Patients with HIV have higher thrombogenicity that correlates with markers of inflammation; both respond to clopidogrel treatment.
Abstract
Persons with HIV infection (PWH) have increased risk for cardiovascular disease (CVD), but the underlying mechanisms remain unclear. Coronary thrombosis is known to provoke myocardial infarctions, but whether PWH have elevated thrombotic propensity is unknown. We compared thrombogenicity of PWH on antiretroviral therapy versus matched controls using the Badimon chamber. Measures of inflammation, platelet reactivity, and innate immune activation were simultaneously performed. Enrolled PWH were then randomized to placebo, aspirin (81 mg), or clopidogrel (75 mg) for 24 weeks to assess treatment effects on study parameters. Thrombogenicity was significantly higher in PWH and correlated strongly with plasma levels of D-dimer, soluble TNF receptors 1 and 2, and circulating classical and nonclassical monocytes in PWH. Clopidogrel significantly reduced thrombogenicity and sCD14. Our data suggest that higher thrombogenicity, interacting with inflammatory and immune activation markers, contributes to the increased CVD risk observed in PWH. Clopidogrel exhibits an anti-inflammatory activity in addition to its antithrombotic effect in PWH.
INTRODUCTION
The clinical use of antiretroviral therapy (ART) has markedly reduced mortality and morbidity for patients with HIV (PWH), leading to prolonged and improved lives. However, accumulating evidence suggests that the risk of myocardial infarction is increased in PWH relative to the general population (1). More than 50% of all PWH in the United States will be >50 years old, substantially increasing the impact of cardiovascular diseases (CVDs) in this group (2, 3). While ART toxicity and traditional risk factors contribute to the high CVD risk in HIV infection, chronic immune activation and inflammation are independently associated with CVD events after controlling for these traditional risk factors, suggesting that inflammation is a key driver of increased CVD risk in HIV infection (4).
Although early subclinical atherosclerosis and elevated markers of immune activation and inflammation have been shown in PWH (5, 6), the impact of HIV infection on blood thrombogenicity of these patients is unknown. Increased platelet reactivity has been reported in ART-treated HIV infection (7–10). Given the key role of platelet activation and thrombus formation in the onset of acute coronary syndromes (ACSs), we sought to determine whether the blood thrombogenicity of ART-treated PWH is elevated relative to HIV-seronegative controls and to assess the potential therapeutic effects of platelet inhibitors. To achieve our aims, we used an ex vivo model of thrombosis that allows measurement of thrombus formation at high shear rate, representative of stenosed arterial vasculature, and low shear rate, representative of venous flow conditions (11, 12). Concurrently, we measured clot kinetics, platelet reactivity, and markers of inflammation and immune activation to determine whether these parameters were associated with thrombogenicity. As a pilot study, we then evaluated the effects of 24 weeks of treatment with antiplatelet drugs aspirin and clopidogrel on blood thrombogenicity and related measures in ART-treated PWH.
RESULTS
Characteristics of participants
A total of 29 participants were enrolled (15 PWH and 14 seronegative controls). One participant with HIV enrolled in the pilot trial did not complete the study treatment due to hospitalization for a surgery, deemed unrelated to the study. Baseline characteristics are summarized in Table 1. The percentage of smokers (current and past) was higher in the PWH group. Of the PWH, two (13%) were on an abacavir-containing ART regimen and six (40%) were on an integrase inhibitor–containing regimen. Self-reported adherence to study treatments was high, with approximately 90% of study participants reporting 100% adherence. Study drugs were safe and well tolerated; there were no serious adverse events. In the PWH randomized to study drugs, plasma HIV-1 RNA levels did not change during treatment and remained below the limits of quantification.
Table 1. Baseline demographics of study participants.
LDL, low-density lipoprotein; HDL, high-density lipoprotein; NSTI, integrase strand transfer inhibitors; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; pi, protease inhibitor.
|
Seronegative controls (n = 14) |
PWH (n = 15) | |
| Age (years) | 44 (21) | 46 (13) |
| Male, no. (%) | 7 (50%) | 8 (53%) |
| Race | ||
| White non-Hispanic | 5 (35.5%) | 3 (20.0%) |
| Black non-Hispanic | 4 (29.0%) | 6 (40.0%) |
| Hispanic | 5 (35.5%) | 6 (40.0%) |
| Body mass index (kg/m2) | 25.6 (2.5) | 27.9 (10.7) |
| CD4 T cell count | – | 708 (380) |
| Smoking | ||
| Never | 11 (78.6%) | 7 (46.7%) |
| Past | 1 (7.1%) | 4 (26.7%) |
| Current | 2 (14.3%) | 4 (26.7%) |
| ART, no. (%) | ||
| Abacavir-based | – | 2 (13.0%) |
| INSTI + 2 NRTI | – | 6 (40.0%) |
| NNRTI + 2 NRTI | – | 2 (13.0%) |
| PI + 2 NRTI | – | 6 (40.0%) |
| LDL (mg/dl) | 114 (30) | 99 (21) |
| HDL (mg/dl) | 52 (20) | 47 (22) |
| Total cholesterol (mg/dl) | 181 (53) | 169 (55) |
| Platelet count (×109/liter) | 221 (95) | 232 (124) |
Thrombogenicity is increased in ART-treated HIV infection
PWH had significantly higher thrombus formation than seronegative controls at both low [median (interquartile range, IQR) size of 6349 (1547) μm2/mm versus 5237 (1929) μm2/mm, P = 0.006] and high shear rates [9256 (3192) μm2/mm versus 7421 (3513) μm2/mm, P = 0.013] (Fig. 1A). Excluding smokers from the analysis did not change these findings (table S1). Breakdown by gender showed that women accounted more for the higher thrombogenicity in PWH versus controls at both low [7047 (1326) μm2/mm versus 4717 (2321) μm2/mm, P = 0.006] and high shear rates [10,268 (4724) μm2/mm versus 7557 (3572) μm2/mm, P = 0.012]. Differences in thrombus size among male patients and controls did not achieve statistical significance at either low [6071 (1574) μm2/mm versus 5626 (1933) μm2/mm] or high shear rate [9135 (2441) μm2/mm versus 7326 (4321) μm2/mm; Fig. 1B].
Fig. 1. Blood thrombogenicity.
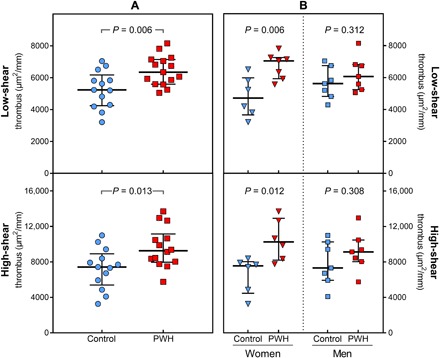
(A) Thrombus formation at low shear rate (top) and high shear (bottom) rate in PWH versus age- and sex-matched seronegative controls. (B) Thrombus formation at low and high shear rates in PWH versus seronegative controls, separated by gender.
Thrombus kinetics in ART-treated HIV infection
Coagulation plays a major role in thrombosis; the impact of HIV infection on thrombus kinetics was assessed using thromboelastometry in this study. PWH exhibited trends toward shorter coagulation times [161.0 (17.0) s versus 168.5 (20.0) s, P = 0.257] and clot formation times [63.0 (35.0) s versus 66.0 (24.0) s, P = 0.328], but the differences were not statistically significant. Maximum clot firmness [62.0 (10.0) mm versus 61.5 (6.0) mm, P = 0.780] and α angles [78.0° (6.0°) versus 76.5° (4.0°), P = 0.207] were similar between PWH and controls. Breakdown by gender showed that the tendency for higher coagulability in PWH appeared to be more marked among females (table S2).
Platelet reactivity is higher in ART-treated HIV infection
As previously described, submaximal concentrations of platelet-activating agonists have been used to identify a hyperreactive platelet phenotype (7, 13). PWH exhibited higher median spontaneous platelet aggregation (SPA) (3% versus 2%, P = 0.008) and also higher median aggregation in response to submaximal agonist concentrations than seronegative controls [collagen (0.05 μg/ml), 3% versus 2%, P = 0.019; arachidonic acid (500 μM), 86% versus 73%, P = 0.013]. Differences in aggregation to low doses of epinephrine and adenosine diphosphate (ADP) between HIV-infected participants and seronegative controls were not significant in this study. At higher agonist concentrations, much of the differences observed between PWH and seronegative controls were attenuated (Table 2).
Table 2. Platelet reactivity of PWH versus seronegative controls.
Maximum platelet aggregation in response to various agonists tested using platelet aggregometry and summarized as median (IQR).
|
Seronegative controls (n = 14) |
PWH (n = 15) | P | |
|
Spontaneous aggregation |
2.0 (2.3) | 3.0 (1.0) | 0.008 |
| ADP | |||
| 0.4 μM | 5.0 (10.8) | 5.0 (5.0) | 0.946 |
| 1.0 μM | 18.0 (24.5) | 14.5 (9.8) | 0.701 |
| 20 μM | 85.5 (13.3) | 84.0 (19.8) | 0.701 |
| Arachidonic acid | |||
| 150 μM | 1.0 (2.5) | 3.0 (4.5) | 0.133 |
| 500 μM | 73.0 (22.0) | 86.5 (14.8) | 0.013 |
| Collagen | |||
| 0.05 μg/ml | 2.0 (2.0) | 3.0 (5.0) | 0.019 |
| 2.0 μg/ml | 82.5 (15.3) | 78.0 (17.0) | 0.571 |
| Epinephrine | |||
| 0.0 5 μM | 5.5 (17.5) | 5.5 (5.3) | 0.635 |
| 0.1 μM | 7.0 (49.8) | 6.5 (6.5) | 0.701 |
| 5.0 μM | 82.5 (22.0) | 88.0 (39.0) | 0.488 |
sCD14 levels are elevated in ART-treated HIV infection, but other markers of inflammation are similar to seronegative controls
PWH showed significantly higher mean values of sCD14 than did seronegative controls [2094 (420) pg/ml versus 1771 (543) pg/ml, P = 0.014]. There were no differences between the two groups in plasma sCD163 [549 (192) versus 463 (333), P = 0.562], D-dimer [1766 (1321) versus 1969 (917), P = 0.601], soluble tumor necrosis factor receptor 1 (sTNFR1) [693 (149) versus 746 (478), P = 0.984], sTNFR2 [4452 (805) versus 4418 (1886), P = 0.581], and soluble interleukin-6 (sIL-6) [2.0 (2.0) versus 2.1 (4.1), P = 0.936)]. The two groups also did not differ significantly in monocyte-platelet aggregate levels (22.0% versus 21.6%, P = 0.97) or in monocyte subsets defined as classical (CD14++CD16−: 66.4% versus 57.4%, P = 0.14), intermediate (CD14++CD16+: 25.6% versus 29.5%, P = 0.07), or nonclassical (CD14dimCD16+: 6.8% versus 7.6%, P = 0.99).
Thrombogenicity in ART-treated HIV infection strongly correlates with D-dimer, sTNFR1 and sTNFR2, and classical and nonclassical monocytes
While no significant associations between thrombus size at low shear rate and other variables were observed, there were significant positive correlations between high shear rate thrombus and D-dimer (r = 0.67, P = 0.017), sTNFR1 (r = 0.75, P = 0.005), sTNFR2 (r = 0.61, P = 0.037), and nonclassical monocytes (r = 0.62, P = 0.031) and a significant negative association between high-shear thrombus and classical monocytes (r = −0.71, P = 0.005; Fig. 2) in PWH. These correlations were not observed in the control group (table S3).
Fig. 2. Correlations of thrombus size with markers of inflammation.
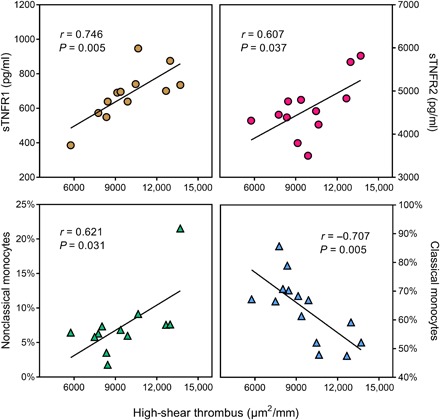
Thrombus formation at high shear rate showed strong correlation with sTNFR1, sTNFR2, and nonclassical monocytes and a strong negative correlation with classical monocytes, tested using Pearson’s correlation.
Clopidogrel, but not aspirin, reduced thrombogenicity and sCD14
Treatment with clopidogrel significantly reduced blood thrombogenicity at low shear rate, with a reduction in median thrombus size of 11.3%, from 6743 (1223) μm2/mm to 5981 (1030) μm2/mm (P = 0.047). At high shear rate, there was a marked trend toward reduced thrombogenicity that did not reach statistical significance [19.6% reduction in median thrombus from 8788 (1325) μm2/mm to 7066 (2516) μm2/mm, P = 0.176] (Fig. 3A). Plasma sCD14 levels were also reduced with clopidogrel treatment by 16.3% at 12 weeks [from 2245 (292) pg/ml to 1879 (326) pg/ml, P = 0.010] and 9% at 24 weeks [from 2245 (292) pg/ml to 2045 (143) pg/ml, P = 0.049]. Of interest, neither placebo nor aspirin reduced thrombogenicity or sCD14 (Fig. 3B).
Fig. 3. Effects of antiplatelet treatments on thrombogenicity and inflammation.
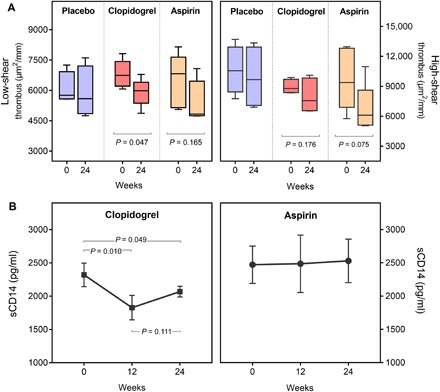
(A) Thrombus formation at low and high shear rates in PWH treated with study drugs, at baseline (0) and after 24 weeks. (B) sCD14 levels measured in study groups at baseline (0) and 12 and 24 weeks of study treatment.
None of the three treatment groups displayed significant changes in clot kinetics; sCD163; D-dimer; sTNFR1; sTNFR2; sIL-6; classical (CD14++CD16−), intermediate (CD14++CD16+), or nonclassical (CD14dimCD16+) monocyte subsets; or monocyte-platelet aggregates from beginning to end of treatment period. Platelet reactivity was significantly reduced by both aspirin and clopidogrel. As expected, aspirin practically abolished arachidonic acid–induced platelet aggregation (76 to 2%, P < 0.001), and clopidogrel significantly reduced platelet aggregation in response to ADP (97 to 68%, P = 0.04). Epinephrine-induced aggregation was significantly inhibited by aspirin (88 to 28%, P = 0.03) but not clopidogrel, and neither treatment significantly affected collagen-mediated aggregation.
DISCUSSION
It is estimated that currently there are more than 37 million people worldwide living with HIV (14). In high-income countries, 50% of the HIV-infected population is aged 50 years or older (15). ART has had a major impact on the life span of PWH, with some studies now estimating that the life span of PWH who achieve virologic suppression may approximate that of the general population (16, 17). However, there is evidence of increased immune activation and resultant residual inflammation contributing to excess non-AIDS conditions and comorbidities including CVD even among those with complete virologic suppression. As in the general population, CVD event rates increase with age in PWH, and given the increasing longevity of the HIV population, the prevalence of CVD is likely to rise unless effective therapeutic strategies are promptly developed. A better understanding of the specific mechanisms responsible for the higher cardiovascular risk reported in PWH on ART will yield specific and effective therapeutic interventions to ameliorate that risk.
Given the critical role of thrombosis in the onset and severity of ACS, we investigated the blood thrombogenicity of ART-treated PWH versus age- and sex-matched HIV-seronegative controls in this study. Our findings of significantly higher levels of thrombus formation in PWH as compared to seronegative controls strongly suggest the presence of a hyperthrombotic state in HIV infection. The increased blood thrombogenicity was observed at both low– and high–shear rate conditions. An interesting finding was that women with HIV accounted for the greatest differences; however, given the limited sample sizes available for gender analysis, it would be prudent to exercise caution in interpreting this result.
We measured clot formation kinetics, platelet reactivity, soluble markers of inflammation, and percent of circulating monocyte subsets and monocyte platelet aggregates to determine which of these variables might be associated with blood thrombogenicity. While baseline measurements of these variables, comparing HIV-infected with seronegative participants in our study, differed only when comparing platelet reactivity to low-dose platelet-activating agents and plasma sCD14, as we previously demonstrated (7), we newly observed that high–shear rate thrombogenicity in PWH strongly correlated with D-dimer, sTNFR1, sTNFR2, and percent nonclassical monocytes. Both IL-6 and D-dimer have been shown to be independently associated with serious non-AIDS conditions among PWH with suppressed virus. D-dimer is a fibrin degradation product present in the blood after a blood clot is degraded by fibrinolysis; therefore, elevated D-dimer levels suggest increased fibrin formation. sTNFR1 and sTNFR2 have also been reported to predict non-AIDS morbidity and mortality (18) and are associated with carotid atherosclerosis (19). TNF-α has been shown to accelerate thrombus formation in vivo, and its prothrombotic effects require TNFR2 (20). In addition, TNF-α has also been shown to contribute to platelet activation (21). Nonclassical monocytes, which also correlated strongly with high–shear rate thrombogenicity in PWH in this study, have been shown to highly express tissue factor in patients infected with HIV (22), and tissue factor is a highly potent activator of blood coagulation and platelet activation.
Our pilot study evaluating the effects of two of the most commonly prescribed antiplatelet drugs on thrombogenicity and inflammatory markers in PWH showed that only the P2Y12 inhibitor clopidogrel, but not aspirin, significantly reduced low–shear rate thrombogenicity and sCD14 in these participants. The lack of effect observed in the aspirin arm of this study is in concordance with the results of AIDS Clinical Trials Group A5331, which demonstrated no significant benefit on immune activity or vascular health from aspirin in PWH (23).
Two major conclusions can be drawn from our study. First, PWH suppressed on ART have higher blood thrombogenicity than HIV-seronegative controls, as shown by the increased platelet thrombus formation, with no demonstrable differences in coagulation (thromboelastometry). Second, treatment with clopidogrel can reduce blood thrombogenicity in this patient population and therefore could offer potential benefits in reducing both the hyperthrombotic and hyperinflammatory status that is postulated to be responsible for the higher cardiovascular risk seen in PWH. Our findings of the antithrombotic and anti-inflammatory activities of clopidogrel in the PWH warrants further investigation to confirm these findings.
Limitations
The sample size of our prospective study was small, especially given the interventional nature of the study. However, this was an exploratory pilot study that included an array of technically challenging and highly specialized assays on HIV-infected and control study participants that were closely matched demographically. In addition, despite the small number of participants, a robust difference in thrombus formation at both shear rates in PWH as compared to seronegative control participants was attained. Furthermore, we observed strong correlations between thrombogenicity and markers of coagulation and inflammation, thus providing a strong mechanistic link between these variables.
MATERIALS AND METHODS
The study was conducted in accordance with the Institutional Review Board policies of Icahn School of Medicine at Mount Sinai (ISMMS) and with the Declaration of Helsinki. All participants provided written informed consent before the start of any study procedures. The clinical trial was registered with www.clinicaltrials.gov (NCT02578706).
Study design
The study consisted of two parts (Fig. 4): (i) a cross-sectional, case-control study to compare the blood thrombogenicity of PWH with age- and sex-matched seronegative controls, and (ii) a pilot trial (randomized, double-blind, parallel-group) to investigate the effects of two differentially acting antiplatelet drugs on inflammation (sCD14) and thrombus formation in PWH. For the pilot trial, PWH enrolled in the case-control study were randomized to placebo, aspirin, or clopidogrel treatment for 24 weeks.
Fig. 4. Study design.
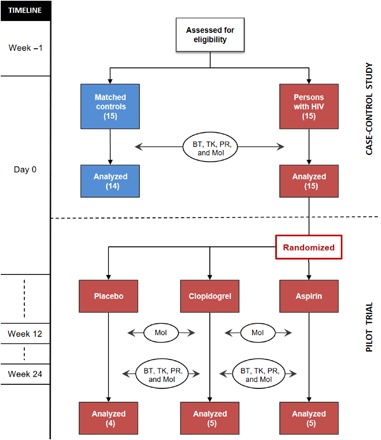
Flow chart of the study design showing the cross-sectional, case-control study in the top part and the randomized, double-blind pilot trial in the bottom part. BT, blood thrombogenicity; TK, thrombus kinetics; PR, platelet reactivity; MoI, markers of inflammation.
Study participants
We enrolled 15 adult participants with chronic HIV infection on suppressive ART (HIV RNA below quantification limit for ≥48 weeks, transiently detectable blips <500 copies/ml were allowed if flanked by undetectable values) and 14 age- and sex-matched seronegative controls in the study. All study participants underwent screening tests (for renal and liver function, hemoglobin, platelet count, pregnancy test when applicable, HIV RNA-1 in PWH, and HIV serology testing in participants without known HIV) to confirm eligibility. Major exclusion criteria (established prospectively) included a history of gastrointestinal or central nervous system bleeding, anemia or thrombocytopenia, recent severe illness, liver or kidney disease, uncontrolled diabetes, known CVD, coexisting cancer, pregnancy or breastfeeding, use of statin, use of nonsteroidal anti-inflammatory drug (including aspirin), and use of immunosuppressive medications.
For the pilot trial, the 15 enrolled PWH were randomized using a double-blind, parallel-group, three-arm design to placebo, aspirin (81 mg once daily; Mylan Pharmaceuticals Inc.), or clopidogrel (75 mg once daily; Bristol-Myers Squibb/Sanofi Pharmaceuticals) treatment for 24 weeks. A permuted block randomization algorithm with a 1:1:1 allocation ratio, block size = 6, was generated by the study biostatistician to determine treatment assignments. Unique study identification numbers (SIDs) were allocated to study participants, and the ISMMS Investigational Drug Service (IDS) pharmacist (unblinded) mapped SIDs to their corresponding treatment regimen. Placebo and study drugs were over-encapsulated by IDS to mask identification and were stored at the IDS facility. Participants, care providers, and study team members were blinded to the intervention assignment.
Following enrollment, all study participants underwent the Badimon perfusion study to assess thrombogenicity and blood sampling for the measurement of thrombus kinetics, platelet reactivity, and markers of inflammation. PWH were then randomized into the pilot trial and began their assigned study treatments. At the midpoint of treatment (12 weeks), blood samples were collected for the measurement of inflammatory markers. At the conclusion of the treatment (24 weeks), all study assessments performed at baseline were repeated. Safety assessments including clinical evaluations, detailed bleeding questionnaires, safety laboratory testing, and blood collection for serum and plasma specimen storage for batched assays were performed at all time points.
Evaluations
Blood thrombogenicity
Assessments of thrombogenicity were made using the Badimon perfusion system, an ex vivo model of thrombosis suitable for such applications (11, 12). The high-shear chambers (shear rate, 1690 s−1) mimic the rheological conditions of a moderately stenosed coronary artery, while the low-shear chamber (shear rate, 212 s−1) simulates venous flow conditions. Surgically dissected porcine aorta was used as the substrate to trigger thrombus formation over its surface. Native (non-anticoagulated) blood was perfused over this tissue directly from an 18-gauge intravenous cannula in the antecubital vein of the participant for 5 min at a rate of 10 ml min−1. Thereafter, perfused segments were fixed in 4% paraformaldehyde and stained with combined Masson trichrome elastin. Total thrombus area (μm2/mm) was quantified by planimetry using a DM5000B microscope (Leica GmbH, Wetzlar, Germany) under ×10 magnification and Image-Pro Plus software (Media Cybernetics, MD, USA) (24).
Thrombus kinetics
The viscoelastic properties of thrombus during formation and early phase of autolysis (thrombus retraction) were assessed by thromboelastometry (ROTEM Gamma; Pentapharm GmbH, Munich, Germany). The time to 2-mm clot amplitude in seconds (coagulation time), the time from 2- to 20-mm clot amplitude in seconds (clot formation time), the maximum amplitude in clot size in millimeters (maximum clot firmness), and the tangent to the clotting curve through the 2-mm point (α angle) were measured (25, 26).
Platelet reactivity
Light transmission aggregometry was performed according to the manufacturer’s specification using a Chrono-log 570 VS aggregometer (Chrono-log Corporation, Havertown, PA). As previously described (7), functional assays of platelet reactivity in response to agonists at various concentrations were performed as follows: arachidonic acid (150 and 500 μM), ADP (0.4, 1.0, and 20 μM), collagen (0.05 and 2.0 μg/ml), epinephrine (0.05, 0.1, and 5 μM), and no agonist (SPA). Maximum percent aggregation at 10 min was recorded. All aggregation studies were completed within 2 hours of blood collection.
Monocyte subsets and monocyte-platelet aggregates
Monocyte subsets were measured in whole blood within 1 hour of collection in EDTA tubes using flow cytometry as previously described (27). Blood was incubated for 15 min on ice with FACS Lyse buffer (BD Biosciences) and then washed in wash buffer [phosphate-buffered saline (PBS) with 1% bovine serum albumin and 0.1% sodium azide]. Cells were stained for 30 min in the dark on ice, washed in wash buffer, and then fixed in 1% formaldehyde.
Monocyte-platelet aggregates were measured in whole blood using flow cytometry within 30 min of collection in sodium citrate tubes. Blood was incubated for 15 min with 1% buffered formalin and then stained at room temperature in the dark for 10 min. Water was then added to lyse red blood cells, the incubation was continued for 10 min in the dark, and then PBS was added.
Cells were acquired using a Fortessa LSR II flow cytometer (BD Biosciences). FlowJo software and Prism version 5.0 software (GraphPad) were used to organize and analyze the data. For monocyte subset analysis, monocytes were identified by size, granularity, and expression of CD14 and CD16. CD14 expression was based on a population gating strategy, with both the lymphocyte population and the florescence minus one (FMO) serving as the lower limit for determining which cells were CD14+ versus CD14−. The upper limit of CD14+ and the lower limit for CD14++ expression are based on the CD14++ population. Expression of CD16 was based on a conservative FMO gating strategy and was confirmed by fluorescence intensity of the CD16− lymphocyte population. For monocyte-platelet aggregate analysis, monocytes were identified by size, granularity, and expression of CD14 and CD61. CD61 FMO was used to set the gate for CD14+CD61+cells. Cell surface molecule expression was monitored by staining cells with the following: fluorochrome-labeled antibodies anti-CD14–Pacific Blue and anti-CD16–PE (phycoerythrin) (both BD Biosciences) for monocyte subsets and anti-CD14–APC (allophycocyanin) and anti-CD61–FITC (fluorescein isothiocyanate) for monocyte-platelet aggregates.
Systemic biomarkers
Assays for systemic biomarkers were run at the end of the study in plasma samples stored at −80°C during the project after centrifuging at 1550g for 5 min. Plasma samples were thawed immediately before testing for sCD14 and sCD163 using sandwich enzyme-linked immunosorbent assays (R&D Systems, UK) and for D-dimer, sTNFR1, sTNFR2, and sIL-6 using Luminex.
Statistical analysis
Demographic and baseline characteristics are summarized for all participants. Data are summarized as median (IQR) unless specified otherwise. Unpaired two-tailed Student’s t tests or Mann-Whitney U tests were used for comparing results of PWH and seronegative controls, as appropriate. Two-tailed paired t tests or Wilcoxon matched-pairs signed-rank test were used for comparing results for PWH before and after study treatments, as appropriate. To examine the biological effects of antiplatelet therapy, as-treated analyses were used, limiting the analysis to participants on treatment for the duration of the study (n = 14). Relationships among variables were assessed using Pearson or Spearman correlation, depending on normality of distribution. All statistical tests were two-sided with a nominal α level of 0.05 with no adjustment for multiple testing. Analyses were performed with GraphPad Prism (version 7.0).
Supplementary Material
Acknowledgments
We thank the medical staff at ISMMS-affiliated HIV clinics for the referral of study participants. We also thank the staff of the Clinical and Translational Research Center (M. Carrington, M. Cespedes, J. Forcht, E. Lam, A. M. Paolino, H. M. Seedhom, and H. Zhan) for clinical trial services and the staff of the Vaccine and Cell Therapy Laboratory (N. Bhardwaj, R. Sabado, M. Meseck, B. S. Oner, M. Aziz, H. Diop, R. Gopal, M. Oke, and M. Diop) for performing blood processing and flow cytometry. Funding: This work was supported by the National Heart, Lung, and Blood Institute at the NIH (grant R56HL127995). Part of this work was also supported by Project DTS16/00133 from the ISCIII, Spanish Government. Author contributions: M.P.O., M.U.Z., J.A.A., and J.J.B. conceived and designed the research. M.P.O., A.W., and A.H. performed statistical analysis. M.P.O., M.U.Z., J.C.R., I.O., A.H., K.C., and G.R.-C. acquired the data. M.P.O., M.U.Z., G.E., J.A.A., and J.J.B. drafted the manuscript and made critical revisions. Competing interests: J.A.A. received clinical research support from Gilead Sciences and GlaxoSmithKline/ViiV Healthcare and received scientific advisory board fees from Gilead Sciences, Janssen, Merck, and ViiV Healthcare, all unrelated to the present study. The authors declare no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/6/eaav5463/DC1
Table S1. Thrombus size [median (IQR) μm2/mm] of PWH versus seronegative controls: Subgroup analyses.
Table S2. Coagulation parameters [median (IQR)] of PWH versus seronegative controls: Gender analyses.
Table S3. Correlation of high-shear thrombus with parameters of inflammation and thrombosis in PWH versus seronegative controls.
REFERENCES AND NOTES
- 1.Drozd D. R., Kitahata M. M., Althoff K. N., Zhang J., Gange S. J., Napravnik S., Burkholder G. A., Mathews W. C., Silverberg M. J., Sterling T. R., Heckbert S. R., Budoff M. J., van Rompaey S., Delaney J. A. C., Wong C., Tong W., Palella F. J., Elion R. A., Martin J. N., Brooks J. T., Jacobson L. P., Eron J. J., Justice A. C., Freiberg M. S., Klein D. B., Post W. S., Saag M. S., Moore R. D., Crane H. M., Increased risk of myocardial infarction in HIV-infected individuals in north america compared with the general population. J. Acquir. Immune Defic. Syndr. 75, 568–576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boccara F., Mary-Krause M., Teiger E., Lang S., Lim P., Wahbi K., Beygui F., Milleron O., Gabriel Steg P., Funck-Brentano C., Slama M., Girard P.-M., Costagliola D., Cohen A.; Prognosis of Acute Coronary Syndrome in HIV-infected patients , Acute coronary syndrome in human immunodeficiency virus-infected patients: Characteristics and 1 year prognosis. Eur. Heart J. 32, 41–50 (2011). [DOI] [PubMed] [Google Scholar]
- 3.D’Ascenzo F., Cerrato E., Biondi-Zoccai G., Moretti C., Omedé P., Sciuto F., Bollati M., Modena M. G., Gaita F., Sheiban I., Acute coronary syndromes in human immunodeficiency virus patients: A meta-analysis investigating adverse event rates and the role of antiretroviral therapy. Eur. Heart J. 33, 875–880 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg M. S., Chang C.-C. H., Kuller L. H., Skanderson M., Lowy E., Kraemer K. L., Butt A. A., Bidwell Goetz M., Leaf D., Oursler K. A., Rimland D., Rodriguez Barradas M., Brown S., Gibert C., McGinnis K., Crothers K., Sico J., Crane H., Warner A., Gottlieb S., Gottdiener J., Tracy R. P., Budoff M., Watson C., Armah K. A., Doebler D., Bryant K., Justice A. C., HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med. 173, 614–622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKibben R. A., Margolick J. B., Grinspoon S., Li X., Palella F. J. Jr., Kingsley L. A., Witt M. D., George R. T., Jacobson L. P., Budoff M., Tracy R. P., Brown T. T., Post W. S., Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J. Infect. Dis. 211, 1219–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo J., Abbara S., Shturman L., Soni A., Wei J., Rocha-Filho J. A., Nasir K., Grinspoon S. K., Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 24, 243–253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien M., Montenont E., Hu L., Nardi M. A., Valdes V., Merolla M., Gettenberg G., Cavanagh K., Aberg J. A., Bhardwaj N., Berger J. S., Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: A pilot study. J. Acquir. Immune Defic. Syndr. 63, 280–288 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayne E., Funderburg N. T., Sieg S. F., Asaad R., Kalinowska M., Rodriguez B., Schmaier A. H., Stevens W., Lederman M. M., Increased platelet and microparticle activation in HIV infection: Upregulation of P-selectin and tissue factor expression. J. Acquir. Immune Defic. Syndr. 59, 340–346 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green S. A., Smith M., Hasley R. B., Stephany D., Harned A., Nagashima K., Abdullah S., Pittaluga S., Imamichi T., Qin J., Rupert A., Ober A., Lane H. C., Catalfamo M., Activated platelet-T-cell conjugates in peripheral blood of patients with HIV infection: Coupling coagulation/inflammation and T cells. AIDS 29, 1297–1308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauguel-Moreau M., Boccara F., Boyd A., Salem J.-E., Brugier D., Curjol A., Hulot J.-S., Kerneis M., Galier S., Cohen A., Montalescot G., Collet J.-P., Silvain J., Platelet reactivity in human immunodeficiency virus infected patients on dual antiplatelet therapy for an acute coronary syndrome: The EVERE2ST-HIV study. Eur. Heart J. 38, 1676–1686 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Zafar M. U., Vorchheimer D. A., Tewar M. P., Giannarelli C., Crippa M., Sartori S., Rodriguez D., Baber U., Mehran R., Badimon J. J., Ticagrelor reduces thrombus formation more than clopidogrel, even when co-administered with bivalirudin. Thromb. Haemost. 112, 1069–1070 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zafar M. U., Farkouh M. E., Osende J., Shimbo D., Palencia S., Crook J., Leadley R., Fuster V., Chesebro J. H., Potent arterial antithrombotic effect of direct factor-Xa inhibition with ZK-807834 administered to coronary artery disease patients. Thromb. Haemost. 97, 487–492 (2007). [PubMed] [Google Scholar]
- 13.Berger J. S., Becker R. C., Kuhn C., Helms M. J., Ortel T. L., Williams R., Hyperreactive platelet phenotypes: Relationship to altered serotonin transporter number, transport kinetics and intrinsic response to adrenergic co-stimulation. Thromb. Haemost. 109, 85–92 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Global Health Observatory data. https://www.who.int/gho/en/. (Accessed 3-June-2019).
- 15.Wing E. J., HIV and aging. Int. J. Infect. Dis. 53, 61–68 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Rodger A. J., Lodwick R., Schechter M., Deeks S., Amin J., Gilson R., Paredes R., Bakowska E., Engsig F. N., Phillips A.; INSIGHT SMART, ESPRIT Study Groups , Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 27, 973–979 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord, Lewden C., Bouteloup V., De Wit S., Sabin C., Mocroft A., Wasmuth J. C., van Sighem A., Kirk O., Obel N., Panos G., Ghosn J., Dabis F., Mary-Krause M., Leport C., Perez-Hoyos S., Sobrino-Vegas P., Stephan C., Castagna A., Antinori A., d’Arminio Monforte A., Torti C., Mussini C., Isern V., Calmy A., Teira R., Egger M., Grarup J., Chêne G., Collaboration of Observational et al., All-cause mortality in treated HIV-infected adults with CD4 ≥ 500/mm3 compared with the general population: Evidence from a large European observational cohort collaboration. Int. J. Epidemiol. 41, 433–445 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Tenorio A. R., Zheng Y., Bosch R. J., Krishnan S., Rodriguez B., Hunt P. W., Plants J., Seth A., Wilson C. C., Deeks S. G., Lederman M. M., Landay A. L., Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J. Infect. Dis. 210, 1248–1259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkind M. S., Cheng J., Boden-Albala B., Rundek T., Thomas J., Chen H., Rabbani L. R. E., Sacco R. L., Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke 33, 31–37 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pircher J., Merkle M., Wörnle M., Ribeiro A., Czermak T., Stampnik Y., Mannell H., Niemeyer M., Vielhauer V., Krötz F., Prothrombotic effects of tumor necrosis factor alpha in vivo are amplified by the absence of TNF-alpha receptor subtype 1 and require TNF-alpha receptor subtype 2. Arthritis Res. Ther. 14, R225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pignatelli P., de Biase L., Lenti L., Tocci G., Brunelli A., Cangemi R., Riondino S., Grego S., Volpe M., Violi F., Tumor necrosis factor-α as trigger of platelet activation in patients with heart failure. Blood 106, 1992–1994 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Funderburg N. T., Mayne E., Sieg S. F., Asaad R., Jiang W., Kalinowska M., Luciano A. A., Stevens W., Rodriguez B., Brenchley J. M., Douek D. C., Lederman M. M., Increased tissue factor expression on circulating monocytes in chronic HIV infection: Relationship to in vivo coagulation and immune activation. Blood 115, 161–167 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien M. P., Hunt P. W., Kitch D. W., Klingman K., Stein J. H., Funderburg N. T., Berger J. S., Tebas P., Clagett B., Moisi D., Utay N. S., Aweeka F., Aberg J. A., A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus-infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect. Dis. 4, ofw278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafar M. U., Ibáñez B., Choi B. G., Vorchheimer D. A., Piñero A., Jin X., Sharma R. K., Badimon J. J., A new oral antiplatelet agent with potent antithrombotic properties: Comparison of DZ-697b with clopidogrel a randomised phase I study. Thromb. Haemost. 103, 205–212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reikvam H., Steien E., Hauge B., Liseth K., Hagen K. G., Størkson R., Hervig T., Thrombelastography. Transfus. Apher. Sci. 40, 119–123 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Rivard G. E., Brummel K., Mann K. G., Fan L., Hofer A., Cohen E., Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. J. Thromb. Haemost. 3, 2039–2043 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funderburg N. T., Zidar D. A., Shive C., Lioi A., Mudd J., Musselwhite L. W., Simon D. I., Costa M. A., Rodriguez B., Sieg S. F., Lederman M. M., Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 120, 4599–4608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/6/eaav5463/DC1
Table S1. Thrombus size [median (IQR) μm2/mm] of PWH versus seronegative controls: Subgroup analyses.
Table S2. Coagulation parameters [median (IQR)] of PWH versus seronegative controls: Gender analyses.
Table S3. Correlation of high-shear thrombus with parameters of inflammation and thrombosis in PWH versus seronegative controls.


