Abstract
Plant-emitted volatile organic compounds (VOCs) play fundamental roles in atmospheric chemistry and ecological processes by contributing to aerosol formation1 and mediating species interactions2. Rising temperatures and the associated shifts in vegetation composition have been shown to be the primary drivers of plant VOC emissions in Arctic ecosystems3. Although herbivorous insects also strongly alter plant VOC emissions2, no studies have addressed the impact of herbivory on plant VOC emissions in the Arctic. Here, we show that warming dramatically increases the amount and alters the blend of VOCs released in response to herbivory. We observed that a tundra ecosystem subjected to warming, by open-top chambers, for 8 or 18 years showed a 4-fold increase in leaf area eaten by insect herbivores. Herbivory by autumnal moth (Epirrita autumnata) larvae and herbivory-mimicking methyl jasmonate application on the widespread circumpolar dwarf birch (Betula nana) both substantially increased emissions of terpenoids. The long-term warming treatments and mimicked herbivory caused, on average, a 2- and 4-fold increase in monoterpene emissions, respectively. When combined, emissions increased 11-fold, revealing a strong synergy between warming and herbivory. The synergistic effect was even more pronounced for homoterpene emissions. These findings suggest that in the rapidly warming Arctic, insect herbivory may be a primary determinant of VOC emissions during periods of active herbivore feeding.
Plants emit a complex blend of volatile organic compounds (VOCs) to the atmosphere, which can account for up to 10% of photosynthetically fixed carbon4. These VOCs have multiple eco-physiological functions, such as mediating interactions with herbivores, attracting natural enemies of herbivores, eliciting pre-emptive defence in neighbouring branches and plants, and protecting plants against abiotic stress2,5–8. Furthermore, plant VOCs impact atmospheric chemistry by modulating the oxidative capacity of the atmosphere, lead to the formation of secondary organic aerosols (SOAs) that scatter solar radiation, and act as cloud condensation nuclei1,7,9,10. As global climate change alters abiotic (e.g., light and temperature) and biotic (e.g., herbivores and pathogens) factors that influence plant VOC emissions7, the quantity and quality of VOCs are likely to change. These changes have the potential to create feedbacks on climate and ecosystem processes and interactions.
Arctic ecosystems have been warming by approximately 1°C per decade for the past 30 years with future temperatures predicted to increase at twice the rate of the global average11. Recent studies suggest that this rapid warming, and the associated changes in vegetation composition and biomass, are transforming the naturally low-emitting Arctic ecosystems to a stronger VOC source3,12–14. For example, warming by only 2-4°C was found to increase biogenic VOC emissions several fold3,14. However, the impacts of biotic factors, such as insect herbivory, are not well studied. Insect feeding can not only rupture VOC storage deposits, leading to emission bursts of preformed (constitutive) VOCs15, it can also trigger de novo biosynthesis and release of novel VOCs (induced)8,16. While VOCs released following physical damage are typically transient and are restricted to the damaged site, herbivore-induced VOCs contain a larger number of compounds, are released in greater concentrations from both damaged and undamaged tissues, and are sustained even after herbivory8,15,16. Therefore, insect herbivory has greater potential than physical damage to augment VOC emission rates, change relative proportions of VOC blends, and thereby influence local air quality, as illustrated recently in boreal forests9,17. Yet, no empirical studies have investigated the effects of insect herbivory on plant VOC emissions from Arctic ecosystems.
Insect herbivory pressure is expected to increase with ongoing climate warming because higher temperature may substantially relieve physiological constraints on ectothermic insects18,19. In cold climates such as the Arctic, warming accelerates insect metabolic rates and population growth18–20, although exceptions exist21. In turn, this could increase the frequency and severity of background insect herbivory and outbreaks, ultimately inflicting more damage to vegetation. Empirical and modelling studies suggest that in the northern hemisphere, global warming leads to insect range expansion towards higher elevation and latitude, and increased foliage loss to insect herbivory18,20,22. During 2002-2008, outbreak range expansion of two moth species (Epirrita autumnata; Operophtera brumata) caused severe defoliation (approximately 1 million hectares) in the birch forest-tundra ectone of northern Fennoscandia22. However, we still know little about how Arctic warming and a concomitant increase in herbivory pressure alter VOC emission patterns.
Here, we used long-term in situ experimental warming to assess how warming and insect herbivory interactively affect VOC emissions from B. nana, a dominant circumpolar shrub and a major player in ongoing Arctic greening23,24. The experiment used open-top chambers (OTCs) in place during summer on a tundra heath in northern Sweden (68°21'N, 18°49'E) for 8 and 18 years in a randomized complete block design (see Methods for a complete description of the experimental design). VOC emissions were collected by the push-pull technique on adsorbent cartridges from enclosed branches of B. nana, and analysed by gas chromatography-mass spectrometry following thermal desorption.
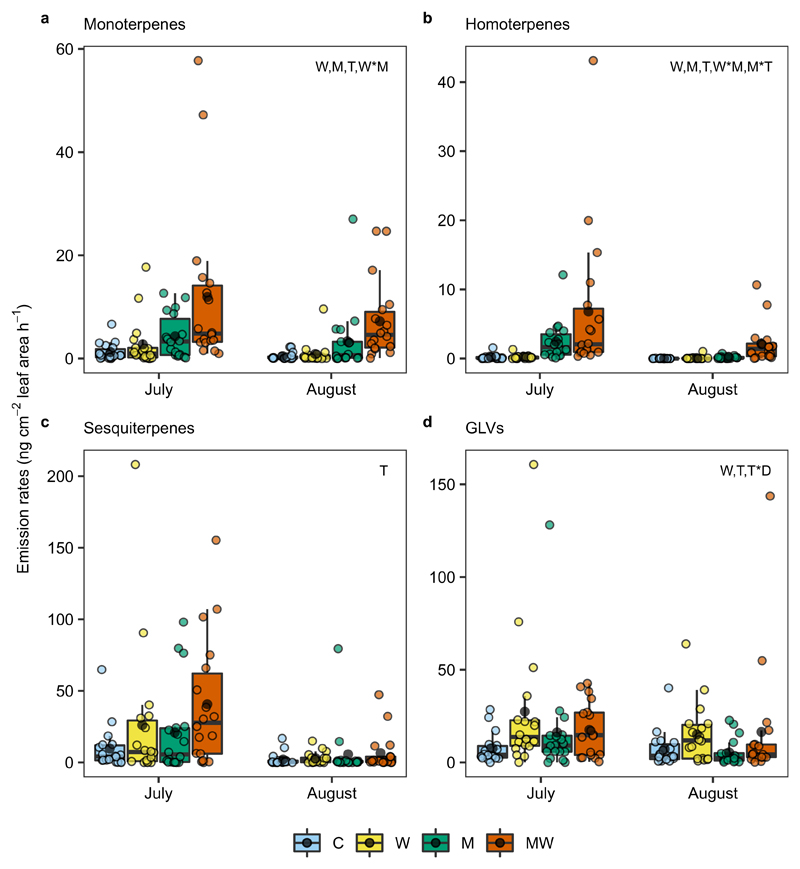
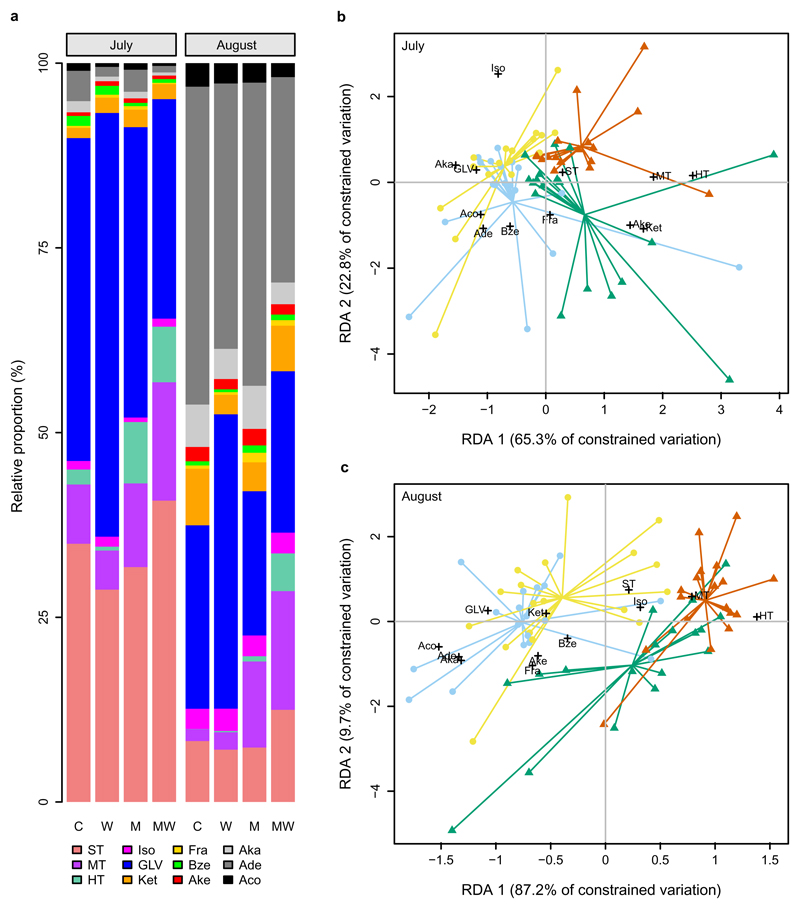
We found that the 2°C canopy warming obtained by the OTCs significantly increased emissions of total terpenoids, and so did the application of methyl jasmonate (MeJA), a plant hormone involved in plant defence against herbivory25. Interestingly, combined warming and MeJA application led to a much stronger increase in total terpenoid emissions (Supplementary Tables 1-3), primarily driven by monoterpenes and homoterpenes (Fig. 1a, b). When averaged over the season, mean monoterpene emissions increased approximately 2-fold under warming, 4-fold under MeJA application, and 11-fold under their combination, while mean homoterpene emissions increased about 2-, 13-, and 46-fold, respectively. Warming and MeJA application both showed marginally significant effects on sesquiterpene emissions (Fig. 1c), which increased approximately 2-, 2-, and 4-fold, respectively, under warming, MeJA application, and their combination. In addition, we found significant warming effects on the emissions of green leaf volatiles (GLVs) (Fig. 1d) and isoprene, significant MeJA effects on the emissions of alkenes, benzenoids, furans, and ketones, and significant interactions on benzenoid and ketone emissions (Supplementary Figs. 1 and 2). Apart from enhancing VOC emission rates, warming and MeJA treatment together significantly altered VOC compositions, rendering monoterpenes and homoterpenes more abundant in VOC blends compared to other treatments (Fig. 2a). Redundancy analysis (RDA), using warming and MeJA treatment as constraining factors, revealed clear separation in VOC blends among treatments, especially between the combination treatment group and control group (Fig. 2b, c; Supplementary Fig. 3). Together, these findings indicate a strong synergy between warming and herbivory on VOC emissions.
Fig. 1. Individual and joint impacts of in situ warming and mimicked herbivory on VOC emissions.
a-d, Emissions of monoterpenes (a), homoterpenes (b), sesquiterpenes (c) and green leaf volatiles (GLVs) (d) are shown for July and August. Each coloured dot represents a single experimental plant; the box and whisker plots show the median, the 25th and 75th percentiles (boxes) and the 10th and 90th percentiles (error bars), with the mean shown with a black dot (n = 18). Colours indicate different treatments [light blue: control (C); yellow: warming treatment (W); dark green: herbivory mimicked by methyl jasmonate application (M); orange: combination of warming and mimicked herbivory (MW)]. The capital letters indicate significant effects (linear mixed-effects model, P < 0.05; Supplementary Table 2) of the corresponding treatments and their interactions, where D and T represent the duration of warming and sampling time, respectively. The pairwise Wilcoxon-Mann-Whitney tests (Supplementary Table 3) produced similar results to the linear-mixed effect models. Note different y-axis scales and that data points are not separated by warming duration. See Supplementary Figs. 1 and 2 for other compound classes.
Fig. 2. Impacts of warming and mimicked herbivory on VOC blends.
a, Changes in relative proportion of different classes of VOCs showing significant interactive effects on monoterpenes and homoterpenes. C: control; W: warming; M: methyl jasmonate application; MW: combination of methyl jasmonate and warming. b, c, Constrained redundancy analyses (RDA) of VOC profiles in July (b) and August (c) showing clear separation (n = 18, P < 0.001, permutation tests) in VOC blends among treatments (light blue dots: control, yellow dots: warming, dark green triangles: methyl jasmonate, orange triangles: combination of warming and methyl jasmonate), as well as the association of treatments with VOC compound classes. Aco: alcohols; Ade: aldehydes; Aka: alkanes; Ake: alkenes; Bze: benzenoids; Fra: furans; Ket: ketones; Iso: isoprene; HT: homoterpenes; MT: monoterpenes; ST: sesquiterpenes. See Supplementary Fig. 3 for RDA analyses based on individual compounds.
There are several potential explanations for the observed synergistic interactions. First, warming can elevate the activity of enzymes involved in VOC biosynthesis and stimulate photosynthesis, which provides substrates for VOC formation7. Second, warming promotes volatilization and diffusion of VOCs. Third, insect feeding is known to modulate the production and release of VOCs through the regulation of gene expression and enzyme production and activity, with higher VOC emissions under greater herbivory pressure5,25. Therefore, synergistic responses may be expected to occur more often in arctic ecosystems, where physiological constraints on plant and insect growth will be reduced with ongoing arctic warming and where plants will face increasing insect herbivory.
We also found higher VOC emissions in July than August, but stronger synergistic effects in August between warming and herbivory (Fig. 1). The higher VOC emissions in July were most likely due to higher light intensities and temperatures, and more active plant growth13. The stronger synergistic interactions observed in August were probably due to a “memory” effect in plant defensive responses, which refers to the capacity of plants to remember past stress events and launch a stronger response to recurrent stress5.
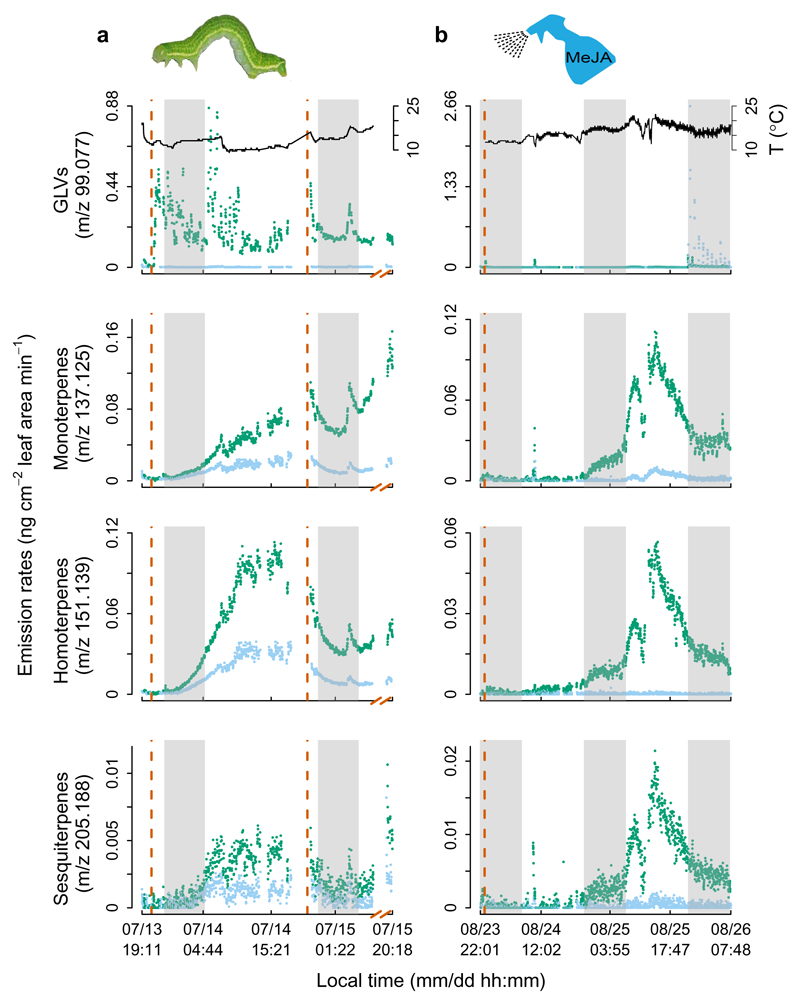
To evaluate whether MeJA application mimics the effects of insect herbivory on VOC release from B. nana, we subjected detached shoots to MeJA or feeding damage by E. autumnata larvae, and tracked VOC emissions in real time using high-resolution proton-transfer-reaction time-of-flight mass spectrometry. We conducted these experiments under semi-controlled field conditions with relatively constant light but varying air temperature and humidity. We found that both larval feeding and MeJA application induced emission of several classes of compounds that stem from different biosynthetic pathways and exhibit different emission dynamics (Fig. 3; Supplementary Figs. 4 and 5). Emissions of GLVs began immediately after herbivore damage and dropped rapidly after larval feeding stopped (Fig. 3a). Unlike herbivore damage, the MeJA treatment did not elicit GLV release (Fig. 3b), which is consistent with the fact that GLVs are typically emitted after physical leaf damage through enzymatic degradation of membrane lipids8,15.
Fig. 3. Time course of VOC induction.
a, VOC emissions induced after real herbivory by Epirrita autumnata larvae. b, VOC emissions induced upon mimicked herbivory by exogenous application of methyl jasmonate (MeJA). Shown are the temporal emission dynamics of GLVs, monoterpenes, homoterpenes and sesquiterpenes from measurements on a single B. nana individual, representing well the four measured replicates (Supplementary Figs. 4 and 5). Dots in light blue and dark green depict control and herbivory treatments, respectively. The dashed brown vertical lines depict the start and end time of the treatments, and the grey shaded areas indicate the time period when lights were turned off. Air temperatures (T) inside the shoot enclosures are shown with black lines.
Emissions of monoterpenes, homoterpenes, and the benzenoid, methyl salicylate (MeSA), did not follow insect feeding activity, but increased gradually and peaked 15–24 hours after herbivore damage (Fig. 3a; Supplementary Fig. 4). During peak induction, the amounts of monoterpenes and homoterpenes released by damaged twigs were approximately four times higher than those from undamaged controls. A similar induction pattern was found when MeJA was applied (Fig. 3b; Supplementary Fig. 5). Herbivore damage did not consistently impact sesquiterpene emissions across the four independent measurements (Supplementary Fig. 4). This disagrees with most studies that typically show a significantly elevated sesquiterpene emission within hours to days after herbivory, followed by a slow decline after its termination5,8. The lack of response we observed was likely due to the low air temperature (15.0 °C) and light intensity (50 µmol m-2 s-1) during measurements. However, the MeJA application, which was performed at a higher light level (120 µmol m-2 s-1) and during a warmer period (21.5 °C), induced sesquiterpene emissions in a similar manner to monoterpene and homoterpene induction (Supplementary Fig. 5).
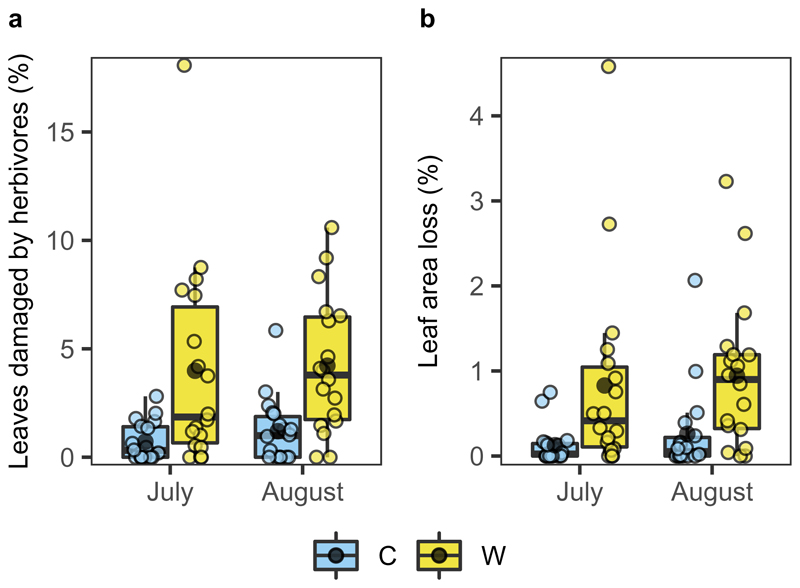
To assess whether climate warming increases herbivory pressure on plants, we surveyed foliar damage (measured as percentage leaf damage and leaf area loss) of B. nana growing in the field experimental plots. We found that in ambient control plots, herbivorous insects damaged 1.2% of the leaves (Fig. 4a) and consumed 0.26% of the total leaf area (Fig. 4b) on average, which agrees with the generally low background loss (approximately 1.4%) reported for woody plant foliage in the Arctic26,27. However, in experimentally warmed plots, the percentage of leaves damaged and the leaf area lost increased to 4.2% and 0.94%, respectively, a 4-fold increase relative to controls (Fig. 4). We also observed that the number of leaf rollers per plot and the percentage of dead leaves caused by them were both approximately 200-fold higher in warmed than control plots (Supplementary Fig. 6), providing further support that rising temperatures are likely to increase herbivory damage. Furthermore, a recent study examining background invertebrate herbivory on a Betula glandulosa-nana complex also revealed a positive correlation between insect herbivory and temperature26. The increased herbivory damage observed in OTCs stem most likely from the temperature-driven rise in insect metabolic rates, overwinter survival and population growth, all of which have been shown to increase the insect consumption rate and thus, foliage damage in cold regions18,20. However, the benign microenvironment created by OTCs, such as increased air humidity and reduced wind, may have also facilitated the establishment of insect herbivores such as leaf rollers and thereby led to enhanced leaf damage.
Fig. 4. Impacts of in situ warming on background insect herbivory.
a, Percentage of leaves damaged by naturally occurring insect herbivores in July and August. b, Percentage of leaf area consumed in July and August. Light blue and yellow bars represent control (C) and experimentally warmed (W) plots, respectively. The box and whisker plots show the median, the 25th and 75th percentiles (boxes) and the 10th and 90th percentiles (error bars), with the mean shown with a black dot. Warming (n = 18, P < 0.05) significantly enhanced herbivory damage according to linear mixed-effects models.
Our results reveal that warming and insect herbivory in an arctic tundra ecosystem interact synergistically to alter plant VOC emissions, both quantitatively and qualitatively. Plant VOCs convey information of plant physiological status and when plants are under attack by herbivores, provide specific information of the identity of the attacking herbivore2,5. Several compounds observed here, including the monoterpenes, linalool and (E)-β-ocimene, the homoterpene, (E)-DMNT [(3E)-4,8-dimethyl-1,3,7-nonatriene], the benzenoid, MeSA, and the GLV, (Z)-3-hexenyl acetate, have been shown to be either attractive or repellent to herbivorous, predatory, or parasitic insects, which may all use VOCs as olfactory cues for locating their required food, prey, or hosts2,5,8,28. Thus, increased VOC emission rates driven by warming, herbivory, and/or their interaction may enhance the detectability of plants by their associated community members; altered relative proportions of individual compounds may affect the reliability or fidelity of the whole VOC blend. Furthermore, enhanced VOC emission rates may protect plants from the oxidative stress caused by insect feeding by maintaining the integrity and fluidity of cell membranes. Some VOCs, especially terpenoids, have been documented to function as antioxidants in plant defences against oxidative stress6,7.
Many stress-induced VOCs, such as sesquiterpenes and MeSA, are more reactive and have a stronger SOA-forming potential than constitutive VOCs, such as α-pinene29. Enhanced emission of these compounds during the periods of active insect feeding will promote SOA formation and growth9,17,29, which may subsequently lead to a local or regional cooling effect30. Moreover, GLVs and isoprene suppress SOA formation by depleting OH radicals29 and as the emissions of other compounds than these were increased more by warming and herbivory, the SOA-mediated cooling effects can be further promoted. Our study shows that climate warming may substantially increase the emission of climate-relevant VOCs by increasing the severity of background herbivory and the herbivore-induced emissions.
Methods
Experimental site
This study took place in a wet arctic tundra heath located in Abisko, northern Sweden (68°21’N, 18°49’E, elevation 385 m). Mean annual temperature is around 0.2°C (1986–2015 average)31, with July and February being the warmest (11.9°C) and coldest (-10.0°C) months, respectively. Mean annual precipitation is 370 mm (1986-2015 average)31. Snowmelt occurs from late April to mid-May, and the snow-free period typically lasts from late May to early October. From early June to mid-July, daylight persists for 24 hours. The vascular plant growing season is from early June to late August. The heath vegetation is composed of evergreen and deciduous dwarf shrubs, graminoids, forbs, mosses, and lichens.
The manipulation experiment simulating climate change-induced summer warming and increased leaf litter fall was established on the heath in 1999 and has been maintained since, i.e. 18 years before the current study began (thereafter called long-term warming). The experimental set-up consisted of 24 field plots (1.2 × 1.2 m) including four treatments: control, warming, litter addition, and their combination in a factorial design, each replicated in six blocks (Supplementary Fig. 7). The warming plots were covered by dome-shaped, open-top chambers (OTCs) made of clear plastic, which were erected yearly in mid-Jun and removed in late August. The litter addition plots received 90 g m-2 air-dried mountain birch (Betula pubescens ssp. tortuosa) litter yearly in late August or early September, which corresponds to the annual leaf litter fall of the nearby mountain birch forest. Since 2009, another six warming plots have been maintained in the six blocks similarly to the long-term warming plots, yielding a treatment with 8 years of warming (thereafter called medium-term warming). In July 2017, six new control plots were paired with the 8-year warming plots. Thus, the experiment consisted of 24 plots for the long-term warming manipulation and 12 later added plots for the medium-term warming manipulation.
The deciduous shrub Betula nana L. was present in all plots. B. nana is one of the dominant circumpolar Arctic shrubs that play a major role in the ‘greening of the Arctic’ and its growth has been shown to respond positively to experimental warming24.
Impacts of experimental warming and mimicked herbivory on Betula nana VOC emissions
VOC emissions were measured twice (July 17–24 and August 23–30) over the growing season in 2017. On July 17, two twigs with no visible signs of insect damage or disease, each being 19.8 ± 1.5 cm in length (mean ± standard error of mean, n = 72) and carrying 76.4 ± 2.5 leaves, were labelled in each plot. The two twigs in each plot were randomly assigned to mimicked herbivory or control treatments. This resulted in a split-plot experimental design with warming and litter addition as the whole plot factor and mimicked herbivory as the subplot factor. Mimicked insect herbivory was achieved by using methyl jasmonate (MeJA), a volatile plant hormone known to induce changes in plant defensive chemistry in the absence of any actual herbivory25. To apply MeJA treatments and minimize cross-contamination to neighbouring plants, each twig was enclosed into a polyethylene terephthalate (PET) bag and one spray of approximately 750 µl of 1mM MeJA dissolved in water was applied to the twig with a spray bottle. Control twigs were sprayed with water in the same way. Immediately after the spray application, the PET bag was removed. The spray treatments continued for three consecutive days, with one spray per day, after which VOC collections were conducted.
VOCs were collected using a push-pull headspace sampling system32. In brief, pre-cleaned (120 °C for 1 h) disposable PET bags (25 × 38 cm; Rul-Let, Abena A/S, Aabenraa) were fastened to the stems with wire tags to enclose the twigs. Air was circulated through the bags by battery-operated pumps connected via Teflon tubes. The incoming air was purified by an activated charcoal filter to remove particles and VOCs, and a copper tube coated with potassium iodide to scrub for ozone. Air was pumped out of the bags through stainless steel adsorbent cartridges filled with 150 mg of Tenax TA and 200 mg of Carbograph 1TD (Markes International, Llantrisant, UK) at 200 ml min-1. Before each measurement, the bags were ventilated for at least five minutes with an inflow rate of 1,000 ml min-1, and during measurements the inflow was set to 300 ml min-1. VOC collection onto each adsorbent cartridge lasted for 30 min. VOCs from control and MeJA-sprayed plants were sampled concurrently whilst sampling from control and warmed plots was staggered by about 17 min. VOC sampling from plots assigned to the long- and medium-term warming manipulation (48 and 24 plant samples, respectively) was done on two different days. Blank samples were collected from empty PET bags in situ. In total, 72 plant samples and six blank samples were taken. Temperature and humidity inside the bags were measured during collection using data loggers (DS1923, iButton Hygrochron, Maxim Integrated, San Jose, CA, USA). Photosynthetically active radiation (PAR) was recorded with a PAR sensor that was enclosed in a PET bag and positioned at a similar height as the measured twigs.
To express VOC emissions per leaf area, the total leaf area of each sampled twig was non-destructively estimated as destructive sampling in the long-term experiment was to be avoided. One large leaf (0.886 cm2) and one small leaf (0.518 cm2) were chosen as the reference leaves to reflect the most common leaf sizes. The total number of large and small leaves in the sampled twigs were counted. To compare the estimated and the actual leaf areas, 31 twigs were selected outside the experimental plots, leaf area was first estimated in situ based on the two reference leaves, and then the twigs were harvested for destructive leaf area measurement. All leaves were scanned and the total leaf area was calculated using Image J. A strong linear correlation was found between the predicted and measured leaf area (measured = 0.9808*predicted, r2 = 0.985; Supplementary Fig. 8a).
In August, the spray treatments, VOC collections, and the leaf area estimations were repeated on the same twigs and in the same manner as in July. Afterwards, the leaves were removed from each twig, scanned to determine leaf area and oven-dried at 60°C for three days. Again, a strong linear correlation was observed between the predicted and measured leaf area (measured = 1.004*predicted, r2 = 0.981; Supplementary Fig. 8b).
VOC samples were analysed by gas chromatography coupled with mass spectrometry (GC-MS, Agilent 7890A GC and 5975C VL MSD; New York, USA). Trapped compounds were thermally desorbed (UNITY2; Markes International, Llantrisant, UK) at 250°C for 10 min, cryofocused at -10°C and injected onto an HP-5 capillary column (50 m × 0.2 mm; film thickness 0.5 µm) with helium as a carrier gas. The column temperature was held at 40°C for 1 min, then ramped at 5°C min-1 to 210°C, and ramped again at 20°C min-1 to 250°C. Chromatograms were analysed using PARADISe33. Individual VOCs were tentatively identified by comparing mass spectra with those in NIST 2014 mass spectral library, verified by chromatography with authentic standards where available, and quantified based on external calibration curves generated with authentic standards. The actual emission rates of individual compounds are expressed in ng cm-2 leaf area h-1 following blank subtraction. In addition, the actual emission rates of monoterpenes, sesquiterpenes, and homoterpenes were standardised to a temperature of 30°C, but not to a fixed PAR level due to relatively little available information on the light dependence of these compounds. Algorithms and formulae developed by Guenther et al.34 were used for the temperature standardization. Since the normalised data showed similar treatment responses as the actual emission data, only the actual emission data are presented here.
VOC emission dynamics of Betula nana in response to real and mimicked insect herbivory
The temporal evolution of Betula nana VOC emissions in response to insect herbivory by the autumnal moth larvae (Epirrita autumnata) was monitored in real time using high-resolution proton-transfer-reaction time-of-flight mass spectrometer (PTR-ToF-MS) (PTR-TOF 1000 ultra, Ionicon Analytik, Innsbruck, Austria), which was placed in a cabin at the experimental site. The experiment was performed in a semi-controlled environment, with constant dim light (about 50 µmol m-2 s-1) during the day but varying ambient temperature and humidity monitored by iButton Hygrochrons (DS1923, Maxim Integrated, San Jose, CA, USA). E. autumnata larvae were collected in a nearby mountain birch forest (68º18' N, 19º12' E) on July 12 and reared on mountain birch leaves on petri dishes during the experimental period. It should be noted that the effects of larval feeding are likely to represent a conservative estimate as most larvae were either approaching pupation or had been naturally parasitized, and did not therefore feed actively (personal observation).
Detached twigs of B. nana growing in the vicinity of the experimental site but outside of the warming treatment plots were used. To minimize the potential genetic and environmental effects on VOC emissions, we chose two adjacent twigs of similar size that were positioned on the same branch and did not exhibit visible damage by natural herbivores or diseases. Each twig was cut under water and immediately inserted in a 50-ml Erlenmeyer flask filled with 30 ml water. The two twigs were then kept inside the PTR-ToF-MS cabin for 1-2 hours before VOC measurements. Polyethylene terephthalate drinking cups (500 ml) were used as enclosure cuvettes35 (Supplementary Fig. 9). A cup was placed upside down over the distal end of each 15-cm-long twig. The opening of the cup was covered with an aluminium foil lid. Care was taken so that the twig was not wounded during fixation. A constant stream of zero air (500 ml min-1) (Parker® ChromGas® Zero Air Generator) was channelled into each cup, so a complete exchange of the air within the cup was achieved in one minute. The outlets of the two cups were directly connected to the inlet of PTR-ToF-MS through an automated three-way valve (Swagelok, Inc.) switching system that alternated between the cups every 10 min. All tubing was made of PTFE and air flow rates were controlled by mass flow controllers. Within 2 hours after the start of VOC measurements, one of the two twigs was randomly assigned to the herbivory treatment in which three E. autumnata larvae were added onto the twig via partly opening the aluminium foil seal. The other twig was left as a control. Thereafter, VOC emissions were continuously recorded for a minimum of 30 hours. After measurements, the leaves were scanned to determine total leaf area and leaf area consumed by E. autumnata larvae, and then oven-dried at 60°C for three days to determine the dry mass.
Measurements were repeated four times during July 13–21 using new pairs of twigs from new independent plants, new larvae, and cups each time (see Supplementary Table 4 for the detailed timeline of each replicate). Owing to the inactivity of larvae, three more larvae were added to replicates 2 and 3, and six larvae were used for replicate 4. Each measurement round started with a background measurement of the empty cup for 40 min. In addition, a blank with an empty cup was measured at least twice during each measurement round.
To investigate whether the exogenous application of MeJA used in the field experiment affects B. nana VOC emissions in a similar manner to insect herbivory, the temporal variation of VOC emissions in response to MeJA application was also monitored using PTR-ToF-MS. The experiment was conducted from August 23–September 1 (Supplementary Table 4) in the same way as described above for the real herbivory experiment, except that the twigs received MeJA spray rather than real insects and that light intensity of about 120 µmol m-2 s-1 was used. Two continuous sprays (750 µl each) of 1mM MeJA dissolved in water were applied to the MeJA twig using a spray bottle while the control twig received two sprays of water. Measurements were repeated four times with new plants and cups.
The PTR-ToF-MS was operated at 2.3 mbar drift tube pressure, 550 V drift tube voltage, and 60 °C drift tube temperature, resulting in a field density ratio (E/N) of about 120 Td. Compounds up to m/z of 280 Da were monitored at 0.2 Hz temporal resolution. The signals were corrected for instrumental transmission coefficients before, during, and after the experiments using a gas standard (about 1ppmv; Ionicon Analytik, Innsbruck, Austria). A detailed description about the PTR-ToF-MS is available in Graus et al.36. The data were analysed using PTRwid software37. PTRwid was used to detect and identify mass peaks in the measurement spectrum, to calibrate the mass scale automatically, to convert the count rates of the detected compounds to VOC mixing ratios, and finally to provide output files with VOC mixing ratios of all detected mass peaks37. VOC concentrations in blank samples were subtracted from those in branch samples. In this study we reported six ion peaks corresponding to methanol (m/z 33), isoprene (m/z 69), the GLV hexenal (m/z 99), monoterpenes (m/z 137), homoterpenes (m/z 151), methyl salicylate (m/z 153) and sesquiterpenes (m/z 205) (Supplementary Table 5).
Insect herbivory survey
To assess whether experimental warming alters background insect herbivory, we surveyed foliar damage of B. nana on July 24 and August 28, corresponding to the peak and late growing season. Three shoots (50-100 leaves) were randomly selected and leaf area loss on each shoot was visually inspected following a widely used methodology38, with slight modifications. We only considered damage caused by external leaf feeders (chewing or skeletonization) since they were the primary insect herbivores found in the experimental plots. Total number and the number of damaged leaves were counted. Each of the damaged leaves was assigned to one of the following damage classes according to the proportion of leaf area lost: 0.01–1, 1–5, 5–10, 10–30, 30–50, 50–75 and 75–100%. The total leaf area loss was calculated by first multiplying the number of leaves in each damage class by the medium value of the class (i.e., 0.5% for the damage class 0.01-1%) and then summing up the obtained values for all damage classes. Then, we counted the number of dead leaves on these shoots caused by leaf rollers, which were the dominant external leaf feeders (personal observation). In addition, we counted the number of leaf rollers in each plot.
Statistical analyses
All statistical analyses were performed in R39. We first performed a univariate linear mixed effects analysis using the lme function in the package nlme, assigning the actual emission rates of individual VOC compounds or compound classes as response variables. Warming, warming duration (8 vs. 18-year warming manipulation), mimicked herbivory (MeJA application), sampling time, and their interactions were treated as fixed effects; plants nested within plots nested within blocks as random effects. Additionally, qqPlots, Shapiro–Wilk normality tests, and Levene’s tests were conducted before performing linear mixed effects analysis to evaluate the assumption of normality and homogeneity of variance. Owing to the violation of assumptions of normality and homogeneity of VOC data, a log(X+1) transformation was applied to all VOC data to adjust for the rightward skew. Full models were fitted for each VOC and VOC class. Leaf herbivory data were analysed with warming, warming duration, sampling time, and their interactions considered as fixed effects and plants nested within plots nested within blocks as random effects. To account for the possibility of considerable bias arising from data transformation, we also used the Kruskal-Wallis tests, a non-parametric method to assess the overall treatment effects (i.e., warming, herbivory, and their combination), followed by pairwise Wilcoxon-Mann-Whitney tests with P-values adjusted using the Benjamini and Hochberg method. The nonparametric tests were performed separately for data collected in July and August. In general, the two statistical approaches were found to produce similar results (Supplementary Tables 2 and 3).
The initial models also included fixed effect of litter addition, but these were omitted as we found no main effects of litter addition, nor warming × litter addition interactions, on VOC emissions (Supplementary Fig. 10; Supplementary Table 6). There was a significant interactive effect only between litter addition and mimicked herbivory on emissions of total sesquiterpenes and a few individual sesquiterpenes. Therefore, we pooled the data across litter treatments to focus on the effects of warming and mimicked herbivory on VOC emissions.
We further employed redundancy analysis (RDA), a constrained multivariate approach using the rda function within the vegan package40, to quantify and visualize how VOC blends responded to warming and mimicked herbivory. VOC blends were expressed as the percentage of individual compound (or compound class) emission of the total VOC emission. We then used permutation analyses that generate pseudo-F values (MVA.synt and MVA.anova, and pairwise.factorfit commands in the RVAideMemoire package) to evaluate the model significance as well as the significance of treatments41. Visualization is similar to Principal Component Analysis (PCA), but the first canonical axes are constrained only to represent the variation explained by the linear predictors in the model (here, warming, mimicked herbivory). VOC blend data were log-transformed to conform to the assumption of multivariate normality and standardised by scaling to a variance of 1.
Supplementary Material
Supplementary Information is available in the online version of the paper.
Acknowledgements
We thank Miika Jylkkä for providing the image of Epirrita autumnata and Cleo Lisa Davie-Martin for language editing. We gratefully acknowledge financial support from the Danish Council for Independent Research | Natural Sciences, the Danish National Research Foundation (CENPERM DNRF100), the Marie Sklodowska-Curie grant (agreement No. 751684), and the European Research Council (ERC, grant agreement No.771012) under the European Union’s Horizon 2020 research and innovation programme. The Abisko Scientific Research Station (Sweden) is thanked for housing and logistics support.
Footnotes
Data availability All VOC and background herbivory data that support the findings of this study are available in Figshare with the data DOI identifier: 10.6084/m9.figshare.7879340.
Author contributions
T.L. and R.R. designed the experiment. A.M. established the experimental site. T.L. and T.H. developed the methodology for the volatile emission time course experiments. T.L. collected, analysed and interpreted the data, and wrote the manuscript with contributions from all authors.
Competing interests
The authors declare no competing interests.
References
- 1.Jokinen T, et al. Production of extremely low volatile organic compounds from biogenic emissions: Measured yields and atmospheric implications. Proceedings of the National Academy of Sciences. 2015;112:7123–7128. doi: 10.1073/pnas.1423977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turlings TCJ, Erb M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annual Review of Entomology. 2018;63:433–452. doi: 10.1146/annurev-ento-020117-043507. [DOI] [PubMed] [Google Scholar]
- 3.Kramshoj M, et al. Large increases in Arctic biogenic volatile emissions are a direct effect of warming. Nature Geoscience. 2016;9:349–352. [Google Scholar]
- 4.Kesselmeier J, et al. Volatile organic compound emissions in relation to plant carbon fixation and the terrestrial carbon budget. Global Biogeochemical Cycles. 2002;16:73-71–73-79. [Google Scholar]
- 5.Dicke M, van Loon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nature Chemical Biology. 2009;5:317–324. doi: 10.1038/nchembio.169. [DOI] [PubMed] [Google Scholar]
- 6.Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature Chemical Biology. 2009;5:283. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- 7.Peñuelas J, Staudt M. BVOCs and global change. Trends in Plant Science. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Kessler A, Baldwin IT. Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 9.Holopainen JK, Kivimäenpää M, Nizkorodov SA. Plant-derived Secondary Organic Material in the Air and Ecosystems. Trends in Plant Science. 2017;22:744–753. doi: 10.1016/j.tplants.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Ehn M, et al. A large source of low-volatility secondary organic aerosol. Nature. 2014;506:476–479. doi: 10.1038/nature13032. [DOI] [PubMed] [Google Scholar]
- 11.Allen MR, et al. Climate Change 2014 Synthesis Report: Summary for policymakers. 2014. [Google Scholar]
- 12.Potosnak MJ, et al. Isoprene emissions from a tundra ecosystem. Biogeosciences. 2013;10:871–889. [Google Scholar]
- 13.Valolahti H, Kivimäenpää M, Faubert P, Michelsen A, Rinnan R. Climate change-induced vegetation change as a driver of increased subarctic biogenic volatile organic compound emissions. Global Change Biology. 2015;21:3478–3488. doi: 10.1111/gcb.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang J, Valolahti H, Kivimäenpää M, Michelsen A, Rinnan R. Acclimation of Biogenic Volatile Organic Compound Emission From Subarctic Heath Under Long-Term Moderate Warming. Journal of Geophysical Research: Biogeosciences. 2018;123:95–105. [Google Scholar]
- 15.Ameye M, et al. Green leaf volatile production by plants: a meta-analysis. New Phytologist. 2018;220:666–683. doi: 10.1111/nph.14671. [DOI] [PubMed] [Google Scholar]
- 16.Rowen E, Kaplan I. Eco-evolutionary factors drive induced plant volatiles: a meta-analysis. New Phytologist. 2016;210:284–294. doi: 10.1111/nph.13804. [DOI] [PubMed] [Google Scholar]
- 17.Bergström R, Hallquist M, Simpson D, Wildt J, Mentel TF. Biotic stress: a significant contributor to organic aerosol in Europe? Atmos Chem Phys. 2014;14:13643–13660. [Google Scholar]
- 18.Deutsch CA, et al. Increase in crop losses to insect pests in a warming climate. Science. 2018;361:916–919. doi: 10.1126/science.aat3466. [DOI] [PubMed] [Google Scholar]
- 19.Post E, et al. Ecological Dynamics Across the Arctic Associated with Recent Climate Change. Science. 2009;325:1355–1358. doi: 10.1126/science.1173113. [DOI] [PubMed] [Google Scholar]
- 20.Lesk C, Coffel E, D’Amato AW, Dodds K, Horton R. Threats to North American forests from southern pine beetle with warming winters. Nature Climate Change. 2017;7:713. doi: 10.1038/nclimate3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrio IC, Bueno CG, Hik DS. Warming the tundra: reciprocal responses of invertebrate herbivores and plants. Oikos. 2016;125:20–28. doi: 10.1111/oik.02190. [DOI] [Google Scholar]
- 22.Jepsen JU, et al. Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub-Arctic birch. Global Change Biology. 2011;17:2071–2083. [Google Scholar]
- 23.Sistla SA, et al. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature. 2013;497:615. doi: 10.1038/nature12129. [DOI] [PubMed] [Google Scholar]
- 24.Hollesen J, et al. Winter warming as an important co-driver for Betula nana growth in western Greenland during the past century. Global change biology. 2015;21:2410–2423. doi: 10.1111/gcb.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuman MC, Heinzel N, Gaquerel E, Svatos A, Baldwin IT. Polymorphism in jasmonate signaling partially accounts for the variety of volatiles produced by Nicotiana attenuata plants in a native population. New Phytologist. 2009;183:1134–1148. doi: 10.1111/j.1469-8137.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- 26.Barrio IC, et al. Background invertebrate herbivory on dwarf birch (Betula glandulosa-nana complex) increases with temperature and precipitation across the tundra biome. Polar Biology. 2017;40:2265–2278. [Google Scholar]
- 27.Kozlov MV, Lanta V, Zverev V, Zvereva EL. Global patterns in background losses of woody plant foliage to insects. Global Ecology and Biogeography. 2015;24:1126–1135. [Google Scholar]
- 28.Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. Shared signals –‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecology Letters. 2008;11:24–34. doi: 10.1111/j.1461-0248.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 29.Mentel TF, et al. Secondary aerosol formation from stress-induced biogenic emissions and possible climate feedbacks. Atmos Chem Phys. 2013;13:8755–8770. [Google Scholar]
- 30.Paasonen P, et al. Warming-induced increase in aerosol number concentration likely to moderate climate change. Nature Geoscience. 2013;6:438–442. [Google Scholar]
- 31.Station ASR. Abisko Scientific Research Station, Polarforskningssekretariatet; 2015. [Google Scholar]
- 32.Tholl D, et al. Practical approaches to plant volatile analysis. The Plant Journal. 2006;45:540–560. doi: 10.1111/j.1365-313X.2005.02612.x. [DOI] [PubMed] [Google Scholar]
- 33.Johnsen LG, Skou PB, Khakimov B, Bro R. Gas chromatography – mass spectrometry data processing made easy. Journal of Chromatography A. 2017;1503:57–64. doi: 10.1016/j.chroma.2017.04.052. [DOI] [PubMed] [Google Scholar]
- 34.Guenther AB, Zimmerman PR, Harley PC, Monson RK, Fall R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. Journal of Geophysical Research: Atmospheres. 1993;98:12609–12617. [Google Scholar]
- 35.Schuman MC, Allmann S, Baldwin IT. Plant defense phenotypes determine the consequences of volatile emission for individuals and neighbors. eLife. 2015;4:e04490. doi: 10.7554/eLife.04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graus M, Müller M, Hansel A. High resolution PTR-TOF: Quantification and formula confirmation of VOC in real time. Journal of the American Society for Mass Spectrometry. 2010;21:1037–1044. doi: 10.1016/j.jasms.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Holzinger R. PTRwid: A new widget tool for processing PTR-TOF-MS data. Atmos Meas Tech. 2015;8:3903–3922. [Google Scholar]
- 38.Kozlov MV, Filippov BY, Zubrij NA, Zverev V. Abrupt changes in invertebrate herbivory on woody plants at the forest–tundra ecotone. Polar Biology. 2015;38:967–974. [Google Scholar]
- 39.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2009. [Google Scholar]
- 40.Oksanen JF, et al. vegan: Community ecology package. R package version 2.0-5. 2012. [Google Scholar]
- 41.Brückner A, Heethoff M. A chemo-ecologists’ practical guide to compositional data analysis. Chemoecology. 2017;27:33–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.