Summary
Animal pathogens attract attention in both the livestock and public health sectors for their impacts on socio-economics, food safety and security, and human health. These impacts are felt at the household, national, regional and global levels. Whereas the World Organisation for Animal Health (OIE) has identified 118 animal diseases as notifiable, based on their potential for impact on trade, there is a selected subset that have been classified as posing a greater threat to countries due to unique characteristics, such as being highly transmissible, spreading rapidly within and between countries, and requiring cooperation between several countries to control their spread or exclude them. While these ‘transboundary diseases’ are endemic in much of the world, particularly the developing nations, many countries are classified as disease free.
Following the terrorist events of 11 September 2001 in the United States, a small group of zoonotic pathogens and a group of animal-specific pathogens (those that cause what are referred to as `high-consequence foreign animal diseases’), were classified as high-risk, biothreat ‘select agents’. Rather than providing a comprehensive review of all animal pathogens, the authors briefly review the impact of these high-risk biothreat agents on animal health, the economy, food security and safety, and public health, using highly pathogenic avian influenza, foot and mouth disease and brucellosis as examples. They focus on the impact of these diseases in the context of high-income countries and low- and middle-income countries, comparing and contrasting their impact at the national and individual household levels.
Keywords: Animal pathogen, Biothreat agent, Brucellosis, Disease outbreak, Foot and mouth disease, Highly pathogenic avian influenza, Impact
Introduction
Over the past 15 years, the possibility of an intentionally released biological agent (bioterrorism/agroterrorism event) spreading rapidly through a human or animal population has raised the world’s consciousness about diseases that have existed in the animal realm for, in some cases, recorded history. Excluding plant pathogens, of the 55 listed disease agents on the United States (US) ‘select agent’ list, 36 are either zoonotic or animal-specific pathogens capable of causing significant clinical illness in humans, animals, or both (1). The World Organisation for Animal Health (OIE) lists 118 notifiable diseases (2). Divided into pathogens that infect multiple species, and those that infect specific terrestrial and aquatic animal species (i.e. cattle, sheep, goats, swine, equines, poultry, bees, fish, rabbits/hares, molluscs, crustaceans and amphibians), the list includes diseases that pose a threat to trade in animals and animal products. In total, 27 of the biothreat agents listed in the US select agent list are also OIE-notifiable diseases. Most were included in the former List A diseases as they are highly transmissible, have the potential to spread rapidly across borders and cause significant socio-economic and public health consequences. It is these characteristics that make them at once both high-risk biothreat agents and significant threats to animal and human health and well-being.
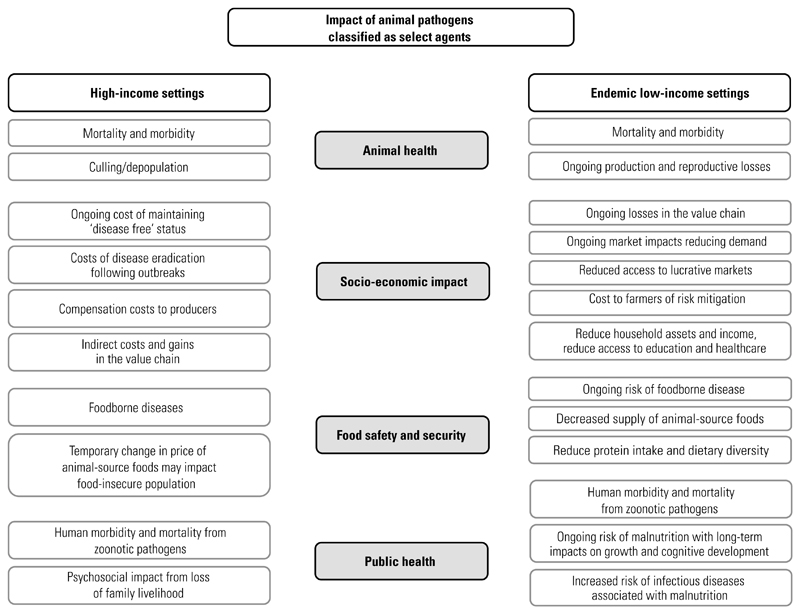
A number of good reviews on the impact of livestock and poultry infectious diseases and zoonoses have been published, focusing on a range of topics, including burden analysis, economics and epidemiology, and their role in pro-poor policies (3, 4, 5, 6, 7). The different characteristics of animal diseases, including the range of hosts affected, transmission modes and ease of spread, their impact on production and survival, and the costs of their prevention and control across different geographical settings, make their review in one manuscript a difficult task. Further, the impact of an outbreak of a listed biothreat agent, while always significant, differs based on where it occurs. For example, exotic Newcastle disease can have a significant impact at the household level in an endemic, low-income setting that depends on eggs from a small flock of chickens as a source of high-quality protein and/or income stream, whereas it may be negligible at the household level in a high-income setting, where higher egg prices may be more of a temporary inconvenience. In contrast, in a high-income setting, the cost of response, control and recovery from a disease outbreak can have a significant impact on the producer and the local community and at the government regulatory level, but less impact on individual households, for which nutritional alternatives are readily available. The general pathways by which animal pathogens affect human health and well-being in high-income and low-income settings are depicted in Figure 1.
Fig. 1. Schematic diagram showing a summary of the impacts of animal pathogens classified as select agents in high-income and endemic low-income settings.
Here, the authors focus on the impact of animal pathogens defined as high-risk, biothreat agents (so-called ‘select agents’ in the US) (1) in four areas: animal health, economics, food safety and security, and public health. A comprehensive review of disease impacts is beyond the scope of this article. Instead, the authors use a few, selected, high-risk disease agents – highly pathogenic avian influenza (HPAI), foot and mouth disease (FMD) and brucellosis – to illustrate the impacts of these diseases in two settings – developed, high-income settings and developing, low-income settings.
Impact of animal pathogens in high-income countries
The development of the select agent list in the US after the events of 11 September 2001 (‘9/11’) highlighted the human, animal and plant biothreats considered by the US to be at the highest risk of deliberate introduction (1). For livestock and poultry pathogens, this list of select agents mirrored the list of ‘foreign animal diseases’ that were already immediately notifiable to the US Department of Agriculture (USDA), including HPAI. The 2014–2015 outbreak of HPAI in the US is an informative case study for the impact of a biothreat agent newly introduced into a susceptible population of animals.
Highly pathogenic avian influenza outbreak: animal health impacts
Highly pathogenic avian influenza was first confirmed in the Pacific North-West of the US (in the state of Washington) in December 2014, shortly after the identification of HPAI in Canada in November 2014 (8). Three strains of influenza (H5N2, H5N8 and a single isolate of H5N1) were eventually identified in the Pacific North-West and California in wild birds, backyard flocks and commercial poultry, with spread presumed to be by wild bird movement. Then, in March 2015, the H5N2 strain was identified in the Midwestern US (along both the Mississippi and Central wild bird flyways), where the major impact on commercial flocks of turkeys and egg layers occurred (8). Disease spread in the Mid-West did not appear to be associated with wild birds. Instead, mechanical and natural vectors (vehicles, wind, feed and others) likely played a major role. While the last identification of an infected flock was in June 2015, response and recovery efforts continued for many months.
The 2014–2015 outbreak of HPAI was the largest foreign animal disease outbreak in the history of the US, in both its economic impact and geographic spread. In total, 7.5 million turkeys and over 42 million egg layers were depopulated to control the disease spread (8). Reaching broadly across the US, the 2014–2015 HPAI outbreak illustrates the potential for a newly introduced disease to severely affect a developed country, with extraordinary morbidity and mortality of infected birds and a loss of life, albeit humane, for millions of healthy birds depopulated in infected and exposed flocks.
Economic impacts
Recent studies on the economic consequences of the HPAI outbreak were published as a themed series in Choices, an online publication of the Agricultural and Applied Economics Association (8). The series examined the impact at multiple levels, from government response efforts to the local communities that were affected. At the national level, the USDA reportedly spent US$ 879 million on identification, response and recovery activities (9, 10). This is equivalent to 1.82% of the total poultry production value in the US. Approximately US$ 200 million of the total was spent on indemnity payments to affected producers, with the rest composed mostly of cleaning, disinfection and disposal costs.
At the local level, communities experienced both gains and losses during the outbreak. For example, while individual producers lost their entire production units and income streams (direct losses), support businesses, such as equipment providers, hotels, restaurants and transportation providers, realised short-term gains from the influx of disease responders, journalists and others. In contrast, indirect losses were experienced at the community level through decreased tax revenue; decreased need for the local agricultural support industry, such as feed companies and processing plants; transportation disruptions; loss of tourism; and various other issues.
In a country as large and diverse as the US, the size and economic complexities of the livestock and poultry industries can mean that disease outbreaks have unexpected consequences, even in one as large as the 2014–2015 HPAI outbreak. For example, the turkey industry suffered an overall loss of US$ 214 million (a decrease in returns of nearly 7%) as a result of being heavily regionalised in the Midwestern states, with far less turkey production elsewhere in the US (11). In contrast, returns to the egg-layer industry nationwide, with significant production outside the disease outbreak region, were higher, as a result of increases in egg prices. There was an overall estimated increase of US$ 52 million in returns (representing a nearly 27% increase). In fact, returns to producers who continued to produce eggs in parts of the US that were unaffected by the outbreak more than offset the overall losses experienced by affected producers in the Mid-West (11). Related support industries, such as soybean processors, suffered large losses. If all related livestock, poultry, feed, land valuation and ancillary industries are considered in total, the loss in value and returns is estimated at more than US$ 1 billion (11).
Detailed data on the impact of the 2014–2015 outbreak of HPAI at the household level are not available. Producers affected by the outbreak were indemnified for their losses – unlike individual producers in resource-constrained countries, where the loss of an entire flock of chickens without indemnity represents a severe economic shock. Nevertheless, it is reasonable to infer that individual producer income was reduced, and that producers whose livelihoods were disrupted, even if temporarily, suffered a keen psychological impact.
The loss of animals with significant genetic value was limited in the 2014–2015 HPAI outbreaks and thus did not impact individual producers in that aspect. However, for some livestock industries, such as cattle producers, biothreat agents such as FMD virus can not only result in a temporary loss of livelihood for the producer, but can also permanently destroy generations of breeding in which genetically superior livestock are lost to the disease or control efforts.
Food safety and security
At the population level, it appears that the overall consumption of eggs in US households was not significantly affected, despite a considerable increase in egg prices, starting in the second quarter of 2015 (12). Egg shortages in affected regions of the US were offset by the importation of eggs from other parts of the country – partly as a result of fewer exports, due to the imposition of international trade restrictions. In general, consumers weathered the short-term rise in egg prices without changing their eating habits (11). Despite a lack of data, it is reasonable to assume that the very poor in the US changed their eating habits during the last three-quarters of the 2015 calendar year, when egg prices were high. However, a short-term change in diet, coupled with the availability of alternative sources of high-quality protein, would likely have had little, if any, long-term health effects. Again, this stands in contrast to some resource-poor settings where the availability of high-quality protein sources is limited and people, particularly children, often live their daily lives at or below the nutritional level required to stave off general malnutrition, stunting and wasting.
Public health
In retrospect, the strains of influenza A responsible for the 2014–2015 outbreak in the US were not serious threats to human health. However, at the inception of the outbreak and in the following months, public health officials worked closely with animal health regulatory authorities to monitor exposed individuals in an effort to establish firmly that human infection with these viruses was not occurring. The public health response also included a significant educational component to reassure the population throughout the US that the devastating outbreak in poultry, featured daily in both formal, established media and social media, was not of concern to them. It is fortunate that, in this case, the outbreak virus strains were benign for humans. The impact of a dual human and animal virulent strain newly introduced (naturally or intentionally) would be significantly more serious for public health. Nevertheless, the 2014–2015 HPAI outbreak in the US is a good example of the public health impact that even a newly introduced, non-zoonotic disease can have.
The impact of animal pathogens classified as biothreat agents differs by type of agent (bacterial versus viral), manner of spread (vector-borne versus airborne), whether introduced as a single-point source or at multiple locations, the susceptibility of the population, population dynamics, and many other factors. It is beyond the scope of this short review to comprehensively examine biothreat agent impact. However, the 2014–2015 outbreak of HPAI in the US serves as a recent and informative example of the impact of an animal pathogen newly introduced into a susceptible population in a developed country.
Continuing with the example of HPAI as a significant biothreat agent, the authors now examine the impact of HPAI H5N1 in low-income settings, using the African continent as an example.
Impact of animal pathogens: highly pathogenic avian influenza H5N1 in low-income settings
The direct impact that animal diseases have on survival and productivity is a major constraint to livestock-keeping in the developing world (13). In rural settings, the proportion of livestock mortality attributable to infectious diseases can be greater than 90% in susceptible populations, and, among those that survive, the consequences of infection can extend to affecting their productivity and reproduction (14, 15).
Economic impacts
Highly pathogenic avian influenza was first detected in Kaduna State in Nigeria in early 2006, and shortly after spread to Niger, Egypt, Cameroon, Burkina Faso, Côte d’Ivoire, Djibouti, Ghana and Togo (16, 17). By the end of 2009, although human cases had only been reported in three countries – Djibouti (1 case), Nigeria (2 cases) and Egypt (354 cases) (18) – the threat of HPAI had a significant effect, both on the economies of those countries affected and of those suspected of having the disease.
These costs have been classified into three areas:
-
i)
direct losses to producers and players in the value chain, through mortality and morbidity and private costs associated with risk mitigation
-
ii)
costs incurred by governments, including investments in surveillance and infrastructure when a notifiable disease occurs
-
iii)
impacts of the disease associated with market reactions to the perception of public health risks (19).
Highly pathogenic avian influenza in Africa resulted in demand shocks (occasioned by consumer panic, leading to reduced prices in the value of poultry and eggs) and supply shock (a reduced supply of poultry and poultry products due to HPAI-associated mortality and panic culling or reduction in flock sizes) (19, 20, 21).
A few studies have attempted to estimate the cost of HPAI in countries in Africa where the virus has been detected, as well as in countries that were unaffected. By the end of 2006, 900,000 birds on commercial farms in Nigeria had died or been culled due to HPAI, with an estimated cost of US$ 4.82 million (22, 23). Further estimates showed that a mild infection affecting 10% of commercial flocks would cost the Nigerian economy US$ 245 million and a worse scenario would cost the economy up to US$ 700 million, in the layer industry alone (24). When other potential losses (in traditional, extensive backyard poultry production systems; control costs for increased biosecurity, vaccinations, diagnostics and surveillance; market/consumer reactions; value chain losses from feed manufacturers to hatcheries up to wholesalers, retailers, restaurants and the catering industry) are all taken into account, estimates of losses associated with HPAI in Nigeria could be significantly higher than reported (19).
Food security and safety
Public perceptions of the health risks during the HPAI outbreaks and scare resulted in a drastic decrease in the consumption of poultry meat and eggs. In Nigeria, the outbreak resulted in up to an 80% reduction in the consumption of poultry at restaurants and in households (22, 25). This is a good example of where a food safety concern, whether legitimate or not, could lead to food insecurity. Although no positive cases of HPAI were detected in Kenya, incorrect media reports fuelled a panic that resulted in a sharp reduction in the consumption of poultry meat and eggs, and a loss of income for the 65% of rural farmers who kept poultry (20, 26). It is estimated that these losses in income could lead to reduced diversity in household food consumption and a decreased intake of nutritionally high-quality proteins from poultry. This in turn could have caused an estimated 3.9% increase in the prevalence of stunting in children aged between six and 36 months (27).
Public health
In contrast to the 2014–2015 outbreak of HPAI in the US, by July 2016, HPAI had been associated with human cases in three countries – Djibouti (one case), Nigeria (one case, one death), and Egypt (354 cases, 117 deaths) (18). In addition to Egypt, where the disease is considered endemic, HPAI H5N1 is endemic in poultry in Bangladesh, China, Indonesia and Vietnam. Although zoonotic in nature, the public heath impact of HPAI is thought to have been limited by the inefficiency of the HPAI H5N1 virus transmission from person to person.
The short case studies above have focused on the impact of a newly introduced biothreat agent into a developed country and a low-income country, using the example of HPAI. While there is no doubt that animal pathogens in general have significant ongoing impact in developed countries in all four areas of animal health, economics, food safety and security, and public health, the endemic animal diseases that cause the most significant impacts in high-income countries are generally not considered high-risk biothreat agents. Nor are they included on the select agents list in the US or similar high-risk agent lists in other developed nations.
Exceptions to this clearly exist. For example, African swine fever has become endemic outside the African continent, including in developed countries. Additionally, select agents are generally not considered ongoing food safety concerns, due to the production systems and post-harvest processing of foods in most developed countries. Again, exceptions exist. Certainly an outbreak of anthrax would raise the food safety risk, at least temporarily.
In contrast to most of the developed world, in low-income and some middle-income countries, animal pathogens considered biothreat agents in the US, European Union member States, and other developed nations are endemic. The authors now examine the impact of these agents at the household level, in a setting in which animal production is much less centralised, and where individual households are increasingly and directly reliant on the animals they own for their livelihood.
Impact of animal pathogens in endemic low-income settings
The control of animal diseases in many countries classified as low- and middle-income is underfunded, with many diseases listed as biothreat agents in developed economies being endemic in such low-income countries. Among many others, these include diseases such as FMD, brucellosis and anthrax. Whereas, in the developed economies, investments are made into surveillance and biosecurity to prevent the introduction of these ‘high-consequence foreign animal diseases’ (with elimination through depopulation when outbreaks occur), in most low-income economies there is little or no surveillance, so the elimination of these diseases is still very much a matter for the distant future. The consequences of the high prevalence and incidence of these infections are felt down to the household level. Livestock production systems in these settings are largely small scale, with farmers directly dependent on their livestock for household economics, nutrition and health.
In this context, high-impact diseases include those that increase the vulnerability of livestock keepers (for example, those that cause mortality in large numbers when they occur, such as anthrax and exotic Newcastle disease), or reduce the value of their livestock (for example, Rift Valley fever); those that reduce livestock productivity and reproductive success, such as FMD and brucellosis; and those that reduce access to local and international markets, such as FMD and avian influenza (4, 13). The relationships between livestock health and production, and household health, economics and nutritional status, have been postulated and demonstrated (28, 29, 30, 31, 32).
Foot and mouth disease is endemic in most of Africa, with the exception of a few regions in southern Africa considered free of the disease. This is not the case in developed economies. Although the impact of FMD in endemic areas has scarcely been studied, a recent review has examined what is known about the impact of FMD on smallholders (33). Direct losses have been reported (34), with up to a 15% reduction in the milk production of clinically infected animals during lactation. Among households that are directly dependent on livestock for access to nutritious animal-based foods, such direct losses in milk production can reduce their access to animal-source foods. These foods are positively associated with a reduced risk of malnutrition, better cognitive development and improved school performance (32, 35, 36). Even in the absence of clinical disease, chronic forms of FMD have been reported as accounting for more than a quarter of total FMD losses (37). Besides affecting the efficiency of production on farms, FMD limits the use of high-producing breeds of cattle, which are more susceptible to the disease. Additionally, its control comes with more costs to individual farmers and public resources, including the costs of vaccination, surveillance, fencing and movement restrictions (33).
Another illustrative example of the impact of endemic disease at the household level is brucellosis. Brucellosis, caused by Brucella spp., such as B. abortus and B. melitensis, has been eliminated from domestic cattle and small ruminants in many high-income countries, but remains endemic in many countries in Africa (38, 39). It is a complex disease affecting multiple livestock species, including cattle, goats, sheep, swine and camels, and is easily transmitted to humans through the consumption of livestock products or contact with infected material. The disease in animals is characterised by abortions and infertility, which are non-specific signs and may not be observed in all infected animals. In naïve herds, the introduction of infected animals may result in ‘abortion storms’, but these are less likely to be observed in subsequent pregnancies, if they occur.
These characteristics make brucellosis a difficult disease to identify and report in low-income countries, as well as a disease for which accurate estimates of the true impact on animal health and production are difficult to obtain (40). Nevertheless, several studies have reviewed the available literature on the economics of brucellosis in low-income countries (38, 41, 42, 43). The disease impacts have been divided into the:
-
–
costs of the illness
-
–
costs of preventing the disease in individuals and/or households and livestock herds
-
–
costs to the health sector
-
–
costs to the economy (41).
At the household level, losses due to lowered milk production and long calving intervals associated with infertility or abortions may directly affect household access to animal-source foods and predispose them to the negative effects of protein malnutrition. The costs of treating infected herds, or healthcare costs incurred by family members infected with the disease, in addition to the loss of working days associated with long periods of illness, may add a significant burden to households in low-income countries (41, 42). In rural households, studies have shown a strong likelihood of there being a brucellosis-seropositive person in a household that has a seropositive animal in the herd; risk factors that increase exposure to brucellosis include drinking unpasteurised milk or handling hides and skins (44). The endemic nature of brucellosis in many low-income countries, its mode of transmission, and the range of hosts that the pathogen can infect, including humans, make brucellosis a significant daily threat to food safety and public health at the household level in low-income settings.
Conclusion
The subset of animal pathogens classified by the OIE as being high-risk threats, due to their highly transmissible nature and their potential to spread rapidly across borders and cause significant socio-economic and public health consequences, are, for the same reasons, also recognised as high-risk agents for deliberate introduction into non-endemic countries. These biothreat agents are pathogens that affect animals and, in some cases, zoonotic pathogens that have been eliminated from many countries but which remain endemic in much of the developing world. Their impact differs, depending on the setting in which they are endemic or have been introduced.
In this short review, the authors have highlighted the impact of biothreat agents in low-income and high-income settings, using several examples in which they are newly introduced or endemic. Where relevant, the similarities and differences in the impact on animal health, the economy, food security and safety, and public health in these disparate settings have been noted. Figure 1 captures some of these similarities and differences.
The 2014–2015 outbreak of HPAI H5N2 in the US illustrates the impact of a newly introduced disease agent into a developed country. Similarly, the HPAI H5N1 outbreak in Africa (reviewed here as an illustrative example) and Asia significantly affected many developing nations. In contrast to these examples of newly recognised or introduced animal and zoonotic pathogens, many high-risk, animal health biothreat agents have a continuing daily impact at the household level in countries where they are endemic. Diseases such as FMD, a feared high-consequence foreign animal disease in non-endemic areas, have a direct impact on household well-being in a low-income area, resulting in reduced access to high-quality proteins from animal-source foods, reduced income from milk and meat production, and reduced overall household assets/savings. Anthrax and brucellosis have similar impacts, with the added ongoing burden of foodborne illnesses in low-income settings. These are not impacts felt by the overwhelming majority of households in a high-income country when an outbreak occurs.
Acknowledgements
S.M. Thumbi is supported by the Wellcome Trust, Grant No. 110330/Z/15/Z. The authors thank Gati Wambura at the Kenya Medical Research Institute for the initial analysis of the literature and for providing useful comments, and the Paul G. Allen School for Global Animal Health for continued support of both S.M. Thumbi and T.F. McElwain.
References
- 1.Centers for Disease Control and Prevention & United States Department of Agriculture. Federal select agents and toxins. Code of Federal Regulations. United States Government; Washington, DC: 2014. [accessed on 28 September 2016]. Available at: www.selectagents.gov/regulations.html. [Google Scholar]
- 2.World Organisation for Animal Health (OIE) OIE-listed diseases. OIE; Paris: 2016. [accessed on 28 September 2016]. Available at: http://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2016. [Google Scholar]
- 3.Perry BD, Grace D, Sones K. Current drivers and future directions of global livestock disease dynamics. Proc Natl Acad Sci USA. 2013;110(52):20871–20877. doi: 10.1073/pnas.1012953108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry B, Grace D. The impacts of livestock diseases and their control on growth and development processes that are pro-poor. Philos Trans Roy Soc Lond, B, Biol Sci. 2009;364(1530):2643–2655. doi: 10.1098/rstb.2009.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich KM, Perry BD. The economic and poverty impacts of animal diseases in developing countries: new roles, new demands for economics and epidemiology. Prev Vet Med. 2011;101(3–4):133–147. doi: 10.1016/j.prevetmed.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Rushton J, Heffernan C. A literature review of livestock diseases and their importance in the lives of poor people. In: Thornton PK, Kruska RL, Henninger N, Kristjanson PM, Reid RS, Atieno F, Odero AN, Ndegwa T, editors. Mapping poverty and livestock in the developing world. International Livestock Research Institute; Nairobi: 2002. pp. 1–85. [Google Scholar]
- 7.Tomley FM, Shirley MW. Livestock infectious diseases and zoonoses. Philos Trans Roy Soc Lond, B, Biol Sci. 2009;364(1530):2637–2642. doi: 10.1098/rstb.2009.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagerman AD, Marsh TL. Theme overview: economic consequences of the 2014-2015 US highly pathogenic avian influenza outbreak. [accessed on 28 September 2016];Choices. 2016 31(Quarter 2) Available at: www.choicesmagazine.org/choices-magazine/theme-articles/economic-consequences-of-highly-pathogenic-avian-influenza/theme-overview-economic-consequences-of-highly-pathogenic-avian-influenza-sthash.nns5CATW.dpuf. [Google Scholar]
- 9.Johnson KK, Seeger RM, Marsh TL. Local economies and highly pathogenic avian influenza. [accessed on 28 September 2016];Choices. 2016 31(Quarter 2) Available at: http://www.choicesmagazine.org/choices-magazine/theme-articles/economic-consequences-of-highly-pathogenic-avian-influenza/local-economies-and-highly-pathogenic-avian-influenza-sthash.U1pzU20N.dpuf. [Google Scholar]
- 10.Johansson RC, Preston WP, Hillberg Seitzinger A. Government spending to control highly pathogenic avian influenza. [accessed on 28 September 2016];Choices. 2016 31(Quarter 2) Available at: www.choicesmagazine.org/choices-magazine/theme-articles/economic-consequences-of-highly-pathogenic-avian-influenza/government-spending-to-control-highly-pathogenic-avian-influenza-sthash.8aWkXkQC.dpuf. [Google Scholar]
- 11.Hillberg Seitzinger A, Parlberg PL. Regionalization of the 2014 and 2015 highly pathogenic avian influenza outbreaks. [accessed on 28 September 2016];Choices. 2016 31(Quarter 2) Available at: www.choicesmagazine.org/choices-magazine/theme-articles/economic-consequences-of-highly-pathogenic-avian-influenza/regionalization-of-the-2014-and-2015-highly-pathogenic-avian-influenza-outbreaks-sthash.JMa8syfO.dpuf. [Google Scholar]
- 12.Huang W, Hagerman AD, Bessler DA. The impact of highly pathogenic avian influenza on table egg prices. [accessed on 28 September 2016];Choices. 2016 31(Quarter 2) Available at: www.choicesmagazine.org/choices-magazine/theme-articles/economic-consequences-of-highly-pathogenic-avian-influenza/the-impact-of-highly-pathogenic-avian-influenza-on-table-egg-prices-sthash.kGVFG3CE.dpuf. [Google Scholar]
- 13.Perry B, Sones K. Science for development. Poverty reduction through animal health. Science. 2007;315(5810):333–334. doi: 10.1126/science.1138614. [DOI] [PubMed] [Google Scholar]
- 14.Thumbi SM, Bronsvoort BM, Kiara H, Toye PG, Poole J, Ndila M, Conradie I, Jennings A, Handel IG, Coetzer JAW, Steyl J, et al. Mortality in East African shorthorn zebu cattle under one year: predictors of infectious-disease mortality. BMC Vet Res. 2013;9:175. doi: 10.1186/1746-6148-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thumbi SM, Bronsvoort BM, Poole EJ, Kiara H, Toye P, Ndila M, Conradie I, Jennings A, Handel IG, Coetzer JA, Hanotte O, et al. Parasite co-infections show synergistic and antagonistic interactions on growth performance of East African zebu cattle under one year. Parasitology. 2013;140(14):1789–1798. doi: 10.1017/S0031182013001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joannis T, Lombin LH, De Benedictis P, Cattoli G, Capua I. Confirmation of H5N1 avian influenza in Africa. Vet Rec. 2006;158(9):309–310. doi: 10.1136/vr.158.9.309-b. [DOI] [PubMed] [Google Scholar]
- 17.Cattoli G, Monne I, Fusaro A, Joannis TM, Lombin LH, Aly MM, Arafa AS, Sturm-Ramirez KM, Couacy-Hymann E, Awuni JA, Batawui KB, et al. Highly pathogenic avian influenza virus subtype H5N1 in Africa: a comprehensive phylogenetic analysis and molecular characterization of isolates. PLoS ONE. 2009;4(3):e4842. doi: 10.1371/journal.pone.0004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. WHO; Geneva: 2016. [accessed on 30 September 2016]. Available at: www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en. [Google Scholar]
- 19.Otte J. Impacts of avian influenza virus on animal production in developing countries. CAB Rev, Perspect Agric Vet Sci Nutr Nat Resour. 2008;3:1–18. doi: 10.1079/PAVSNNR20083080. [DOI] [Google Scholar]
- 20.Omiti JM, Okuthe S. Background paper. Africa/Indonesia Region Report No. 4. International Food Policy Research Institute; Washington, DC: 2009. An overview of the poultry sector and status of highly pathogenic avian influenza (HPAI) in Kenya; p. 106. [Google Scholar]
- 21.Birol E, Asare-Marfo D, Ayele G, Mensah-Bonsu A, Ndirangu L, Okpukpara B, Devesh R, Yakhshilikov Y. The impact of avian flu on livelihood outcomes in Africa: evidence from Ethiopia, Ghana, Kenya and Nigeria. Afr J Agric Resour Econ. 2013;8(4):275–288. [Google Scholar]
- 22.United Nations Development Programme (UNDP) Socio-economic impact of avian influenza in Nigeria. UNDP; Nairobi: 2006. p. 599. [Google Scholar]
- 23.Roeder P, Masiga W, Bastiaensen P. Joint FAO/OIE/AU–IBAR mission to Nigeria on highly pathogenic avian influenza, 3–13 October. Report submitted to the Food and Agriculture Organization of the United Nations (FAO), World Organisation for Animal Health (OIE) and African Union Interafrican Bureau for Animal Resour. (AU-IBAR) 2006. [accessed 22 September 2017]. p. 30. Available at: www.oie.int/doc/ged/D5361.PDF.
- 24.Fasina FO, Sirdar MM, Bisschop SPR. The financial cost implications of the highly pathogenic notifiable avian influenza H5N1 in Nigeria. Onderstepoort J Vet Res. 2008;75(1):39–46. doi: 10.4102/ojvr.v75i1.86. [DOI] [PubMed] [Google Scholar]
- 25.Obayelu AE. Socio-economic analysis of the impacts of avian influenza epidemic on households poultry consumption and poultry industry in Nigeria: empirical investigation of Kwara State. [accessed on 11 June 2017];Livest Res Rural Dev. 2007 19(1) Available at: www.lrrd.org/lrrd19/1/obay19004.htm. [Google Scholar]
- 26.Kimani T, Obwayo M, Muthui L. Avian flu threat: socio-economic assessment of the impacts on poultry related livelihoods in selected districts in Kenya. Pan-African Program for the Control of Epizoonotics; Nairobi: 2008. p. 106. [Google Scholar]
- 27.Iannotti L, Roy D. Nutritional impact of highly pathogenic avian influenza in Kenya. Food Nutr Bull. 2013;34(3):299–309. doi: 10.1177/156482651303400302. [DOI] [PubMed] [Google Scholar]
- 28.Bradford GE. Contributions of animal agriculture to meeting global human food demand. Livest Prod Sci. 1999;59(2–3):95–112. doi: 10.1016/S0301-6226(99)00019-6. [DOI] [Google Scholar]
- 29.Randolph TF, Schelling E, Grace D, Nicholson CF, Leroy JL, Cole DC, Demment MW, Omore A, Zinsstag J, Ruel M. Invited review: role of livestock in human nutrition and health for poverty reduction in developing countries. J Anim Sci. 2007;85(11):2788–2800. doi: 10.2527/jas.2007-0467. [DOI] [PubMed] [Google Scholar]
- 30.Iannotti L, Lesorogol C. Animal milk sustains micronutrient nutrition and child anthropometry among pastoralists in Samburu, Kenya. Am J Phys Anthropol. 2014;155(1):66–76. doi: 10.1002/ajpa.22547. [DOI] [PubMed] [Google Scholar]
- 31.Thumbi SM, Njenga MK, Marsh TL, Noh S, Otiang E, Munyua P, Ochieng L, Ogola E, Yoder J, Audi A, Montgomery JM, et al. Linking human health and livestock health: a ‘One-Health’ platform for integrated analysis of human health, livestock health, and economic welfare in livestock dependent communities. PLoS ONE. 2015;10:e0120761. doi: 10.1371/journal.pone.0120761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosites E, Thumbi SM, Otiang E, McElwain TF, Njenga MK, Rabinowitz PM, Rowhani-Rahbar A, Neuhouser ML, May S, Palmer GH, Walson JL. Relations between household livestock ownership, livestock disease, and young child growth. J Nutr. 2016;146(5):1118–1124. doi: 10.3945/jn.115.225961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knight-Jones TJD, McLaws M, Rushton J. Foot-and-mouth disease impact on smallholders: what do we know, what don’t we know and how can we find out more? Transbound Emerg Dis. 2016 doi: 10.1111/tbed.12507. Epub:11 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyons NA, Alexander N, Stärk KDC, Dulu TD, Sumption KJ, James AD, Rushton J, Fine PEM. Impact of foot-and-mouth disease on milk production on a large-scale dairy farm in Kenya. Prev Vet Med. 2015;120(2):177–186. doi: 10.1016/j.prevetmed.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Demment M, Allen L, editors. Animal source foods to improve micronutrient nutrition and human function in developing countries. Proc. of the Conference on Animal Source Foods in Developing Countries, 24–26 June 2002, Washington, DC. J Nutr. 2003;133(11, Suppl. 2):3875S–4061S. doi: 10.1093/jn/133.11.3875S. [DOI] [PubMed] [Google Scholar]
- 36.Darapheak C, Takano T, Kizuki M, Nakamura K, Seino K. Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. Int Arch Med. 2013;6:29. doi: 10.1186/1755-7682-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barasa M, Catley A, MacHuchu D, Laqua H, Puot E, Kot DT, Ikiror D. Foot-and-mouth disease vaccination in South Sudan: benefit–cost analysis and livelihoods impact. Transbound Emerg Dis. 2008;55(8):339–351. doi: 10.1111/j.1865-1682.2008.01042.x. [DOI] [PubMed] [Google Scholar]
- 38.McDermott JJ, Arimi SM. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol. 2002;90(1–4):111–134. doi: 10.1016/S0378-1135(02)00249-3. [DOI] [PubMed] [Google Scholar]
- 39.Moreno E. Retrospective and prospective perspectives on zoonotic brucellosis. Front Microbiol. 2014;5:213. doi: 10.3389/fmicb.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ducrotoy M, Bertu WJ, Matope G, Cadmus S, Conde-Álvarez R, Gusi AM, Welburn S, Ocholi R, Blasco JM, Moriyón I. Brucellosis in sub-Saharan Africa: current challenges for management, diagnosis and control. Acta Trop. 2015;165:179–193. doi: 10.1016/j.actatropica.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 41.McDermott J, Grace D, Zinsstag J. Economics of brucellosis impact and control in low-income countries. In: Plumb GE, Pappas G, Olsen SC, editors. Brucellosis: recent developments towards One Health Rev Sci Tech Off Int Epiz. 1. Vol. 32. 2013. pp. 249–261. [DOI] [PubMed] [Google Scholar]
- 42.Rubach MP, Halliday JEB, Cleaveland S, Crump JA. Brucellosis in low-income and middle-income countries. Curr Opin Infect Dis. 2013;26(5):404–412. doi: 10.1097/QCO.0b013e3283638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Njeru J, Wareth G, Melzer F, Henning K, Pletz MW, Heller R, Neubauer H. Systematic review of brucellosis in Kenya: disease frequency in humans and animals and risk factors for human infection. BMC Public Hlth. 2016;16(1):853. doi: 10.1186/s12889-016-3532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osoro EM, Munyua P, Omulo S, Ogola E, Ade F, Mbatha P, Mbabu M, Ng’ang’a Z, Kairu S, Maritim M, Thumbi SM, et al. Strong association between human and animal Brucella seropositivity in a linked study in Kenya, 2012–2013. Am J Trop Med Hyg. 2015;93(2):224–231. doi: 10.4269/ajtmh.15-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]