INTRODUCTION
RET rearrangements are identified in 1% to 2% of non–small-cell lung cancers (NSCLCs).1,2 In patients with advanced, RET-rearranged lung cancers, systemic therapy can be highly active. We demonstrated previously that pemetrexed-based chemotherapy can achieve an objective response rate of 45% and a median progression-free survival (PFS) of 19 months.3 Furthermore, the activity of targeted therapy has improved dramatically with the introduction of selective RET inhibitors to the clinic. In early-phase testing, objective response rates with LOXO-2924 and BLU-6675 are 68% (26 of 38) and 50% (seven of 14), respectively. These outcomes exceed the modest activity observed previously with multikinase inhibitors such as cabozantinib6 and vandetanib.7
In contrast, the activity of immunotherapy in RET-rearranged lung cancers has not been well characterized. This represents a clear unmet need, given that all prior regulatory approvals of immune checkpoint inhibitors, either alone or in combination with chemotherapy, and in stage III or IV disease, have technically included patients with RET-rearranged lung cancers.8,9 Furthermore, although increasing levels of programmed death-ligand 1 (PD-L1) expression and high tumor mutational burden (TMB) have been associated with benefit from immune checkpoint blockade,10 the immunophenotype of RET-rearranged lung cancers and the role of PD-L1 and TMB status in relation to benefit with immunotherapy remain poorly described. We set out to characterize these factors.
METHODS
In this retrospective study, patients from Memorial Sloan Kettering Cancer Center with RET-rearranged lung cancers diagnosed between 2009 and 2017 were identified under an institutional review board–approved waiver. Clinical characteristics including age, sex, and smoking history were collected. Patients who received immunotherapy, defined as a monoclonal antibody against programmed cell death protein 1 (PD-1) or PD-L1, were included in an analysis of treatment history.
RET rearrangements were identified using targeted next-generation sequencing of DNA (Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets [MSK-IMPACT] or Foundation One) or RNA (anchored multiplex polymerase chain reaction [PCR]; Memorial Sloan Kettering Solid Fusion Panel) in more contemporary samples, and fluorescence in situ hybridization (10q11 and 6q22 break apart probe, Metasystems, Altussheim, Germany) or reverse-transcriptase PCR in older samples.11,12 PD-L1 immunohistochemistry was evaluated using the E1L3N antibody (Cell Signaling Technology, Danvers, MA), our institutional standard, which has been validated against a 22C3 kit performed in a commercial laboratory with comparable results.13 For uniformity, TMB (reported as the number of nonsynonymous mutations per megabase) was analyzed only for samples sequenced using MSK-IMPACT.14,15 The median TMB of RET-rearranged was compared with that of RET wild-type NSCLCs (Mann-Whitney U test).
In patients with both baseline and serial on-treatment imaging, the best objective response to therapy (Response Evaluation Criteria in Solid Tumors [RECIST] v1.1) was determined by a study radiologist. Time to treatment discontinuation (TTD) was defined as the time from therapy initiation to the last dose.16 PFS was defined as the time from therapy initiation to radiologic progression or death. Overall survival (OS) was defined as the time from diagnosis of metastatic disease to death. For TTD, PFS, and OS analyses, Kaplan-Meier curves were compared using the Mantel-Cox log-rank test. Hazard ratios were calculated using the Mantel-Haenszel method.
RESULTS
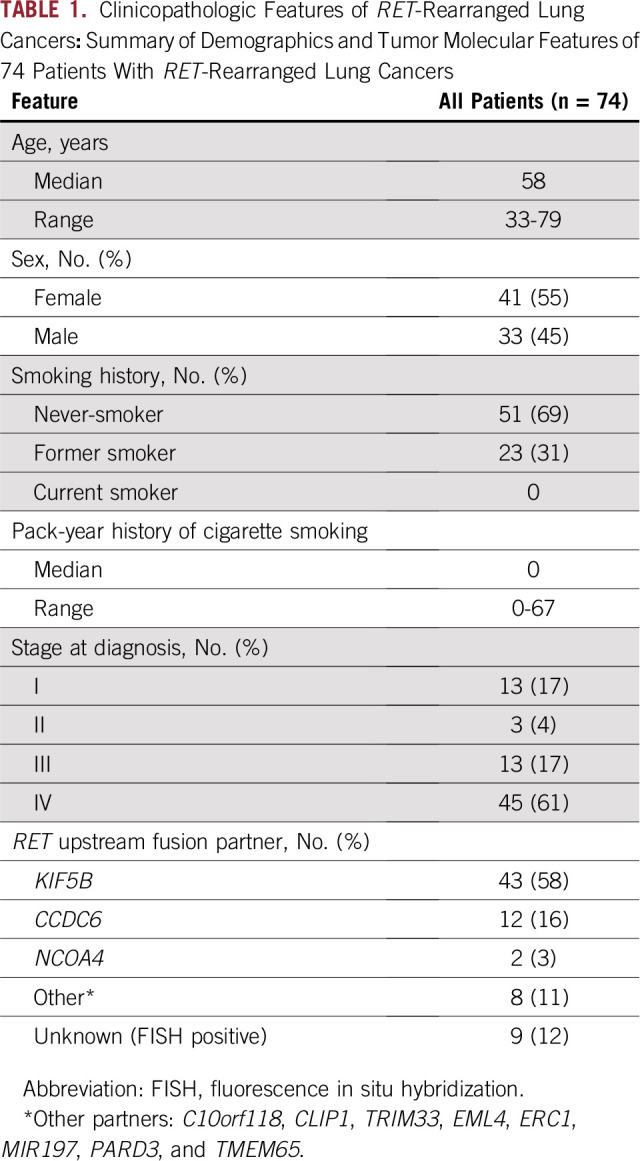
Seventy-four patients with RET-rearranged lung cancers were identified. Clinicopathologic features are summarized in Table 1. The median age was 58 years, 55% were female, and 69% were never-smokers. RET rearrangement was identified as follows: DNA-based next-generation sequencing (80% [n = 59]), RNA-based anchored multiplex PCR (1% [n = 1]), fluorescence in situ hybridization (15% [n = 11]), and reverse-transcriptase PCR (4% [n = 3]). Consistent with previous reports, the most common RET fusion partner was KIF5B (66% [n = 43 of 65]), followed by CCDC6 (18% [n = 12 of 65]) when known.
TABLE 1.
Clinicopathologic Features of RET-Rearranged Lung Cancers: Summary of Demographics and Tumor Molecular Features of 74 Patients With RET-Rearranged Lung Cancers
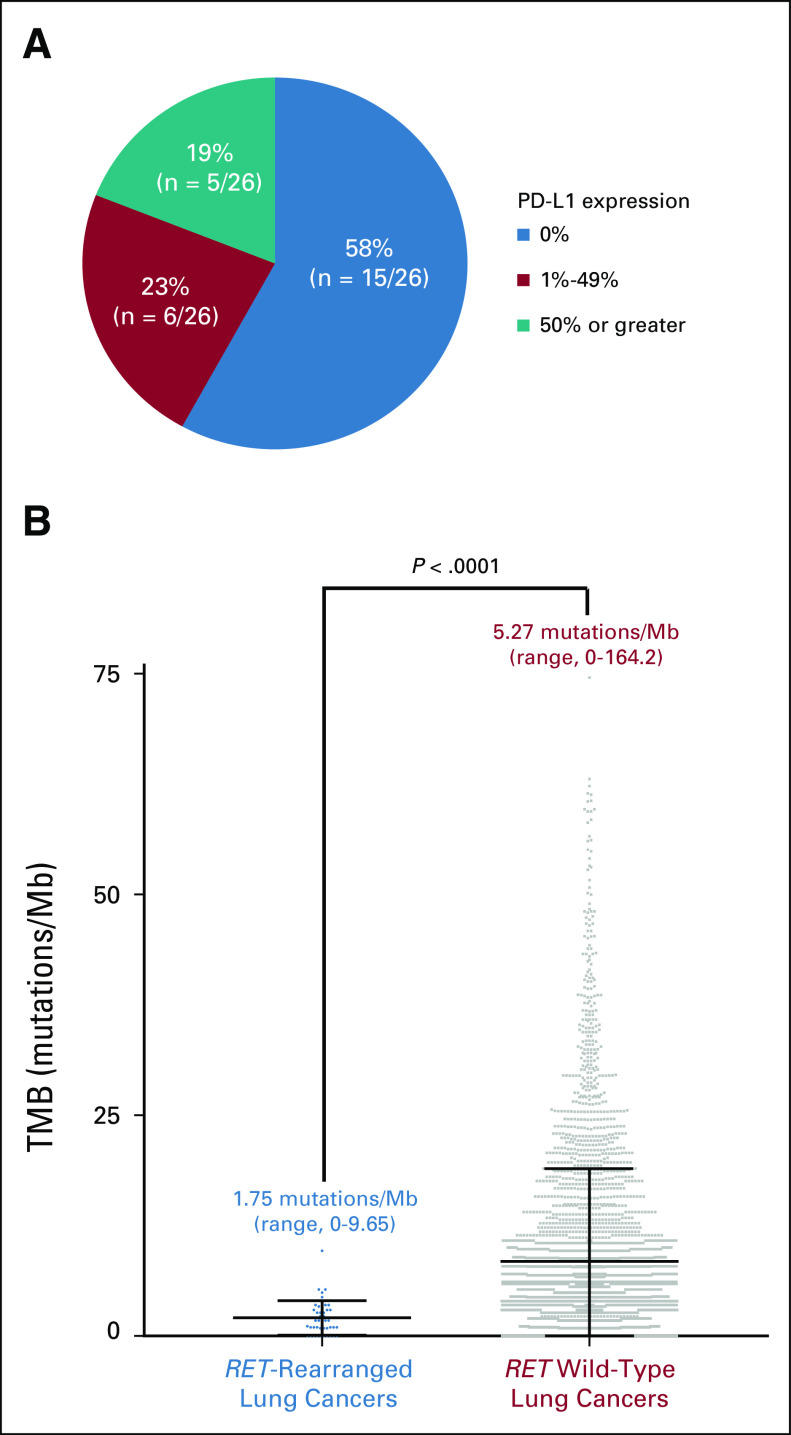
In patients with sufficient tissue for PD-L1 testing, tumor PD-L1 expression was 0%, 1% to 49%, and 50% or greater in 58% (n = 15 of 26), 23% (n = six of 26), and 19% (n = five of 26) of cases, respectively (Fig 1A). PD-L1 expression was absent or below 50% in the majority of tumors (81% [n = 21 of 26]). No major differences in PD-L1 expression were observed when stratified by upstream fusion partner (Appendix Table A1). In 44 patients with sufficient tissue for TMB analysis, the median TMB was 1.75 mutations/Mb (range, 0 to 9.65 mutations/Mb), significantly lower (P < .0001) than the median TMB of 5.27 mutations/Mb (range, 0 to 164.20 mutations/Mb) in 3,631 patients with RET wild-type NSCLCs (Fig 1B).
FIG 1.
Immunophenotype of RET-rearranged lung cancers. (A) The programmed death-ligand 1 (PD-L1) expression (E1L3N, Cell Signaling) of 26 RET-rearranged lung cancers with sufficient tissue for testing is shown. The majority (81%) of tumors had either no PD-L1 expression (0%) or low levels of PD-L1 expression (1% to 49%). (B) The tumor mutational burden (TMB) of 44 RET-rearranged lung cancers is displayed (left) relative to the TMB of 3,631 RET wild-type lung cancers (right). Above each plot, the median TMB and TMB range are indicated. The median TMB of RET-rearranged lung cancers was significantly lower than the median TMB of RET wild-type lung cancers (Mann-Whitney; P < .0001). For ease of representation, three outlier RET wild-type lung cancer samples with TMB greater than 75 mutations/Mb that were included in the statistical analysis were excluded in this plot.
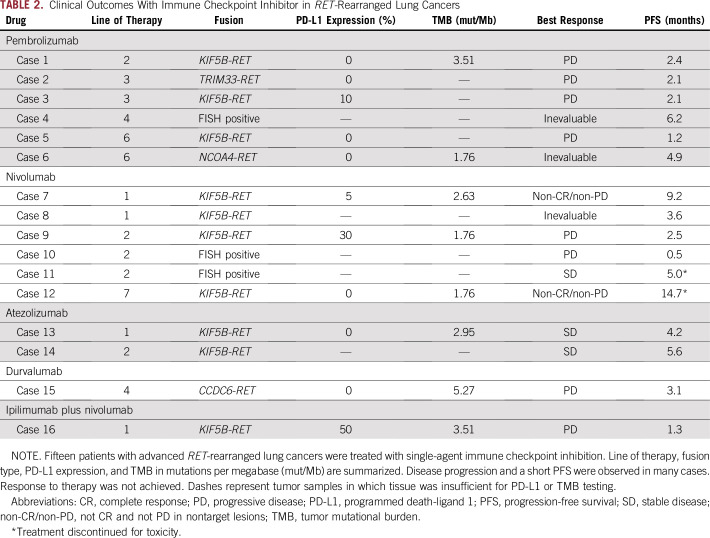
The clinical outcomes of immunotherapy in patients with advanced RET-rearranged lung cancers are summarized in Table 2. Patients received pembrolizumab (n = 6), nivolumab (n = 6), atezolizumab (n = 2), durvalumab (n = 1), or ipilimumab plus nivolumab (n = 1). The median line of therapy at which immunotherapy was administered was 2 (range, 1 to 7). In cases with sufficient tissue for testing, PD-L1 expression ranged from 0% to 50% and TMB ranged from 1.76 to 5.27 mutations/Mb.
TABLE 2.
Clinical Outcomes With Immune Checkpoint Inhibitor in RET-Rearranged Lung Cancers
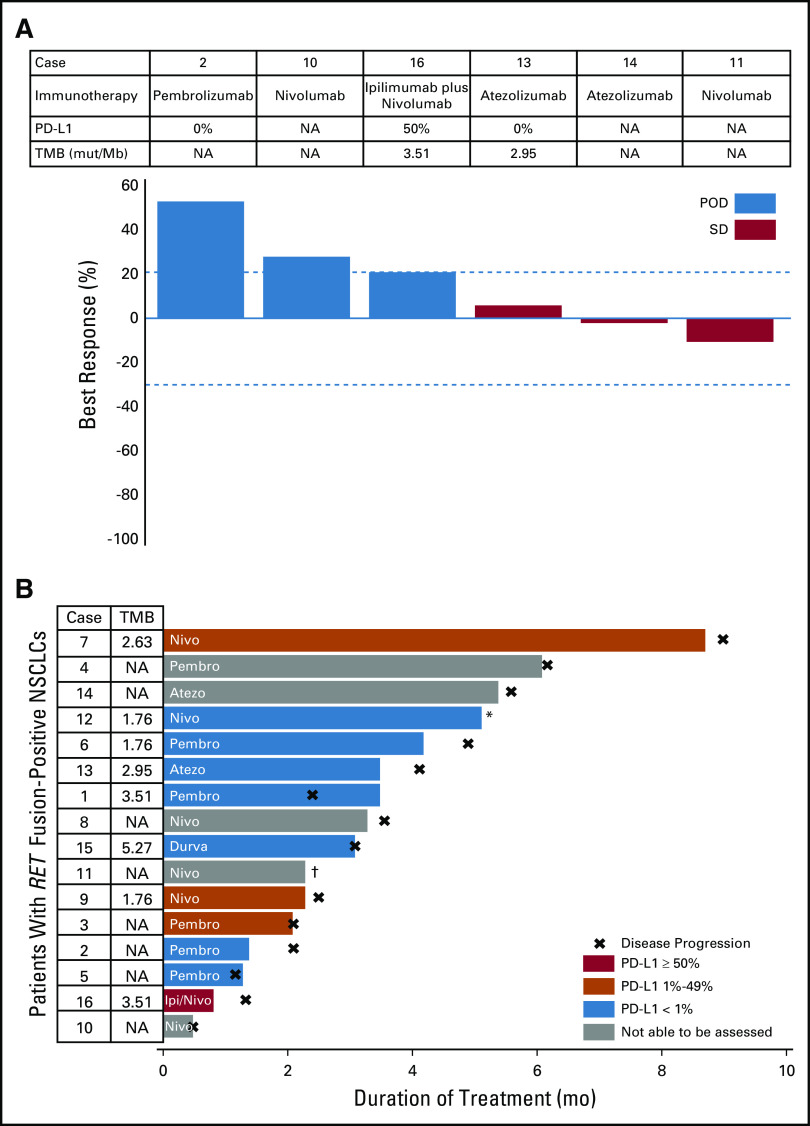
A total of 13 patients with RET-rearranged lung cancers were assessed for clinical and/or radiologic response. Response to immunotherapy was not observed. Progression of disease was observed in 62% of cases (n = eight of 13; Table 2); disease progression involved new or worsening brain metastases in three patients (cases 4, 6, and 15). Stable disease was achieved in 23% (three of 13) and non-CR/non-PD (not complete reponse and not progressive disease in nontarget lesions) in 15% (two of 13). A waterfall plot of best objective response in the six patients with measurable disease is presented in Fig 2A.
FIG 2.
Response to immunotherapy and treatment duration. (A) A waterfall plot of best objective response to single-agent immune checkpoint inhibition is shown. Only patients with measurable disease (n = 6) were included in this analysis. No complete or partial responses were observed. (B) A swimmer’s plot of time to treatment discontinuation is shown for all patients who received immunotherapy (n = 16). None of the patients who received immunotherapy remain on treatment. For both figures, the agent administered and the immunophenotype (programmed death-ligand 1 [PD-L1]) and tumor mutational burden [TMB], when known) are indicated for each patient. (*) Immune checkpoint inhibitor stopped for toxicity; continued stable disease for a PFS of 14.7 months. (†) Immune checkpoint inhibitor stopped for toxicity; continued stable disease for PFS of 5 months, but died as a result of pneumonitis. mut, mutations; NA, not available; NSCLC, non–small-cell lung cancer; POD, progression of disease.
The median PFS in all patients was 3.4 months (95% CI, 2.1 to 5.6 months). No association was seen between PD-L1 or TMB status and PFS. In patients with the highest levels of PD-L1 expression (50% and 30%), PFS was short (1.3 months and 2.5 months, respectively). Similarly, in patients with the highest TMB (5.27 and 3.51 mutations/Mb), PFS was short (3.1 and 1.3 months, respectively).
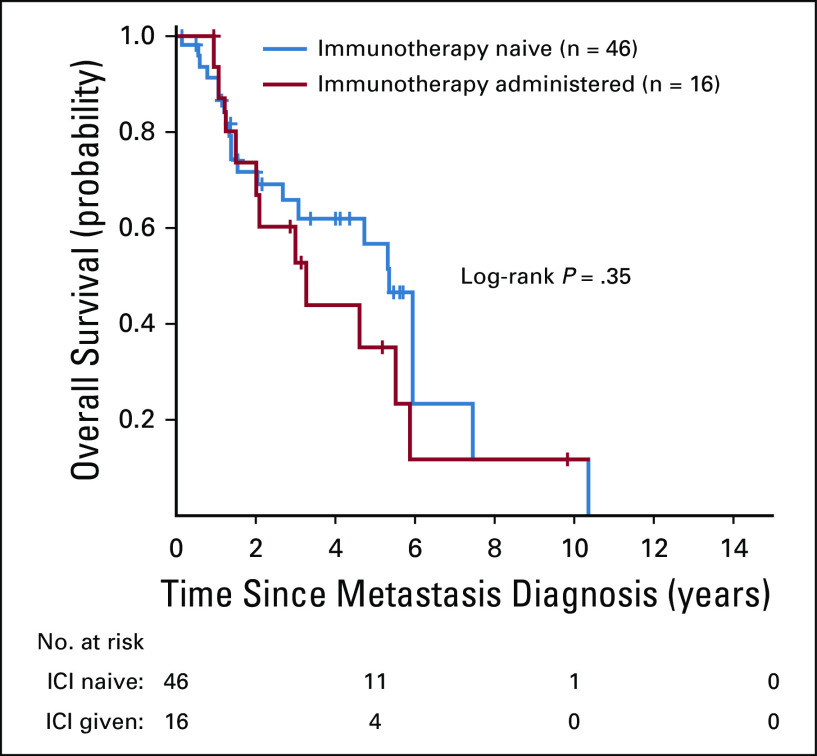
A swimmer’s plot of TTD is presented in Fig 2B. The median TTD in all patients was 3.2 months (range, 0.5 to 14.7 months). Treatment was discontinued for toxicity in two of 16 patients (fatigue [case 11] and pneumonitis [case 8]). When patients with advanced RET-rearranged lung cancers who received immunotherapy (n = 16) were compared with those who did not receive immunotherapy (n = 46), there was no difference in OS (hazard ratio, 1.4 [95% CI, 0.7 to 2.9]; log-rank P = .35; Appendix Fig A1).
DISCUSSION
In this series, we demonstrate that the immunophenotype of RET-rearranged lung cancers is characterized by low levels of PD-L1 expression and low TMB in the majority of patients. This raises the possibility that these tumors are biologically cold (ie, less likely to be highly responsive to immunotherapy relative to other cancers). Consistent with this hypothesis, overall outcomes with single- and dual-agent immunotherapy were poor. No responses were observed, and the best objective response to therapy in most patients was progressive disease. Furthermore, median PFS was short.
These findings are consistent with a growing body of evidence uncovering poor outcomes with immune checkpoint inhibition in select oncogene-addicted lung cancers. In EGFR-mutant and ALK-rearranged lung cancers, early data on the decreased activity (compared with unselected cancers) of immunotherapy resulted in the exclusion of patients with these tumors in registration-enabling studies. Furthermore, we showed previously that MET exon 14–altered NSCLCs had low TMB and poor outcomes with immunotherapy.17 Finally, an ongoing global registry (Immunotarget) and independent series have shown similarly low response rates and short median PFS with single-agent immune checkpoint inhibition in lung cancers with oncogenic drivers.18 Immunotarget had 16 patients in the RET-rearranged cohort and reported a response rate of 6% and median PFS of 2.1 months, comparable to the findings in our study.
The clinical implications of these observations relate to the sequence with which immunotherapy is used and the type of immunotherapy strategy selected. Regarding the former, it is becoming increasingly clear that specific, targeted therapies (selective RET inhibitors)2,4,19 and chemotherapy agents (pemetrexed-containing regimens)3 can achieve superior outcomes compared with immunotherapy in this series. Thus, it is reasonable to consider the use of checkpoint inhibition only after select targeted therapies and platinum doublet-containing chemotherapy have been administered.
Note that high PD-L1 expression (50% or more) was uncommon in RET-rearranged lung cancers in this study. Only one case treated with immunotherapy highly expressed PD-L1 (50%) and, despite this, still responded poorly to dual immune checkpoint inhibitor therapy (case 16). A recommendation regarding the use of pembrolizumab in treatment-naïve, advanced RET-rearranged lung cancers with high PD-L1 expression cannot be made on the basis of our findings, although its use should be approached with caution. In MET exon 14–altered lung cancers, TMB is similarly low; although a higher proportion of tumors have high PD-L1 expression, this was not associated with increased benefit with single-agent immunotherapy.17
Finally, no patients in this series received the combination chemotherapy and immunotherapy that is currently approved for the treatment of EGFR and ALK wild-type advanced NSCLC. Given the benefit of pemetrexed-containing regimens in RET-rearranged lung cancers, a combination of a platinum agent, pemetrexed, and pembrolizumab is reasonable to consider. Prospective trials of immunotherapy combinations should incorporate comprehensive molecular profiling to establish prospective data in driver-positive subpopulations of patients.
In summary, most RET-rearranged lung cancers have low PD-L1 expression and low TMB, and response to immunotherapy was not observed in this series. Although this study is limited by a small sample size, given the rarity of RET-rearranged NSCLCs, these findings remain meaningful and support evolving literature showing that outcomes with immune checkpoint inhibition are poor in select oncogene-addicted NSCLCs. Other systemic therapy strategies should instead be considered in patients with advanced RET-rearranged lung cancers before administering immunotherapy alone.
Appendix
Fig A1.
Overall survival of RET-rearranged lung cancers. Overall survival from the diagnosis of metastatic disease was compared between patients who received an immune checkpoint inhibitor (n = 16) and those who did not (n = 46). There was no difference in overall survival between these groups (hazard ratio, 1.4 [95% CI, 0.7 to 2.9]; log-rank P = .35). ICI, immune checkpoint inhibitor.
Table A1.
PD-L1 Status by RET Upstream Fusion Partner

Footnotes
Supported in part by the National Cancer Institute of the National Institutes of Health (T32 CA009207, P30 CA008748).
AUTHOR CONTRIBUTIONS
Conception and design: Michael Offin, Robin Guo, Stephanie L. Wu, Joshua Sabari, Josiah D. Land, Larry W. Buie, Terry Pak, Dazhi Liu, Matthew D. Hellmann, Maria Arcila, Mark G. Kris, Bob T. Li, Alexander Drilon
Financial Support: Mark G. Kris
Administrative support: Mark G. Kris
Provision of study material or patients: Gregory J. Riely, Mark G. Kris, Charles M. Rudin, Marc Ladanyi, Natasha Rekhtman
Collection and assembly of data; Michael Offin, Robin Guo, Stephanie L. Wu, Joshua Sabari, Josiah D. Land, Joseph Montecalvo, Larry W. Buie, Dazhi Liu, Matthew D. Hellmann, Ryma Benayed, Maria Arcila, Mark G. Kris, Charles M. Rudin, Marc Ladanyi, Natasha Rekhtman, Alexander Drilon
Data analysis and interpretation: Michael Offin¸ Robin Guo, Stephanie L. Wu, Joshua Sabari, Josiah D. Land, Ai Ni, Darragh F. Halpenny, Larry W. Buie, Dazhi Liu, Gregory J. Riely, Matthew D. Hellmann, Maria Arcila, Mark G. Kris, Bob T. Li, Marc Ladanyi, Natasha Rekhtman, Alexander Drilon
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Michael Offin
Consulting or Advisory Role: PharmaMar
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme
Larry W. Buie
Consulting or Advisory Role: Amgen, Jazz Pharmaceuticals, Heron, Pfizer
Gregory J. Riely
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Millennium Pharmaceuticals (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Infinity Pharmaceuticals (Inst), ARIAD Pharmaceuticals (Inst), Mirati Therapeutics (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Patent application submitted covering pulsatile use of erlotinib to treat or prevent brain metastases (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Matthew D. Hellmann
Stock and Other Ownership Interests: Shattuck Labs
Honoraria: AstraZeneca, Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech, AstraZeneca/MedImmune, Novartis, Janssen Pharmaceuticals , Nektar, Syndax Pharmaceuticals, Mirati Therapeutics, Shattuck Labs
Research Funding: Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: A patent has been filed by Memorial Sloan Kettering (PCT/US2015/062208) for the use of tumor mutation burden for prediction of immunotherapy efficacy, and which is licensed to Personal Genome Diagnostics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol-Myers Squibb
Maria Arcila
Honoraria: Invivoscribe
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Invivoscribe, Raindance Technologies
Mark G. Kris
Consulting or Advisory Role: AstraZeneca, Regeneron Pharmaceuticals, Pfizer
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: Memorial Sloan Kettering Cancer Center
Charles M. Rudin
Consulting or Advisory Role: Bristol-Myers Squibb, AbbVie, Seattle Genetics, Harpoon Therapeutics, Genentech/Roche, AstraZeneca, Ascentage Pharma, Bicycle Therapeutics, Celgene, Daiichi Sankyo, Ipsen, Loxo, PharmaMar, Elucida Oncology
Research Funding: AbbVie/Stemcentrx (Inst), Viralytics (Inst), Merck (Inst), Daiichi Sankyo (Inst)
Bob T. Li
Consulting or Advisory Role: Roche, Biosceptre International, Thermo Fisher Scientific, Mersana, Guardant Health, Hengrui Therapeutics
Research Funding: Roche/Genentech (Inst), Illumina (Inst), BioMed Valley Discoveries (Inst), AstraZeneca (Inst), GRAIL (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Guardant Health (Inst)
Marc Ladanyi
Honoraria: Merck (I)
Consulting or Advisory Role: National Comprehensive Cancer Network/AstraZeneca Tagrisso Request for Proposal Advisory Committee, Takeda Pharmaceuticals, Bristol-Myers Squibb, Bayer AG, Merck (I)
Research Funding: Loxo (Inst), Helsinn Therapeutics
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, Peerview
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer AG, Tyra Biosciences, Verastem, Takeda Pharmaceuticals/ARIAD Pharmaceuticals/Millenium Pharmaceuticals, BerGenBio, MORE Health
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, TEVA Pharmaceuticals Industries, Taiho Pharmaceutical
No other potential conflicts of interest were reported.
REFERENCES
- 1.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15:151–167. doi: 10.1038/nrclinonc.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drilon A, Bergagnini I, Delasos L, et al. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol. 2016;27:1286–1291. doi: 10.1093/annonc/mdw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxnard GR, Subbiah V, Park K, et al. Clinical activity of LOXO-292, a highly selective RET inhibitor, in patients with RET fusion+ non-small cell lung cancer: An update from ASCO 2018. IASLC 19th World Conference on Lung Cancer; Toronto, Canada. September 23-26, 2018. [Google Scholar]
- 5.Subbiah V, Taylor M, Lin J, et al. Abstract CT043: Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in a phase I study of advanced, RET-altered solid tumors. http://cancerres.aacrjournals.org/content/78/13_Supplement/CT043 [Google Scholar]
- 6.Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SH, Lee JK, Ahn MJ, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: A phase II clinical trial. Ann Oncol. 2017;28:292–297. doi: 10.1093/annonc/mdw559. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 10.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 13.Gaule P, Smithy JW, Toki M, et al. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol. doi: 10.1001/jamaoncol.2016.3015. epub ahead of print on August 18, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33:843–852.e4. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Offin M, Rizvi H, Tenet M, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res. 2019;25:1063–1069. doi: 10.1158/1078-0432.CCR-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabari JK, Leonardi GC, Shu CA, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29:2085–2091. doi: 10.1093/annonc/mdy334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazieres J, Drilon AE, Mhanna L, et al. Efficacy of immune-checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) patients harboring activating molecular alterations (ImmunoTarget) J Clin Oncol. 2018;36 (suppl; abstr 9010) [Google Scholar]
- 19.Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8:836–849. doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]