Abstract
The Bulldog is a popular companion breed in the UK despite widely reported disease predispositions. This study aimed to characterise the demography, mortality and common disorders of Bulldogs under veterinary care in the UK during 2013. VetCompass collates anonymised clinical data from UK primary-care veterinary practices for epidemiological research. The clinical records of all Bulldogs available in the VetCompass study dataset were reviewed manually in detail to extract the most definitive diagnoses recorded for all disorders that existed during 2013 and for all deaths. Bulldogs comprised 1621 (0.36%) of 445,557 study dogs. Bulldogs increased from 0.35% of the 2009 birth cohort to 0.60% in 2013. Median longevity was 7.2 years, which was lower in males (6.7 years) than females (7.9 years) (P = 0.021). The most prevalent fine-level precision disorders recorded were otitis externa (n = 206, prevalence 12.7%, 95% CI: 11.1–14.4), pyoderma (142, 8.8%, 95% CI: 7.4–10.2) and overweight/obesity (141, 8.7%, 95% CI: 7.4–10.2). The most prevalent disorder groups were cutaneous (n = 463, prevalence: 28.6%, 95% CI: 26.4–30.8), ophthalmological (292, 18.0%, 95% CI: 16.2–20.0), aural (211, 13.0%, 95% CI: 11.4–14.8), enteropathy (188, 11.6%, 95% CI: 10.1–13.3) and upper respiratory tract (171, 10.5%, 95% CI: 9.1–12.1). Provision of an evidence base on the most common disorders and causes of mortality within breeds can support owners, breeders and the veterinary profession to improve health and welfare within these breed.
Introduction
Arguably the most iconic of British dog breeds, the Bulldog (British Bulldog) descends from dogs originally used for blood sports in the 16th century [1]. These early bulldogs were small, thick-set dogs with powerful jaws that were used for bull-baiting [1]. After bull baiting was banned in 1835, the bulldog sank into obscurity, regarded as ‘a relic of a barbarous and bygone age’ [2, 3]. However, when the Kennel Club was founded in 1873, the rehabilitated Bulldog was among the first breeds it recognised [4]. The show Bulldog was selectively bred to resemble an idealised physical ‘breed standard’, in which various features, such as a head ‘the larger the better’ and a protruding lower jaw, were intended to define a dog suitably shaped for bull-baiting, despite the sport’s abolition [3]. Various subsequent changes, both in the breed standard and in its interpretation by breeders, have since further modified the shape of the Bulldog; the short ‘screw’ tail often seen today, for example, was a controversial novelty in the 1890s [5]. In recent years, the UK Kennel Club breed standard has been reworded to discourage extreme conformation [6].
In recent years, in line with a general rise in popularity of the brachycephalic (flat faced) breeds, the Bulldog has risen in popularity [7]. In 2017, the Bulldog was the sixth most common breed registered by the Kennel Club [8]. Although the Bulldog has not experienced such a dramatic surge in popularity as the French Bulldog and Pug have shown over the past decade, annual registration data from the UK Kennel Club still depicts over a two-fold increase in registrations during this time-scale, from 4543 (1.7% of all registrations) in 2008 to 9450 (3.9% of all registrations) in 2017 [9].
The distinctive physical appearance of brachycephalic breeds, including the Bulldog, is a key factor influencing their popularity [10]. However, some aspects of the Bulldog conformation, such as short muzzles and wrinkled faces, are associated with health problems [11–13]. Bulldogs are reported as predisposed to several health disorders, including brachycephalic obstructive airway syndrome (BOAS) [11, 14–16], dystocia [17], patellar luxation [18], seizures [19], corneal ulcers [12, 20] and spinal disease associated with vertebral malformations [21]. Indeed, Bulldogs have been reported with 39 breed predispositions to disease [22].
These disease predispositions are not new developments. There is ample archival evidence that a similar range of disorders already troubled Bulldogs over a century ago. Then, some Bulldog enthusiasts and judges, concerned that selection for exaggerated conformation had rendered the breed ‘a mere caricature’ of its bull-baiting ancestors [23] complained that the show Bulldog of c. 1900 had ‘a sadly shortened duration of life’ [24] and was beset by exercise intolerance and dystocia [23, 25]. They described features which today would be considered as linked to BOAS, such as nostrils ‘so small and pinched that it would be [a] hard job to pass a toothpick’ [26], upper jaws so short ‘as to render them incapable of getting a firm hold on anything more aggressive than a beefsteak’ [27], and that Bulldogs were ‘peculiarly susceptible’ to ‘heat apoplexy’ (hyperthermia) [28]. Canine veterinarians of that era recognised the increased risk of anaesthetising short-nosed breeds [29, 30]. Nonetheless, they advised early caesarian section for Bulldog bitches with dystocia, in the hope of at least saving the puppies, if not the bitch [29–32]. Breed activists, themselves often breeders, lamented these various issues. They urged judges not to award prizes to ‘cripples, deformities and grotesques … [with] exaggerated points’, and recommended breeding from ‘hardy and active’ dogs [33].
However, more than a century later, many Bulldogs are still struggling with similar difficulties [34, 35]. The UK Kennel Club lists the Bulldog as a Category 3 breed (the highest category) in its ‘Breed Watch’ system [36] with points of concern that focus on the airways, skin, tail, weight, eyes and gait [37].
Using veterinary clinical data from the VetCompass Programme [38], this study aimed to characterise the demography, longevity and common disorders of the general population of Bulldogs under veterinary care in the UK. The study additionally aimed to compare results between males and females, and specifically report the proportion of dogs recorded as blue or merle colour. Based on both the direct content of the current study and on comparability to other VetCompass breed studies, these results could support initiatives aimed at improving breeding and clinical management practices that ultimately contribute to better health and welfare of Bulldogs [39, 40].
Materials and methods
The study population included all dogs under primary veterinary care at clinics participating in the VetCompass Programme during 2013. Dogs under veterinary care were defined as those with either a) at least one electronic patient record (EPR) (VeNom diagnosis term, free-text clinical note, treatment or bodyweight) recorded during 2013 or b) at least one EPR recorded both before and after 2013. VetCompass collates de-identified EPR data from primary-care veterinary practices in the UK for epidemiological research [38]. Data fields available to VetCompass researchers include a unique animal identifier along with species, breed, date of birth, colour, sex, neuter status and bodyweight, and also clinical information from free-form text clinical notes, summary diagnosis terms [41] and treatment with relevant dates.
A prevalence study design derived from the cohort clinical data of dogs registered at participating practices was used to estimate the one-year period prevalence of the most commonly diagnosed disorders [42]. Sample size calculations estimated that 1,696 dogs would be sampled to estimate a disorder that had 5% prevalence with 1% acceptable margin of error at a 95% confidence level (assuming Bulldogs comprised 0.3% of a UK population of 8 million dogs [43, 44]. Ethics approval was obtained from the RVC Ethics and Welfare Committee (reference number 2015/1369).
Dogs recorded as Bulldog breed at their final available record were categorised as Bulldog and all remaining dogs were categorised as non-Bulldog. Breed classification was thus ultimately based on owner and/or veterinary practice identification that could be refined over time as the dog revisited the practice. No distinction was made between show, Kennel Club registered or unregistered Bulldogs. Animals that were recorded as entirely or partly blue were categorised as ‘blue’. Adult Bodyweight described the mean bodyweight (Kg) recorded from all bodyweight data for dogs aged over 18 months at the time of weighing and was categorised into 6 groups (< 20.0, 20.0 to < 24.0, 24.0 to < 28.0, 28.0 to < 32.0, 32.0 to < 36.0, ≥ 36.0). Neuter described the status of the dog (entire or neutered) at the final EPR. Age described the age (years) at the final date under veterinary care during 2013 (December 31st, 2013) and was categorised into 8 groups (< 1.0, 1.0 to < 2.0, 2.0 to < 4.0, 4.0 to < 6.0, 6.0 to < 8.0, 8.0 to < 10.0, 10.0 to < 12.0, ≥ 12.0).
The list of unique Bulldog animal identification numbers was randomly ordered and the clinical records of all animals were reviewed manually in detail to extract the most definitive diagnoses recorded for all disorders that existed during 2013 [15]. Elective (e.g. neutering) or prophylactic (e.g. vaccination) clinical events were not included. No distinction was made between pre-existing and incident disorder presentations. Disorders described within the clinical notes using presenting sign terms (e.g. ‘vomiting’ or 'vomiting and diarrhoea'), but without a formally recorded clinical diagnostic term, were included using the first sign listed (e.g. vomiting). These disorders included in the study did not need to be the reason that the dog presented for veterinary care; awareness of the existence of many of these disorders was first raised as part of veterinary examinations during routine visits for prophylactic care such as vaccination or during visits that were precipitated by an altogether different condition. Mortality data (recorded cause, date and method of death) were extracted on all deaths at any date during the available EPR data. The extracted diagnosis terms were mapped to a dual hierarchy of diagnostic precision for analysis: fine-level precision and grouped-level precision as previously described [15]. Briefly, fine-level precision terms described the original extracted terms at the maximal diagnostic precision recorded within the clinical notes (e.g. inflammatory bowel disease would remain as inflammatory bowel disease). Grouped-level precision terms mapped the original diagnosis terms to a general level of diagnostic precision (e.g. inflammatory bowel disease would map to gastro-intestinal). This approach of reporting disorders at two levels of precisions aimed to accommodate for the varying depths of clinical precision across the breadth of primary care clinical caseloads.
Following data checking for internal validity and cleaning in Excel (Microsoft Office Excel 2013, Microsoft Corp.), analyses were conducted using Stata Version 13 (Stata Corporation). The sex, neuter status, age, colour and adult bodyweight for Bulldogs under veterinary care during 2013 were described. Annual proportional birth rates described the relative proportion of Bulldogs compared with all dogs that were born in each year from 2008–2013 from the cohort that were under veterinary care in 2013. All-age bodyweight data with their associated dates were used to generate individual bodyweight growth curves for male and female Bulldogs by plotting age-specific bodyweights and were overlaid with a cross medians line plot using the Stata mband command.
One-year (2013) period prevalence values were reported along with 95% confidence intervals (CI) that described the probability of diagnosis at least once during 2013. The CI estimates were derived from standard errors based on approximation to the normal distribution for disorders with ten or more events [45]. The median age at the end of the study date range was reported for affected animals. Prevalence values were reported overall and also separately for males and females. The chi-square test was used to compare categorical variables and the Students t-test or Mann-Whitney U test to compare continuous variables as appropriate [45]. Statistical significance was set at the 5% level.
From a qualitative research perspective [46], this paper discussed the health concerns that were written about for the Bulldog during its previous peak of popularity (around 1900) in relation to the main concerns of the modern Bulldog. This archival research was carried out through a survey of publications (newspapers, journals and books) serving the British dog breeding and veterinary communities at this time. If was not possible to compare the health status between these periods directly because there was no comparable quantitative data resource available for the breed in 1900.
Results
Demography and mortality
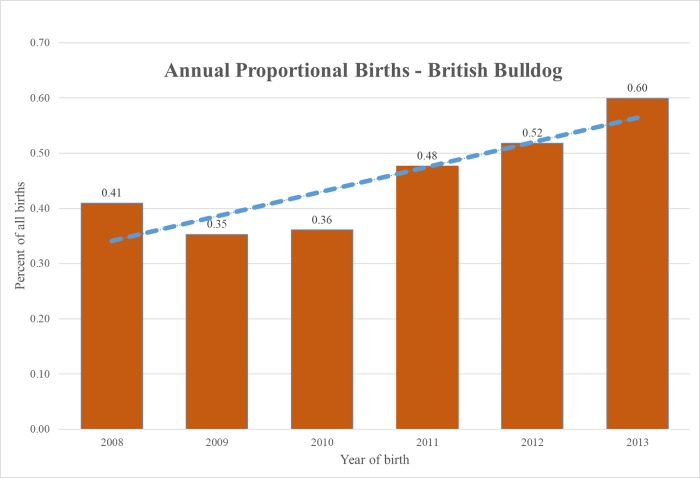
The study population of 455,557 dogs from 304 clinics in the VetCompass database under veterinary care during 2013 included 1,621 (0.36%) Bulldogs. This is a lower percentage than indicated by Kennel Club registration figures, but practice data additionally includes unregistered dogs of all breeds, including those, such as ‘designer’ and other crossbreeds, which are ineligible for Kennel Club registration [15]. Annual proportional birth rates showed gradually rising popularity of Bulldogs in the UK, ranging from 0.35% of all puppy births in 2009 to 0.60% in 2013 (Fig 1).
Fig 1. Annual proportional birth rates (2008–2013) for Bulldogs in the UK.
The analysis included 1,621 Bulldogs from 455,557 dogs attending UK primary-care veterinary clinics participating in the VetCompass Programme.
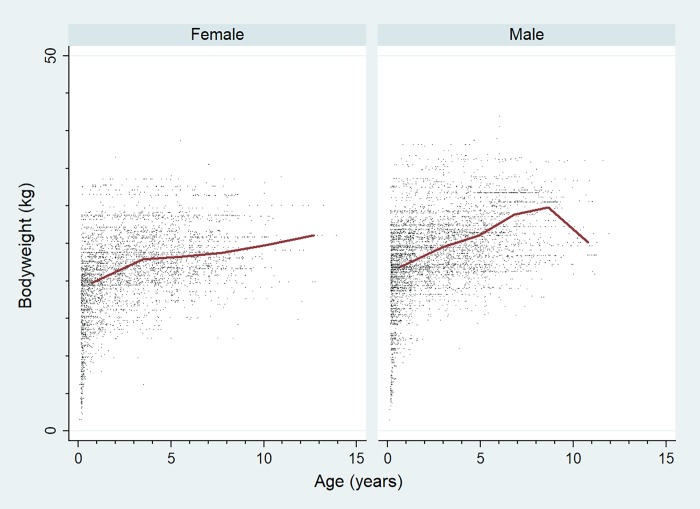
Of the Bulldogs with information available, 805 (49.9%) were female and 410 (25.8%) were neutered. Neuter status did not differ between females and males (25.3% versus 26.6%, P = 0.550). Eight dogs (0.5%) were recorded as blue (partly or completely) and there were no dogs recorded as merle. The median age of the Bulldogs overall was 2.3 years (IQR 1.1–4.5, range 0.1–14.0). The overall mean adult bodyweight was 26.0 kg (standard deviation [SD] 4.7 kg). The mean adult bodyweight of males (27.6 kg, SD 4.6 kg) was heavier than females (24.3 kg, SD 4.1 kg) (P < 0.001) (Table 1). The median bodyweight across all ages for males (23.9 kg, IQR: 21.1–27.3, range: 1.5–42.0) was higher than for females (21.7 kg, IQR: 18.6–24.7, range: 1.6–38.7) (P < 0.001). Bodyweight growth curves based on 3,974 bodyweight values from 662 females and 5,163 bodyweight values from 690 males across all ages showed that Bulldog puppies grow rapidly during their first year but progressive with slower bodyweight increases for most of their adult lives (Fig 2). Data completeness varied across the variables assessed: sex 99.5%, neuter 97.9%, age 97.7%, colour 92.8% and all-age bodyweight 83.8%.
Table 1. Demography of Bulldogs under primary veterinary care at practices participating in the VetCompass Programme in the UK from January 1st, 2013 to December 31st, 2013 (n = 1,621).
| Variable | Category | Count* | Percent |
|---|---|---|---|
| Sex | Female | 805 | 49.9 |
| Male | 808 | 50.1 | |
| Female neuter | Entire | 596 | 74.7 |
| Neutered | 202 | 25.3 | |
| Male neuter | Entire | 573 | 73.4 |
| Neutered | 208 | 26.6 | |
| Colour | Blue (partly or completely) | 8 | 0.5 |
| Merle | 0 | 0.0 | |
| Not blue or merle | 1,497 | 99.5 | |
| Female adult bodyweight (aged ≥ 18 months) (kg) | < 20.0 | 68 | 14.2 |
| 20.0 to < 24.0 | 169 | 35.2 | |
| 24.0 to < 28.0 | 161 | 33.5 | |
| 28.0 to < 32.0 | 59 | 12.3 | |
| 32.0 to < 36.0 | 20 | 4.2 | |
| ≥ 36.0 | 3 | 0.6 | |
| Male adult bodyweight (aged ≥ 18 months) (kg) | < 20.0 | 14 | 2.7 |
| 20.0 to < 24.0 | 98 | 18.9 | |
| 24.0 to < 28.0 | 183 | 35.3 | |
| 28.0 to < 32.0 | 131 | 25.3 | |
| 32.0 to < 36.0 | 67 | 12.9 | |
| ≥ 36.0 | 25 | 4.8 | |
| Age (years) | < 1.0 | 372 | 23.5 |
| 1.0 to < 2.0 | 333 | 21.0 | |
| 2.0 to < 4.0 | 398 | 25.1 | |
| 4.0 to < 6.0 | 271 | 17.1 | |
| 6.0 to < 8.0 | 125 | 7.9 | |
| 8.0 to < 10.0 | 62 | 3.9 | |
| 10.0 to < 12.0 | 19 | 1.2 | |
| ≥ 12.0 | 3 | 0.2 |
* Results from dogs with available data.
Fig 2. Bodyweight growth curves for female and male Bulldogs in the UK.
The analysis included 3,974 bodyweight values from 662 female Bulldogs and 5,163 bodyweight values from 690 male Bulldogs attending UK primary-care veterinary clinics participating in the VetCompass Programme. The bodyweight growth curves are overlaid with a cross medians line plot.
There were 181 deaths recorded during the study. The median longevity of Bulldogs overall was 7.2 years (IQR 4.9–9.3, range 0.0–14.1). The median longevity of females (7.9 years, IQR 4.9–10.2, range 0.7–14.1, n = 73) was greater than males (6.7 years, IQR 4.4–9.1, range 0.0–11.9, n = 107) (P = 0.021). The median longevity of neutered animals (8.0 years, IQR 5.8–10.3, range 1.2–14.1, n = 66) was greater than entire animals (6.7 years, IQR 4.3–9.0, range 0.0–13.0, n = 113) (P = 0.003). The cause of death was not recorded for 71 (39.2%) of deaths. Of the 110/181 dogs with a recorded cause of death, the most common causes of death described at a grouped-precision level were heart disease (n = 13, prevalence 11.8%), neoplasia (12, 10.9%) and brain disorder (10, 9.1%) (Table 2).
Table 2. Grouped causes of mortality in Bulldogs with a recorded cause of death under primary-care veterinary at UK practices participating in the VetCompass Programme from January 1st, 2013 to December 31st, 2013 (n = 110).
| Grouped disorder term | Count | Percent | 95%CI* |
|---|---|---|---|
| Heart disease | 13 | 11.8 | 6.4–19.4 |
| Neoplasia | 12 | 10.9 | 5.8–18.3 |
| Brain disorder | 10 | 9.1 | 4.4–16.1 |
| Collapsed | 7 | 6.4 | 2.6–12.7 |
| Lower respiratory tract disorder | 7 | 6.4 | 2.6–12.7 |
| Mass-associated disease | 7 | 6.4 | 2.6–12.7 |
| Undesirable behaviour | 6 | 5.5 | 2.0–11.5 |
| Upper respiratory tract disease | 6 | 5.5 | 2.0–11.5 |
| Other | 42 | 38.2 | 29.1–47.9 |
| Total | 110 |
* CI confidence interval
Disorder prevalence
The EPRs of all 1,621 Bulldogs were manually examined to extract all recorded disorder data for 2013. There were 1,148 (70.8%) Bulldogs with at least one disorder recorded during 2013 while the remaining 29.2% had no disorder recorded and either presented for prophylactic management only or did not present at all during 2013. The median annual disorder count per Bulldog during 2013 was 1 disorder (IQR 0–3, range 0–15) and was higher in males (median 1.5, IQR 0–3, range 0–15) compared with females (median 1, IQR 0–3, range 0–14) (P = 0.006).
The study included 3,298 unique disorder events recorded during 2013 that encompassed 296 distinct fine-level disorder terms. The most prevalent fine-level precision disorders recorded were otitis externa (n = 206, prevalence 12.7%, 95% CI: 11.1–14.4), pyoderma (142, 8.8%, 95% CI: 7.4–10.2), overweight/obesity/ (141, 8.7%, 95% CI: 7.4–10.2), skin fold dermatitis (126, 7.8%, 95% CI% 6.5–9.2), overlong nails (119, 7.3%, 95% CI 6.1–8.7) and prolapsed gland of third eyelid (cherry eye) (110, 6.8%, 95% CI% 5.6–8.1). Males had higher prevalence than females for 4 of the 29 most common fine-level precision disorders (pyoderma, interdigital cyst, atopic dermatitis and aggression) while females had higher prevalence for 2 disorders (periodontal disease and overweight/obesity). There were 50 dogs (3.1% prevalence, 95% CI: 2.3–4.0) recorded with a condition that was paradoxically labelled as ‘normal for the breed’ (Table 3).
Table 3. Prevalence of the most common disorders at a fine-level of diagnostic precision recorded in Bulldogs (n = 1,621) attending UK primary-care veterinary practices participating in the VetCompass Programme from January 1st, 2013 to December 31st, 2013.
The P-value reflects prevalence comparison between females and males. The median age described the age of diagnosed cases on the end of the study date range.
| Fine-level disorder | Count | Overall prevalence % | 95% CI* | Female prevalence % | Male prevalence % | P-Value | Median age at diagnosis (years) |
|---|---|---|---|---|---|---|---|
| Otitis externa | 206 | 12.7 | 11.1–14.4 | 11.6 | 14.0 | 0.143 | 3.8 |
| Pyoderma | 142 | 8.8 | 7.4–10.2 | 6.5 | 11.1 | 0.001 | 2.4 |
| Overweight/ obesity | 141 | 8.7 | 7.4–10.2 | 10.7 | 6.8 | 0.006 | 2.7 |
| Skin fold dermatitis | 126 | 7.8 | 6.5–9.2 | 7.1 | 8.5 | 0.275 | 2.4 |
| Overlong nails | 119 | 7.3 | 6.1–8.7 | 7.0 | 7.8 | 0.518 | 2.8 |
| Prolapsed gland of third eyelid | 110 | 6.8 | 5.6–8.1 | 6.2 | 7.4 | 0.333 | 1.2 |
| Cryptorchidism (males only) | 45 | 5.6 | 4.1–7.4 | ~ | ~ | ~ | 1.1 |
| Conjunctivitis | 88 | 5.4 | 4.4–6.6 | 4.8 | 6.1 | 0.281 | |
| Pododermatitis | 88 | 5.4 | 4.4–6.6 | 4.5 | 6.4 | 0.083 | 4.0 |
| Alopecia | 86 | 5.3 | 4.3–6.5 | 4.5 | 6.2 | 0.125 | 2.2 |
| Diarrhoea | 79 | 4.9 | 3.9–6.0 | 4.1 | 5.7 | 0.138 | 1.1 |
| Pyotraumatic dermatitis | 70 | 4.3 | 3.4–5.4 | 3.5 | 5.2 | 0.090 | 2.2 |
| Interdigital cyst | 60 | 3.7 | 2.8–4.7 | 2.2 | 5.2 | 0.002 | 3.7 |
| Entropion | 58 | 3.6 | 2.7–4.6 | 3.9 | 3.3 | 0.583 | 2.8 |
| Vomiting | 58 | 3.6 | 2.7–4.6 | 2.7 | 4.5 | 0.063 | 1.5 |
| Brachycephalic obstructive airway syndrome (BOAS) | 57 | 3.5 | 2.7–4.5 | 3.1 | 4.0 | 0.353 | 2.7 |
| Atopic dermatitis | 57 | 3.5 | 2.7–4.5 | 2.1 | 5.0 | 0.002 | 3.6 |
| Corneal ulceration | 51 | 3.1 | 2.4–4.1 | 3.0 | 3.3 | 0.679 | 2.8 |
| Normal for breed | 50 | 3.1 | 2.3–4.0 | 2.4 | 3.8 | 0.087 | 1.3 |
| Prognathism | 47 | 2.9 | 2.1–3.8 | 3.6 | 2.2 | 0.101 | 1.0 |
| Umbilical hernia | 45 | 2.8 | 2.0–3.7 | 2.9 | 2.6 | 0.750 | 1.1 |
| Lameness | 44 | 2.7 | 2.0–3.6 | 2.1 | 3.3 | 0.130 | 1.8 |
| Malassezia dermatitis | 40 | 2.5 | 1.8–3.3 | 2.0 | 3.0 | 0.204 | 4.2 |
| Aggression | 39 | 2.4 | 1.7–3.3 | 1.6 | 3.2 | 0.036 | 4.1 |
| Keratoconjunctivitis sicca | 36 | 2.2 | 1.6–3.1 | 2.1 | 2.4 | 0.745 | 6.1 |
| Periodontal disease | 34 | 2.1 | 1.5–2.9 | 2.9 | 1.4 | 0.037 | 5.5 |
| Demodicosis | 33 | 2.0 | 1.4–2.8 | 1.4 | 2.7 | 0.054 | 1.3 |
| Distichiasis | 32 | 2.0 | 1.4–2.8 | 2.1 | 1.9 | 0.713 | 2.6 |
| Upper respiratory tract infection | 25 | 1.5 | 1.0–2.2 | 1.6 | 1.5 | 0.833 | 1.0 |
* CI confidence interval
The study included 52 distinct grouped-level precision disorder terms. The most prevalent grouped-level precision disorders were cutaneous (n = 463, 28.6%, 95% CI: 26.4–30.8), ophthalmological (292, 18.0%, 95% CI: 16.2–20.0), aural (211, 13.0%, 95% CI: 11.4–14.8) and enteropathy (188, 11.6%, 95% CI: 10.1–13.3). Males had higher prevalence than females for cutaneous and enteropathy disorders while females had higher prevalence for overweight/obesity (Table 4).
Table 4. Prevalence of the most common grouped-level disorders recorded in Bulldogs (n = 1,621) attending UK primary-care veterinary practices participating in the VetCompassProgramme from January 1st, 2013 to December 31st, 2013.
The P-value reflects prevalence comparison between females and males. The median age described the age of cases on the end of the study date range.
| Grouped-level disorder | Count | Overall prevalence | 95% CI* | Female prevalence % | Male prevalence % | P-Value | Median age (years) |
|---|---|---|---|---|---|---|---|
| Cutaneous | 463 | 28.6 | 26.4–30.8 | 25.5 | 31.9 | 0.004 | 2.5 |
| Ophthalmological | 292 | 18.0 | 16.2–20.0 | 17.6 | 18.6 | 0.630 | 1.8 |
| Aural | 211 | 13.0 | 11.4–14.8 | 11.8 | 14.4 | 0.128 | 4.0 |
| Enteropathy | 188 | 11.6 | 10.1–13.3 | 9.8 | 13.5 | 0.021 | 1.3 |
| Upper respiratory tract | 171 | 10.5 | 9.1–12.1 | 9.6 | 11.6 | 0.177 | 1.4 |
| Overweight/obesity | 141 | 8.7 | 7.4–10.2 | 10.7 | 6.8 | 0.006 | 2.7 |
| Claw/nail | 136 | 8.4 | 7.1–9.8 | 7.7 | 9.2 | 0.292 | 2.7 |
| Musculoskeletal | 110 | 6.8 | 5.6–8.1 | 6.3 | 7.3 | 0.441 | 3.6 |
| Male reproductive (males only) | 55 | 6.8 | 5.2–8.8 | ~ | ~ | ~ | 1.2 |
| Female reproductive (females only) | 45 | 5.6 | 4.1–7.4 | ~ | ~ | ~ | 2.9 |
| Parasitic | 74 | 4.6 | 3.6–5.7 | 4.2 | 5.0 | 0.485 | 1.1 |
| Behavioural | 65 | 4.0 | 3.1–5.1 | 3.4 | 4.7 | 0.168 | 3.0 |
| Neoplastic | 58 | 3.6 | 2.7–4.6 | 3.1 | 4.1 | 0.291 | 4.0 |
| Urinary | 58 | 3.6 | 2.7–4.6 | 4.0 | 3.2 | 0.414 | 2.4 |
* CI confidence interval
Discussion
This study of over one thousand six hundred animals is the largest analysis to date in the UK of breed health in Bulldogs based on primary-care veterinary records. The results highlight a gradual rise in Bulldog ownership in the UK. Bulldogs comprised over 0.6% of all dogs born in 2013 attending the participating veterinary practices, although some of these dogs may have been born outside the UK. Given that the longevity data presented in this paper shows a relative short median longevity for Bulldogs of 7.2 years, it is possible that their rising ownership may be exaggerated as an artefact of their short lifespan that promotes left truncation of the data [47, 48]. However, annual registration data from the UK Kennel Club similarly depicts a two-fold increase in registrations over the past ten years, so the popularity of Bulldogs, along with other small-medium sized, flat-faced breeds, appears to be truly rising. This may be because potential owners consider them physically appealing but this increasing popularity is concerning because several of the most common disorders in Bulldogs are linked with their physical conformation [10].
Much of the previous evidence on health issues in Bulldogs was derived from referral, insurance and especially owner questionnaire data sources [49]. In contrast, the current study relies on de-identified clinical data recorded by primary-care veterinary teams and therefore offers a new perspective on the health issues that can supplement these other sources to build a more complete picture of breed health [50]. Research using veterinary clinical records that are recorded contemporaneously at the time of the clinical event and with no prior awareness of the subsequent specific research topics reduces recall and selection biases [49]. Results from breed-health studies such as the current study are contributing to modern breed health strategies such as those of the Kennel Club’s Breed Health and Conservation Plans project [40] and the strategic framework aims of the Brachycephalic Working Group [39].
The Kennel Club only permits registration of Bulldogs of a defined list of fifteen colourings: include brindle, fawn, red, red fawn, red brindle, as well as each of these colours combined with white [51]. The Kennel Club does not recognise blue, an autosomal recessive dilution which produces a slate-grey colour phenotype or merle, the blotched grey and black colouration seen in dogs heterozygous for the merle gene, colours in Bulldogs [52]. Merle is associated with both auditory and ophthalmologic abnormalities, particularly in homozygotes [52, 53]. Although there have been reports of a rise in public demand for blue (and, very recently, merle) Bulldogs [54], the current study reported that 0.5% were blue and 0.0% were merle in the general Bulldog population in 2013.
The 7.2 years median longevity of the Bulldog reported here was almost five years shorter than the 12.0 years median reported previously for dogs overall in England [55]. It is interesting to note that 7 years was still described as ‘quite an old age for show Bulldogs’ in 1901 [56]. Common causes of death in the current study included heart disease, neoplasia, brain disorders and respiratory tract (lower and upper) disorders. The relatively short median longevity of this breed may be a reflection of a generally high disorder burden, with the majority of Bulldogs (70.8%) recorded to have at least one disorder during 2013. These veterinary-derived results are markedly higher than the disorder prevalence estimated in an owner self-reported study in 2017, where only half (48.11%) of Bulldogs were reported as having 1 or more disorders [16]. This disparity may in part be due to the different data collection methodologies, with owners being susceptible to recognition biases when they consider disorders such as dyspnoea as ‘normal for the breed’ and therefore fail to acknowledge these as true health problems in their dog [57]. However, such normalising phenomena are also shown by veterinary surgeons; in the present study, 3.1% of dogs had a condition that was classified as ‘normal for the breed’ recorded in their clinical notes. Such normalisation may paradoxically result in underestimated prevalence figures for disorders that are common in a breed. While desensitisation of veterinary professionals to breed-related problems is problematic from a research perspective, it may also lead to potential under-treatment of clinical patients.
The most common disorders identified in Bulldogs in the current study were otitis externa, pyoderma, overweight/obesity and skin fold dermatitis. These results can provide a framework to identify health priorities in Bulldogs that can contribute positively to improved health and welfare within the breed. Some health distinctions were also identified between the sexes in this population of Bulldogs. Female Bulldogs lived over one year longer than males, with a similar female longevity advantage previously recorded in breeds such as the Rottweiler [58] and German Shepherd Dog [59] but not in others such as the French Bulldog [60]). It is unclear why a consistent sex-based longevity effect does not exist across breeds but differences may be explained by differing sex-associated disorder prevalence, inbreeding effects and early deaths across breeds [61–63]. Marked sex differences in disorder prevalence were observed in the current study, with males more likely than females to be diagnosed with 5 of the 29 most common fine-level precision disorders, and 2 of the 14 most common grouped-level precision disorders. The only disorders at fine- or grouped-level precision that were more common in female dogs were overweight/obesity. Whether this apparent female predisposition to overweight/obesity is a biological effect (e.g. hormonal), husbandry-related (e.g. owner influence) or assessment based (e.g. differing use of body condition scoring between males and females) is not clear [64]. These additional sex-based prevalence data can highlight those disorders that would benefit from special focus within specific sexes in order to contribute to improved Bulldog health and welfare as well as assisting decision-making by veterinarians and owners on the most appropriate sex selection when first deciding on puppy purchase [15, 65].
Skin disorders were the most common grouped-level category in the Bulldog, with an overall prevalence of 28.6%. At the fine-level diagnosis level, common skin disorders included pyoderma (8.8%), skin fold dermatitis (7.8%), pododermatitis (5.4%), alopecia (5.3%), pyotraumatic dermatitis (4.3%) and atopic dermatitis (3.5%) among the top 20 most common fine-level disorders in the breed. Skin disease is well recognised as a problem in Bulldogs; as long ago as 1897, concern for the high ‘general prevalence of eczema’ in the breed occasioned specific comment [66]. Kennel Club Breed Watch raises points of concern related to the skin, including hair loss or scarring from previous dermatitis, and lists several anatomical features of Bulldogs that may predispose to skin fold dermatitis including heavy overnose wrinkle (roll), inverted tail and tight tail [37]. Skin fold dermatitis may occur at any body site where excessive skin wrinkling causes skin-on-skin contact, including the facial region of brachycephalic dogs or in skin folds around absent, short or screw-tails [67]. High levels of fold-related skin disease have been documented in other small brachycephalic breeds, such as the Pug and French Bulldog [60, 68], which share the characteristic facial wrinkling and short or screw-tails features of the Bulldog. The Kennel Club breed standard for the Bulldog currently describes facial folds as “Over nose wrinkle, if present, whole or broken, must never adversely affect or obscure eyes or nose” [51], while the American Kennel Club standard more explicitly encourages a wrinkled look; “The head and face should be covered with heavy wrinkles” [69]. With skin conditions so prevalent in the Bulldog, it would be sensible to avoid breeding for exaggerated skin fold conformations that promote high welfare risks without advantages for the dog.
In addition to skin disorders that are directly related to conformation, atopic dermatitis (3.5%) featured in the top 20 most common disorders of the Bulldog in agreement with previous reports in the veterinary dermatology literature that the Bulldog breed is predisposed to canine atopic dermatitis [70]. Additionally, the most common fine-level disorder was otitis externa (12.7%). With previous research demonstrating that 43% of otitis externa cases result from underlying atopic dermatitis [71], and 59% of recurrent pyoderma cases reported as sequelae to allergic skin disease [72] it is possible that some or many of these otitis and pyoderma cases are associated with allergic skin disease pathology.
Ophthalmological disorders were the second most common grouped-level disorder of Bulldogs (18.0%), with prolapsed nictitans gland (6.8%), conjunctivitis (5.4%), entropion (3.6%) and corneal ulceration keratitis (3.1%) in the top 20 most common fine-level disorders in the breed. Many of these problems also have a long history of being reported in the breed; entropion, ectropion and conjunctivitis were described as prevalent in Bulldogs in 1908 [73]. A previous VetCompass study of corneal ulcers identified Bulldogs as the fifth most commonly affected breed (prevalence: 2.41%) and with six-times higher odds of corneal ulceration compared with crossbreeds [20]. In a prospective clinical study, 18% of Bulldogs were affected by corneal ulcers [12], with conformational risk factors for corneal ulceration identified including the presence of a nasal fold and brachycephalic skull shape which are both characteristic features of the Bulldogs [12]. Although Bulldogs do not share the same exophthalmic eye conformation as other brachycephalic breeds predisposed to corneal ulcers such as the Pug [12, 20], the marked nasal skin fold of the Bulldog may lead to medial canthal entropion and nasal fold trichiasis due to direct contact between the nasal fold and cornea. The Kennel Club (UK) has recently taken efforts to reduce this problem, with Breed Watch points of concern for the Bulldog including ‘sore eyes due to damage or poor eyelid conformation’, ‘Excessive amounts of loose facial skin with conformational defects of the upper and/or lower eyelids so that the eyelid margins are not in normal contact with the eye’ and ‘Heavy overnose wrinkle (roll)’ [37].
Upper respiratory tract (URT) disorders were commonly recorded in Bulldogs, being the fifth most common disorder at grouped-level diagnostic precision and affecting 10.5% of dogs. At fine-level diagnostic precision, the most common respiratory tract disorder was brachycephalic obstructive airway syndrome (BOAS) (3.5%; median age 2.7 years). With clinical signs that include chronic breathlessness, exercise intolerance, eating difficulties and disrupted sleeping including periods of apnoea, BOAS is a disorder of major animal welfare concern that adversely affects animals both while awake and asleep [74]. Archival sources describe a similar presentation extending back over a century. In 1902, a breed activist complained that the new-style Bulldog was ‘so exaggerated that … [it] can only move with difficulty, and pants painfully with the least exertion’ [24]. The apparently low prevalence of BOAS recorded in the current retrospective study contrasts with much higher prevalence reported in other prospective and disorder-specific studies. Sixty-three percent of Bulldogs attending a UK veterinary referral hospital were diagnosed with BOAS based on clinical history, owner questionnaire and clinical examination [11]. A study using whole-body barometric plethysmography reported that 84.8% of tested Bulldogs were affected by BOAS to some extent, with 44.0% exhibiting clinically relevant disease [75]. Finally, an earlier VetCompass study reported that 19.5% of Bulldogs had at least one URT disorder over a 4.5 year study period [76], compared to the one year period of the current study. Recognition of BOAS by both owners and veterinarians may also be hampered by perceptions that this disorder is ‘normal for the breed’; indeed, nearly 60% of owners of dogs affected by BOAS did not perceive that their dog had a breathing problem [57, 77]. Efforts to mitigate airway disease in the breed have been introduced via the Kennel Club Breed Watch scheme, where points of concern cover dogs showing respiratory distress including difficulty in breathing or laboured breathing, and pinched nostrils (a risk factor for BOAS [75]) [37]. Such measures, however, only directly influence those breeders who engage with Kennel Club initiatives and it may take some time for such progress to spill over to the wider population of dogs that are not registered with the Kennel Club. Moreover, the data used for the current study were collected relatively soon after the 2009 launch of Breed Watch, and therefore describes a Bulldog population that may yet not have benefitted much by its recommendations. As BOAS is intimately linked with conformation, major reductions in prevalence are unlikely without accompanying conformational change and therefore high levels of awareness and willingness to engage is critically needed from breed communities to ensure success [11].
This study had some limitations. Because primary-care practice data seldom records such information, the study did not directly assess the conformation of the dogs involved nor differentiate between the show, registered and unregistered populations. Therefore, while comparison of disease levels between these groups would be illuminating, it was consequently beyond the scope of this paper. Research using primary-care veterinary clinical data has some inherent limitations that have been previously reported [78]. Studies based on reviews of animal medical records may under-estimate true disease burdens by predominantly including those more severely affected animals that warrant veterinary management and there may be reduced reporting of less severely affected animals that may be less likely to be clinically presented [79]. Clinical variants of some diseases may be recorded using several distinct disorder terms and therefore the combined prevalence for these diseases may be fragmented into separate prevalence values for each of multiple more-specific diagnostic term, giving the illusion of lower prevalence [15]. The practices participating in the study were a convenience sample and may not be fully representative of the overall veterinary practice structure and caseloads in in the UK.
Conclusions
This study of over sixteen hundred Bulldogs documents rising ownership of this breed in the UK and provides important disorder prioritisation based on the general population of Bulldogs. The most common disorders in Bulldogs were otitis externa, pyoderma and overweight/obesity. The skin was the most affected body region, with pyoderma, skin fold dermatitis and pododermatitis among the top ten most common disorders of Bulldogs. Many common disorders of Bulldogs are linked with their appearance, including skin fold disease, BOAS and corneal ulceration. By contextualising these data within a longer historical timeframe, this paper observes that the high frequency of conformation-related disease in Bulldogs is a long-standing problem that also led breeders themselves to question conformational breeding goals over a century ago. These results provide a framework of health priorities for Bulldogs and therefore can contribute positively to improved health and welfare within the breed.
Acknowledgments
Thanks to Noel Kennedy (RVC) for VetCompass software and programming development. We acknowledge the Medivet Veterinary Partnership, Vets4Pets/Companion Care, Goddard Veterinary Group, Independent Vet Care, Beaumont Sainsbury Animal Hospital, Vets Now and the other UK practices who collaborate in VetCompass. Archival material was accessed through the Kennel Club Library, RCVS Knowledge and the British Library. We are grateful to The Kennel Club and The Kennel Club Charitable Trust for supporting VetCompass.
Abbreviations
- BOAS
brachycephalic obstructive airway syndrome
- CI
confidence interval
- EPR
electronic patient record
- IQR
interquartile range
- OR
odds ratio
- URT
upper respiratory tract
Data Availability
The dataset underlying the current study have been uploaded to the RVC open access database and are freely available at: http://researchonline.rvc.ac.uk/11945/.
Funding Statement
This study was supported at the RVC by an award from the Kennel Club Charitable Trust. Neither the Kennel Club Charitable Trust nor the Kennel Club had any input in the design of the study, the collection, analysis and interpretation of data or in writing the manuscript. RP is supported by BBSRC grant number BB/P010881/1. AS is supported by Wellcome Trust grant number 203385/Z/16/Z. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Leighton R. The Complete Book Of The Dog. London: Cassell and Company, Ltd; 1924. [Google Scholar]
- 2.Ritvo H. The animal estate: the English and other creatures in the Victorian Age. Cambridge, Mass.: Harvard University Press; 1987. [Google Scholar]
- 3.Dalziel H. British Dogs: their varieties, history, characteristics, breeding, management, and exhibition Illustrated with portraits of dogs of the day. By Hugh Dalziel … assisted by eminent fanciers. London: L. Upcottt Gill; 1889. [Google Scholar]
- 4.Worboys M, Strange J-M, Pemberton N. The Invention of the Modern Dog: Breed and Blood in Victorian Britain. Baltimore: John Hopkins University Press; 2018. [Google Scholar]
- 5.Warland TH. Bulldog Points. The Stock-keeper and Fanciers’ Chronicle. 1898. [Google Scholar]
- 6.The Kennel Club. Breed Standards Information: Dog Breeds & Groups: The Kennel Club; 2019. [Available from: https://www.thekennelclub.org.uk/activities/dog-showing/breed-standards/. [Google Scholar]
- 7.The Kennel Club. Comparative Table of Registrations, 1908–2017. In: Library. TKC, editor. London2018. [Google Scholar]
- 8.The Kennel Club. Breed registration statistics: The Kennel Club Limited; 2019. [Available from: http://www.thekennelclub.org.uk/registration/breed-registration-statistics/. [Google Scholar]
- 9.Kennel Club T. Breed Registration Statistics 2018. [Available from: https://www.thekennelclub.org.uk/registration/breed-registration-statistics. [Google Scholar]
- 10.Packer RMA, Murphy D, Farnworth MJ. Purchasing popular purebreds: investigating the influence of breed-type on the pre-purchase motivations and behaviour of dog owners. Animal Welfare. 2017;26(2):191–201. [Google Scholar]
- 11.Packer RMA, Hendricks A, Tivers MS, Burn CC. Impact of facial conformation on canine health: Brachycephalic Obstructive Airway Syndrome. PLoS ONE. 2015;10(10):e0137496 10.1371/journal.pone.0137496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Packer RMA, Hendricks A, Burn CC. Impact of Facial Conformation on Canine Health: Corneal Ulceration. PLOS ONE. 2015;10(5):e0123827 10.1371/journal.pone.0123827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MS, Cummings SL, Payton ME. Effect of brachycephaly and body condition score on respiratory thermoregulation of healthy dogs. Journal of the American Veterinary Medical Association. 2017;251(10):1160–5. 10.2460/javma.251.10.1160 [DOI] [PubMed] [Google Scholar]
- 14.Liu N-C, Sargan DR, Adams VJ, Ladlow JF. Characterisation of Brachycephalic Obstructive Airway Syndrome in French Bulldogs Using Whole-Body Barometric Plethysmography. PLOS ONE. 2015;10(6):e0130741 10.1371/journal.pone.0130741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS ONE. 2014;9(3):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiles BM, Llewellyn-Zaidi AM, Evans KM, O’Neill DG, Lewis TW. Large-scale survey to estimate the prevalence of disorders for 192 Kennel Club registered breeds. Canine Genetics and Epidemiology. 2017;4(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans KM, Adams VJ. Proportion of litters of purebred dogs born by caesarean section. J Small Anim Pract. 2010;51. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill DG, Meeson RL, Sheridan A, Church DB, Brodbelt DC. The epidemiology of patellar luxation in dogs attending primary-care veterinary practices in England. Canine Genetics and Epidemiology. 2016;3(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erlen A, Potschka H, Volk HA, Sauter-Louis C, O'Neill DG. Seizure occurrence in dogs under primary veterinary care in the UK: prevalence and risk factors. Journal of Veterinary Internal Medicine. 2018;32(5):1665–76. 10.1111/jvim.15290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill DG, Lee MM, Brodbelt DC, Church DB, Sanchez RF. Corneal ulcerative disease in dogs under primary veterinary care in England: epidemiology and clinical management. Canine Genetics and Epidemiology. 2017;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan R, Gutierrez-Quintana R, ter Haar G, De Decker S. Prevalence of thoracic vertebral malformations in French Bulldogs, Pugs and English Bulldogs with and without associated neurological deficits. The Veterinary Journal. 2017;221:25–9. 10.1016/j.tvjl.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 22.Gough A, Thomas A, O'Neill D. Breed Predispositions to Disease in Dogs and Cats. 3rd ed Chichester, West Sussex: Wiley-Blackwell; 2018. 398 p. [Google Scholar]
- 23.Redwar H. A Plea for the Bulldog. The Stock-keeper and Fanciers’ Chronicle. 1901:113. [Google Scholar]
- 24.Redwar H. The Breeding of Bulldogs. Illustrated Kennel News. 1902:399. [Google Scholar]
- 25.St. John Cooper H. The Evolution of the Bulldog. The Stock-keeper and Fanciers’ Chronicle. 1902. [Google Scholar]
- 26.Crowther FW. Bulldogs. The Kennel Gazette. 1893:10–2. [Google Scholar]
- 27.Preston Beecher J. The Evolution of the Bulldog. The Stock-keeper and Fanciers’ Chronicle. 1902:8. [Google Scholar]
- 28.Clarke CFC. “The Kennel” Bulldog Section. The Kennel. 1911:211 & 62. [Google Scholar]
- 29.Hobday F. Canine and Feline Surgical Operations. Veterinary Record. 1897;10(November 20):281–2. [Google Scholar]
- 30.Smith S. A Few Points in Canine Practice. Veterinary Record. 1910;23(July 23):55–9. [Google Scholar]
- 31.Sewell AJ. Parturition. The Kennel Gazette. 1895:247. [Google Scholar]
- 32.Hobday FTG. Surgical Diseases of the Dog and Cat: with chapters on anaesthetics and obstetrics. 2nd ed ed. Chicago: Chicago Medical Book Company; 1906. [Google Scholar]
- 33.Chaundler CS. Bulldogs. The Stock-keeper and Fanciers’ Chronicle. 1901. February 22. [Google Scholar]
- 34.Brodbelt DC, Pfeiffer DU, Young LE, Wood JLN. Results of the Confidential Enquiry into Perioperative Small Animal Fatalities regarding risk factors for anesthetic-related death in dogs. Journal of the American Veterinary Medical Association. 2008;233(7):1096–104. 10.2460/javma.233.7.1096 [DOI] [PubMed] [Google Scholar]
- 35.O'Neill DG, O'Sullivan AM, Manson EA, Church DB, Boag AK, McGreevy PD, et al. Canine dystocia in 50 UK first-opinion emergency-care veterinary practices: prevalence and risk factors. Veterinary Record. 2017;181(4). [DOI] [PubMed] [Google Scholar]
- 36.The Kennel Club. Breed Watch: The Kennel Club,; 2019. [Available from: https://www.thekennelclub.org.uk/services/public/breed/watch/Default.aspx. [Google Scholar]
- 37.The Kennel Club. Breed Watch: Bulldog 2018. [Available from: https://www.thekennelclub.org.uk/services/public/breed/watch/display.aspx?breed=4084. [Google Scholar]
- 38.VetCompass. VetCompass™ Programme London: RVC Electronic Media Unit; 2019 [Available from: http://www.rvc.ac.uk/VetCOMPASS/.
- 39.BWG. The Brachycephalic Working Group: The Brachycephalic Working Group; 2018 [Available from: http://www.ukbwg.org.uk/.
- 40.The Kennel Club. The Kennel Club’s Breed Health and Conservation Plans project: The Kennel Club Limited; 2019. [Available from: https://www.thekennelclub.org.uk/health/breed-health-and-conservation-plans/. [Google Scholar]
- 41.The VeNom Coding Group. VeNom Veterinary Nomenclature: VeNom Coding Group; 2019. [Available from: http://venomcoding.org. [Google Scholar]
- 42.Pearce N. Classification of epidemiological study designs. International Journal of Epidemiology. 2012;41(2):393–7. 10.1093/ije/dys049 [DOI] [PubMed] [Google Scholar]
- 43.Asher L, Buckland E, Phylactopoulos CL, Whiting M, Abeyesinghe S, Wathes C. Estimation of the number and demographics of companion dogs in the UK. BMC Veterinary Research. 2011;7(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epi Info 7 CDC. Centers for Disease Control and Prevention (US): Introducing Epi Info 7 Atlanta, Georgia: CDC; 2019. [Available from: http://wwwn.cdc.gov/epiinfo/7. [Google Scholar]
- 45.Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd ed Oxford: Blackwell Science; 2003. [Google Scholar]
- 46.Stake RE. Qualitative research: studying how things work. New York: The Guilford Press; 2010. 244 p. [Google Scholar]
- 47.Urfer SR. Bias in canine lifespan estimates through right censored data. Journal of Small Animal Practice. 2011;52(10):555–. 10.1111/j.1748-5827.2011.01125.x [DOI] [PubMed] [Google Scholar]
- 48.O'Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of owned dogs in England. The Veterinary Journal. 2013;198(3):638–43. 10.1016/j.tvjl.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 49.O'Neill D, Church D, McGreevy P, Thomson P, Brodbelt D. Approaches to canine health surveillance. Canine Genetics and Epidemiology. 2014;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGreevy PD, Nicholas FW. Some practical solutions to welfare problems in dog breeding. Anim Welfare. 1999;8:329–41. [Google Scholar]
- 51.The Kennel Club. Bulldog Breed Standard 2018. [ [Google Scholar]
- 52.Schmutz SM. Genes affecting coat colour and pattern in domestic dogs: a review. Anim Genet. 2007;38(6):539 [DOI] [PubMed] [Google Scholar]
- 53.Charon K M., Lipka K R. The Effect of a Coat Colour-Associated Genes Polymorphism on Animal Health–A Review. Annals of Animal Science. 2015;15(1):3–17. [Google Scholar]
- 54.The Bulldog Breed Council. Kennel Club Bulldog Breed Standard: The Bulldog Breed Council; 2016. [Available from: http://www.bulldogbreedcouncil.co.uk/standard.html. [Google Scholar]
- 55.O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of owned dogs in England. Vet J. 2013;198 10.1016/j.tvjl.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 56.Anon. Whispers of Fancy. The Stock-keeper and Fanciers’ Chronicle. 1902. February 1. [Google Scholar]
- 57.Packer RMA, Hendricks A, Burn CC. Do dog owners perceive the clinical signs related to conformational inherited disorders as ‘normal’ for the breed? A potential constraint to improving canine welfare. Anim Welfare. 2012;21. [Google Scholar]
- 58.O’Neill DG, Seah WY, Church DB, Brodbelt DC. Rottweilers under primary veterinary care in the UK: demography, mortality and disorders. Canine Genetics and Epidemiology. 2017;4(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Neill DG, Coulson NR, Church DB, Brodbelt DC. Demography and disorders of German Shepherd Dogs under primary veterinary care in the UK. Canine Genetics and Epidemiology. 2017;4(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Neill DG, Baral L, Church DB, Brodbelt DC, Packer RMA. Demography and disorders of the French Bulldog population under primary veterinary care in the UK in 2013. Canine Genetics and Epidemiology. 2018;5(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffman JM, O'Neill DG, Creevy KE, Austad SN. Do Female Dogs Age Differently Than Male Dogs? The Journals of Gerontology: Series A. 2018;73(2):150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyd C, Jarvis S, McGreevy PD, Heath S, Church DB, Brodbelt DC, et al. Mortality resulting from undesirable behaviours in dogs aged under three years attending primary-care veterinary practices in England. Anim Welfare. 2018;27(3):251–62. [Google Scholar]
- 63.Leroy G, Phocas F, Hedan B, Verrier E, Rognon X. Inbreeding impact on litter size and survival in selected canine breeds. The Veterinary Journal. 2015;203(1):74–8. 10.1016/j.tvjl.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 64.Montoya-Alonso JA, Bautista-Castaño I, Peña C, Suárez L, Juste MC, Tvarijonaviciute A. Prevalence of canine obesity, obesity-related metabolic dysfunction, and relationship with owner obesity in an obesogenic region of spain. Frontiers in Veterinary Science. 2017;4(59). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeo GSH. Genetics of obesity: can an old dog teach us new tricks? Diabetologia. 2016:1–6. 10.1007/s00125-016-4046-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox H. Bulldogs. The Kennel Gazette. 1897:428. [Google Scholar]
- 67.Nuttall T, Harvey R, McKeever P. A colour handbook of skin disease of the dog and cat. 2nd ed London: Manson Publishing Ltd; 2009. [Google Scholar]
- 68.O’Neill DG, Darwent EC, Church DB, Brodbelt DC. Demography and health of Pugs under primary veterinary care in England. Canine Genetics and Epidemiology. 2016;3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.American Kennel Club. Bulldog Breed Standard 2018 [Available from: https://www.akc.org/dog-breeds/bulldog/.
- 70.Griffin CE, DeBoer DJ. The ACVD task force on canine atopic dermatitis (XIV): clinical manifestations of canine atopic dermatitis. Veterinary Immunology and Immunopathology. 2001;81(3–4):255–69. [DOI] [PubMed] [Google Scholar]
- 71.Saridomichelakis MN. Aetiology of canine otitis externa: a retrospective study of 100 cases. Veterinary Dermatology. 2007;18(5):341–7. 10.1111/j.1365-3164.2007.00619.x [DOI] [PubMed] [Google Scholar]
- 72.Seckerdieck F, Mueller RS. Recurrent pyoderma and its underlying primary diseases: a retrospective evaluation of 157 dogs. Veterinary Record. 2018;182(15):434–7. 10.1136/vr.104420 [DOI] [PubMed] [Google Scholar]
- 73.Gray H. Diseases of the Eye. Veterinary Record. 1908;20(February 15):570–8. [Google Scholar]
- 74.Beausoleil NJ, Mellor DJ. Introducing breathlessness as a significant animal welfare issue. N Z Vet J. 2015;63. [DOI] [PubMed] [Google Scholar]
- 75.Liu N-C, Adams VJ, Kalmar L, Ladlow JF, Sargan DR. Whole-Body Barometric Plethysmography Characterizes Upper Airway Obstruction in 3 Brachycephalic Breeds of Dogs. Journal of Veterinary Internal Medicine. 2016;30(3):853–65. 10.1111/jvim.13933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Neill DG, Jackson C, Guy JH, Church DB, McGreevy PD, Thomson PC, et al. Epidemiological associations between brachycephaly and upper respiratory tract disorders in dogs attending veterinary practices in England. Canine Genet Epidemiol. 2015;2 10.1186/s40575-015-0013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fawcett A, Barrs V, Awad M, Child G, Brunel L, Mooney E, et al. Consequences and Management of Canine Brachycephaly in Veterinary Practice: Perspectives from Australian Veterinarians and Veterinary Specialists. Animals. 2018;9(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS ONE. 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roedler FS, Pohl S, Oechtering GU. How does severe brachycephaly affect dog’s lives? Results of a structured preoperative owner questionnaire. Vet J. 2013;198 10.1016/j.tvjl.2013.02.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset underlying the current study have been uploaded to the RVC open access database and are freely available at: http://researchonline.rvc.ac.uk/11945/.