Abstract
Background and objectives
Encouraging awareness of fetal movements is a common strategy used to prevent stillbirths. Information provided to pregnant women about fetal movements is inconsistent perhaps due to limited knowledge about normal fetal movement patterns in healthy pregnancies. We aimed to describe maternally perceived fetal movement strength, frequency, and pattern in late pregnancy in women with subsequent normal outcomes.
Methods
Participants were ≥28 weeks’ gestation, with a non-anomalous, singleton pregnancy who had been randomly selected from hospital booking lists and had consented to participate. Fetal movement data was gathered during pregnancy via a questionnaire administered face-to-face by research midwives. Participants remained eligible for the study if they subsequently gave birth to a live, appropriate-for-gestational-age baby at ≥37 weeks.
Results
Participants were 274 women, with normal pregnancy outcomes. The majority (59.3%, n = 162) of women reported during antenatal interview that the strength of fetal movements had increased in the preceding two weeks. Strong fetal movements were felt by most women in the evening (72.8%, n = 195) and at night-time including bedtime (74.5%, n = 199). The perception of fetal hiccups was also reported by most women (78.8%). Women were more likely to perceive moderate or strong fetal movements when sitting quietly compared with other activities such as having a cold drink or eating.
Conclusions
Our data support informing women in the third trimester that as pregnancy advances it is normal to perceive increasingly strong movement, episodes of movements that are more vigorous than usual, fetal hiccups, and a diurnal pattern involving strong fetal movement in the evening. This information may help pregnant women to better characterise normal fetal movement and appropriately seek review when concerned about fetal movements. Care providers should be responsive to concerns about decreased fetal movements in the evening, as this is unusual.
Introduction
Maternal perception of fetal movements is reassuring of fetal wellbeing. It is well established that perception of decreased fetal movements (DFM) is associated with stillbirth and pregnant women are routinely asked about fetal movements during antenatal visits [1,2]. However, association of DFM with stillbirth is only moderately strong (odds ratio 2.4–14.1)[3,4] and the majority of presentations for DFM are followed by a normal pregnancy outcome [5]. A large UK trial has reported that encouraging awareness of fetal movement, coupled with a management protocol involving a low threshold for induction of labour, led to increased intervention and no reduction in stillbirths [5]. Some commentators have concluded that encouraging awareness of fetal movements is harmful and should be discouraged [6,7]. Others have pointed out that maternal concern about DFM remains a risk factor for adverse outcome and argued for renewed focus of researchers’ efforts to understand this important clinical sign [8].
In a survey, 99.9% of pregnant women reported that it was important for them to feel their baby move every day [9]. Studies report that women would like to receive more information about fetal movements [1,10], preferably written and face-to-face from midwives [1]. However, between 25–60% of pregnant women do not recall receiving any information about fetal movements [1,11,12]. Despite a lack of evidence for the effectiveness of fetal movement counting [13], women in many parts of the world continue to be advised about normal fetal movements in terms of movement counts [10,14–16]. In the past decade, researchers have come to define DFM as the qualitative perception of a decrease in fetal movements, as determined by the pregnant woman, rather than any numeric definition [17]. And some maternity care providers also acknowledge the importance of the mother’s subjective perception of fetal movements [18]. However, it remains the case that clinically relevant definitions of normal fetal movements have yet to be made. Thus, there is little agreement about what is normal or expected and women can receive conflicting or inadequate information [10,14].
The significance of changes in perceived fetal movements at term is another area of debate. Some studies have shown that fetal movements are reduced slightly at term [19,20], whilst others show no reduction [21,22]. Commentators on the AFFIRM trial have suggested limiting campaigns to encourage awareness of fetal movements to women who are >37 weeks’ gestation to minimise risk of iatrogenic harm [6]. However, association between decreased frequency of fetal movements and stillbirth has been shown to be stronger between 28 and 36+6 weeks’ gestation than after 37 weeks [3].
Improved understanding of maternally perceived fetal movements in normal pregnancies, including qualitative features such as strength, pattern, and changes at term may assist maternity care providers in providing information to pregnant women about what to expect. Informing pregnant women about fetal movements has been demonstrated to reduce stillbirths in Norway [23]. The women’s experience of fetal movement includes qualitative aspects such as fetal responses to maternal position, activity, meals, and noise and touch [24] but few data exist about these features. For women with a normally progressing pregnancy, providing information about the typical strength and pattern of fetal movements may provide reassurance and reduce unnecessary presentations for assessment.
Our aim was to describe maternal perception of fetal movements (strength, frequency, and pattern) in a group of women ≥28 weeks’ gestation with non-anomalous, singleton pregnancies, to better understand normal fetal movement in late pregnancy. A secondary aim was to describe any variation in perceived fetal movements at term as compared to early third trimester. We also sought to explore maternal report of variation in fetal movement strength in relation to factors that are commonly believed to provide a stimulus to fetal movement such as noise, touch, and ingestion of food and drinks [25].
Materials and methods
This cross-sectional study was conducted across seven healthcare regions in New Zealand. Participants were initially recruited as controls in a larger study on late stillbirth and were randomly selected from hospital booking lists based on gestation-matching with stillbirths in that locality [26]. At recruitment eligible participants were ≥28 weeks’ gestation, with a non-anomalous singleton pregnancy, and provided written consent to participate. Women were interviewed antenatally between February 2012 and December 2015. The findings of the stillbirth study have been reported elsewhere [26,27]. Birth outcome data were collected from the medical records following birth. Ethical approval was obtained from the Northern X Region Ethics Committee: NTX/06/05/054.
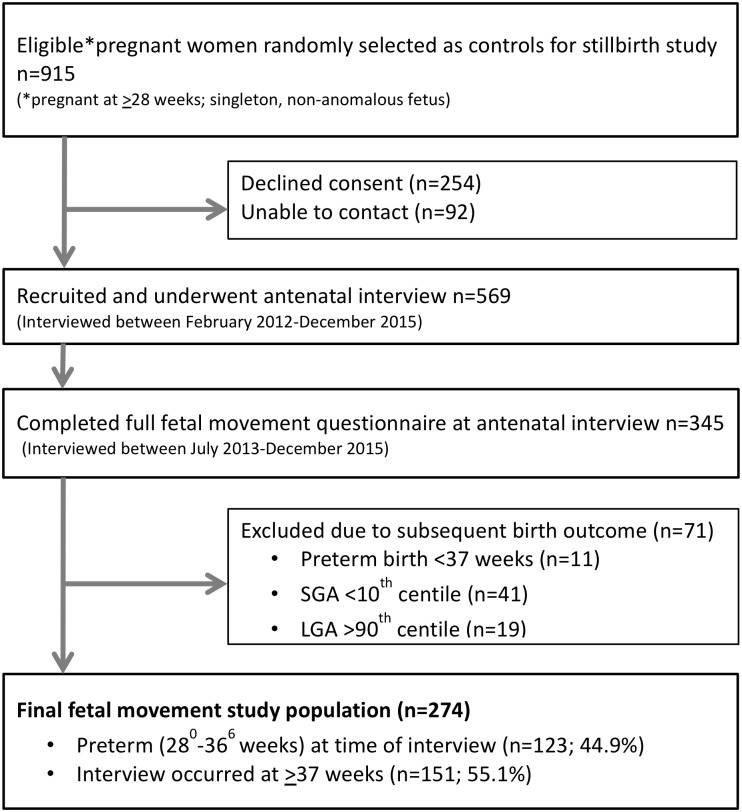
Participants were included in the present cross-sectional study if they; were recruited after the 1st of July 2013 and had completed the detailed fetal movement questionnaire (published as Supplementary Information in McCowan et al), and subsequently delivered a live, term infant (≥37 weeks’ gestation based on early scan or the first day of last menstrual period) of an appropriate-for-gestational-age birthweight (customised birthweight centile between the 10th and the 90th centile calculated using the New Zealand version of GROW) [28] (Fig 1). For women who declined to participate or who were not contactable: age, parity and ethnicity data was collected without identifying information. Interpreters were arranged for women who had difficulty with reading or speaking English.
Fig 1. Study population flow chart.
Data were collected antenatally via a questionnaire administered face-to-face by trained research midwives in the setting of the woman’s choosing, usually her home. The antenatal interview included a broad range of questions about lifestyle factors such as diet and sleep in addition to the fetal movement questions. Data were collected on a range of fetal movement variables perceived by the woman in the last two weeks. These variables included: fetal movement strength and frequency; fetal hiccups; movements that were ‘more vigorous than usual’; perception of fetal movement clusters or ‘busy times’; and fetal movement quality in relation to time of day, maternal position and activity, consumption of food and drinks, and environmental stimuli, including loud noises and touching the abdomen.
Maternal perception of fetal movement strength and frequency over the last two weeks was categorised as ‘increased’, ‘decreased’, ‘stayed the same’ or ‘unsure’. ‘Busy times’ were defined for participants as ‘a period where there is a group of movements, rather than single isolated movements, which might be short (15–45 seconds), or prolonged and involving many movements for up to 20 minutes.’ Participants indicated how many times in a day, on average, they perceived fetal ‘busy times’. Changes in duration of ‘busy times’ in the last two weeks were categorised as ‘longer than before’, ‘about as long as before’ or ‘shorter than before’. If participants were unsure, the fetal hiccups sensation was described as ‘regular jerking movements happening at 1–2 second intervals over a period of 1–5 minutes’. Fetal movement quality in relation to maternal position, activities, and time of day was categorised as ‘notably quiet’, ‘subtle or light movement’, ‘moderate movement’, ‘strong movement’, ‘jumps or startles’ and ‘unsure/don’t notice’.
The category ‘notably quiet’ was rarely selected and was associated with ‘subtle or light movement’. Similarly, the category ‘jumps or startles’ was uncommon and was associated with ‘strong movement’. Therefore, these categories were collapsed into a ‘quiet or light movement’ category and a ‘strong or jumps/startles’ category. Times of day were defined as ‘on waking and before rising’, ‘during the morning’, ‘during the afternoon’, ‘during the evening’, and ‘night-time including bedtime’.
Analysis was performed in SAS version 9.4 (SAS institute Inc., Cary, NC, USA). Frequency tabulations are presented, illustrating perceived changes in strength, frequency, more vigorous than usual movements, fetal hiccups and ‘busy times’. The likelihood of the woman indicating that fetal movements were either ‘quiet or light’, ‘moderate’, or ‘strong including jumps/startles’ in relation to a given fetal movement variable was calculated using chi-square, with p = 0.05 considered statistically significant. Finally, a comparison was conducted between women who were interviewed preterm (28+0 to 36+6 weeks’ gestation, n = 123) and those interviewed at term (≥37 weeks’ gestation, n = 151), subsequently referred to as preterm and term, in order to consider possible differences in fetal movements according to gestational age.
Results
In total, 274 women met the study inclusion criteria (Fig 1). Mean (SD) maternal age at interview was 29.8 (5.2) years and nulliparous women comprised 42.7% of the sample. Mean (SD) gestation at interview was 36.2 (3.3) weeks with approximately half (44.9%) interviewed between 28+0 and 36+6 weeks’ gestation and the remainder interviewed at ≥37 weeks’ gestation (Table 1). Demographics characteristics were similar in this sub-study to the larger control group (data not shown). Comparison of demographic characteristics between eligible non-participants and recruited participants has already been reported [26]. Briefly, women of high parity were slightly under-represented, whilst women of Indian ethnicity were over-represented and New Zealand Māori women were under-represented in the recruited population[26].
Table 1. Participant characteristics.
| Characteristics | Participants N = 274 |
|---|---|
| Age (years) | 29.8 (5.2)* |
| Ethnicity | |
| Māori | 29 (10.6) |
| Pacific | 31 (11.3) |
| Indian | 47 (17.1) |
| Other Asian | 31 (11.3) |
| European | 131 (47.8) |
| Other | 5 (1.8) |
| Parity | |
| 0 | 117 (42.7) |
| 1–3 | 153 (55.8) |
| ≥4 | 4 (1.5) |
| BMI (booking) (kg/m2) | |
| <25 | 147 (53.6) |
| 25–29.9 | 72 (26.3) |
| ≥30 | 55 (20.1) |
| Smoker | 25 (9.1) |
| In paid work (last month) | 161 (61.5) |
| Preterm at interview (28+0 to 36+6 weeks) | 123 (44.9) |
| Term at interview (≥37 weeks) | 151 (55.1) |
| Gestation at interview (weeks) | 36.2 (3.33)* |
| Gestation at birth (weeks) | 39.5 (1.14)* |
| Birthweight (grams) | 3556 (372)* |
| Female infant sex | 143 (52.2%) |
Data are n (%) or *mean (standard deviation).
Participants commonly perceived that the strength of fetal movements had increased in the last two weeks (Table 2). Few women indicated that fetal movements had decreased in either strength (6.2%) or frequency (13.9%). Multiple episodes of fetal movements that were more vigorous than usual were commonly reported. Perception of fetal hiccups was typical (92.2%), either occasionally (44.5%) or daily (42.5%). Most participants (65.9%) indicated that they perceived fetal ‘busy times’ 3 to 9 times per day, and that the duration of these busy times in the last two weeks was either unchanged (56.1%) or longer than before (36.8%) (Table 2).
Table 2. Fetal movement strength, frequency, hiccups and ‘busy times’ (N = 274).
| Interview question | N = 274 |
|---|---|
| In the last two weeks did the strength of your baby’s movements? | |
| Increase | 162 (59.3) |
| Decrease | 17 (6.2) |
| Stay the same | 89 (32.6) |
| Unsure | 5 (1.8) |
| In the last two weeks did the frequency of your baby’s movements? | |
| Increase | 107 (39.1) |
| Decrease | 38 (13.9) |
| Stay the same | 125 (45.6) |
| Unsure | 4 (1.5) |
| During the last two weeks did you notice any time that your baby was more vigorous than usual? | |
| No | 127 (47.6) |
| Yes, once | 15 (5.6) |
| Yes, more than once | 118 (44.2) |
| Yes, unsure frequency | 7 (2.6) |
| During the last two weeks did you feel your baby having hiccups? | |
| No | 63 (23.1) |
| Yes | 200 (73.3) |
| Unsure | 10 (3.7) |
| If yes, how often? | |
| Unsure if hiccups | 10 (5.0) |
| Yes, once | 11 (5.5) |
| Yes, occasionally | 89 (44.5) |
| Yes, daily | 85 (42.5) |
| Yes, unsure frequency | 5 (2.5) |
| In the last two weeks, how many busy times did your baby have in a day? | |
| 0–2 | 60 (22.0) |
| 3–9 | 180 (65.9) |
| 10+ | 33 (12.1) |
| In the last two weeks, on average, how long did these busy times last? | |
| Longer than before | 99 (36.8) |
| About as long as before | 151 (56.1) |
| Shorter than before | 19 (7.1) |
Data are n (%).
We asked about perceived fetal movement strength in relation to time of day, maternal position, meals, and stimuli in the environment. Data in relation to time of day indicated a clear diurnal pattern characterised by an increasing likelihood of strong fetal movement as the day advanced and corresponding decrease in the likelihood of quiet movement (Table 3). On waking, just 22.0% of women reported strong fetal movement, which increased to 74.5% by night-time (P<0.001).
Table 3. Perceived strength of fetal movements and time of day (N = 268).
| Time of day | Missing | Reported fetal movement strength in the last two weeks n(%) | Chi-square, P value | ||
|---|---|---|---|---|---|
| Quiet | Moderate | Strong | |||
| On waking (before rising) | 5 | 112 (42.6) | 93 (35.4) | 58 (22.0) | reference |
| During the morning | 7 | 101 (38.0) | 107 (40.2) | 58 (21.8) | 1.53, 0.46 |
| During the afternoon | 7 | 43 (16.0) | 129 (48.1) | 96 (35.8) | 45.89, <0.001 |
| During the evening | 7 | 10 (3.7) | 63 (23.5) | 195 (72.8) | 165.20, <0.001 |
| Night-time (including bedtime) | 8 | 19 (7.1) | 49 (18.3) | 199 (74.5) | 165.99, <0.001 |
Data are n (%). P value is for row comparison with referent. Chi-square tests were calculated where there was complete data for both variables for a subject.
Quiet fetal movement was commonly reported both before and after meals (Table 4). Compared to ‘before meals’ there was an increase in strength of fetal movements ‘within fifteen minutes of eating’ (p<0.001) and ‘an hour after eating’ (p<0.001) (Table 4). However, fewer than a third of women reported strong fetal movement after eating.
Table 4. Perceived strength of fetal movements in relation to meals (N = 239).
| Prandial stage | Missing | Reported fetal movement strength in the last two weeks n (%) | Chi-square, P value | ||
|---|---|---|---|---|---|
| Quiet | Moderate | Strong | |||
| When you are hungry | 31 | 114 (52.3) | 63 (28.9) | 41 (18.8) | 2.3, 0.31 |
| Before a usual meal-time | 17 | 132 (59.5) | 53 (23.9) | 37 (16.7) | reference |
| While you are eating | 24 | 140 (58.6) | 58 (24.3) | 41 (17.1) | 0.04, 0.98 |
| Within 15 minutes of eating | 28 | 89 (37.6) | 74 (31.2) | 74 (31.2) | 23.7, <0.001 |
| An hour after eating | 33 | 90 (39.8) | 93 (41.2) | 43 (19.0) | 19.32, <0.001 |
Data are n (%). P value is for row comparison with referent. Chi-square tests were calculated where there was complete data for both variables for a subject. Missing data is for referent comparisons.
Most women reported moderate (39.9%) or strong (42.9%) fetal movements when sitting quietly (Table 5). In contrast, women typically perceived fetal movements to be quiet when walking (61.0%) or standing (57.8%), which was significantly different when compared to the sitting position (all p<0.001) (Table 5). Fetal movement strength was not significantly different between sitting and side-lying (p = 0.06) (Table 5).
Table 5. Perceived strength of fetal movements in relation to maternal position (N = 266).
| Fetal movement variable | Missing | Reported fetal movement strength in the last two weeks n (%) | Chi-square, P value | ||
|---|---|---|---|---|---|
| Quiet | Moderate | Strong | |||
| Maternal position | |||||
| Sitting quietly | 0 | 46 (17.3) | 106 (39.9) | 114 (42.9) | reference |
| When you lie on your side | 8 | 67 (25.7) | 92 (35.3) | 102 (39.1) | 5.51, 0.06 |
| Walking around at home or at work | 15 | 155 (61.0) | 66 (26.0) | 33 (13.0) | 112.83, <0.001 |
| Standing in one spot | 12 | 149 (57.8) | 76 (29.5) | 33 (12.8) | 103.88, <0.001 |
| Fetal stimulus | |||||
| Rub or prod belly/baby | 11 | 71 (27.3) | 78 (30.0) | 111 (42.7) | 9.57, 0.008 |
| Sitting in a cramped position | 38 | 79 (34.1) | 77 (33.2) | 76 (32.8) | 18.67, <0.001 |
| Unexpected loud noise | 73 | 84 (43.1) | 49 (25.1) | 62 (31.8) | 37.38, <0.001 |
| Cold drink | 39 | 79 (34.2) | 67 (29.0) | 85 (36.8) | 19.36, <0.001 |
| Within 15 minutes of eating | 32 | 89 (37.6) | 74 (31.2) | 74 (31.2) | 26.31, <0.001 |
Data are n (%). P value is for row comparison with referent. Chi-square tests were calculated where there was complete data for both variables for a subject.
We asked about maternally perceived strength of fetal movements in a number of situations that are commonly believed to promote fetal activity, including abdominal prodding, sitting in a cramped position, loud noises, consuming a cold drink or eating (Table 5). In all situations, fetal movement strength was less likely to be perceived as strong than when women were sitting quietly (all p<0.001) (Table 5).
We also considered variation in fetal movement strength, frequency, hiccups, and ‘busy times’ by gestation at the time of interview. The most frequent response relating to strength of fetal movements in the last two weeks in both the preterm and term interview groups was an increase in strength (70.7% and 50.0%, respectively) (Table 6). Most (48.0%) women interviewed preterm reported an increase in frequency, whilst most (50.3%) women interviewed at term reported that frequency had ‘stayed the same’ (P = 0.02). Women interviewed at term were more likely to perceive hiccups, compared to women interviewed preterm (78.8% vs 66.4%, P = 0.04). Frequency of ‘busy times’ did not differ by gestation, but women interviewed at term, compared with those interviewed preterm, were less likely to indicate that busy times were longer than before (31.9% vs 42.7%, P = 0.01). However, in both groups, women typically reported busy times that were ‘about as long as before’ (Table 6).
Table 6. Fetal movement strength, frequency, hiccups and busy times by gestation at interview.
| Interview question | Preterm at interview (28+0–36+6 weeks) N = 123 | Term at interview (≥37 weeks) N = 151 | P |
|---|---|---|---|
| In the last two weeks did the strength of your baby’s movements? | |||
| Increase | 87 (70.7) | 75 (50.0) | 0.005 |
| Decrease | 4 (3.3) | 13 (8.7) | |
| Stay the same | 30 (24.4) | 59 (39.3) | |
| Unsure | 2 (1.6) | 3 (2.0) | |
| In the last two weeks did the frequency of your baby’s movements? | |||
| Increase | 59 (48.0) | 48 (31.8) | 0.02 |
| Decrease | 12 (9.8) | 26 (17.2) | |
| Stay the same | 49 (39.8) | 76 (50.3) | |
| Unsure | 3 (2.4) | 1 (0.7) | |
| During the last two weeks did you notice any time that your baby was more vigorous than usual? | |||
| No | 52 (43.3) | 75 (51.0) | 0.37 |
| Yes, once | 7 (5.8) | 8 (5.4) | |
| Yes, more than once | 56 (46.7) | 62 (42.2) | |
| Yes, unsure frequency | 5 (4.2) | 2 (1.4) | |
| During the last two weeks did you feel your baby having hiccups? | |||
| No | 34 (27.9) | 29 (19.2) | 0.04 |
| Yes | 81 (66.4) | 119 (78.8) | |
| Unsure | 7 (5.7) | 3 (2.0) | |
| If yes, how often? | |||
| Unsure if hiccups | 7 (8.3) | 3 (2.6) | 0.07 |
| Yes, once | 8 (9.5) | 3 (2.6) | |
| Yes, occasionally | 36 (42.9) | 53 (45.7) | |
| Yes, daily | 31 (36.9) | 54 (46.5) | |
| Yes, unsure frequency | 2 (2.4) | 3 (2.6) | |
| In the last two weeks, how many busy times did your baby have in a day? | |||
| 0–2 | 25 (20.3) | 35 (23.3) | 0.29 |
| 3–9 | 79 (64.2) | 101 (67.3) | |
| 10+ | 19 (15.4) | 14 (9.3) | |
| In the last two weeks, on average, how long did these busy times last? | |||
| Longer than before | 67 (42.7) | 58 (31.9) | 0.01 |
| About as long as before | 85 (54.1) | 106 (58.2) | |
| Shorter than before | 5 (3.2) | 18 (9.9) | |
Data are n(%). P value is for comparison between preterm and term.
The pattern of increasing likelihood of strong fetal movements in the evening and at night-time was observed in both the preterm and term interview groups. The only difference observed was that compared to those interviewed preterm, women interviewed at term were more likely to report quiet fetal movement during the afternoon (21.9% vs 9.0%; p = 0.008). However, the likelihood of strong movement in the evening was not different between women interviewed preterm and women interviewed at term (71.5% vs 74.0%; p = 0.65) (S1 Table).
Similarly, fetal movement strength in relation to prandial state was not different between women interviewed preterm and at term, with the exception that strong fetal movement ‘an hour after eating’ was less likely to be reported by women interviewed at term (13.1% vs 27.1%, p = 0.02). There were no differences by gestation at interview in regard to maternal position or purported fetal stimulus. Compared to women interviewed preterm, more women at term reported strong movements when touching their abdomen (47.9% vs 36.2%), but this difference was not statistically significant (p = 0.12) (S1 Table).
Discussion
This cross-sectional study of a representative sample of pregnant women who subsequently gave birth to live appropriate-for-gestational-age babies at term, provides novel quantitative data on aspects of fetal movement that are observed by pregnant women but not currently well described in the literature. We found that women typically perceived fetal movements in the third trimester to be increasingly strong, likely to include fetal hiccups, and exhibiting a clear diurnal pattern involving strong fetal movements in the evening.
Our findings are consistent with a number of qualitative studies of maternal perception of fetal movement where perception of strong fetal movement was a notable feature of women’s descriptions, particularly at term [18,24,29]. Increased strength of perceived fetal movements has been shown in case-control studies conducted in the United Kingdom and in New Zealand to be associated with lower risk of late-stillbirth [3,4]. In our study, decreased strength of fetal movements occurred infrequently in at both preterm and term gestations, although decreased frequency was more likely to be reported by women interviewed at term than those interviewed preterm. This may reflect a change in fetal behavioural state development with longer periods of quiescence at term gestation.
Almost all women in our study reported perception of fetal hiccups, with hiccups perceived more often at term. Hiccups (or hiccoughs) appear to be universal in mammals, but their origin and purpose remain unknown [30]. Ultrasound studies have demonstrated that fetal hiccups are a normal aspect of fetal life and occur less frequently later in pregnancy [20,31]. Increased perception of fetal hiccups at term in our study may be due to physiological changes in late gestation making the sensation of hiccups easier for women to identify, or it may indicate increased recognition of the sensations by the mother. Regardless, fetal hiccups are a normal aspect of fetal behaviour and maternal perception of fetal hiccups is associated with reduced risk of stillbirth [3,32]. A number of hypotheses have been advanced for the purpose of hiccups in fetal life including; developing respiratory muscles, preparing the infant for suckling [30], and regulating amniotic fluid in early gestation [33]. Regardless, the association of perception of hiccups with reduced risk of stillbirth suggests maternal perception of fetal hiccups towards term is indicative of fetal wellbeing.
Our data demonstrate a clear diurnal pattern in the strength of fetal movements. Of all factors considered in this study, strong or moderate fetal movements were most commonly perceived in the evening and night-time including bed-time. This finding is consistent with other studies where women report increased perception of fetal movements in the evening [18,24,34,35]. One commonly advanced explanation is that women are more likely to be sitting or lying down in the evening, which we also found were associated with increased perception of fetal movement strength. However, Minors and Waterhouse (1979) showed that perceived fetal movements increased in the evening, independent of maternal sitting position [36]. Furthermore, studies in instrumented fetal lambs have shown a significant increase in fetal activity in the evening, even though ewes remained in a standing position [37].
Another commonly advanced explanation is that women are less attentive to fetal movements during the day due to competing distractions [18]. However, ultrasound studies of fetal behaviour have objectively demonstrated increased fetal activity in the evening and greater likelihood of fetal quiescence during the morning [31,38,39]. We have previously reported that fetal quiescence in the evening is associated with a more than three-fold increased risk of stillbirth [27]. Thus, maternal perception of decreased fetal movement strength in the evening, especially if different from their usual diurnal pattern, may be an indicator of a compromised fetus. Women should be encouraged to present in the evening if such a change occurs, rather than waiting until the morning, as delay in presentation for DFM has been associated with increased risk of stillbirth [40].
It has been suggested that a perceived change in the pattern of fetal movements can indicate fetal compromise [41]. However, the term ‘pattern’ has not been well defined for the purposes of antenatal education. Ultrasound studies have documented a number of normal fetal movement patterns including: a sequence of movements collectively known as a ‘general movement’ [20]; an ultradian or short-term fetal movement pattern involving cycling between alternating periods of activity and rest which is comparable to the behavioural states seen in infants [42]; and a circadian or 24-hour movement pattern characterised by increased movement in the evening [38,43]. In the normal healthy fetus near term, the alternating periods of activity and rest that occur in ultradian cycling lengthen and periods of inactivity can last an hour or more. This normal development may explain some benign presentations with DFM at term. Indeed, clinical practice guidelines recommend pregnant women are informed about these changes in fetal movements at term [17]. In contrast, the diurnal fetal movement pattern of strong movements in the evening demonstrated in our study was consistently reported by women interviewed both preterm and at term. Therefore, it is reasonable to inform pregnant women that perception of a pattern of increased strength of fetal movements in the evening is common throughout late pregnancy and may be reassuring of fetal wellbeing.
Studies in the UK, Europe and Australia have found that pregnant women are frequently advised to drink cold water or eat sugary foods when concerned about fetal movements [10,14,44]. However, we found that consuming a cold drink or eating were significantly less likely to promote strong or moderate fetal movement than simply sitting quietly. Our results are consistent with a systematic review that found that the use of glucose did not make fetal testing more effective [45]. Furthermore, a study of fetal heart rate reactivity on cardiotocograph in women given oral glucose (n = 42) compared to women given water (n = 40) found no difference between the two groups in mean time to reactivity [46]. An evidence-based clinical guideline for management of women with DFM has emphasised that advising women to drink water or eat sugary foods has no basis in evidence and may delay women from seeking fetal wellbeing assessment [17].
We found more women perceived strong fetal movements when sitting compared to side-lying, however, this difference was not statistically significant (p = 0.06). A study of maternal position and cardiotocograph reported significantly shorter time to demonstrate fetal heart rate reactivity when women were sitting, compared to lying supine [47]. Studies using magnetic resonance imaging have demonstrated that maternal supine position is associated with reduced diameter of the inferior vena cava and reduced blood flow in the uterine artery when compared to side-lying [48–50]. When the mother is in the supine position the fetus has been reported to spend more time in fetal behavioural state one or ‘quiescence’ compared to side-lying [51]. Maternal sitting position is under-explored in relation to fetal movement observation. This may be due to early fetal movement research being conducted primarily in the hospital setting where, women were more often observed in hospital beds or on examination tables. In day-to-day life some pregnant women may find sitting for a period to observe fetal movements more practical than side-lying. Our data suggest either sitting or side-lying may be effective, although supine position should be avoided.
A strength of this study is that we have collected detailed fetal movement data from a group of women during pregnancy with subsequent normal outcome. This was a national study with participants recruited from multiple sites within New Zealand. The reported strength and frequency data are similar to those in an earlier Auckland study and a large UK study [3,4], suggesting the findings are likely to be generalisable to other populations. Participants in this study were representative of New Zealand society, including ethnic distribution. Although women with high parity were under-represented in our study, this does not reduce the utility of the information presented as parity has been shown to have no effect on perception of fetal movements in the third trimester [9]. Furthermore, sharing information about fetal movements may be beneficial, specifically for women in a first pregnancy [23].
The women in this study were experiencing normal ongoing pregnancy at the time of interview and had no reason to interpret their experience differently in relation to pregnancy outcome, limiting bias. An acknowledged source of potential selection bias in fetal movement studies is that women who normally do not perceive fetal movements may decline to participate. Women approached to participate in this study were informed that the study was about pregnancy and the term ‘fetal movements’ was not used in the preamble to gain consent, making this source of selection bias unlikely. A possible limitation of the study design is that fetal movement information was derived from maternal report and is therefore subject to bias. However, maternal report of altered fetal movements may be clinically important as subjective perception of DFM is associated with adverse pregnancy outcomes.
We took measures to minimise the risk of interviewer inference by using a structured questionnaire to gather the fetal movement data. Midwife interviewers were trained to administer the questionnaire and instructed to ask the questions exactly as written. To ensure women’s comfort with responding to questions, interviewers began the section of detailed fetal movement questions with the words “there are no right or wrong answers to any of these questions”. We anticipated that women may ask midwife interviewers for their opinion on factors being investigated in the study and instructed interviewers to respond that ‘evidence was inconclusive and that is why we are carrying out this study’. Feedback from participants about involvement with the study was overwhelmingly positive. With the exception of BB and RC, who conducted a small proportion of the interviews, interviewers had no role in generating the hypotheses being explored, or the subsequent data analysis.
One limitation of this study is that we did not have access to more detailed birth outcome data such as Apgar score or neonatal intensive care admission. Thus, the study group may have included some women who experienced a less than optimal birth outcome. Nevertheless, by only including women who gave birth to a live appropriate-for-gestational age infant at term, our data are likely to be broadly representative of women with normal outcome.
Conclusions
Pregnant women have indicated they would like more information about fetal movements. Our data support informing women in the third trimester that as pregnancy advances it is normal to perceive increasingly strong movement, episodes of movements that are more vigorous than usual, fetal hiccups, and a diurnal pattern involving strong fetal movement in the evening. This information may help pregnant women to better characterise normal fetal movement and appropriately seek review when concerned about fetal movements. This study should also inform care providers that it is important to be responsive to reports of fetal movement concerns in the evening as such reports are unusual.
Supporting information
(DOCX)
Acknowledgments
We would like to thank the research midwives who have conducted interviews for this project. We also would like to thank all the participants.
Data Availability
Due to ethical restrictions, data underlying the results presented in the study can not be shared publicly. Data will be available to approved researchers under the data-sharing arrangements provided by the Maternal and Perinatal Central Coordinating Research Hub (CCRH), based at the University of Auckland (https://wiki.auckland.ac.nz/researchhub). Metadata, along with instructions for data access, are available at the University of Auckland’s research data repository, Figshare (http://auckland.figshare.com/). Data access requests are to be submitted to the Data Access Committee via researchhub@auckland.ac.nz. De-identified data will be shared with researchers who provide a methodologically sound proposal and have appropriate institutional approval. Due to ethical restrictions, provision of data will be subject to receiving appropriate New Zealand ethical approval. Researchers must sign and adhere to a Data Access Agreement that includes a commitment to using the data only for the specified proposal, to refrain from any attempt to identify individual participants, to store data securely and to destroy or return the data after completion of the project. The CCRH reserves the right to cover the costs of making data available, if required. Contact Information for NZ Ethics Committee: Email: hdec@moh.govt.nz; Postal address: Ministry of Health, Health and Disability Ethics Committees, PO Box 5013, Wellington 6140, New Zealand; Attention: Ethics Committee manager for Protection, Regulation and Assurance.
Funding Statement
Funding was provided by: The Health Research Council of New Zealand (ref: 12/372, http://www.hrc.govt.nz/); Cure Kids (Grant number:5357, http://curekids.org.nz/); Mercia Barnes Trust (https://www.ranzcog.edu.au/about/regional/nz/Mercia-Barnes-Trust); Nurture Foundation (http://www.nurture.org.nz/); University of Auckland, Faculty Research Development Fund (Grant 3700696). Funding sources had no role in study design, data collection, analysis, interpretation, writing the report, or decision to submit the paper for publication.
References
- 1.McArdle A, Flenady V, Toohill J, Gamble J, Creedy D. How pregnant women learn about foetal movements: Sources and preferences for information. Women Birth. 2015;28: 54–59. 10.1016/j.wombi.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 2.Peat AM, Stacey T, Cronin R, McCowan LME. Maternal knowledge of fetal movements in late pregnancy. Aust N Z J Obstet Gynaecol. 2012;52: 445–449. 10.1111/j.1479-828X.2012.01462.x [DOI] [PubMed] [Google Scholar]
- 3.Stacey T, Thompson JMD, Mitchell E, Ekeroma A, Zuccollo J, McCowan L. Maternal perception of fetal activity and late stillbirth risk; findings from the Auckland stillbirth study. Birth. 2011;38: 1–6. 10.1111/j.1523-536X.2010.00448.x [DOI] [PubMed] [Google Scholar]
- 4.Heazell AEP, Warland J, Stacey T, Coomarasamy C, Budd J, Mitchell EA, et al. Stillbirth is associated with perceived alterations in fetal activity—findings from an international case control study. BMC Pregnancy Childbirth. 2017;17 10.1186/s12884-017-1555-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norman JE, Heazell AEP, Rodriguez A, Weir CJ, Stock SJE, Calderwood CJ, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge, cluster-randomised trial. The Lancet. 2018; 10.1016/S0140-6736(18)31543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker KF, Thornton JG. Encouraging awareness of fetal movements is harmful. The Lancet. 2018;392: 1601–1602. 10.1016/S0140-6736(18)31720-3 [DOI] [PubMed] [Google Scholar]
- 7.Gidlöf S. When will we stop encouraging awareness of fetal movements? Acta Obstet Gynecol Scand. 2019;98: 137–138. 10.1111/aogs.13517 [DOI] [PubMed] [Google Scholar]
- 8.Flenady V, Ellwood D, Bradford B, Coory M, Middleton P, Gardener G, et al. Beyond the headlines: Fetal movement awareness is an important stillbirth prevention strategy. Women Birth. 2018; 10.1016/j.wombi.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 9.Saastad E, Ahlborg T, Frøen J. Low maternal awareness of fetal movement is associated with small for gestational age infants. J Midwifery Womens Health. 2008;53: 345–352. 10.1016/j.jmwh.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 10.Smyth RMD, Taylor W, Heazell AE, Furber C, Whitworth M, Lavender T. Women’s and clinicians perspectives of presentation with reduced fetal movements: a qualitative study. BMC Pregnancy Childbirth. 2016;16 10.1186/s12884-016-1074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berndl AML, O’Connell CM, McLeod NL. Fetal Movement Monitoring: How Are We Doing as Educators? J Obstet Gynaecol Can. 2013;35: 22–28. 10.1016/S1701-2163(15)31044-6 [DOI] [PubMed] [Google Scholar]
- 12.Olagbuji B, Igbarumah S, Akintayo A, Olofinbiyi B, Aduloju P, Alao O. Maternal understanding of fetal movement in third trimester: A means for fetal monitoring and reducing stillbirth. Niger J Clin Pract. 2014;17: 489 10.4103/1119-3077.134049 [DOI] [PubMed] [Google Scholar]
- 13.Mangesi L, Hofmeyr GJ, Smith V, Smyth RM. Fetal movement counting for assessment of fetal wellbeing In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2015. http://doi.wiley.com/10.1002/14651858.CD004909.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warland J, Glover P. Fetal movements: What are we telling women? Women Birth. 2016;30: 23–28. 10.1016/j.wombi.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 15.Farrant K, Heazell AEP. Online information for women and their families regarding reduced fetal movements is of variable quality, readability and accountability. Midwifery. 2016;34: 72–78. 10.1016/j.midw.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 16.Unterscheider J, Horgan RP, Greene RA, Higgins JR. The management of reduced fetal movements in an uncomplicated pregnancy at term: Results from an anonymous national online survey in the Republic of Ireland. J Obstet Gynaecol. 2010;30: 578–582. 10.3109/01443615.2010.481733 [DOI] [PubMed] [Google Scholar]
- 17.Daly LM, Gardener G, Bowring V, Burton W, Chadha Y, Ellwood D, et al. Care of pregnant women with decreased fetal movements: Update of a clinical practice guideline for Australia and New Zealand. Aust N Z J Obstet Gynaecol. 2018; 10.1111/ajo.12762 [DOI] [PubMed] [Google Scholar]
- 18.Raynes-Greenow CH, Gordon A, Li Q, Hyett JA. A cross-sectional study of maternal perception of fetal movements and antenatal advice in a general pregnant population, using a qualitative framework. BMC Pregnancy Childbirth. 2013;13: 32 10.1186/1471-2393-13-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson J, Weaver J. Fetal activity and fetal wellbeing: an evaluation. Br Med J. 1976;1: 1305–1307. 10.1136/bmj.1.6021.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roodenburg PJ, Wladirimoff A, van Es A, Prechtl HFR. Classification and quantitative aspects of fetal movements during the second half of normal pregnancy. Early Hum Dev. 1991;25. [DOI] [PubMed] [Google Scholar]
- 21.Valentin L, Marŝál K. Fetal movement in the third trimester of normal pregnancy. Early Hum Dev. 1986;14: 295–306. 10.1016/0378-3782(86)90192-1 [DOI] [PubMed] [Google Scholar]
- 22.Roberts AB, Griffin D, Mooney R, Cooper DJ, Campbell S. Fetal activity in 100 normal third trimester pregnancies. Br J Obstet Gynaecol. 1980;87: 480–484. [DOI] [PubMed] [Google Scholar]
- 23.Tveit J, Saastad E, Stray-Pedersen B, Børdahl PE, Flenady V, Fretts R, et al. Reduction of late stillbirth with the introduction of fetal movement information and guidelines—a clinical quality improvement. BMC Pregnancy Childbirth. 2009;9: 32 10.1186/1471-2393-9-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford B, Maude R. Maternal perception of fetal movements in the third trimester: A qualitative description. Women Birth. 2018;31: e287–e293. 10.1016/j.wombi.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 25.Bradford B, Maude R. Fetal response to maternal hunger and satiation—novel finding from a qualitative descriptive study of maternal perception of fetal movements. BMC Pregnancy Childbirth. 2014;14: 288 10.1186/1471-2393-14-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCowan LME, Thompson JMD, Cronin RS, Li M, Stacey T, Stone PR, et al. Going to sleep in the supine position is a modifiable risk factor for late pregnancy stillbirth; Findings from the New Zealand multicentre stillbirth case-control study. Crispi F, editor. PLOS ONE. 2017;12: e0179396 10.1371/journal.pone.0179396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford B, Cronin R, McCowan L, McKinlay CJD, Mitchell E, Thompson JMD. Maternal Perception of Fetal Movement Quality and Risk of Late Stillbirth. Journal of Paediatrics and Child Health. 2018. pp. 10–10. 10.1111/jpc.14325 [DOI] [PubMed] [Google Scholar]
- 28.Anderson N, Sadler L, Stewart A, McCowan L. Maternal and pathological pregnancy characteristics in customised birthweight centiles and identification of at-risk small-for-gestational-age infants: a retrospective cohort study: Maternal characteristics in customised birthweight centiles. BJOG Int J Obstet Gynaecol. 2012;119: 848–856. 10.1111/j.1471-0528.2012.03313.x [DOI] [PubMed] [Google Scholar]
- 29.Rådestad I, Lindgren H. Women’s perceptions of fetal movements in full-term pregnancy. Sex Reprod Healthc. 2012;3: 113–116. 10.1016/j.srhc.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 30.Howes D. Hiccups: A new explanation for the mysterious reflex. BioEssays. 2012;34: 451–453. 10.1002/bies.201100194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrick J, Campbell K, Carmicheal L, Natale R, Richardson B. Patterns of gross fetal body movements over 24-hour observation intervals during the last 10 weeks of pregnancy. Am J Obstet Gynecol. 1982;142: 363–371. [DOI] [PubMed] [Google Scholar]
- 32.Heazell AEP, Budd J, Li M, Cronin R, Bradford B, McCowan LME, et al. Alterations in maternally perceived fetal movement and their association with late stillbirth: findings from the Midland and North of England stillbirth case–control study. BMJ Open. 2018;8: e020031 10.1136/bmjopen-2017-020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murchison AG. Hiccups and amniotic fluid regulation in early pregnancy. Med Hypotheses. 2015;84: 448–450. 10.1016/j.mehy.2015.01.040 [DOI] [PubMed] [Google Scholar]
- 34.Fisher M. Reduced fetal movements: a research based project. Br J Midwifery. 1999;7: 733–737. [Google Scholar]
- 35.Ehrström C. Orcadian Rhythm of Fetal Movements. Acta Obstet Gynecol Scand. 1984;63: 539–541. 10.3109/00016348409156716 [DOI] [PubMed] [Google Scholar]
- 36.Minors DS, Waterhouse JM. The effect of maternal, posture, meals and time of day on fetal movements. Br J Obstet Gynaecol. 1979;86: 717–723. [DOI] [PubMed] [Google Scholar]
- 37.King V. The effects of chronic inflammation on circadian rhythms in the preterm fetus. MSc Physiology, University of Auckland. 2018.
- 38.de Vries JIP, Visser GHA, Prechtl HFR. The emergence of fetal behaviour. III. Individual differences and consistencies. Early Hum Dev. 1988;16: 85–103. [DOI] [PubMed] [Google Scholar]
- 39.Roberts AB, Little D, Cooper D, Campbell S. Normal Patterns of Fetal Activity in the Third Trimester. BJOG Int J Obstet Gynaecol. 1979;86: 4–9. 10.1111/j.1471-0528.1979.tb10674.x [DOI] [PubMed] [Google Scholar]
- 40.Saastad E, Tveit J, Flenady V, Stray-Pedersen B, Fretts RC, Børdahl PE, et al. Implementation of uniform information on fetal movement in a Norwegian population reduced delayed reporting of decreased fetal movement and stillbirths in primiparous women—a clinical quality improvement. BMC Res Notes. 2010;3: 2 10.1186/1756-0500-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warland J, Heazell AEP, Mitchell EA, The STARS Consortium. An international internet survey of the experiences of 1,714 mothers with a late stillbirth: the STARS cohort study. BMC Pregnancy Childbirth. 2015;15 10.1186/s12884-015-0602-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillai M, James D, Parker M. The development of ultradian rhythms in the human fetus. Am J Obstet Gynecol. 1992;167: 172–177. [DOI] [PubMed] [Google Scholar]
- 43.de Vries JI, Visser GH, Mulder EJ, Prechtl HF. Diurnal and other variations in fetal movements and heart rate patterns at 20–22 weeks. Early Hum Dev. 1987;15: 333–348. [DOI] [PubMed] [Google Scholar]
- 44.Georgsson S, Linde A, Pettersson K, Nilsson R, Rådestad I. To be taken seriously and receive rapid and adequate care—Womens’ requests when they consult health care for reduced fetal movements. Midwifery. 2016;40: 102–108. 10.1016/j.midw.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 45.Tan KH, Sabapathy A. Maternal glucose administration for facilitating tests of fetal wellbeing. Cochrane Database Syst Rev. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Druzin M, Foodim J. Effect of maternal glucose ingestion compared with maternal water ingestion on the nonstress test. Obstet Gynecol. 1986;67: 425–426. [PubMed] [Google Scholar]
- 47.Cito G, Luisi S, Mezzesimi A, Cavicchioli C, Calonaci G, Petraglia F. Maternal position during non-stress test and fetal heart rate patterns. Acta Obstet Gynecol Scand. 2005;84: 335–338. 10.1111/j.0001-6349.2005.00644.x [DOI] [PubMed] [Google Scholar]
- 48.Higuchi H, Takagi S, Zhang K, Furui I, Ozaki M. Effect of Lateral Tilt Angle on the Volume of the Abdominal Aorta and Inferior Vena Cava in Pregnant and Nonpregnant Women Determined by Magnetic Resonance Imaging: Anesthesiology. 2015;122: 286–293. 10.1097/ALN.0000000000000553 [DOI] [PubMed] [Google Scholar]
- 49.Jeffreys R, Stepanchak W, Lopez B, Hardis J, Clapp J. Uterine blood flow during supine rest and exercise after 28 weeks of gestation. BJOG Int J Obstet Gynaecol. 2006;113: 1239–1247. 10.1111/j.1471-0528.2006.01056.x [DOI] [PubMed] [Google Scholar]
- 50.Humphries A, Mirjalili SA, Tarr GP, Thompson JMD, Stone P. The effect of supine positioning on maternal hemodynamics during late pregnancy. J Matern Fetal Neonatal Med. 2018; 1–8. 10.1080/14767058.2018.1478958 [DOI] [PubMed] [Google Scholar]
- 51.Stone PR, Burgess W, McIntyre JPR, Gunn AJ, Lear CA, Bennet L, et al. Effect of maternal position on fetal behavioural state and heart rate variability in healthy late gestation pregnancy: Effect of maternal position on fetal behavioural state. J Physiol. 2017;595: 1213–1221. 10.1113/JP273201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Due to ethical restrictions, data underlying the results presented in the study can not be shared publicly. Data will be available to approved researchers under the data-sharing arrangements provided by the Maternal and Perinatal Central Coordinating Research Hub (CCRH), based at the University of Auckland (https://wiki.auckland.ac.nz/researchhub). Metadata, along with instructions for data access, are available at the University of Auckland’s research data repository, Figshare (http://auckland.figshare.com/). Data access requests are to be submitted to the Data Access Committee via researchhub@auckland.ac.nz. De-identified data will be shared with researchers who provide a methodologically sound proposal and have appropriate institutional approval. Due to ethical restrictions, provision of data will be subject to receiving appropriate New Zealand ethical approval. Researchers must sign and adhere to a Data Access Agreement that includes a commitment to using the data only for the specified proposal, to refrain from any attempt to identify individual participants, to store data securely and to destroy or return the data after completion of the project. The CCRH reserves the right to cover the costs of making data available, if required. Contact Information for NZ Ethics Committee: Email: hdec@moh.govt.nz; Postal address: Ministry of Health, Health and Disability Ethics Committees, PO Box 5013, Wellington 6140, New Zealand; Attention: Ethics Committee manager for Protection, Regulation and Assurance.