Abstract
The benefit of revascularization of chronic total occlusion (CTO) in percutaneous coronary intervention (PCI) is controversial. On the other hand, left ventricular (LV) global longitudinal strain (GLS) is a more sensitive marker of LV myocardial ischemia and LV function than LV ejection fraction (EF). The purpose of this study was to investigate the impact of revascularization of CTO on LV function using LV GLS. A total of 70 consecutive patients (65.1±8.9 years, 59 males, LVEF 51.0±12.0%) with CTO who had a positive functional ischemia and underwent PCI, were included in this study. Echocardiography was performed before and 9 months after the procedure with conventional assessment including LV end-diastolic and end-systolic volume (LVEDV, LVESV), LVEF, and with 2DSTE analysis of GLS. Successful PCI was obtained in 60 patients (86%). There were no stent thromboses during follow-up. GLS showed a significant improvement 9 months after successful PCI (pre-PCI -12.4±4.1% vs. post-PCI -14.5±4.1%, P< 0.01), whereas in failed PCI group that did not change significantly (pre-PCI -13.2±4.2% vs. post-PCI -14.0±4.7%, P = 0.64). LVEF, LVEDV and LVESV did not change significantly during follow-up in both successful and failed groups. Successful PCI for CTO improved LV function, assessed by LV GLS.
Introduction
Chronic total occlusions (CTO) are defined as lesions with TIMI (Thrombolysis in Myocardial Infarction) grade 0 flow for more than three months. CTO lesions are identified in 18.4% in patients undergoing elective percutaneous coronary intervention (PCI) in the absence of previous coronary artery bypass surgery or those presenting with acute myocardial infarction [1]. Several previous studies reported the effect of successful PCI for CTO, such as improvement of quality of life, exercise capacity, and reducing the need for late CABG surgery [2, 3]. However the benefits of revascularization using PCI for CTO are still controversial. Two-dimensional speckle-tracking echocardiography (2DSTE) is emerging as a novel technique to allow the assessment of LV systolic and diastolic function through the quantification of active myocardial deformation [4–6]. The global longitudinal strain (GLS) assessed with 2DSTE, which evaluates the longitudinal myocardial deformation, is more reproducible than left ventricular ejection fraction (LVEF) or wall motion score index (WMSI), is advantageous over the color kinesis technique and is proven to be effective in detecting the LV myocardial ischemia [7–13]. Moreover in patients with cardiovascular disease, myocardial dysfunction occurs even if overall LVEF is preserved, and that may be associated with impaired LV longitudinal deformation [14]. Accordingly, the purpose of this study was to investigate the impact of revascularization of CTO on LV function using LV GLS.
Methods
Patients
We conducted a retrospective study in a cohort of 70 consecutive patients with CTO who had attempted PCI at Himeji Cardiovascular Center between May 2009 and February 2014. All patients had single vessel coronary artery disease with CTO and had a positive functional ischemia study. We excluded patients who had an anticipated noncompliance with dual antiplatelet treatment for at least 12 months, the history of coronary artery bypass surgery, severe valvular disease, other co-morbid systemic disease and atrial fibrillation. CTO was defined as a coronary artery obstruction with thrombolysis in myocardial infarction (TIMI) grade 0 and all patients had a native vessel occlusion estimated to be of at least 3 months duration based on the time from diagnosis made on coronary angiography [15]. PCI and stent implantation were performed in a standard manner. Drug-eluting stents (DESs) were used in all of the PCI procedures. After the PCI, all of the patients were prescribed lifelong aspirin and clopidogrel for at least 12 months. Collateral channels and their Rentrop classification were analyzed in the pre-procedural coronary angiography. Successful PCI was defined as follows; the residual stenosis < 50% by visual estimation, a restoration of TIMI flow 3 in the target vessel after stent implantation and no immediate angiographic complications. Failed PCI was defined as failure to cross the occlusion or reduce obstruction to less than 50% in the target CTO [16].
All of the patients underwent an extensive baseline clinical history taking and physical examination, 12-lead electrocardiography, and transthoracic echocardiography. This study was approved by the research ethics committee of Himeji Cardiovascular Center and carried out in accordance with approved guidelines. Written informed consent was obtained from all patients.
Transthoracic echocardiography
Echocardiography was performed before and 9 months after the procedure (Fig 1). Comprehensive transthoracic echocardiography was performed by experienced research sonographers by using commercially available Aplio (Toshiba Medical Systems, Tokyo, Japan). Two-dimensional and color Doppler echocardiography were performed in standard parasternal and apical views. LV end-diastolic volume (EDV), end-systolic volume (ESV), and LVEF were measured using a modified Simpson method. All images were stored online and measured with offline software later by independent investigators who were blinded to the clinical data.
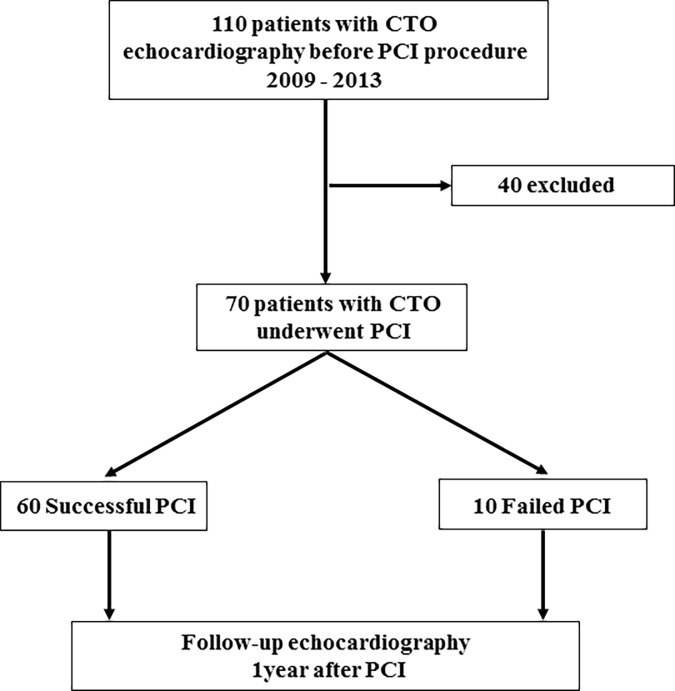
Fig 1. Patient flow chart.
LV strain measurements
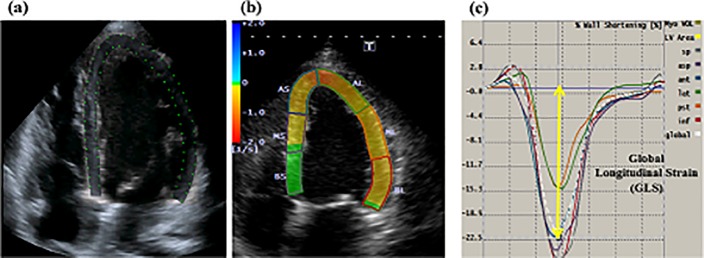
The LV GLS was obtained by using 2DST software (Toshiba Medical Systems, Tokyo, Japan). The endocardial border was traced manually in the end-diastolic frame. The software automatically tracked the myocardium throughout the cardiac cycle. The peak values of segmental longitudinal strain were obtained from greyscale-recorded images in the apical four-chamber, two-chamber, and long-axis views with a frame rate between 50 and 70 frames per second, and GLS was obtained by averaging the peak values [17] (Fig 2). The coefficients of variation of interobserver variability for GLS was 8%. The coefficients of variation of intraobserver variability for GLS was 3% in our previous study [18].
Fig 2. Example of the measurement of left ventricular (LV) global longitudinal strain (GLS) with a two-dimensional speckle-tracking echocardiography (2DSTE) in a patient with chronic total occlusion who underwent successful percutaneous coronary intervention (PCI).
Figure (a) and (b) showed apical four-chamber view. White broken lines indicated the LV global strain curves for GLS. Yellow arrow indicated the mean LV global peak longitudinal strain; GLS: −22.5% (c).
Statistical analysis
Data are expressed as mean ± standard deviation for continuous variables, and median and interquartile range for non-normally distributed continuous variables. Data from the successful and failed PCI groups were compared by using the Student’s t-test and one-way analysis of variance (ANOVA) for normally distributed continuous variables, the Wilcoxon–Mann–Whitney test for non-normally distributed continuous variables, and the χ2 test or Fisher’s exact test for categorical variables. Changes in the LVEDV, LVESV, LVEF and GLS data between the before and the 9 months after the procedure were assessed by ANOVA. A value of p <0.05 were considered statistically significant. MedCalc Version 12.7.8 (Acacialaan 22, B-8400 Ostend, Belgium) was used for all analyses.
Results
Between January 2009 and December 2013, a total of 70 CTO lesions (in 70 patients) were targeted, and 60 lesions were successfully revascularized with PCI, and 10 lesions were failed (Fig 1). No procedural complications (coronary perforation, cardiac tamponade or emergent cardiac surgery) were observed in any patients undergoing CTO-PCI attempt. During the 9-month from the procedure, there were no changes in prescribed medical treatment, such as beta-blockers, angiotensin receptor blockers or angiotensin converting enzyme inhibitors, and nitrates, and no cardiac resynchronization therapy and pacemaker were implanted.
Clinical and angiographic data
Baseline characteristics are shown in Table 1. The mean age of the patients was 65.1 ± 8.9 years. Eighty-four percent of the patients were male, 57% of the patients had a history of diabetes and 91% of the patients had hypertension. There were no significant difference in baseline characteristics between successful and failed PCI group. The angiographic characteristics of CTO lesions are shown in Table 2. The most common location of CTO was right coronary artery (RCA), followed by left anterior descending artery (LAD) and left circumflex artery (LCX). A total of 60 CTOs were revascularized, 19 CTOs in LAD, 31 CTOs in RCA, and 10 CTOs in LCX. Location of CTO and grade of collateral flow (Rentrop score) did not differ significantly between successful and failed PCI group. There were no stent thromboses during the 9 month follow-up.
Table 1. Patient baseline characteristics.
| All Patients (n = 70) |
Successful PCI (n = 60) |
Failed PCI (n = 10) |
p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 65.1 ± 8.9 | 65.2 ± 9.0 | 64.2 ± 8.6 | 0.74 |
| Male, n (%) | 59 (84) | 51 (85) | 8 (80) | 0.90 |
| Hypertension, n (%) | 64 (91) | 55 (92) | 9 (90) | 0.86 |
| Diabetes Mellitus, n (%) | 40 (57) | 33 (55) | 7 (70) | 0.38 |
| Dyslipidemia, n (%) | 62 (89) | 53 (88) | 9 (90) | 0.89 |
| Smoking, n (%) | 35 (50) | 30 (50) | 5 (50) | 0.66 |
| Systolic BP (mm Hg) | 131 ± 24 | 132 ± 25 | 125 ± 18 | 0.45 |
| Heart rate (beats/min) | 68±11 | 67±11 | 71±14 | 0.30 |
| Laboratory data | ||||
| Serum creatinine (mg/dL) | 1.1 ± 1.0 | 1.1 ± 1.0 | 1.0 ± 0.7 | 0.66 |
| Medications | ||||
| ACEI and/ or ARB, n (%) | 41 (58) | 35 (58) | 6 (60) | 0.92 |
| Beta-blockers, n (%) | 37 (53) | 32 (53) | 5 (50) | 0.85 |
| Nitrates, n (%) | 27 (39) | 22 (37) | 5 (50) | 0.65 |
| Statin, n (%) | 61 (87) | 52 (87) | 9 (90) | 0.78 |
ARB, angiotensin II receptor blocker; ACEI, angiotensin converting enzyme inhibitor; BP, blood pressure; and PCI, percutaneous coronary intervention
Table 2. Lesion characteristics.
| All Patients (n = 70) |
Successful PCI (n = 60) |
Failed PCI (n = 10) |
p Value | |
|---|---|---|---|---|
| Chronic total occlusion location | ||||
| RCA/LCX/LAD, n (%) | 37/11/22 | 31/10/19 | 6/1/3 | 0.79 |
| Rentrop score (0/1/2/3) | 0/1/60/9 | 0/1/51/8 | 0/0/9/1 | 0.89 |
| Number of treated with stents, n (%) | N/A | 57 (95) | N/A | N/A |
| Number of stents per lesion, n (%) | N/A | 1.5 ± 0.5 | N/A | N/A |
| Stent length, (mm. per stented lesion) | N/A | 38 ± 16 | N/A | N/A |
| Stent thrombosis, n (%) | N/A | 0 (0) | N/A | N/A |
LAD, left anterior descending artery; LCX, left circumflex artery; and RCA, right coronary artery; other abbreviations are as defined in Table 1.
Echocardiographic measurement
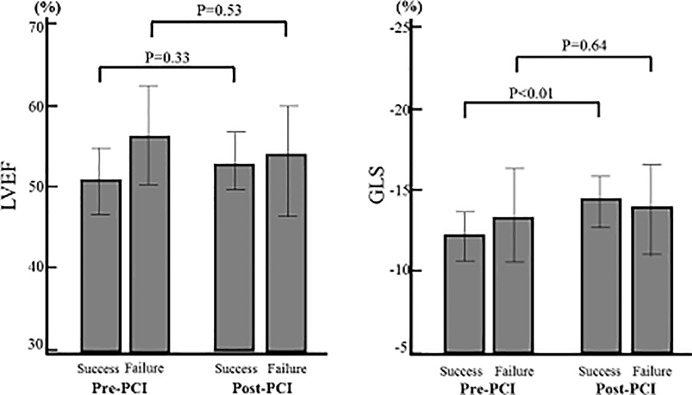
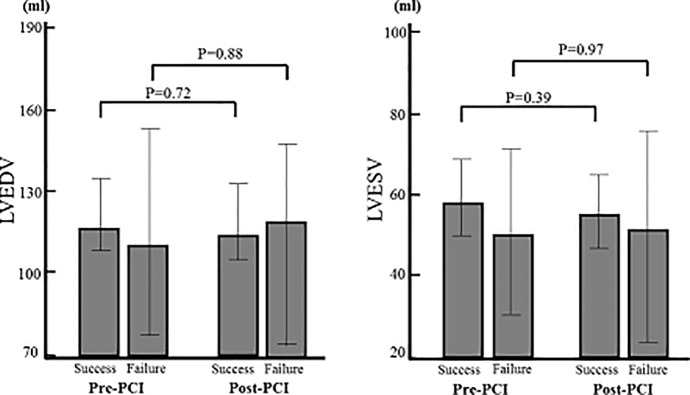
Echocardiographic parameters are shown in Table 3. There were no significant differences in baseline LV function and volume between successful and failed PCI groups. GLS showed a significant improvement 9 months after successful PCI group (pre-PCI -12.4±4.1% vs. post-PCI -14.5±4.1%, P< 0.01), whereas that did not change significantly after failed PCI group (pre-PCI -13.2±4.2% vs. post-PCI -14.0±4.7%, P = 0.64). On the other hands, LVEF (pre-PCI 50.2±12.3% vs. post-PCI 52.4±10.7%, P = 0.33, pre-PCI 55.9±8.9% vs. post-PCI 53.2±9.9%, P = 0.53), LVEDV (pre-PCI 122.9±39.1ml vs. post-PCI 121.1±41.1ml, P = 0.72, pre-PCI 116.1±51.2ml vs. post-PCI 117.6±49.7ml, P = 0.88) and LVESV (pre-PCI 58.5±32.3ml vs. post-PCI 55.7±33.9ml, P = 0.39, pre-PCI 48.1±30.0ml vs. post-PCI 49.3±36.3ml, P = 0.97) showed no significant changes both successful and failed PCI groups during the 9 month follow-up, respectively (Figs 3 and 4).
Table 3. Echocardiographic measures.
| All Patients (n = 70) |
Successful PCI (n = 60) |
Failed PCI (n = 10) |
p Value | |
|---|---|---|---|---|
| Echocardiographic data | ||||
| LVEDV (mL) | 121.9 ± 40.7 | 122.9 ± 39.1 | 116.1 ± 51.2 | 0.63 |
| LVESV (mL) | 57.0 ± 31.9 | 58.5 ± 32.3 | 48.1 ± 30.0 | 0.35 |
| LVEF (%) | 51.0 ± 12.0 | 50.2 ± 12.3 | 55.9 ± 8.9 | 0.11 |
| E/A ratio | 0.72 ± 0.20 | 0.72 ± 0.19 | 0.73 ± 0.21 | 0.85 |
| GLS (%) | -12.2 ± -4.7 | -12.4 ± -4.1 | -13.2 ± -4.2 | 0.47 |
Fig 3. Change of LV function from baseline to 9 months after PCI.
GLS in successful PCI group was significantly improved (P<0.01), whereas in failed PCI group that did not change significantly. LVEF showed no significant improvement in both successful and failed PCI groups.
Fig 4. Change of LV volume from baseline to 9 months after PCI.
LVEDV and LVESV showed no significant improvement in both successful and failed PCI groups.
Discussion
In this study, we investigated the impact of revascularization of CTO on LV function using LV GLS. Successful PCI for CTO improved LV function assessed by LV GLS. To the best of our knowledge, this is the first study to demonstrate the comparison with successful and failed CTO-PCI patients with a positive functional ischemia before PCI, according to LV function evaluated with LV GLS before and after 9 month PCI.
CTO is common, being reported in 30% of patients undergoing coronary angiography [19, 20]. However, the benefits of PCI for CTO are controversial. The procedure lasted several hours with significant radiation exposure and contrast dose. Furthermore the higher complication rates of CTO procedure even in experienced dedicated PCI operators and the higher failure rates of CTO-PCI, were still reported [21–26]. Therefore, it is important to select the patients suitable for CTO-PCI and to assess the impact of CTO revascularization by conventional assessment.
Several investigators reported the effect of revascularization of CTO on LV function. Henriques et al. reported that they did not find an overall benefit for CTO-PCI in terms of LVEF or LVEDV [27]. Mashayekhi et al. reported that no benefit was seen for CTO-PCI in terms of the segmental wall thickening, LVEF and LVEDV [28]. In this study, LVEF and LVEDV also showed no significant changes both successful and failed PCI groups during the 9 month follow-up, respectively. However, since there was report that reduced major adverse coronary event rates at 12 months in CTO-PCI compared with optimal medical therapy [28], we assessed cardiac function evaluated by GLS, with the idea that some improvement in cardiac function that can not be assessed by LVEF or LVEDV in successful CTO-PCI group.
GLS assessed with 2DSTE is a new echocardiographic technique for the evaluation of global myocardial function. This method is based on tracking the characteristics speckle patters created by interference of ultrasound beams in the myocardium. Its accuracy has been confirmed using CMR [29] and it does not require special equipment. Kalam et al. showed that the subendocardial longitudinal fiber is more sensitive to myocardial ischemia and the depressed LV longitudinal function occured at an earlier stage than abnormal radial contraction, as assessed by the LVEF [30]. GLS represents the subendocardial longitudinal fiber movement [31], therefore, in the case of preserved LVFE CTO patients, GLS assessed by 2DSTE is a quite suitable method to evaluate the change of myocardial ischemic burden.
In this study, we studied of preserved LVEF patients with positive functional ischemia of CTO lesion before PCI, and assessed the effect of revascularization of CTO on LV function using GLS assessed by 2DSTE before and 9months after procedure. GLS showed a significant improvement 9 months after successful PCI, whereas that in failed PCI group did not change significantly. These results indicated that CTO revascularization improves LV function.
Limitation
This study is limited by the fact that it was a single center, retrospective study and had a small sample size, which might diminish the power of the drawn statistical inference. A further study with a large scale prospective, multicenter design is thus required to confirm our results. An additional limitation to this study is that radial and circumferential strain were not explored. However, it has been recently reported that longitudinal deformation may be a more sensitive marker of cardiac function compared with radial and circumferential strain, especially in chronic ischemic patients [32], so not assessing the radial and circumferential strain dose not affect the result. And then the assessment of the success or failure of the PCI was done immediately after the PCI; it may not reflect what happened later during the 9 months.
Conclusion
Successful PCI for CTO improved LV function, assessed by LV GLS.
Acknowledgments
The authors greatly thank the doctors of the Department of Cardiology and the sonographers of Echo Lab at Himeji Cardiovascular Center for their help in this study.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991–997. 10.1016/j.jacc.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 2.Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: Results from the FlowCardia's Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes 2010;3:284–290 10.1161/CIRCOUTCOMES.108.825760 [DOI] [PubMed] [Google Scholar]
- 3.Mehran R, Claessen BE, Godino C, Dangas GD, Obunai K, Kanwal S, et al. Multinational Chronic Total Occlusion Registry. Long-term outcome of percutaneous coronary intervention for chronic total occlusions. JACC Cardiovasc Interv 2011;4:952–961 10.1016/j.jcin.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 4.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 2004;17:1021–1029 10.1016/j.echo.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 5.Ng AC, Tran da T, Newman M, Allman C, Vidaic J, Kadappu KK, et al. Comparison of myocardial tissue velocities measured by two-dimensional speckle tracking and tissue Doppler imaging. Am J Cardiol 2008;102:784–789 10.1016/j.amjcard.2008.05.027 [DOI] [PubMed] [Google Scholar]
- 6.Blessberger H, Binder T. NON-invasive imaging: Two dimensional speckle tracking echocardiography: basic principles. Heart 2010;96:716–722 10.1136/hrt.2007.141002 [DOI] [PubMed] [Google Scholar]
- 7.Liou K, Negishi K, Ho S, Russell EA, Cranney G, Ooi SY. Detection of Obstructive Coronary Artery Disease Using Peak Systolic Global Longitudinal Strain Derived by Two-Dimensional Speckle-Tracking: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr 2016;29:724–735 10.1016/j.echo.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Li J, Ren M, Wang ZZ, Li ZY, Gao F, et al. Multilayer longitudinal strain at rest may help to predict significant stenosis of the left anterior descending coronary artery in patients with suspected non-ST-elevation acute coronary syndrome. Int J Cardiovasc Imaging 2016;32:1675–1685 [DOI] [PubMed] [Google Scholar]
- 9.Dimitriu-Leen AC, Scholte AJ, Katsanos S, Hoogslag GE, van Rosendael AR, van Zwet EW, et al. Influence of Myocardial Ischemia Extent on Left Ventricular Global Longitudinal Strain in Patients After ST-Segment Elevation Myocardial Infarction. Am J Cardiol 2017;119:1–6 10.1016/j.amjcard.2016.08.091 [DOI] [PubMed] [Google Scholar]
- 10.Dattilo G, Imbalzano E, Lamari A, Casale M, Paunovic N, Busacca P, et al. Ischemic heart disease and early diagnosis. Study on the predictive value of 2D strain. Int J Cardiol 2016;215:150–156 10.1016/j.ijcard.2016.04.035 [DOI] [PubMed] [Google Scholar]
- 11.Liel-Cohen N, Tsadok Y, Beeri R, Lysyansky P, Agmon Y, Feinberg MS, et al. A new tool for automatic assessment of segmental wall motion based on longitudinal 2D strain: a multicenter study by the Israeli Echocardiography Research Group. Circ Cardiovasc Imaging 2010;3:47–53 10.1161/CIRCIMAGING.108.841874 [DOI] [PubMed] [Google Scholar]
- 12.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr 2004;17:630–633 10.1016/j.echo.2004.02.011 [DOI] [PubMed] [Google Scholar]
- 13.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364 10.1161/CIRCIMAGING.109.862334 [DOI] [PubMed] [Google Scholar]
- 14.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J 2012;33:1716–1717 10.1093/eurheartj/ehs124 [DOI] [PubMed] [Google Scholar]
- 15.Di Mario C, Werner GS, Sianos G, Galassi AR, Büttner J, Dudek D, et al. European perspective in the recanalisation of Chronic Total Occlusions (CTO): consensus document from the EuroCTO Club. EuroIntervention 20073:30–43 [PubMed] [Google Scholar]
- 16.George S, Cockburn J, Clayton TC, Ludman P, Cotton J, Spratt J, et al. British Cardiovascular Intervention Society; National Institute for Cardiovascular Outcomes Research. Long-term follow-up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. Central Cardiac Audit Database. J Am Coll Cardiol 2014;64:235–243 10.1016/j.jacc.2014.04.040 [DOI] [PubMed] [Google Scholar]
- 17.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014; 100:1673–1680 10.1136/heartjnl-2014-305538 [DOI] [PubMed] [Google Scholar]
- 18.Chimura M, Onishi T, Tsukishiro Y, Sawada T, Kiuchi K, Shimane A, et al. Longitudinal strain combined with delayed-enhancement magnetic resonance improves risk stratification in patients with dilated cardiomyopathy. Heart 2017;103:679–686 10.1136/heartjnl-2016-309746 [DOI] [PubMed] [Google Scholar]
- 19.Kahn JK. Angiographic suitability for catheter revascularization of total coronary occlusions in patients from a community hospital setting. Am Heart J 1993;126:561–564 [DOI] [PubMed] [Google Scholar]
- 20.Werner GS, Gitt AK, Zeymer U, Juenger C, Towae F, Wienbergen H, et al. Chronic total coronary occlusions in patients with stable angina pectoris: impact on therapy and outcome in present day clinical practice. Clin Res Cardiol 2009;98:435–441 10.1007/s00392-009-0013-5 [DOI] [PubMed] [Google Scholar]
- 21.Prasad A, Rihal CS, Lennon RJ, Wiste HJ, Singh M, Holmes DR Jr. Trends in outcomes after percutaneous coronary intervention for chronic total occlusions: A 25-year experience from the Mayo Clinic. J Am Coll Cardiol 2007;49:1611–1618 10.1016/j.jacc.2006.12.040 [DOI] [PubMed] [Google Scholar]
- 22.Rathore S, Matsuo H, Terashima M, Kinoshita Y, Kimura M, Tsuchikane E, et al. Procedural and in-hospital outcomes after percutaneous coronary intervention for chronic total occlusions of coronary arteries 2002 to 2008: Impact of novel guidewire techniques. JACC Cardiovasc Interv 2009;2:489–497 10.1016/j.jcin.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Reifart NJ, Moussa I, Hoye A, Cox DA, Colombo A, et al. Percutaneous recanalization of chronically occluded coronary arteries: A consensus document: part II. Circulation 2005;112:2530–2537 10.1161/CIRCULATIONAHA.105.583716 [DOI] [PubMed] [Google Scholar]
- 24.Elezi S, Kastrati A, Wehinger A, Walter H, Schühlen H, Hadamitzky M, et al. Clinical and angiographic outcome after stent placement for chronic coronary occlusion. Am J Cardiol 1998;82:803–806 [DOI] [PubMed] [Google Scholar]
- 25.Violaris AG, Melkert R, Serruys PW. Long-term luminal renarrowing after successful elective coronary angioplasty of total occlusions. A quantitative angiographic analysis. Circulation 1995;91:2140–2150 [DOI] [PubMed] [Google Scholar]
- 26.Galassi AR, Tomasello SD, Reifart N, Werner GS, Sianos G, Bonnier H, et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. EuroIntervention 2011;7:472–479 10.4244/EIJV7I4A77 [DOI] [PubMed] [Google Scholar]
- 27.Henriques JP, Hoebers LP, Råmunddal T, Laanmets P, Eriksen E, Bax M, et al. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients With STEMI: The EXPLORE Trial. J Am Coll Cardiol. 2016;15:1622–1632. [DOI] [PubMed] [Google Scholar]
- 28.Mashayekhi K, Nührenberg TG, Toma A, Gick M, Ferenc M, Hochholzer W, et al. A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion: The REVASC Trial. JACC Cardiovasc Interv. 2018;19:1982–1991 [DOI] [PubMed] [Google Scholar]
- 29.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006;47:789–793 10.1016/j.jacc.2005.10.040 [DOI] [PubMed] [Google Scholar]
- 30.Sabbah HN, Marzilli M, Stein PD. The relative role of subendocardium and subepicardium in left ventricular mechanics. Am J Physiol 1981;240:H920–926 10.1152/ajpheart.1981.240.6.H920 [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto I, Li X, Hejmadi Bhat A, Jones M, Zetts AD, Sahn DJ. Myocardial strain rate is a superior method for evaluation of left ventricular subendocardial function compared with tissue Doppler imaging. J Am Coll Cardiol 2003;42:1574–1583 [DOI] [PubMed] [Google Scholar]
- 32.Sengupta PP, Narula J. Reclassifying heart failure: predominantly subendocardial, subepicardial, and transmural. Heart Fail Clin 2008;4:379–382 10.1016/j.hfc.2008.03.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.