs. We also characterized fetal cytochrome P450 (CYP) mRNA expression and its associations with PBDE exposures.
Methods.
We collected paired samples of maternal serum and fetal liver (n=86) with a subset having matched placenta (n=50). We measured PBDEs, OH-PBDEs, and mRNA expression of CYP genes (e.g. CYP1A1, −2E1, −2J2, −2C9) in all samples. As a sensitivity analysis, we measured PBDEs and OH-PBDEs in umbilical cord serum from a subset (n=22).
Results.
BDE-47 was detected in ≥ 96% of all tissues. Unadjusted ∑PBDEs concentrations were highest in fetal liver (geometric mean (GM) = 0.72 ng/g); whereas lipid-adjusted concentrations were highest in cord serum (111.12 ng/g lipid). In both cases, fetal concentrations were approximately two times higher than maternal serum levels (GM = 0.33 ng/g or 48.75 ng/g lipid). ΣOH-PBDEs were highest in maternal and cord sera and 20–200 times lower than PBDE concentrations. In regression models, maternal BDE-47 explained more of the model variance of liver than of placenta BDE-47 concentrations (adjusted R2 = 0.79 vs 0.48, respectively). In adjusted logistic regression models, ∑PBDEs were positively associated with expression of CYP2E1 and −2J2 (placenta), and −1A1 (liver) (p < 0.05).
Conclusion.
Our findings suggest that under normal conditions of mid gestation, the human fetus is directly exposed to concentrations of PBDEs that may be higher than previously estimated based on maternal serum and that these exposures are associated with the expression of mRNAs coding for CYP enzymes. These results will help frame and interpret findings from studies that use maternal or cord blood as proxy measures of fetal exposures, and will inform the molecular pathways by which PBDEs affect human health.
Keywords: Brominated flame retardants, fetal metabolism, prenatal exposures, OH-PBDEs, endocrine disrupters, cytochrome P-450 enzymes, placenta transport, organohalogen chemicals
Introduction
Polybrominated diphenyl ethers (PBDEs) are persistent organic pollutants that have been widely used as flame retardants in consumer products since the 1970s. Although use of PBDEs is being phased out, their ongoing presence in durable consumer products, in food, and in indoor dust suggest that humans will continue to be exposed to PBDEs and these exposures will continue to bioaccumulate (Frederiksen et al. 2009; Zota et al. 2013). Consequently, people worldwide will remain exposed to these chemicals for decades (Mitro et al. 2015).
Biomonitoring studies show that greater than 90% of pregnant women are exposed to at least one of the PBDE congeners (Woodruff et al. 2011). Developmental PBDE exposure is associated with cognitive deficits in children (e.g., (Lam et al. 2017)). These studies report that developmental exposure to PBDEs is associated with mental and physical development (Herbstman et al. 2010), Full-Scale Intelligence Quotient, reading skills and externalizing behaviors (Vuong et al. 2017c; Zhang et al. 2017), executive function (Vuong et al. 2017b) and other neurobehaviors (Lam et al. 2017). Some of these reports focus on prenatal exposure by measuring PBDEs in maternal and/or cord blood, while others focus on post-natal exposures by measuring PBDE exposures in children’s blood (e.g., (Vuong et al. 2017a)).
While these studies provide sufficient evidence to support an association of PBDE exposure and cognitive deficits in children, there are important uncertainties. Specifically, it is not clear that PBDE congeners and/or the metabolites that prenatally mediate neurobehavioral effects actually cross the placenta to reach the fetus. Although maternal exposure to PBDEs may affect maternal or placental physiology such that adverse effects are observed in the offspring, it is important to determine whether PBDEs and/or their metabolites could also exert adverse effects by acting directly on the fetus. However, we have limited information about fetal exposures to PBDEs that occur over the course of pregnancy or to target organs of concern. One study demonstrates that PBDEs can be measured in human fetal liver (Schecter et al. 2007b). However, these fetuses were stillborn; thus, their pathology may have contributed to placental passage of PBDEs. Moreover, PBDEs were not measured in maternal serum, thereby making it difficult to determine the relationship between maternal and fetal exposures.
There are also existing data gaps on fetal metabolism of PBDEs, which is critical to evaluating developmental PBDE toxicity since PBDE metabolites may be more biologically active than parent PBDEs within some domains (e.g., (Stapleton et al. 2011)). Cytochrome (CYP) p450 enzymes metabolize both xenobiotic and endogenous substances and may be critical to PBDE-mediated toxicity. In vitro, CYPs such as CYP2B6, metabolize PBDEs to form hydroxylated metabolites (OH-PBDEs) (Erratico et al. 2012). Additionally, PBDEs may alter CYP expression in adult human liver cells (Stapleton et al. 2009). However, much of the data on CYPs is based on studies of adult human cells and rodent studies, and CYP levels and activity will not be the same during human fetal development due to the age-dependent expression of CYPs in the fetal liver and unique contributions of placental CYP expression (Hakkola et al. 1998). There is evidence in rats that perinatal exposures to BDE-47 or −99 can increase expression of CYP enzymes in the fetal and postnatal liver (Blanco et al. 2012; Suvorov and Takser 2010), but we do not have similar data in humans.
Therefore, the goal of this study was to evaluate maternal/fetal transfer of PBDEs and their metabolites during the second trimester of pregnancy, a period of rapid biologic development that can result in increased sensitivity to environmental stressors. To accomplish this, we measured several PBDEs and their metabolites in normal fetal liver, placenta, and maternal and cord sera. In addition, we measured the expression of several mRNAs coding for CYP enzymes to identify those that may be related to PBDE exposure and metabolism. We focus on mRNAs coding for CYP enzymes, rather than CYP enzyme activity, because environmental chemicals can induce the transcriptional activity of genes coding for these enzymes; therefore, mRNA levels are more directly related to induction by environmental factors.
Materials and Methods
Study Population.
In 2011–2012 and 2014, we recruited and consented English-and Spanish-speaking patients between 15 and 24 weeks of pregnancy seeking medical care from the University of California, San Francisco (UCSF) Women’s Options Center at San Francisco General Hospital in San Francisco, California. The Women’s Options Center is an outpatient clinic providing pregnancy terminations and serving an ethnically diverse and predominantly lower income population from the San Francisco Bay Area and other parts of California. Eligible study participants were identified by reviewing the patient’s medical record only after they had 1) consulted with a trained counselor for an elective second trimester termination procedure and 2) consented to the procedure as documentation of intent to proceed with the elective pregnancy termination. The 2011–2012 cohort (Cohort 1) consists of 36 smoking and non-smoking women and the 2014 cohort (Cohort 2) consists of 50 former or non-smoking women. For both cohorts, we excluded participants if they were seeking a termination because of fetal anomalies. All study protocols were approved by the UCSF Committee on Human Research.
Sample Collection and Preparation.
Maternal blood and fetal liver samples were collected in both study populations. Placenta was collected in Cohort 2 (n=50), and as a sensitivity analysis, cord blood was collected in a subset of Cohort 1 (n=22). Maternal blood was collected from each participant prior to medical procedures in red top Vacutainer tubes. The time between blood collection and prior food or fluid consumption ranged from 0.5–23 hours (mean = 13 hours; n=65). Women’s Options Center medical staff collected umbilical cord blood with assistance from our study team. Umbilical cord blood was drained directly into red top collection tubes during the procedure to avoid contact with medical devices and the environment. However, minimal contamination from maternal blood on the umbilical cord could not be precluded. After collection, both the maternal and umbilical cord blood samples were centrifuged at 3000 RPM for 10 min at 4 °C. Serum was aliquoted using glass pipettes into sterilized amber vials, which were pre-screened for environmental contamination, and stored at −80°C until analysis. At the end of the dilation and evacuation procedure, research assistants collected samples of placenta and fetal liver for chemical and RNA expression analyses using dissecting forceps. The samples collected for chemical analyses were washed in ice-cold phosphate-buffer solution (PBS) and then stored in pre-screened amber jars at −80°C. In Cohort 1, tissues collected for RNA expression analyses were washed in cold PBS, soaked and incubated in RNAlater solution in RNAlater Tissue Protector Tubes (2–8°C, overnight), and stored at −80°C. Tissues for RNA analyses in Cohort 2 were washed in cold PBS, flash frozen, and stored at −80°C.
Environmental Chemical Analysis.
We analyzed 19 PBDE congeners and eight OH-PBDEs at the Department of Toxic Substances Control (DTSC) (Berkeley, CA, USA) within its clean laboratory facility, where human specimens are exclusively processed. In this study, we focus our analysis on the congeners detected in >50% in maternal serum (BDE-28, −47, −99, −100, −153) and the four most commonly detected OH-PBDEs (5-OHBDE-47, 6-OHBDE-47, 5-OHBDE-99, 4-OHBDE-49) in maternal serum, fetal liver, and placenta. We also present preliminary data on a smaller subset of cord blood samples.
Liquid-liquid extraction and phase separation were used to separate the phenolic compounds from neutral compounds and are described in detail elsewhere (Zota et al. 2011). Initial sample preparation steps differed depending on the matrix. Briefly, placenta was lyophilized and homogenized with sterile scalpels and a 10 minute sonication was added during the protein denaturalization and liquid-liquid extraction step to maximize extraction efficiency. Fetal liver, once homogenized with a scalpel, behaved similarly to serum and was extracted in the same manner. After liquid-liquid extraction, placenta and liver extracts were measured for lipid content. Re-suspension and phase separation of phenolic compounds followed.
PBDEs and OH-PBDEs were analyzed in separate sample aliquots. Samples for OH-PBDEs analysis were concentrated to 50 µL to improve sensitivity. PBDE quantitation was accomplished by isotope dilution. Each set of isomers (i.e., tri-, tetra-, penta-, etc.) had a labeled surrogate. A complete analyte list with labeled surrogates is available in the Supplemental Materials, Table S1. OH-PBDE aliquots were spiked with labeled 6-OH-BDE47 prior to derivatization as a marker to facilitate reaction yield estimation and recovery correction. PBDEs were measured using gas chromatography/high resolution double-focusing sector mass spectrometry (GC-HRMS, DFS, ThermoFisher, Bremen, Germany). OH-PBDEs were methylated with diazomethane and quantitated using GC-NCI/MS (Varian 1200 GC/MS operated in negative chemical ionization, Walnut Creek, CA, USA) for Cohort 1, and GC-HRMS for Cohort 2. To quantify PBDEs, we used a DB-5MS column (30 m x 0.25 mm I.D. x 0.25 µm film thickness, J & W Scientific, Folsom, CA) for Cohort 1, and a similar but shorter column for Cohort 2.
The instrument detection threshold (IDT) was defined by the peak height/area. The method detection limit (MDL) was calculated as three times the standard deviation (SD) of the blank concentrations (MDLs by cohort and matrix are provided in Supplemental Materials, Table S2). Precision and accuracy of PBDEs from reference material (SRM 1589a, National Institute of Standards and Technology) and in-house control samples were within reasonable analytical error ranges. Quality control data for Cohort 1 serum are described elsewhere (Zota et al. 2013). Quality control data for all other biological specimens are presented in Supplemental Materials (Table S3). Chemical concentrations in both cohorts were corrected by surrogate recoveries.
Lipid Analysis.
Lipid analysis for maternal and cord serum was completed at Boston Children’s Hospital. Levels were estimated from measurements of total cholesterol and triglycerides (Phillips et al., 1989). Lipid content of placenta and liver samples was determined gravimetrically. After liquid-liquid extraction of the tissue sample, extracts were allowed to dry in pre-weighted aluminum dishes. After solvent evaporation the lipid content was determined by the difference. The distribution of lipid measurements by specimen type is presented in Supplemental Materials (Figure S1).
Cytochrome P450 mRNA expression.
We quantified expression of eight mRNAs coding for specific CYP enzymes: CYP1A1, −1A2, −2E1, −2C9, −2J2, −2C19, −3A4, and −2B6. These CYPs were selected a priori since they may be involved in PBDE metabolism and/or response (Dunnick et al. 2012; Erratico et al. 2011; Erratico et al. 2012). Frozen fetal liver (30–50mg) or placenta (200–400mg) tissue was homogenized in RLT Buffer (Qiagen) and RNA was isolated using the RNeasy Plus Mini Kit (Qiagen) following the manufacturer’s instructions. We quantified RNA concentrations by a NanoDrop spectrometer (Thermo Scientific). All RNA samples used in this study had an absorbance (260/280nm) ratio of 1.9–2.1. RNA was converted to cDNA (iScript cDNA Synthesis Kit, Bio-Rad Laboratories). Relative abundance of specific mRNAs was determined by qRT-PCR using gene-specific Taqman Gene Expression Assays (Supplemental Materials, Table S3) with Taqman Universal Master Mix II, no UNG (Life Technologies). For each reaction, 10ng cDNA was used. For each individual Taqman probe, all liver or placental samples were run in parallel to eliminate potential batch (i.e. plate) effects. Cycle thresholds (CT) were determined for each probe in triplicate. Technical replicates were averaged and the geometric mean (GM) of housekeeping genes (GAPDH and ACTB) for each sample was subtracted to calculate the ΔCT (Livak and Schmittgen 2001). All eight CYPs were examined in all liver samples (n = 84), and a subset of placenta samples (n = 4). CYPs with greater than 50% detection frequency (limit of detection (LOD) <40 CT) in the four placenta samples (CYP1A1, −2C9, −2E1, −2J2) were evaluated in all placenta samples (n = 47) (Supplemental Material, Table S4). Three fetal liver samples were removed from the analysis due to the relatively low expression of housekeeping gene expression (CT > mean + 2 SD). Since fetal liver and placenta samples were assayed at different times, CT values are not directly comparable between the two matrices.
Statistical Methods.
We imputed undetected values for each PBDE and OH-PBDE congener based on a log-normal distribution whose parameters were calculated through maximum likelihood estimation assuming that the undetected values fell between zero and (Zota et al. 2011). We calculated the GM and geometric standard deviation for all chemicals with greater than 50% detection frequency in each tissue. To compare PBDE concentrations in maternal and cord sera to those in liver and placenta, we converted serum concentrations into ng/g units assuming a density of 1.03 g/mL (Trudnowski and Rico 1974). These statistics were expressed as ng/g concentrations for OH-PBDEs and as ng/g and lipid-adjusted concentrations for PBDEs, since PBDEs can distribute to the lipid fraction of a tissue. Descriptive statistics for PBDEs and OH-PBDEs in maternal and cord sera are presented in ng/ml units in supplemental material (Table S5). Chemical analytes were log-transformed prior to regression analysis to account for their non-normal distributions. To compare levels between tissues, we calculated chemical ratios by dividing the chemical levels in one tissue by the levels in a second tissue (i.e., cord serum/fetal liver, cord serum/maternal serum, fetal liver/maternal serum, and placenta/fetal liver). We used Spearman correlations to test the correlation between chemical concentrations in different tissues. Only participants with detected chemical levels in both tissues were included in the ratio calculations and correlations.
To examine variation in potential metabolism of PBDEs across participants and matrix, we calculated the percent of metabolites in each tissue as a proportion of their potential precursor using the following equation: (∑OH-PBDEs / (∑OH-PBDEs + ∑PBDEs)), where ∑PBDEs is equal to the sum of BDE-28, −47, −99, −100, and −153. We also compared the composition of ∑OH-PBDEs by matrix since concentrations of some metabolites may be influenced by dietary sources while concentrations of other metabolites may reflect biotransformation of PBDEs (Wiseman et al. 2011).
We used multivariate regression models to identify determinants of liver and placenta concentrations of BDE-47, the most abundant PBDE congener. In order to choose a parsimonious model, we generated all possible subsets of a model including serum BDE-47, serum lipids, parity (0, ≥1), race/ethnicity (Black, White, Latina, other), maternal age, systolic and diastolic blood pressure, cohort, body mass index, smoking status (recent, former, never), fetal sex, and gestational age. We used Mallows’ Cp statistic and R2 to evaluate the models, choosing the model that maximized both statistics while minimizing the number of covariates. Outcomes were modeled as wet weight and lipid-adjusted concentrations. In a subset of models, serum lipids was added into the final model since this approach may reduce measurement error (O’Brien et al. 2016).
We used logistic regression models to examine associations between PBDE exposures and categorical CYP expression and to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Before using the CYP values in regression models, we multiplied the ΔCT values by negative 1 (calculating −ΔCT) so that smaller numbers represented lower ΔCT values. We also created a categorical CYP expression variable by dividing the −ΔCT values at the median or the MDL if the detection frequency was <50%. Cohort-specific medians were used due to slight differences between the −ΔCT values measured in each cohort. Exposure was modeled as BDE-47 or a summary measure of PBDEs (∑PBDEs) for each tissue. Associations were similar between the two exposures so we only present the results for ∑PBDEs. The following covariates were included a priori in our adjusted regression models based on biological or analytical considerations: lipids, gestational age, fetal sex, smoking, and cohort (fetal liver models only). As a sensitivity analysis, we ran all of our final logistic regression models restricting the analysis to never smokers (n=37 for placenta models and n=56 for liver models). All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC). A p-value < 0.05 was considered significant and p < 0.10 marginally significant on two-sided tests.
Results
The study population consisted predominately of young, low-income, U.S.-born, nonwhite women in their second trimester of pregnancy. Approximately one third of the population identified as Latina and another 25% identified as Black. Eighty-five percent used public insurance. The two study populations were demographically similar except for smoking status (fewer recent or former smokers in Cohort 2) and insurance type (more people using private insurance in Cohort 1) (Table 1). A previously published comparison of maternal serum levels of PBDEs and OH-PBDEs found no significant differences in BDE-47, BDE-99, or OHPBDEs between the two cohorts and significantly lower levels of BDE-100 and BDE-153 in Cohort 2 (2014) compared to Cohort 1 (2011–2012) (Parry et al. 2017).
Table 1.
Demographic characteristics of the study population
| Characteristic | Both populations | Cohort 1 (n=35) | Cohort 2 (n=50) | Cohort 1 vs. 2 |
|---|---|---|---|---|
| Age (years) | 0.85 | |||
| 18–22 | 41 (48.2%) | 18 (51.4%) | 23 (46.0%) | |
| 23–30 | 30 (35.3%) | 12 (34.3%) | 18 (36.0%) | |
| 31–42 | 14 (16.5%) | 5 (14.3%) | 9 (18.0%) | |
| Ethnicitya | 0.27 | |||
| Latina | 27 (32.1%) | 7 (20.6%) | 20 (40.0%) | |
| Black | 22 (26.2%) | 10 (29.4%) | 12 (24.0%) | |
| White | 23 (27.4%) | 12 (35.3%) | 11 (22.0%) | |
| Other | 12 (14.3%) | 5 (14.7%) | 7 (14.0%) | |
| Country of Originb | 0.13 | |||
| US | 71 (89.9%) | 33 (97.1%) | 38 (84.4%) | |
| Other | 8 (10.1%) | 1 (2.9%) | 7 (15.6%) | |
| Education | 0.49 | |||
| < HS | 12 (14.1%) | 7 (20.0%) | 5 (10.0%) | |
| HS graduate | 30 (35.3%) | 13 (37.1%) | 17 (34.0%) | |
| Some college | 35 (41.2%) | 13 (37.1%) | 22 (44.0%) | |
| College grad + | 8 (9.4%) | 2 (5.7%) | 6 (12.0%) | |
| BMIc | 0.92 | |||
| < 25 | 37 (44.1%) | 15 (44.1%) | 22 (44.0%) | |
| 25 – 29.9 | 24 (28.6%) | 9 (26.5%) | 15 (30.0%) | |
| ≥ 30 | 23 (27.4%) | 10 (29.4%) | 13 (26.0%) | |
| Smoking | 0.0011 | |||
| Recent | 20 (23.5%) | 15 (42.9%) | 5 (10.0%) | |
| Former | 6 (7.1%) | 1 (2.9%) | 5 (10.0%) | |
| Never | 59 (69.4%) | 19 (54.3%) | 40 (80.0%) | |
| Parity | 0.53 | |||
| 0 | 32 (37.7%) | 11 (31.4%) | 21 (42.0%) | |
| 1 | 31 (36.5%) | 15 (42.9%) | 16 (32.0%) | |
| >1 | 22 (25.9%) | 9 (25.7%) | 13 (26.0%) | |
| Gestational age | 0.36 | |||
| < 19 weeks | 25 (29.4 %) | 13 (37.1%) | 12 (24.0%) | |
| 19 – 21 weeks | 22 (25.9%) | 7 (20.0%) | 15 (30.0%) | |
| > 21 weeks | 38 (44.7%) | 15 (42.9%) | 23 (46.0%) | |
| Fetal Sex | ||||
| Female | 32 (37.7%) | 7 (20.0%) | 25 (50.0%) | 0.005 |
| Male | 53 (62.4%) | 28 (80.0%) | 25 (50.0%) | |
| Insurance3 | 0.0042 | |||
| Medi-Cal | 68 (85.0%) | 27 (79.4%) | 41 (89.1%) | |
| Private/HMO | 6 (7.5%) | 6 (17.7%) | 0 (0%) | |
| Self-Pay | 6 (7.5%) | 1 (2.9%) | 5 (10.9%) |
Missing:1
Missing:6
Missing:5
P-values are from chi-square and Fisher’s exact tests
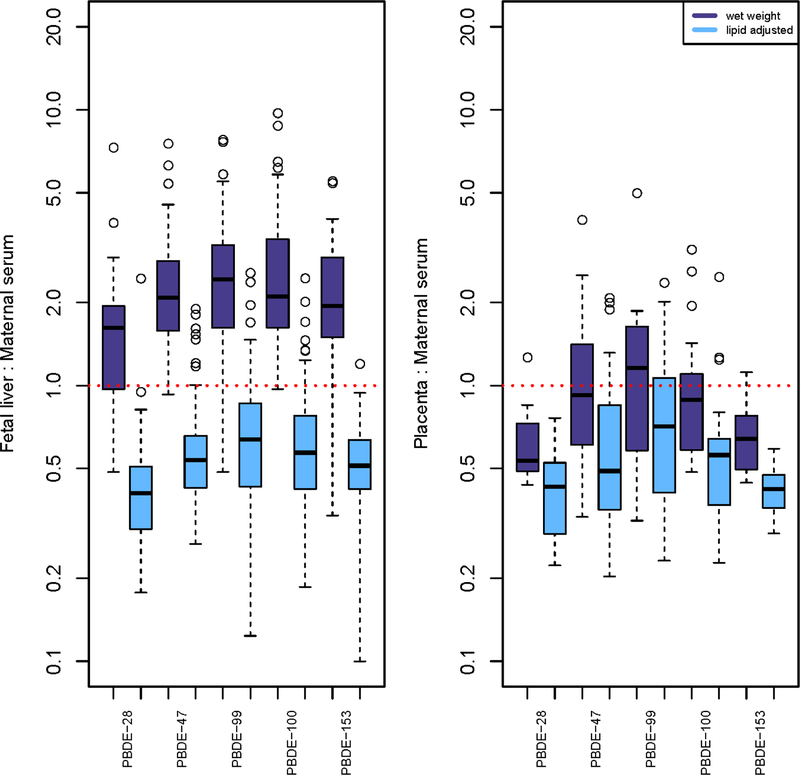
PBDE congeners were widely detected in all four matrices and BDE-47 was consistently the most abundant congener (Table 2). Liver ΣPBDEs concentrations (GM = 0.72 ng/g) were approximately two times higher than maternal serum concentrations in wet weight (GM =0.33 ng/g), while cord serum concentrations (GM = 111.12 ng/g lipid) were approximately two times higher than maternal serum concentrations (48.75 ng/g lipid) in lipid-adjusted weight. Chemical ratios between matrices varied by lipid adjustment (Figure 1). For example, the median wet weight fetal liver:maternal serum ratio for BDE-47 and −153 was 2.0 whereas the lipid-adjusted median ratio was 0.5. We also examined associations between chemical and lipid concentrations by tissue. Tissue-specific PBDE and lipid concentrations were associated only in liver (Supplemental Materials, Table S6).
Table 2.
Descriptive statistics for PBDEs and OH-PBDEs in biological matrices
| GM (GSD)a | GM (GSD)a | |||||||
|---|---|---|---|---|---|---|---|---|
| PBDE | n | % >MDLb | ng/g | ng/g lipid | OH-PBDE | n | % >MDLb | ng/g |
| Maternal Serum (Cohorts 1 and 2) | ||||||||
| PBDE-28 | 85 | 78.8% | 0.015 (2.17) | 2.22 (2.17) | 5-OHBDE-47 | 85 | 55.3% | 0.0035 (3.78) |
| PBDE-47 | 85 | 100% | 0.17 (1.97) | 25.24 (2.02) | 6-OHBDE-47 | 85 | 63.5% | 0.0043 (3.21) |
| PBDE-99 | 85 | 61.2% | 0.035 (2.66) | 5.18 (2.70) | 5-OHBDE-99 | 85 | 30.6% | -- |
| PBDE-100 | 85 | 78.8% | 0.022 (2.74) | 3.34 (2.80) | 4-OHBDE-49 | 85 | 12.9% | -- |
| PBDE-153 | 85 | 77.7% | 0.049 (2.96) | 7.22 (3.04) | ∑OH-PBDEs | 85 | - | 0.015 (2.76) |
| ∑PBDEs | 85 | - | 0.33 (1.91) | 48.75 (1.96) | -- | -- | -- | -- |
| Lipids (mg/ml)c | 85 | - | 6.61 (1.09) | -- | -- | -- | -- | -- |
| Fetal Liver (Cohorts 1 and 2) | ||||||||
| PBDE-28 | 85 | 85.9% | 0.023 (2.38) | 0.88 (2.30) | 5-OHBDE-47 | 80 | 35.0% | -- |
| PBDE-47 | 85 | 100% | 0.36 (2.14) | 14.09 (2.08) | 6-OHBDE-47 | 80 | 23.8% | -- |
| PBDE-99 | 85 | 90.6% | 0.099 (2.56) | 3.88 (2.50) | 5-OHBDE-99 | 80 | 23.8% | -- |
| PBDE-100 | 85 | 94.1% | 0.064 (2.69) | 2.52 (2.58) | 4-OHBDE-49 | 80 | 1.3% | -- |
| PBDE-153 | 85 | 83.5% | 0.095 (3.46) | 3.72 (3.30) | ∑OH-PBDEs | 80 | - | 0.0042 (2.16) |
| ∑PBDEs | 85 | -- | 0.72 (2.03) | 28.34 (1.95) | -- | -- | -- | -- |
| Lipids (mg/g)c | 85 | -- | 25.85 (4.19) | -- | -- | -- | -- | -- |
| Placenta (Cohort 2 only) | ||||||||
| PBDE-28 | 50 | 28.0% | -- | -- | 5-OHBDE-47 | 48 | 39.6% | -- |
| PBDE-47 | 50 | 96.0% | 0.15 (1.96) | 13.21 (2.04) | 6-OHBDE-47 | 48 | 54.2% | 0.0014 (2.80) |
| PBDE-99 | 50 | 60.0% | 0.028 (2.97) | 2.51 (3.13) | 5-OHBDE-99 | 48 | 4.2% | -- |
| PBDE-100 | 50 | 46.0% | -- | -- | 4-OHBDE-49 | 48 | 20.8% | -- |
| PBDE-153 | 50 | 30.0% | -- | -- | ∑OH-PBDEs | 48 | - | 0.0038 (2.45) |
| ∑PBDEs | 50 | - | 0.23 (1.96) | 20.44 (2.05) | -- | -- | -- | -- |
| Lipids (mg/g)c | 50 | - | 11.42 (1.83) | -- | -- | -- | -- | -- |
| Cord Serum (Cohort 1 only)d | ||||||||
| PBDE-28 | 22 | 68.2% | 0.015 (2.42) | 7.97 (1.71) | 5-OHBDE-47 | 22 | 50.0% | 0.0065 (3.27) |
| PBDE-47 | 22 | 100% | 0.22 (1.75) | 67.08 (1.92) | 6-OHBDE-47 | 22 | 27.3% | -- |
| PBDE-99 | 22 | 36.4% | -- | -- | 5-OHBDE-99 | 22 | 4.6% | -- |
| PBDE-100 | 22 | 50.0% | 0.0097 (3.61) | 7.92 (2.59) | 4-OHBDE-49 | 22 | 18.2% | -- |
| PBDE-153 | 22 | 36.4% | -- | -- | ∑OH-PBDEs | 22 | -- | 0.016 (2.51) |
| ∑PBDEs | 22 | -- | 0.31 (1.72) | 111.12 (19.91) | -- | -- | -- | -- |
| Lipids (mg/ml)c | 13 | -- | 2.82 (0.98) | -- | -- | -- | -- | -- |
Notes: PBDEs, polybrominated diphenyl ethers; OH-PBDEs, hydroxylated PBDE metabolites; MDL, method detection limit; GM, geometric mean; GSD, geometric standard deviation; ∑PBDEs, sum of BDE-28, −47, −99, −100, −153; ∑OH-PBDEs, sum of 5-OHBDE-47, 6-OHBDE-47, 5O-HBDE-99, 4-OHBDE-49
GM and GSD only calculated for chemicals with detection frequencies ≥ 50%.
Calculated with cohort-specific MDLs. (MDLs by biological matrix and cohort are provided in Supplemental Materials, Table S1.)
Lipid concentrations are normally distributed, with units mg/mL (cord serum, maternal serum) or mg/g (placenta, liver). Summary statistics show mean (standard deviation).
Data missing for cord lipids (n=9) due to low sample volume; GM (GSD) for lipid-adjusted cord PBDEs (ng/g lipid) is calculated from 13 samples.
Figure 1.
Boxplots of the ratios of PBDE levels between matrices, with and without lipid adjustment. Only values above the method detection limit (MDL) in both matrices were included in the ratios. The red dotted line indicates a ratio equal to 1.
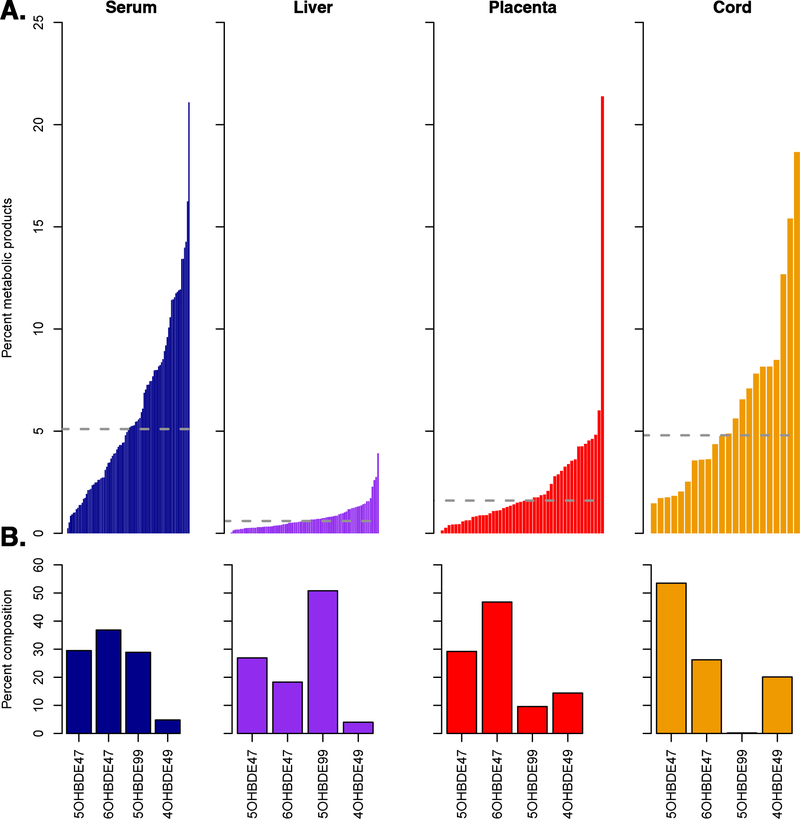
OH-PBDE concentrations were infrequently detected and median ΣOHPBDEs were 20–200 times lower than median ΣPBDEs concentrations. ΣOH-PBDEs concentrations were highest in maternal and cord sera (GM = 0.015–0.016 ng/g) and lowest in the placenta (GM = 0.0038 ng/g) (Table 2). The percent of ΣOH-PBDEs as a proportion of BDE-47 was higher in maternal and cord sera (median: 5–10%) than in placenta or liver (median: 1–5%) and varied considerably across participants. The composition of ΣOH-PBDEs also varied by matrix; 6-OH-BDE47 was the dominant metabolite in maternal serum and placenta while 5-OH-BDE47 and 5-OH-BDE99 were the dominant metabolites in cord seri, and liver, respectively (Figure 2).
Figure 2.
A) Percent of metabolic products in each participant by tissue matrix, calculated using the following equation: (∑OH-PBDEtissue / (∑OH-PBDEtissue+ ∑PBDEtissue )). The grey dotted line indicates the median percent of metabolic products by tissue. B) Percent composition of ∑OH-PBDEs by matrix.
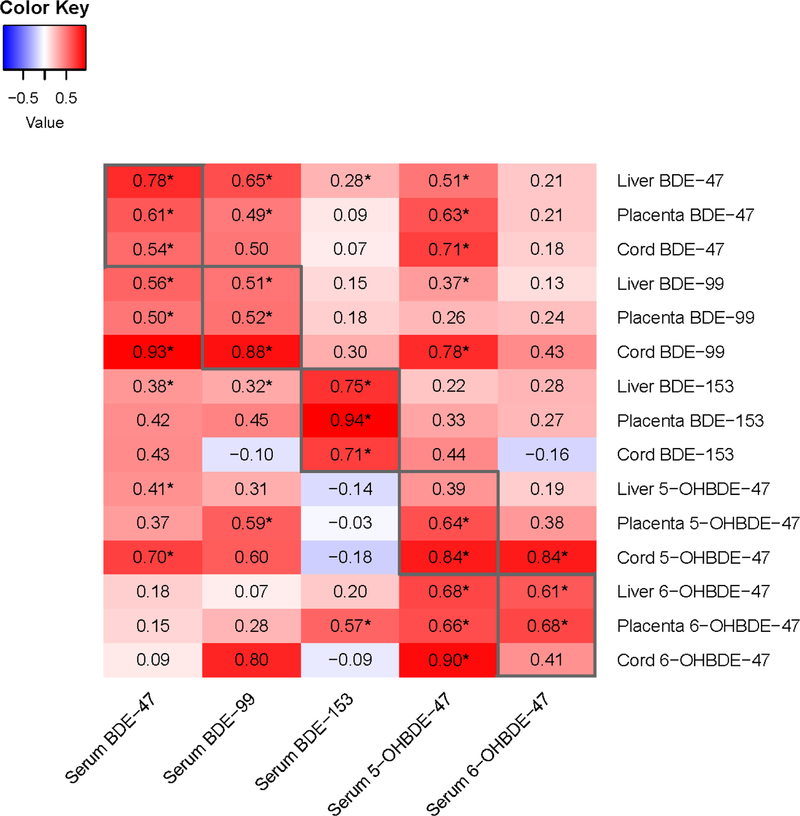
Maternal serum concentrations of each PBDE congener were correlated with concentrations of the same congener in other matrices. Correlations across matrices for BDE-47 ranged from 0.54 to 0.78, while correlations across matrices were slightly higher for BDE-153 and lower for the OH-PBDEs (Figure 3). In multivariate regression models, maternal BDE-47 concentrations explained more of the model variance for liver than for placenta BDE-47 concentrations (adjusted R2 = 0.79 vs 0.48, respectively; Table 3). Associations between maternal and fetal BDE-47 concentrations did not change meaningfully when exposure and/or outcome were lipid-adjusted (Table 3).
Figure 3.
Correlations between individual PBDE and OH-PBDE analytes (wet weight; ng/ml) in maternal serum, fetal liver, placenta, and cord serum. Correlations were calculated using only values above the method detection limit in both matrices. Asterisks indicate p < 0.05.
Table 3.
Associations between maternal serum BDE-47, maternal and fetal characteristics, and fetal (placenta or liver) BDE-47 concentrations.
| Wet weight exposure and outcome (ng/ml) | Lipid-adjusted exposure and outcome (ng/g lipid) | |||||
|---|---|---|---|---|---|---|
| Parameter | Model 1 | Model 2 | Model 3 | Model 1 LA | Model 2 LA | Model 3 LA |
| Outcome: Fetal liver BDE-47 (n = 83) | ||||||
| Maternal serum BDE-47 | 0.92 (0.065)* | 0.92 (0.065)* | 0.93 (0.061)* | 0.87 (0.057)* | 0.90 (0.057)* | 0.90 (0.056)* |
| Cohort | 0.36 (0.090)* | 0.37 (0.091)* | 0.36 (0.090)* | 0.44 (0.081)* | 0.42 (0.080)* | 0.40 (0.082)* |
| Maternal serum lipids | -- | −0.039 (0.042) | −0.018 (0.039) | -- | 0.083 (0.038)* | 0.093 (0.037)* |
| Gestational age | -- | -- | −0.059 (0.021)* | -- | -- | −0.023 (0.019) |
| Systolic blood pressure | -- | -- | 0.0087 (0.0040)* | -- | -- | 0.0072 (0.0036) |
| Sex (male) | -- | -- | −0.14 (0.091) | -- | -- | −0.15 (0.083) |
| Adjusted R2 | 0.72 | 0.72 | 0.76 | 0.76 | 0.77 | 0.79 |
| Outcome: Placenta BDE-47 (n = 48) | ||||||
| Maternal serum BDE-47 | 0.64 (0.11)* | 0.64 (0.11)* | 0.52 (0.13)* | 0.60 (0.12)* | 0.66 (0.12)* | 0.51 (0.13)* |
| Maternal serum lipids | -- | 0.026 (0.064) | −0.00019 (0.062) | -- | 0.15 (0.071)* | 0.097 (0.067) |
| Diastolic blood pressure | -- | -- | 0.016 (0.0070)* | -- | -- | 0.021 (0.0072)* |
| Parity (≥ 1)a | -- | -- | 0.30 (0.18) | -- | -- | 0.39 (0.18)* |
| Adjusted R2 | 0.40 | 0.39 | 0.45 | 0.33 | 0.38 | 0.48 |
Reference group is nulliparity
p < 0.05
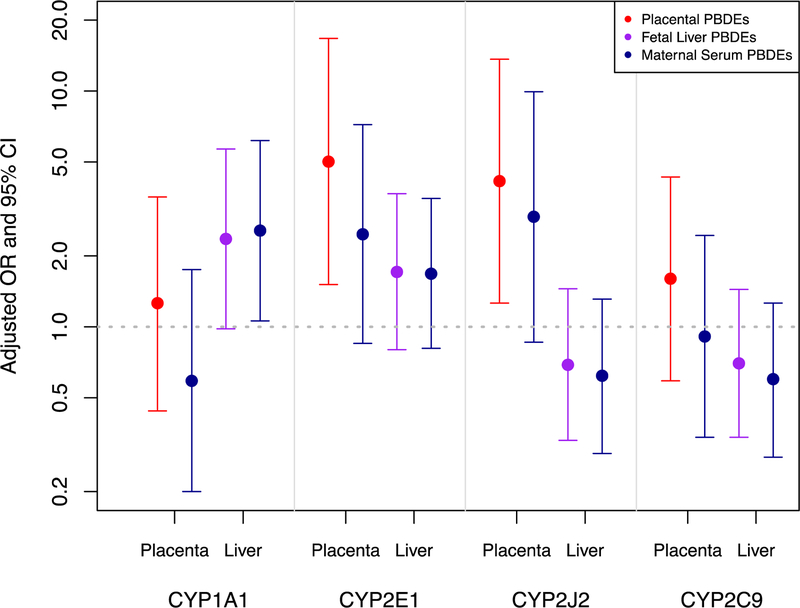
Virtually all fetal liver samples (>98%) had detectable expression of most CYPs except for CYP2B6 and −1A2 (Supplemental Materials, Table S3). CYP2C9, −2E1 and −2J2 were detected in >80% of placentas while CYP1A1 was only detected in 30% of placentas. PBDE levels were associated with altered expression of some CYPs in fetal liver and placenta. Placental ΣPBDEs were positively associated with placental CYP2J2 expression (OR (95% CI) = 4.15 (1.26, 13.64)) and CYP2E1 (OR (95% CI) = 5.02 (1.51, 16.72)) (Figure 4 and Table 4). For the liver CYPs, there was a positive association between liver ΣPBDEs and CYP1A1 (OR (95% CI) = 2.36 (0.98, 5.68)), and a suggestive but non-significant inverse association with CYP2J2 (OR (95% CI) = 0.69 (0.33, 1.45). Associations with liver CYP expression were slightly more pronounced when exposure was modeled as maternal serum ΣPBDEs (e.g. CYP1A1 OR (95% CI) = 2.56 (1.06, 6.17)). In contrast, placental CYPs were more strongly associated with placental ΣPBDEs than maternal serum ΣPBDEs. Associations were similar when chemical concentrations were lipid-adjusted (Table 4). When we restricted our model to never smokers, the relationship between PBDEs and CYPs were generally similar although the magnitude of the association changed for some CYP isoforms (Supplemental Materials, Table S7). Notably, the association between placental ΣPBDEs and placental CYP2J2 expression was lower in magnitude (OR (95% CI) = 2.89 (0.91, 9.12) and the association between liver ΣPBDEs and liver CYP1A1 expression was greater in magnitude (OR (95% CI) = 4.55 (1.21, 17.1)) than associations that included former and current smokers.
Figure 4.
Associations (ORs and 95% CIs) between CYP 450 mRNA expression in placenta (n=45) and fetal liver (n=81) and ∑PBDEs adjusted for gestational age, fetal sex, lipid content, smoking status, and cohort (fetal liver models only).
Table 4.
Association (OR (95%CI) between CYP mRNA expression and PBDE concentrations
| Outcome: Placenta CYPs | ||||
|---|---|---|---|---|
| Placenta | Serum ∑PBDEs (n=45) |
Placenta ∑PBDEs (n=45) |
||
| wet weight | lipid adjusted | wet weight | lipid adjusted | |
| CYP1A1 | ||||
| Non-detect | ref | ref | ref | ref |
| Detect | 0.59 (0.20, 1.75) | 0.58 (0.19, 1.74) | 1.26 (0.44, 3.55) | 1.26 (0.45, 3.55) |
| CYP2E1 | ||||
| Low | ref | ref | ref | ref |
| High | 2.47 (0.85, 7.21)^ | 2.44 (0.83, 7.13) | 5.02 (1.51, 16.72)* | 4.90 (1.48, 16.20)* |
| CYP2C9 | ||||
| Low | ref | ref | ref | ref |
| High | 0.91 (0.34, 2.44) | 0.91 (0.34, 2.45) | 1.60 (0.59, 4.32) | 1.58 (0.59, 4.26) |
| CYP2J2 | ||||
| Low | ref | ref | ref | ref |
| High | 2.93 (0.86, 9.93)^ | 2.91 (0.86, 9.87)^ | 4.15 (1.26, 13.64)* | 4.00 (1.23, 13.05)* |
| Outcome: Liver CYPs | ||||
| Liver | Serum ∑PBDEs (n=81) |
Liver ∑PBDEs (n=81) |
||
| wet weight | lipid adjusted | wet weight | lipid adjusted | |
| CYP1A1 | ||||
| Low | ref | ref | ref | ref |
| High | 2.56 (1.06, 6.17)* | 2.60 (1.07, 6.29)* | 2.36 (0.98, 5.68)^ | 2.29 (0.96, 5.46)^ |
| CYP2E1 | ||||
| Low | ref | ref | ref | ref |
| High | 1.68 (0.81, 3.50) | 1.65 (0.79, 3.41) | 1.71 (0.80, 3.67) | 1.72 (0.80, 3.69) |
| CYP2C9 | ||||
| Low | ref | ref | ref | ref |
| High | 0.60 (0.28, 1.26) | 0.60 (0.28, 1.27) | 0.70 (0.34, 1.44) | 0.69 (0.33, 1.41) |
| CYP2J2 | ||||
| Low | ref | ref | ref | ref |
| High | 0.62 (0.29, 1.31) | 0.61 (0.29, 1.29) | 0.69 (0.33, 1.45) | 0.69 (0.33, 1.44) |
| CYP2C19 | ||||
| Low | ref | ref | ref | ref |
| High | 0.75 (0.36, 1.57) | 0.75 (0.36, 1.57) | 0.91 (0.45, 1.83) | 0.92 (0.45, 1.84) |
| CYP3A4 | ||||
| Low | ref | ref | ref | ref |
| High | 1.58 (0.76, 3.29) | 1.58 (0.76, 3.28) | 1.36 (0.67, 2.78) | 1.34 (0.66, 2.74) |
| CYP2B6 | ||||
| Non-detect | ref | ref | ref | ref |
| Detect | 1.12 (0.53, 2.36) | 1.14 (0.54, 2.40) | 1.23 (0.59, 2.58) | 1.23 (0.59, 2.57) |
All models adjusted for gestational age, fetal sex, lipids, smoking status, and cohort (liver CYP models only)
p< 0.05
p <0.10
Discussion
This work shows that, under normal conditions of mid gestation, the human fetus is widely exposed to PBDEs and their hydroxylated metabolites, that fetal exposures are higher than maternal exposures, and that these exposures are associated with the expression of mRNAs coding for CYP enzymes. These findings collectively indicate the human fetus experiences higher exposures than have been previously estimated based on maternal levels and may be relevant for estimating health risks that are not currently captured by epidemiologic or risk assessment studies.
We report higher maternal serum PBDE concentrations in the current study population compared to three other contemporary populations of pregnant women from various US cities (Chen et al. 2013; Leonetti et al. 2016a; Morello-Frosch et al. 2016). However, our findings are more similar to those measured in US pregnant women from the 2003–2004 NHANES survey, which coincides with peak use of the PentaBDE commercial formulation in the US (Abbasi et al. 2015; Woodruff et al. 2011).
In the current study, PBDE concentrations in fetal liver and cord serum are higher than that in maternal serum, suggesting there may be fetal accumulation of PBDEs during development as previously surmised on the basis of placental PBDE concentrations (Leonetti et al. 2016b). In our case, PBDE concentrations were about two-fold higher in the fetal liver than in maternal serum when using wet weight as the denominator. However, when normalizing with respect to lipid levels, fetal liver concentrations were approximately 50% of that of maternal serum. This means that lipid concentrations in fetal liver tissue are much higher than that of maternal serum, as would be expected. Moreover, it seems likely that much of the PBDEs measured in fetal liver are contained within the highly vascular compartment of the liver, while hepatic lipids are more likely to be intracellular. Thus, while our data may not fully support the conclusion that PBDEs bioaccumulate in the fetal liver, they clearly demonstrate that the normal human fetus is directly exposed to concentrations of PBDEs that may be higher than previously estimated on the basis of maternal serum.
However, it is important to recognize that the “concentration” of chemicals in liver tissue cannot be directly compared to that of serum because the denominator (volume, wet weight versus unit protein or unit lipid) is not the same in both compartments. While approaches for lipid standardization in epidemiologic studies of lipophilic environmental chemicals have been widely discussed (O’Brien et al. 2016; Schisterman et al. 2005), there has been little agreement on best practices for lipid standardization in exposure studies, such as ours, that include multiple tissue types. Though researchers commonly assume that serum concentrations of lipophilic chemicals such as PBDEs are dependent on serum lipid concentrations (Schisterman et al. 2005), we found no association between levels of PBDEs and lipids in maternal serum or placenta, suggesting that the assumption of equilibrium between total PBDE burden and lipid levels may not be appropriate in many circumstances and further methodological work is needed.
Our models estimating BDE-47 levels in fetal liver and placenta suggest that maternal serum levels are a reasonable proxy for concentrations in these tissues although maternal levels were more highly correlated with liver than placenta concentrations. There are few studies to which we can compare our findings. Two prior studies reported PBDE concentrations in fetal livers from mid-gestation; however those studies did not collect matched samples of maternal serum from participants (Doucet et al. 2009; Schecter et al. 2007a). Several other studies evaluated maternal serum-placental pairs, but they were for placentas collected at term, rather than mid-gestation (Frederiksen et al. 2010; Leonetti et al. 2016a). The maternal serum/placenta correlations in those studies ranged from 0.36 to 0.74, which brackets our correlation of 0.61, indicating that there may be some exposure misclassification in epidemiologic studies that use maternal serum as a proxy for placental PBDE exposures.
An additional feature of the current study is that we observed significant associations between PBDE exposure and the expression of mRNAs coding for specific CYPs in the placenta or fetal liver. For example, in the placenta, ΣPBDEs were associated with CYP2E1 mRNA, while in the fetal liver, ΣPBDEs were associated with CYP1A1 mRNA. These associations persisted even when smokers were excluded. In general, CYP1A1 and −2E1 are well-characterized xenobiotic metabolizing enzymes, and are also actively involved in diverse endogenous roles in fatty acid, steroid, and other metabolic pathways (Rendic 2002). These findings demonstrate that the mRNA expression of specific P450 enzymes is correlated with specific chemical exposures in the human fetus. Although we did not measure enzyme activity, there are many studies showing the association of CYP mRNA and enzyme activity (Giera et al. 2011; Lai et al. 2004; Sharlin et al. 2010; Tutelyan et al. 2012), and we are unaware of studies showing a mismatch between CYP mRNA and enzyme activity. Recent evidence demonstrates that CYP1A1 mRNA expression is tightly correlated with the expression of thyroid hormone-regulated genes in the human term placenta, indicating that inducible metabolic enzymes in placenta may bioactivate chemicals such as PBDEs that can, in turn, directly interact with the TH receptor (Wadzinski et al. 2014). Therefore, our results suggest the importance of the metabolic capacity of the fetal compartment in metabolizing environmental chemicals. Future studies examining these relationships in vitro or in vivo in relevant cell populations will assist in understanding the functional importance of these associations. Furthermore, measures of CYP activity such as EROD and PROD in future studies would confirm the relationship between PBDE exposures, mRNA levels, and enzyme activity.
We did not observe significant associations between PBDE exposures and CYP2B6 in the fetal liver. In the adult liver, CYP2B6 is postulated to be the predominant CYP enzyme involved in the formation of OH-PBDEs from oxidative metabolism of PentaBDE congeners such as BDE-47 and −99 (Erratico et al. 2012; Erratico et al. 2013). In this study, CYP2B6 was only detected in 60% of liver samples and OH-PBDEs in the liver were substantially lower than those in maternal or cord sera. These observations support previous findings that CYP2B6 is expressed at low levels in the human fetal liver (Croom et al. 2009) and suggest that metabolism of PBDEs in the fetal liver is probably limited compared to adults.
However, the developing fetus may still encounter different exposures to OH-PBDEs than the pregnant woman since levels and composition of OH-PBDEs can vary by tissue. In our study, ΣOH-PBDEs concentrations in maternal and cord sera were similar and higher than those in fetal liver and placenta. Moreover, 6-OHBDE-47 was more abundant in maternal serum while 5-OHBDE-47 was more abundant in cord serum. Prior studies have generally reported higher levels of most OH-PBDEs in cord relative to maternal serum (Chen et al. 2013; Morello-Frosch et al. 2016; Qiu et al. 2009; Wan et al. 2010). Consistent with our study, the one study that reported measurable levels of 5-OHBDE-47 and 6-OHBDE-47 in paired maternal and cord sera found higher cord:maternal ratios for 5-OH-BDE47 than for 6-OH-BDE47 (Chen et al. 2013). Maternal-fetal partitioning of OH-PBDEs is poorly understood and the observed differences in the profiles of individual OH-PBDEs across specimens may reflect differences in physico-chemical properties and/or external sources of exposure (e.g., dietary sources).
Several elements of the current study require consideration. First, this was a cross-sectional study design and the sample size was modest. Moreover, none of the participants provided data from all four of the compartments measured (maternal and cord sera, fetal liver and placenta). However, it is still the only study to date of PBDE exposures during a normal human mid-gestation that includes paired samples of maternal and fetal specimens. The CYP enzymes under investigation are not specific to PBDEs, and expression may be induced by other exposures. Our study did not adjust for medications or other environmental chemicals that may induce CYP expression, because PBDE exposure is unlikely to be causally associated with these exposures and they are therefore unlikely to be confounders. Instead, other varied sources of CYP induction uncorrelated with PBDEs may be introducing noise into the CYP expression data, which would bias associations towards the null. Our models estimating the association between maternal concentrations and liver and placenta concentrations may not be generalizable to other populations that have significantly different demographic characteristics. The associations between PBDE exposures and CYP mRNA expression were often imprecise with large confidence intervals and warrant replication in larger epidemiologic studies.
Conclusion
In conclusion, our data on chemical levels from four tissues in the maternal-fetal unit shows that exposure to PBDEs and OH-PBDEs is widespread in both the mother and fetus during mid-gestation and that these exposures are associated with specific CYP enzyme transcriptional levels in the placenta and fetal liver. Furthermore, our results show that maternal serum PBDE levels are a better proxy for PBDE levels in the fetal liver than in the placenta. While we should be cautious interpreting these results given the small sample size and the potential residual confounding from other environmental exposures that could impact CYP expression, these results make an important contribution to the field given the novelty of the dataset, particularly the paired maternal-fetal human samples from a sensitive window of development. These findings advance our understanding of human fetal exposures to PBDEs during pregnancy and consequently improve our understanding of population risks to PBDEs as well as other structurally similar environmental chemicals. They may also inform the accuracy and power of epidemiologic investigations by providing novel data to estimate fetal exposure when only maternal specimens are available.
Supplementary Material
Highlights.
PBDE concentrations in some fetal matrices were higher than those in maternal serum.
Maternal serum levels were more highly correlated with levels in fetal liver than placenta.
PBDEs were associated with CYP mRNA expression in both the liver and the placenta.
Acknowledgements:
This study was funded by the National Institute of Environmental Health Sciences (P01ES022841, R21ES022422, R01ES010026, R00ES019881,Virtual Consortium for Translational/Transdisciplinary Environmental Research (ViCTER)) and the U.S. EPA-Science to Achieve Results (STAR) RD (83543301). We thank Dr. Linda Linderholm and Dr. Suhash Harwani for their assistance with the chemical analysis; Gregory Yeh and Wendy Duong for assistance with sample extraction; Dr. Chelsea Snyder for assistance with RNA expression analyses; Dr. Saunak Sen for his input on data analysis; Ruth Geller for assistance with manuscript formatting; and Erin DeMicco, Janet Pan, Carrie Dickenson, and Cheryl Godwin de Medina for sample collection. Competing financial interests declaration: The authors declare that they have no competing financial interests, and that their freedom to design, conduct, interpret, and publish research is not compromised by any controlling sponsor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbasi G, Buser AM, Soehl A, Murray MW, Diamond ML. 2015. Stocks and flows of PBDEs in products from use to waste in the U.S. and Canada from 1970 to 2020. Environ Sci Technol 49:1521–1528. [DOI] [PubMed] [Google Scholar]
- Blanco J, Mulero M, Domingo JL, Sánchez DJ. 2012. Gestational exposure to BDE-99 produces toxicity through up-regulation of CYP isoforms and ROS production in the fetal rat liver. Toxicol Sci:kfs082 [DOI] [PubMed]
- Chen AM, Park JS, Linderholm L, Rhee A, Petreas M, DeFranco EA, et al. 2013. Hydroxylated Polybrominated Diphenyl Ethers in Paired Maternal and Cord Sera. Environ Sci Technol 47:3902–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croom EL, Stevens JC, Hines RN, Wallace AD, Hodgson E. 2009. Human hepatic CYP2B6 developmental expression: the impact of age and genotype. Biochemical pharmacology 78:184–190. [DOI] [PubMed] [Google Scholar]
- Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. 2009. Persistent Organic Pollutant Residues in Human Fetal Liver and Placenta from Greater Montreal, Quebec: A Longitudinal Study from 1998 through 2006. Environ Health Perspect 117:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick J, Brix A, Cunny H, Vallant M, Shockley K. 2012. Characterization of polybrominated diphenyl ether toxicity in Wistar Han rats and use of liver microarray data for predicting disease susceptibilities. Toxicologic Pathology 40:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erratico CA, Moffatt SC, Bandiera SM. 2011. Comparative oxidative metabolism of BDE-47 and BDE-99 by rat hepatic microsomes. Toxicol Sci 123:37–47. [DOI] [PubMed] [Google Scholar]
- Erratico CA, Szeitz A, Bandiera S. 2012. Oxidative metabolism of BDE-99 by human liver microsomes: predominant role of CYP2B6. Toxicol Sci [DOI] [PubMed]
- Erratico CA, Szeitz As, Bandiera SM 2013. Biotransformation of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) by human liver microsomes: identification of cytochrome P450 2B6 as the major enzyme involved. Chemical research in toxicology 26:721–731. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. 2009. Human internal and external exposure to PBDEs -A review of levels and sources. Int J Hyg Environ Health 212:109–134. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Thomsen C, Froshaug M, Vorkamp K, Thomsen M, Becher G, et al. 2010. Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort. Int J Hyg Environ Health 213:233–242. [DOI] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. 2011. Individual polychlorinated biphenyl (PCB) congeners produce tissue-and gene-specific effects on thyroid hormone signaling during development. Endocrinology 152:2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 118:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KP, Wong MH, Wong CK. 2004. Modulation of AhR-mediated CYP1A1 mRNA and EROD activities by 17beta-estradiol and dexamethasone in TCDD-induced H411E cells. Toxicol Sci 78:41–49. [DOI] [PubMed] [Google Scholar]
- Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, et al. 2017. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ Health Perspect 125:086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM. 2016a. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environmental Health 15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti C, Butt CM, Hoffman K, Miranda ML, Stapleton HM. 2016b. Concentrations of polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues. Environ Int 88:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Mitro SD, Johnson T, Zota AR. 2015. Cumulative chemical exposures during pregnancy and early development. Curr Environ Health Rep 2:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Cushing LJ, Jesdale BM, Schwartz JM, Guo W, Guo T, et al. 2016. Environmental Chemicals in an Urban Population of Pregnant Women and Their Newborns from San Francisco. Environ Sci Technol 50:12464–12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR. 2016. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ Health Perspect 124:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Bigsby RM, Hites RA. 2009. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ Health Perspect 117:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S 2002. Summary of information on human CYP enzymes: human P450 metabolism data. Drug metabolism reviews 34:83–448. [DOI] [PubMed] [Google Scholar]
- Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. 2007a. Polybrominated diphenyl ether (PBDE) levels in livers of US human fetuses and newborns. J Toxicol Env Health Part A 70:1–6. [DOI] [PubMed] [Google Scholar]
- Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. 2007b. Polybrominated diphenyl ether (PBDE) levels in livers of U.S. human fetuses and newborns. J Toxicol Environ Health A 70:1–6. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Buck Louis GM, Louis TA. 2005. Lipid Adjustment in the Analysis of Environmental Contaminants and Human Health Risks. Environ Health Perspect 113:853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharlin DS, Gilbert ME, Taylor MA, Ferguson DC, Zoeller RT. 2010. The nature of the compensatory response to low thyroid hormone in the developing brain. J Neuroendocrinol 22:153–165. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Pei R, Letcher RJ, Gunsch C. 2009. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ Health Perspect 117:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. 2011. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect 119:1454–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorov A, Takser L. 2010. Global gene expression analysis in the livers of rat offspring perinatally exposed to low doses of 2,2’,4,4’-tetrabromodiphenyl ether. Environ Health Perspect 118:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudnowski RJ, Rico RC. 1974. Specific gravity of blood and plasma at 4 and 37 C. Clinical chemistry 20:615–616. [PubMed] [Google Scholar]
- Tutelyan VA, Trusov NV, Guseva GV, Beketova NA, Aksenov IV, Kravchenko LV. 2012. Indole-3-carbinol induction of CYP1A1, CYP1A2, and CYP3A1 activity and gene expression in rat liver under conditions of different fat content in the diet. Bull Exp Biol Med 154:250–254. [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Poston KL, Xie C, Webster GM, Sjodin A, et al. 2017a. Prenatal and postnatal polybrominated diphenyl ether (PBDE) exposure and measures of inattention and impulsivity in children. Neurotoxicol Teratol 64:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Poston KL, Xie C, Webster GM, Sjodin A, et al. 2017b. Childhood polybrominated diphenyl ether (PBDE) exposure and executive function in children in the HOME Study. Int J Hyg Environ Health [DOI] [PMC free article] [PubMed]
- Vuong AM, Yolton K, Xie C, Webster GM, Sjodin A, Braun JM, et al. 2017c. Childhood polybrominated diphenyl ether (PBDE) exposure and neurobehavior in children at 8 years. Environ Res 158:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski TL, Geromini K, McKinley Brewer J, Bansal R, Abdelouahab N, Langlois M-F, et al. 2014. Endocrine disruption in human placenta: expression of the dioxin-inducible enzyme, CYP1A1, is correlated with that of thyroid hormone-regulated genes. J Clin Endocrinol Metab 99:E2735–E2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Choi K, Kim S, Ji K, Chang H, Wiseman S, et al. 2010. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: placental transfer and potential risks. Environ Sci Technol 44:5233–5239. [DOI] [PubMed] [Google Scholar]
- Wiseman SB, Wan Y, Chang H, Zhang X, Hecker M, Jones PD, et al. 2011. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: environmental sources, metabolic relationships, and relative toxicities. Marine pollution bulletin 63:179–188. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. 2011. Environmental chemicals in pregnant women in the US: NHANES 2003–2004. Environ Health Perspect 119:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Sjodin A, Calafat AM, Dietrich KN, et al. 2017. Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Environ Health Perspect 125:746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Park J-S, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. 2011. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol 45:7896–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Linderholm L, Park J-S, Petreas M, Guo T, Privalsky ML, et al. 2013. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ Sci Technol 47:11776–11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.