Abstract
Adipocyte development and adipose tissue expansion have many implications for human diseases, including obesity. Obesity is a debilitating disorder and a risk factor for metabolic disorders including insulin resistance and diabetes mellitus, due in part to an overabundance of adipocytes and adipocyte dysfunction. In recent years, obesity has become a global pandemic with approximately one-third of US adults classified as obese. Adipose tissue has recently been identified as a major metabolic organ, classified into white adipose tissue (WAT) and brown adipose tissue (BAT). Other than lifestyle modifications and invasive surgeries, only a very limited number of drugs are available to treat obesity and overweight.
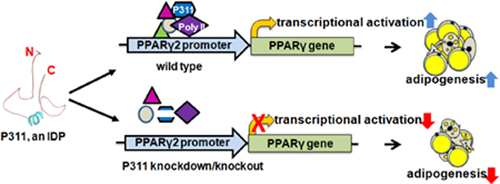
P311 has been shown to play a key role in blood pressure regulation, vascular contractility and tissue remodeling. Here we present a role for P311 in adipogenesis using a 3T3-L1 cell culture model. P311 expression is initiated with the induction of adipogenesis and increased during adipogenesis. This increase correlates with an increase in the expression of the key adipogenic transcriptional factors PPARγ2 and C/EBPα. In addition, siRNA-mediated P311 knockdown inhibits adipogenic differentiation in 3T3-L1 cells. Finally, P311 binds to the PPARγ2 promoter, implicating P311 mediates adipogenesis partly through PPARγ activation.
Keywords: P311, adipogenesis, PPARγ activation, intrinsically disordered protein, white adipose tissue
Graphical Abstract
1. Introduction
Obesity is a complex metabolic disorder affecting one in three adults and one in five children in the United States [1]. It is a risk factor for insulin resistance, type II diabetes mellitus (T2DM), hypertension, cardiovascular disease, stroke, cancer and hepatic steatosis [2]. Adipose tissue (AT) consists of two types: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT and BAT are directly and inversely proportional to body mass index (BMI) and adiposity, respectively. Currently, WAT and BAT are considered as endocrine organs that contribute to systemic metabolic regulation [3,4]. Understanding the molecular mechanisms of adipocyte differentiation of WAT and BAT has many implications for understanding metabolic diseases. An array of cellular events and networks—including transcriptional factors (TF) and cofactors (TCF)—regulate mesenchymal stem cell commitment and preadipocyte differentiation into adipocytes. Key transcriptional regulators of this process include three members of the CCAAT enhancer-binding protein (C/EBPβ, δ and α) family [5] and peroxisome proliferator activator (PPARγ) [6,7]. Despite progress in recent years to identify the mechanisms that control WAT expression, efforts are still needed to study adipocyte origins and differentiation [1,8], to find novel therapeutics to treat obesity and associated disorders.
P311 is an 8-kDa protein that is conserved across species [9,10] and abundantly produced in vascular smooth muscle [9,10], myofibroblasts [11], lung adipoblasts [12], blood mononuclear cells [13], and fibrotic tissues [14]. Recently, Leung et al. showed that P311 overexpression induces lipid accumulation in C3H10T1/2 cells. This study showed that P311 knockdown inhibited retinoic acidmediated lipid accumulation, but no mechanism(s) were identified [12]. Further, Chaudhuri et al. showed a decrease in the expression of P311 in mononuclear cells of obese T2DM individuals treated with exenatide, an antidiabetic drug that also targets weight loss [13]. As no systematic studies have shown the role of P311 in adipogenesis, we initiated this study. Our data demonstrate that P311 regulates adipocyte development. The adipogenic function of P311 is modulated partly through regulating PPARγ activation. Understanding the molecular functions of novel factors like P311, that cross talk across multiple pathologies, is important to the development of monotherapies for multiple diseases.
2. Materials and methods
2.1. Cell culture –
3T3-L1 cells (CL-173™, ATCC) were cultured in Dulbecco’s Modified Eagle Media high medium with 10% fetal calf serum (ATCC) and penstrep and used for the adipogenesis studies as described earlier [15].
2.2. Transfections –
3T3-L1 cells were transfected with pCMV (empty vector), pcP311 (c-terminal myc-tagged) plasmids [9] or other plasmids using Amaxa™ Cell Line Nucleofector™ Kit (VCA-1005, Lonza Biologics, Inc., Portsmouth, NH) following manufacturer’s optimized protocol.
2.3. siRNA studies –
Self delivering P311 siRNA, Accell Mouse Nrep (27528) siRNA - SMART pool (GE Healthcare Dharmacon, Inc., Lafayette, CO), was used for knockdown studies. A pool of four siRNA (75 μM) were delivered using 1X siRNA delivery medium. Non-targeting (NT-) siRNA and siRNA-green were used for experimental control and transfection efficiency studies, respectively.
2.4. Quantitative real-time PCR –
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA). Complimentary DNA (cDNA) was synthesized using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). cDNA was amplified and detected with the iQ SYBR Green Supermix (Bio-Rad Laboratories) according to the manufacturer’s protocol using a C1000 Touch Thermal cycler with CFX96 Real Time detection system (Bio-Rad Laboratories). Onboard software was used to calculate the cycle threshold. Results are expressed as 2−ΔΔCT [9,15].
2.5. Western Blots –
Immunoblot analysis was performed as described earlier [9,15]. Briefly, proteins were extracted using either M-PER lysis buffer (Pierce Protein Biology, Waltham, MA) or chromatin immunoprecipitation (ChIP) lysis buffer (Millipore, Burlington, MA) supplemented with protease inhibitor cocktail, lactacystine (10 μM) and o-phenantroline (1.25 mM). Proteins were resolved on 4–12% Bis-Tris gels and transferred onto Immuno-BlotR PVDF membrane (Bio-Rad). The membranes were incubated with primary antibodies followed by horseradish peroxidase conjugated secondary antibodies (1:10,000 dilution). Then, Amersham™ ECL Western blotting analysis system (GE Healthcare) was used to visualize the chemiluminescence of protein bands. Anti-P311 (Abcam, Eugene, CA), anti-PPARγ (Abcam), anti-RNA polymerase II (RNApolII) (Sigma-Aldrich, St. Louis, MO, EMD Millipore Corp., Billerica, MA) and anti-GAPDH (Abcam) were used.
2.6. Chromatin Immunoprecipitation (ChIP) Assays –
ChIP assays were conducted [9,15] using ChIP Assay Kit (EMD Millipore Corp.) following manufacturer’s instructions. 3T3-L1 cells were transfected with plasmids pcP311 or pCMV (empty vector) and one set was used for ChIP assays at 48 hours post transfection (pc- and pcP311-). Adipogenesis was induced in the second set as described above and mature adipocytes were used after 10 days of adipogenic induction (pc+ and pcP311+). ChIP quality mouse monoclonal anti-RNApolII Clone CTD4HB (EMD Millipore Corp.) was used with SureBeads protein A/G magnetic beads (BioRad) to immunoprecipitate RNApolII. Anti-c-Myc magnetic beads (Pierce) were used to immunoprecipitate c-myc-tagged P311. One sixth of the beads were used to perform western blots to confirm protein immunoprecipitation. Cell lysates were used for the ChIP assays. The remaining beads were used to extract chromatin and perform quantitative real-time PCRs to amplify either the PPARγ promoter or the C/EBPα promoter. Input ChIP lysate samples were saved to perform qPCRs for the PPARγ promoter and C/EBPα promoter for normalization purposes.
2.7. Immunofluorescence (IF) –
IF studies were conducted as described earlier [16]. 3T3-L1 cells were fixed with 4% paraformaldehyde, followed by blocking with 3% BSA with triton X100 made in 1X phosphate buffered saline. Anti-myc-tag antibody (Sigma) for overexpressed c-myc-P311 and anti-P311 antibody (mouse: Invitrogen, and Abcam) were used (1:1000). 4’, 6-diamidino-2-phenylindole (DAPI, Blue) was used to stain nuclei.
2.8. Secrete-Pair Dual Luminescence Assay –
3T3-L1 preadipocytes were transfected with either the PPARγ2 promoter reporter construct pEZX-PG04-PPARγ2 promoter or pEZX-PG04-C/EBPα promoter and plasmids pCMV (empty vector) or pcP311. Adipogenesis was induced as described above for 5 days and luciferase-secreted alkaline phosphatase (SEAP) assays were performed. Promoter reporter constructs contained Gaussia luciferase (GLuc) and SEAP reporter system and used with the assay kit (GeneCopoeia Inc., Rockville, MD). Additional negative control construct (pEZX-PG04 empty vector) with pc-P311 plasmid cotransfections was used as a negative control.
2.9. Oil-Red-O staining –
Oil-Red-O staining was performed as described earlier by Badri et al. [15] using Adipogenesis Assay kit (MilliporeSigma, St Louis, MO). Oil-Red-O staining pictures were taken using an Olympus IX71 microscope. The staining was quantitated using dye extraction solution and absorbance was read at 520 nm following manufacturer’s instructions.
2.10. Protein structure prediction –
The structure of full length P311 protein was predicted using protein structure prediction web server Phyre2. Phyre2 predicted protein structure using homology modeling method.
2.11. Data Analysis –
Data was presented as mean ± standard deviation. Statistical significance was calculated across groups using either student t test or one way analysis of variance, ANOVA.
3. Results
3.1. P311 expression is induced during adipogenesis –
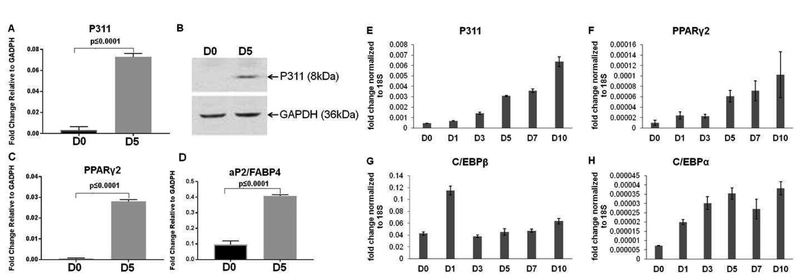
We chose the widely used 3T3-L1 preadipocyte cell culture model for our current studies because it sufficiently represents in vivo white adipogenesis and lipogenesis mechanisms [17]. As shown in Figure 1A, P311 mRNA levels were increased significantly on day 5 (D5) of postadipogenic induction in 3T3-L1 adipocytes compared to uninduced 3T3-L1 fibroblast like preadipocytes (D0). The increased levels of key adipogenic markers, PPARγ2 and adipocyte protein 2 (aP2) in D5 adipocytes served as positive controls (Figure 1C & D). Western blot analysis revealed an increase in P311 proteins levels in adipocytes compared to preadipocytes (Figure 1B). Further, we determined the expression profiles of early key adipogenic markers and adipocyte-specific markers along with P311 to compare the patterns of adipogenic gene expression during adipogenesis. P311 and adipogenic gene expression showed basal expression in uninduced 3T3-L1 preadipocytes (D0). However, P311 expression started rising on day 1 and continuously increased significantly through day 10 after adipogenic induction (p<0.0001). We observed a similar expression pattern of P311 with PPARγ2 (p<0.001) versus the other adipogenic markers tested, C/EBPβ, C/EBPδ (data not shown) and C/EBPα (Figure 2 E–H).
P311 was induced during 3T3-L1 adipogenesis.
A. A qPCR study was used to examine the expression of P311. B. Western blot analysis was performed to verify P311 protein expression. The lower panel shows the loading control, GAPDH. C & D. Expression of the key adipogenic markers PPARγ2 and aP2. E - H. Gene expression profile of 3T3-L1 adipogenesis. qPCR was used to examine the expression of P311 (p<0.0001) (E) and the adipogenic markers PPARγ2 (p<0.001) (F), C/EBPβ (p<0.0001) (G) and C/EBPα (p<0.0001) (H) in 3T3-L1 preadipocytes differentiated for different days (D0 to D10).
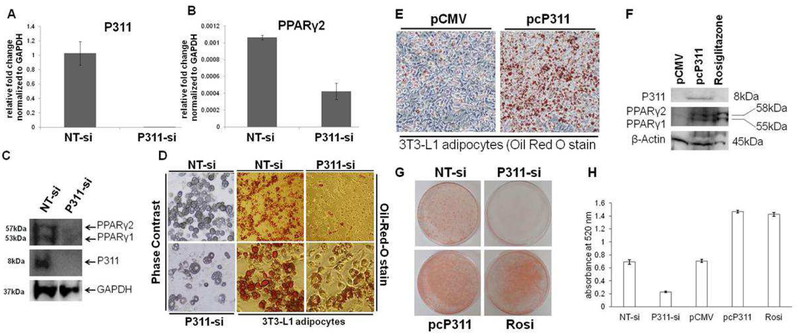
P311 knockdown inhibited 3T3-L1 adipogenesis and P311 overexpression promoted adipogenesis.
A and B. qPCR was used to measure P311 and PPARγ2 mRNA levels in cells induced to differentiate with either non-targeted siRNA (NT-si) or P311 siRNA. C. Western blot analysis of cell lysates of NT-si or P311-siRNA treated 3T3-L1 adipocytes. The lower panel represents the loading control (GAPDH). D. Left panel shows the phase contrast pictures of 3T3-L1 adipocytes treated with NT-si or P311-siRNA. Oil Red O staining was performed to assess lipid accumulation (middle and right panels). E. 3T3-L1 preadipocytes were transfected with control (pCMV) or P311 overexpression (pcP311) plasmid followed by adipogenic induction. Oil Red O staining is shown. F. Western blot analysis showed the overexpression of c-myc-tag-P311 immunodetected with anti-myc-tag-antibody. The P311 overexpression group showed elevated PPARγ expression compared to the control group. The lower panel represents the loading control (β-actin). G & H. Oil Red O staining (10 day postadipogenic induction) was performed to assess lipid accumulation and quantified the lipid accumulation.
3.2. P311 knockout inhibits adipogenic differentiation of 3T3-L1 cells –
To elucidate the requirement of P311 in adipogenesis, we conducted P311 knockdown studies using a novel siRNA technology. Additional NT-siRNA was used as negative control. As shown in Figure 2, P311 siRNA significantly knocked down the endogenous P311 as shown by both PCR and immunoblot. Knockdown of P311 also significantly decreased the levels of PPARγ2 (Figure 2C). Oil-Red-O staining of neutral lipids showed fewer differentiated adipocytes in the P311-siRNA group (Figure 2D) compared to the non-targeting siRNA (NT-si) control group. These results show that P311 knockdown inhibits adipogenesis and reduces lipid accumulation in 3T3-L1 adipocytes.
3.3. P311 overexpression enhanced adipogenic differentiation of 3T3-L1 cells –
To determine whether an increase in P311 expression could enhance adipogenesis in 3T3-L1 preadipocytes, we transiently transfected 3T3-L1 cells with either empty vector (pCMV) or c-myc-tag-P311 plasmid (pcP311) [9], followed by adipogenic induction. At 5 days post adipogenic induction, Oil-Red-O staining showed that P311 overexpression enhanced adipogenesis, as indicated by increased lipid accumulation (Figure 2E). The pcP311 group showed overexpression of myc-P311 and elevated PPARγ2 levels (Figure 2F). By contrast, the empty vector and rosiglitazone (2μM) treated groups showed no/lower and comparable PPARγ2 expression respectively.
3.4. P311 is a cytoplasmic and nuclear protein –
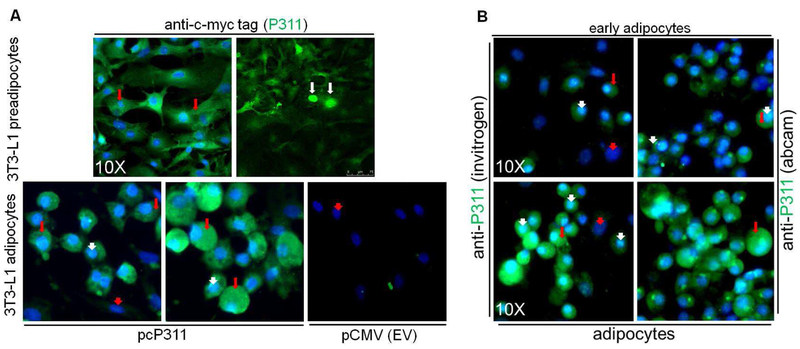
P311 was majorly shown to be present in the cytoplasm. While conducting adipocyte induction studies, we observed nuclear translocation of P311 in 3T3-L1 preadipocytes transfected with pCMV-P311 to overexpress c-myc-tagged P311 in immunofluorescence studies (Figure 3A). In preadipocytes, P311 is mainly found in the cytoplasm (Figure 3A-top panel, red arrows). However, a few cells indicated the presence of P311 in the nucleus (white arrows, Figure 3A-top panel). Also, more P311 is localized to the nucleus in differentiated P311 transfected adipocytes but not in empty vector transfected controls (Figure 3A-lower panel) as indicated by anti-myc-tag antibody immunofluorescence. In untransfected 3T3-L1 adipocytes (Figure 3B), we observed elevated expression of endogenous P311 in the cytoplasm (red arrows, green fluorescence) as well as the nucleus (white arrow heads, aquagreen color). This result indicates that P311 localizes to the nucleus during adipogenesis, potentially to facilitate a transcriptional regulatory/co-regulatory function. Red arrow heads indicate the uninduced preadipocytes with blue nuclear DAPI stain without P311.
P311 is a nuclear and cytoplasmic protein.
A. In pcP311 transfected 3T3-L1 preadipocytes, c-myc-tagged-P311 (green) were mainly localized in the cytoplasm (red arrows). Blue stain (red arrow heads) indicates the DAPI stain for nuclei. In a small population of 3T3-L1 preadipocytes, P311 was translocated to the nucleus (top right panel, white arrows) upon adipogenic induction. Differentiated mature 3T3-L1 adipocytes showed P311 in both the nuclei (aqua green, white arrow heads) and cytoplasm (green, red arrows), as tested with anti-c-myc tag antibody. Empty vector (pCMV) transfected 3T3-L1 adipocytes served as control. B. Differentiated mature 3T3-L1 adipocytes showed P311 in both the nucleus (aqua green, white arrow heads) and cytoplasm (green, red arrows), as tested with two anti-P311 antibodies.
3.5. P311 binds to PPARγ2 promoter and activates it –
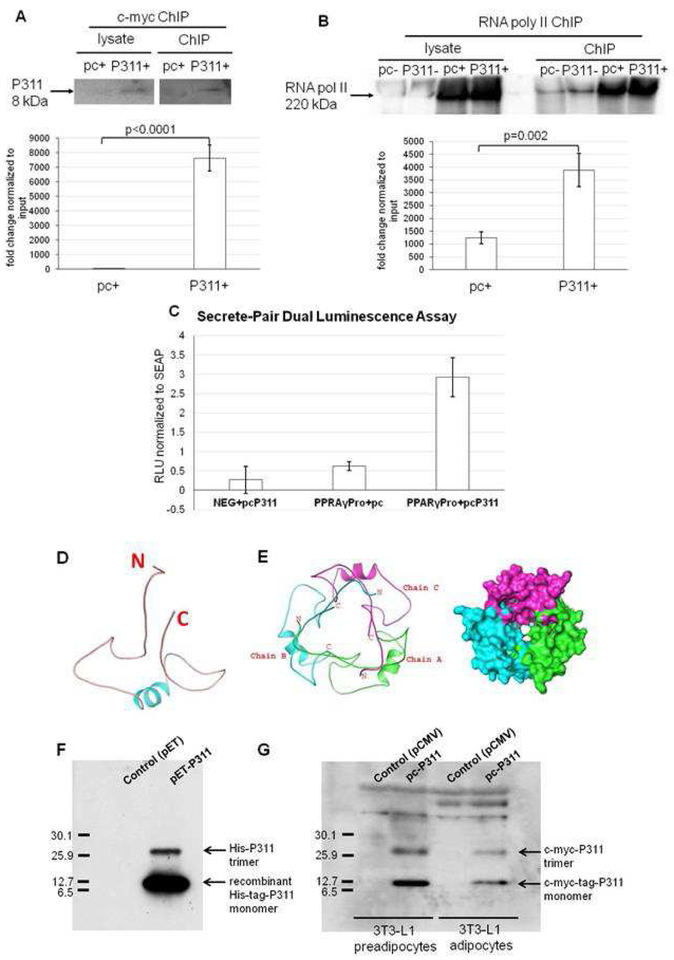
As P311 translocation to the nucleus upon adipogenic induction implies a role for the transcriptional regulation/co-regulation. To determine if P311 binds to and/or regulates the transcription of PPARγ2 and/or C/EBPα in adipogenesis, we conducted ChIP assays using 3T3-L1 preadipocytes and adipocytes transfected with pCMV-P311 that overexpress c-myc-tag-P311 or an empty vector, pCMV. Western blot analysis confirmed the induction and immunoprecipitation of c-myc-tagged P311. The relative amplification of the PPARγ2 promoter in the pCMV and pCMV-P311 transfected groups in 3T3-L1 adipocytes (pc+ and pcP311+) is shown in Figure 4A. In P311 overexpressing adipocytes, P311 recruitment to the PPARγ2 promoter was significantly higher (p≤0.0001) compared to the control group, implying a critical role of P311 in adipocyte development partly through the activation of PPARγ2, the master regulator of adipogenesis. Immunoprecipitates of P311 overexpression in preadipocytes did not amplify the PPARγ promoter. P311 immunocomplexes did not amplify the C/EBPα promoter in either preadipocytes or adipocytes (data not shown).
P311 binds to the PPARγ2 promoter and it is an intrinsically disordered protein.
A. Western blot analysis of chromatin immuno-precipitates (ChIP) of myc-P311 in pcP311 and empty vector (pCMV) transfected 3T3-L1 adipocytes (10 days post induction). The bands represents myc-P311 expression and pulldown (upper panel). qPCR was used to measure PPARγ2 promoter amplification to examine P311 recruitment to PPARγ2 promoter in ChIP complexes in P311 over expressed adipocytes (pc+ and pcP311+) and empty vector transfected controls (lower panel). B. Western blot analysis of ChIP of RNApolII in pcP311 and pCMV transfected 3T3-L1 adipocytes. Uninduced preadipocyte lysates and ChIP samples (pc- and pcP311-) had basal levels of RNApolII whereas adipocytes (pc+ and pcP311+) showed elevated levels of RNA RNApolII (upper panel). qPCR was used to measure PPARγ2 promoter amplification to examine RNApolII recruitment to PPARγ2 promoter in ChIP complexes in P311 over expressed adipocytes empty vector transfected controls (lower panel). C. Secreted-pair dual luminescence assay showed the binding of c-myc-P311 to the PPARγ promoter compared to the empty vector promoter (NEG) and empty vector control (pCMV). D. Predicted structure of monomeric P311. N- and C-represents N- and C-termini of proteins. E. Predicted structure of trimeric P311. F. Western blot analysis showed the overexpression of monomeric (8 kDa) and trimeric his-tag-P311 (~24 kDa) in E. coli (BL21D3) cell lysates immunodetected with P311-antibody. G. Western blot analysis showed the overexpression of monomeric and trimeric c-myc-tag-P311 immunodetected with c-myc-tag-antibody in 3T3-L1 preadipocytes and adipocytes. pET-P311 and pcP311 are bacterial and mammalian expression plasmids of his-tagged and myc-tagged P311 respectively. Empty vectors, pET and pcP311 served as controls.
In addition, we confirmed the recruitment of RNApolII to the PPARγ2 promoter by conducting ChIP assays using anti-RNApolII antibody. As shown in Figure 4B, P311 transfected adipocytes showed increased PPARγ2 promoter amplification. However, undifferentiated preadipocytes, both empty vector and P311 transfected cells (pc- and pcP311-) showed very minimal expression and pull down of RNApolII. Further, P311 transfected 3T3-L1 uninduced preadipocytes failed to amplify the PPARγ2 promoter, suggesting that nuclear translocation of P311 may only occur with adipogenesis induction. This indicated that, in 3T3-L1 preadipocytes, transcriptional complex (RNApolII) is not recruited to the PPARγ2 promoter. We further examined PPARγ2 promoter activation of P311 using secrete-pair dual luminescence promoter (SEAP) reporter assays. We observed that P311 cotransfection with PPARγ promoter shows significantly increased GLuc expression compared to empty vector control cotransfections. This confirms the recruitment of P311 to the PPARγ promoter (Figure 4C).
3.6. Four independent prediction studies including PSIPRED, Kytedoolittle hydropathy plot, SCRATCH (not shown) and Phyre2 (Figure 4) predicted that P311 has intrinsically disordered structure.
Phyre2 demonstrates that P311 have a small helix encompassing aminoacids 35–42 (figure 4D). This region of protein appears to be key in the function of the protein. Further, the protein-protein interaction studies using ZDOCK predicted the trimer for P311 (Figure 4E). These predictions were confirmed by westernblot analysis of lysates of E.coli, and 3T3-L1 preadipocytes and adipocytes expressing pET-P311 and pcP311 expressing N-terminal his-tagged-P311 and c-myc-tagged-P311 respectively (figure 4F–G). Empty vectors pET and pCMV expressing lysates were served as controls.
4. Discussion
In this study, we show that P311 is induced during the adipogenesis process and expressed in mature adipocytes but not in fibroblast like 3T3-L1 preadipocytes. Comparison of P311 expression with some early adipogenic markers showed the correlations in the expression of P311 and the master adipogenic regulator PPARγ. In addition, our data demonstrates nuclear translocation of P311, suggesting a potential transcriptional regulatory/coregulatory role of P311 in adipogenesis process. ChIP assays and luciferase assays confirm that the transcriptional activation of PPARγ is potentially mediated by P311 by binding to PPARγ promoter.
PPARγ2 is a ligand-activated transcription factor that belongs to the nuclear hormone receptor superfamily. Upon ligand activation, it dimerizes with RXRα and binds to PPARγ binding elements in the promoters of target genes. Further, PPARγ2 is the only known factor that is absolutely required and sufficient for adipogenesis [18]. Although very few proteins are known to regulate PPARγ2 nuclear localization [19] or transcriptional activation [20,21], the natural endogenous ligand for PPARγ2 remains unknown. PPARγ activation induces fatty acid uptake and triacylglycerol storage in adipocytes [22]. Human mutational analysis of PPARγ indicates a general role for PPARγ2 in adipogenesis, energy balance, insulin resistance and dyslipidemia [23,24]. The mechanism by which PPARγ activation regulates these pathologies is largely unknown.
Assembly of specific TFs and TCFs along with other key members including RNApolII into the transcriptional complex at regulatory elements on the chromatin has long been regarded as the fundamental mechanism for the regulation of gene expression. However, in spite of extensive studies during past three decades, the molecular mechanisms of gene regulation remain incompletely understood [25,26]. P311 ChIPs failed to amplify the PPARγ promoter in uninduced 3T3-L1 preadipocytes. And lack of RNA polymerase complex indicated that, in the 3T3-L1 system upon adipogenic induction only most of the transcriptional machinery gets activated, otherwise it will be at basal level to maintain housekeeping. This implies that P311 recruitment to PPARγ promoter require adipogenic induction signals and P311 alone may not be sufficient to induce adipogenesis by itself. Here, we demonstrate the existence of a previously-unknown P311-PPARγ2 biological axis and its importance in the regulation of adipocyte development. Of equal importance, this study characterizes the nuclear transcriptional regulatory/co-regulatory functions of P311, implicating P311 as among the few proteins that modulate PPARγ activation. Further, we observed nuclear translocation of P311 upon adipogenic induction and its expression in both the cytoplasm and nuclei of adipocytes, implicating potential complex nuclear and cytoplasmic roles for P311.
Binding of P311 to the PPARγ promoter may be indirect if it has a transcriptional co-regulator function or direct if it has a transcriptional regulator function. We anticipate that interaction of P311 could be co-regulatory function, given its small size; generally, TF are larger (50 kDa) and form large protein complexes of 1–3 MDa in size [27]. Transcriptional activator proteins generally bear a dimerization module along with a DNA binding domain and a separable activation domain [28]. Our additional modeling studies along with circular dichroism studies [data not shown, [29]], indicate that P311 is an Intrinsically Disordered Protein (IDP) [30]. Some IDPs are predicted to be completely disordered and some predicted to have disordered sequences [31]. Intrinsic disorganization or lack of secondary structure will give IDPs leverage to bind to various biomolecules by assuming a tertiary/quaternary structure upon binding to the target [32]. We further observed, in protein expression studies (immunoblots) and modeling studies that P311 form trimers. P311 potentially might act as a TF in spite of its small size due to its intrinsic disorderliness or by forming a trimer/multimer. Inducible cAMP early repressor (ICER) isomers are the smallest transcriptional factors (12 and 13.5 kDa) known [33]. Moreover, synthetic small molecules (4.2 kDa) showed transcriptional regulatory activity in vitro [28]. Further studies are underway in our lab to understand the molecular mechanism and biology of P311.
Previously Badri et al. [9] and Yue et al. [29] identified the novel role of P311 in RNA binding. To verify this, we conducted RNA immunoprecipitation studies using P311 overexpressing adipocytes and empty vector transfected adipocytes for adipogenic gene marker amplification for the key adipogenic markers PPARγ, C/EBPα, C/EBPβ and C/EBPδ. We did not amplify key adipogenic gene marker transcripts (data not shown), implying that P311 mediated adipogenesis may not be RNA regulated rather partly transcription mediated. In conclusion, P311 has a role in adipocyte development, potentially making it a key player in energy and metabolic homeostasis.
Supplementary Material
Highlights.
P311 plays a role in adipogenesis
P311 has transcriptional regulatory/coregulatory function
P311 is an intrinsically disordered protein
Acknowledgements:
We thank Pedanna Kotha for technical assistance. This study was supported by NIMHD/NIH grant funding through S21MD000101 (to Morehouse School of Medicine), G12MD007602 (to Morehouse School of Medicine) and U54MD008621 (Hampton University, Hampton, VA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of competing interest:
The authors declare that there are no conflicts of interest to disclose.
References
- [1].Gupta RK, Adipocytes, Curr Biol 24 (2014) R988–993. [DOI] [PubMed] [Google Scholar]
- [2].Mann JI, Diet and risk of coronary heart disease and type 2 diabetes, Lancet 360 (2002) 783–789. [DOI] [PubMed] [Google Scholar]
- [3].Rosen ED, MacDougald OA, Adipocyte differentiation from the inside out, Nat Rev Mol Cell Biol 7 (2006) 885–896. [DOI] [PubMed] [Google Scholar]
- [4].Eugene Chen Y, Editorial: The Yin and Yang of Perivascular Adipose Tissue in Vascular Disease, Cardiovasc Drugs Ther 32 (2018) 477–479. [DOI] [PubMed] [Google Scholar]
- [5].Darlington GJ, Ross SE, MacDougald OA, The role of C/EBP genes in adipocyte differentiation, J Biol Chem 273 (1998) 30057–30060. [DOI] [PubMed] [Google Scholar]
- [6].Wu Z, Puigserver P, Spiegelman BM, Transcriptional activation of adipogenesis, Curr Opin Cell Biol 11 (1999) 689–694. [DOI] [PubMed] [Google Scholar]
- [7].Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM, PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro, Mol Cell 4 (1999) 611–17. [DOI] [PubMed] [Google Scholar]
- [8].Mota de Sa P, Richard AJ, Hang H, Stephens JM, Transcriptional Regulation of Adipogenesis, Compr Physiol 7 (2017) 635–674. [DOI] [PubMed] [Google Scholar]
- [9].Badri KR, Yue M, Carretero OA, Aramgam SL, Cao J, Sharkady S, Kim GH, Taylor GA, Byron KL, Schuger L, Blood pressure homeostasis is maintained by a P311-TGF-beta axis, J Clin Invest 123 (2013) 4502–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor GA, Hudson E, Resau JH, Vande Woude GF, Regulation of P311 expression by Met-hepatocyte growth factor/scatter factor and the ubiquitin/proteasome system, J Biol Chem 275 (2000) 4215–4219. [DOI] [PubMed] [Google Scholar]
- [11].Pan D, Zhe X, Jakkaraju S, Taylor GA, Schuger L, P311 induces a TGF-beta1-independent, nonfibrogenic myofibroblast phenotype, J Clin Invest 110 (2002) 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leung JK, Cases S, Vu TH, P311 functions in an alternative pathway of lipid accumulation that is induced by retinoic acid, J Cell Sci 121 (2008) 2751–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chaudhuri A, Ghanim H, Makdissi A, Green K, Abuaysheh S, Batra M, D.K. N, P. Dandona, Exenatide induces an increase in vasodilatory and a decrease in vasoconstrictive mediators, Diabetes Obes Metab 19 (2017) 729–733. [DOI] [PubMed] [Google Scholar]
- [14].Badri KR, Kach J, Aramgam S, Sandbo NK, Schuger L, P311, a new key player in the pathogenesis of pulmonary fibrosis, American Journal of Respiratory and Critical Care Medicine 187 (2013) A5602. [Google Scholar]
- [15].Badri KR, Zhou Y, Dhru U, Aramgam S, Schuger L, Effects of the SANT domain of tension-induced/inhibited proteins (TIPs), novel partners of the histone acetyltransferase p300, on p300 activity and TIP-6-induced adipogenesis, Mol Cell Biol 28 (2008) 6358–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi J, Badri KR, Choudhury R, Schuger L, P311-induced myofibroblasts exhibit ameboid-like migration through RalA activation, Exp Cell Res 312 (2006) 3432–3442. [DOI] [PubMed] [Google Scholar]
- [17].F.-R J.M.a..Moreno-Navarrete JM, (Ed.), Adipocyte differentiation, Springer Science + Business Media, LLC, 2012. [Google Scholar]
- [18].Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM, PPAR gamma is required for placental, cardiac, and adipose tissue development, Mol Cell 4 (1999) 585–595. [DOI] [PubMed] [Google Scholar]
- [19].Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC, Adamo ML, Clemmons DR, Rosen CJ, A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation, Proc Natl Acad Sci U S A 107 (2010) 10508–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cho YM, Kwak SN, Joo NS, Kim DH, Lee AH, Kim KS, Seo JB, Jeong SW, Kwon OJ, X-box binding protein 1 is a novel key regulator of peroxisome proliferator-activated receptor gamma2, FEBS J 281 (2014) 5132–5146. [DOI] [PubMed] [Google Scholar]
- [21].Hamm JK, Park BH, Farmer SR, A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes, J Biol Chem 276 (2001) 18464–18471. [DOI] [PubMed] [Google Scholar]
- [22].Stephane Gesta CRK, (Ed.), White Adipose Tissue, Springer Science Business Media LLC, 2017. [Google Scholar]
- [23].Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S, Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension, Nature 402 (1999) 880–883. [DOI] [PubMed] [Google Scholar]
- [24].Gray SL, Dalla Nora E, Vidal-Puig AJ, Mouse models of PPAR-gamma deficiency: dissecting PPAR-gamma’s role in metabolic homoeostasis, Biochem Soc Trans 33 (2005) 1053–1058. [DOI] [PubMed] [Google Scholar]
- [25].Liu Z, Tjian R, Visualizing transcription factor dynamics in living cells, J Cell Biol 217 (2018) 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gottesfeld JM, Carey MF, Introduction to the Thematic Minireview Series: Chromatin and transcription, J Biol Chem 293 (2018) 13775–13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maeshima K, Kaizu K, Tamura S, Nozaki T, Kokubo T, Takahashi K, The physical size of transcription factors is key to transcriptional regulation in chromatin domains, J Phys Condens Matter 27 (2015) 064116. [DOI] [PubMed] [Google Scholar]
- [28].Mapp AK, Ansari AZ, Ptashne M, Dervan PB, Activation of gene expression by small molecule transcription factors, Proc Natl Acad Sci U S A 97 (2000) 3930–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yue MM, Lv K, Meredith SC, Martindale JL, Gorospe M, Schuger L, Novel RNA-binding protein P311 binds eukaryotic translation initiation factor 3 subunit b (eIF3b) to promote translation of transforming growth factor beta1–3 (TGF-beta1–3), J Biol Chem 289 (2014) 33971–33983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Badri KR, Muppuru KM Samuel, R.E., P311, an instrinsically unstructured protein, in adipogenesis, FASEB Journal 31 (2017) lb220. [Google Scholar]
- [31].Wright PE, Dyson HJ, Intrinsically disordered proteins in cellular signalling and regulation, Nat Rev Mol Cell Biol 16 (2015) 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dyson HJ, Wright PE, Intrinsically unstructured proteins and their functions, Nat Rev Mol Cell Biol 6 (2005) 197–208. [DOI] [PubMed] [Google Scholar]
- [33].Borlikova G, Endo S, Inducible cAMP early repressor (ICER) and brain functions, Mol Neurobiol 40 (2009) 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.