Abstract
Respiratory frequency plasticity is a long-lasting increase in breathing frequency due to a perturbation. Mechanisms underlying respiratory frequency are poorly understood, and there is little evidence of frequency plasticity in neonates. This hybrid review/research article discusses available literature regarding frequency plasticity and highlights potential research opportunities. Also, we include data demonstrating a model of frequency plasticity using isolated neonatal rat brainstem-spinal cord preparations. Specifically, substance P (SubP) application induced a long-lasting (> 60 min) increase in spontaneous respiratory motor burst frequency, particularly in brainstem-spinal cords with the pons attached; there were no male/female differences. SubP-induced frequency plasticity is dependent on the application pattern, such that intermittent (rather than sustained) SubP applications induce more frequency plasticity. SubP-induced frequency plasticity was blocked by a neurokinin-1 receptor antagonist. Thus, the newborn rat respiratory control system has the capacity to express frequency plasticity. Identifying mechanisms that induce frequency plasticity may lead to novel methods to safely treat breathing disorders in premature and newborn infants.
Keywords: Neuroplasticity, Frequency plasticity, Neonatal, Respiratory, Development, Substance P, Brain stem, Spinal cord
1. Introduction
The respiratory control system in newborn mammals needs to be responsive to ongoing developmental changes in respiratory mechanics and capable of altering breathing during chemosensory challenges immediately at birth (Greer, 2012). Respiratory neuroplasticity, which is defined as a long-lasting change in breathing due to experience (Mitchell and Johnson, 2003; Fuller and Mitchell, 2017), may play a role in maintaining blood-gas homeostasis during early development. With respect to respiratory neurobiology, it is important to identify specific neuroplasticity phenotypes, determine the optimal stimuli that induce the neuroplasticity, characterize underlying cellular/synaptic mechanisms, and then use that knowledge to develop novel therapies to treat respiratory disease. For example, intermittent-hypoxia induced phrenic long-term facilitation (Millhorn et al., 1980a,b) is induced by intermittent, but not sustained, bouts of hypoxia (Baker and Mitchell, 2000), and details regarding underlying cellular and synaptic mechanisms are well-established and continually updated (Devinney et al., 2013; Xing et al., 2013; Fuller and Mitchell, 2017; Turner et al., 2018). That intermittent hypoxia therapy can augment walking and breathing in spinally injured humans (Gonzalez-Rothi et al., 2015; Fuller and Mitchell, 2017) is a testament to the translational importance of identifying mechanisms of neuroplasticity in basic science studies using rodent models.
Breathing in newborn humans, especially pre-term infants, is marked by irregular rhythm, variable amplitude, and impaired chemosensitivity (Greer, 2012). Common diseases of newborns include apnea of prematurity, congenital hypoventilation, bronchopulmonary dysplasia, respiratory distress syndrome, pneumothorax, and persistent pulmonary hypertension, disorders resulting in long-term developmental deficits and pathology (Gallacher et al., 2016; Patel, 2016). Newborns are also susceptible to infection, which can further disturb irregular and otherwise normal breathing patterns, ultimately leading to death (Chan et al., 2015). Since neonatal inflammation augments apneas and hypopneas in extremely preterm infants compared with premature infants without infection (Hofstetter et al., 2008), there may be a synergistic negative effect of neonatal inflammation on the developing respiratory circuitry. Finally, persistent deleterious changes in respiratory function and control can occur following in utero exposure to nicotine and ethanol (Keegan et al., 2010; Savran and Ulrik, 2018), suggesting windows of respiratory system susceptibility both before and after birth. In preterm and term infants, these diseases and conditions result in recurring bouts of apnea, irregular breathing rhythm, and hypopnea, all of which contribute to chronic intermittent hypoxemia. Intermittent hypoxemic episodes (oxygen saturation < 80%) in preterm infants are associated with a higher risk of death or disability at 18 months of age (Poets et al., 2015).
Caffeine is the most common and effective methylxanthine used to treat apnea of prematurity (Dobson et al., 2014). Caffeine treatment also decreases the risk of bronchopulmonary dysplasia, the duration of positive airway pressure support, and increases the rate of successful extubations (Dobson and Patel, 2016). Although caffeine treatment reduces apneas, nearly 50% of apneas persist despite adequate therapy (Erenberg et al., 2000). In addition, high caffeine dosages (which are more effective at reducing apneas) in preterm infants increase the incidence of cerebellar hemorrhage two years later, with significant changes in motor performance (McPherson et al., 2015). Thus, there is still a need to improve therapies and lower the risk of deleterious caffeine-induced side effects. Identifying and characterizing models of respiratory neuroplasticity that capitalize on endogenous cellular mechanisms to increase breathing frequency and regularity in preterm and term neonates may not only be beneficial by itself, but also as an effective companion therapy with caffeine. The major goal of this review is to focus on one such form of respiratory neuroplasticity known as “frequency plasticity”, which is a long-lasting increase in breathing frequency following a relatively brief stimulus. To date, there are several examples of respiratory frequency plasticity in animal models, but few studies have examined underlying mechanisms. Thus, a second goal of this review is to present results from preliminary studies using a highly accessible in vitro neonatal rat model of frequency plasticity in which mechanisms can be studied.
1.1. Respiratory frequency plasticity
Although frequency plasticity is expressed in adult, juvenile, and older neonatal rodents, the capacity of the respiratory control system to express frequency plasticity in younger neonates remains poorly understood. In addition, the cellular and synaptic mechanisms underlying respiratory frequency plasticity in neither young, nor adult rodents are known.
1.1.1. Intermittent hypoxia-induced frequency plasticity in adult rats
Intermittent hypoxia induces phrenic long-term facilitation, but it can also induce frequency plasticity. In a meta-analysis covering years of experimental results with intermittent hypoxia exposures in adult rats (Baker-Herman and Mitchell, 2008), the key findings were that: (a) intermittent hypoxia induces frequency plasticity more commonly in unanesthetized rats compared to anesthetized rats; (b) frequency plasticity induction is not pattern-sensitive (intermittent and sustained hypoxic exposures induce frequency plasticity); (c) the magnitude of frequency plasticity is typically only 7–11% above baseline frequency; (d) the magnitude of frequency plasticity indirectly correlates with baseline frequency (frequency plasticity is greater in rats with lower baseline breathing frequencies), and directly correlates with the magnitude of phrenic long-term facilitation; and (e) rats that exhibit a greater hypoxic response express more frequency plasticity. The meta-analysis did not include data related to underlying mechanisms, but the authors hypothesized that substance P or orexin may be involved in frequency plasticity induction (Baker-Herman and Mitchell, 2008). This meta-analysis provides a useful framework for comparing the characteristics of frequency plasticity in younger rodents.
1.1.2. Frequency plasticity in intact or in situ juvenile and neonatal rodents
Awake one- and two-month old rats exposed to either twelve 5-min bouts of 12% oxygen (5-min normoxic intervals) expressed ventilatory long-term facilitation 45 min later that was due mainly to a modest 12–16% increase in breath frequency (McGuire and Ling, 2005). Likewise, five 5-min bouts were sufficient to induce ventilatory long-term facilitation only in the one-month old rats due to small (< 10%) increases in breath frequency and tidal volume (McGuire and Ling, 2005). In younger intact and semi-intact rats, intermittent hypoxic exposures that included CO2 administration induced respiratory frequency plasticity. For example, with urethane anesthetized P14-15 rats, intermittent hypoxia induced a 20–40% increase in breathing frequency above of baseline after 60 min (Reid and Solomon, 2014). Similarly, with semi-intact in situ working-heart brainstem preparations obtained from P15-P25 male rats, intermittent hypoxia exposures (three 5-min bouts with 5-min intervals) were tested at three different hypoxic levels (Tadjalli et al. 2007). At baseline conditions, the solution perfusing the working heart brainstem-spinal cord tissue was equilibrated with 95% O2/5% CO2, and the three hypoxic solutions were either severe (10% O2/5% CO2/85% N2), moderate (20% O2/5% CO2/75% N2), or mild (40% O2/5% CO2/55% N2). 60-min after exposure to intermittent severe, moderate, and mild hypoxia, respiratory frequency above baseline was increased by 40%, 40% and 25%, respectively (Tadjalli et al., 2007). In contrast, continuous 15-min exposures to severe, moderate, or mild hypoxia did not induce frequency plasticity. There were also no long-lasting increases in phrenic motor burst amplitude following intermittent or continuous hypoxic exposures (Tadjalli et al., 2007). Thus, in older neonatal rats, frequency plasticity appears to be pattern-sensitive.
In intact newborn P1, P4, and P10 rats, intermittent hypoxic exposures (ten 60-s bouts of 5% O2 with 2-min of reoxygenation, separated by 5-min normoxic intervals) increased respiratory frequency, tidal volume, and ventilation acutely during hypoxic conditions (Julien et al., 2008). However, long-lasting (> 120 min) increases in ventilation and tidal volume were only observed in the P10 rats (Julien et al., 2008). There was no evidence for respiratory frequency plasticity at any age, nor were sex differences detected. Thus, although there was considerable variability in these experiments with respect to experimental approach (e.g., intact animals versus working heart-brainstem preparations), and intermittent hypoxia protocols (e.g., timing, oxygen level, duration and number of exposures), we conclude that frequency plasticity is difficult to induce with intermittent hypoxia in intact rats less than 10 days old.
1.1.3. Frequency plasticity in neonatal rodents in vitro
From the time of birth to one week of age, frequency plasticity in respiratory-related motor output can also be induced in vitro using isolated brainstem-spinal cords or rhythmically active medullary slices. For example, intermittent bath-application of hypoxic (95% N2/5% CO2; PO2 = ~15 kPa) solution (three 3-min bouts with 5-min intervals of 95% O2/5% CO2) to murine P2 brainstem-spinal cord preparations induced a 34% increase in motor burst frequency after 40 min of washout with normoxic (95% O2/5% CO2) solution (Berner et al., 2007). Likewise, frequency plasticity was observed in rhythmically active medullary slices from P0-P7 mice 90 min following intermittent bath application of hypoxic solution (95% N2/5% CO2; Blitz and Ramirez, 2002). Interestingly, the magnitude of the frequency plasticity appeared to depend on the interval duration between hypoxic solution applications because there was a 40–65% increase in motor burst frequency when three 3-min hypoxic solution exposures were separated by 5-min intervals compared to only a 10–30% increase with 10-min intervals (Blitz and Ramirez, 2002). Frequency plasticity induced in intact rodents and in isolated rodent brainstem-spinal cord or medullary slice preparations is summarized in Table 1.
Table 1.
Frequency plasticity in rodent preparations.
| Age, Species, Preparation | Stimulus | Frequency plasticity | Reference |
|---|---|---|---|
| 1–2 month old awake, intact rats | 12 5-min bouts of 12% oxygen (5-min normoxic intervals) | 12–16% increase in breath frequency at 45 min post-stimulus | McGuire and Ling (2005) |
| 1-month old awake, intact rats | 5 5-min bouts of 12% oxygen (5-min normoxic intervals) | < 10% increase in breath frequency at 45 min post-stimulus | |
| urethane anesthetized P14–15 rats | 40% O2/4% CO2 under urethane anesthesia to obtain baseline breathing data | 20–40% increase in breathing frequency above of baseline after 60 min | Reid and Solomon (2014) |
| 3 5-min bouts of 8% O2/4% CO2 with 5-min intervals of 40% O2/4% CO2 | |||
| semi-intact in situ working-heart brainstem preparations from P15–P25 rats | Baseline conditions, solution equilibrated with 95% O2/5% CO2, | Intermittent severe, moderate, and mild hypoxia increase respiratory frequency above baseline by 40%, 40% and 25%, respectively, 60-min after the exposure | Tadjalli et al. (2007) |
| 3 5-min bouts of hypoxic solution with 5-min intervals at different hypoxic levels: Severe – 10% O2/5% CO2/85% N2 Moderate – 20% O2/5% CO2/75% N2 Mild – 40% O2/5% CO2/55% N2 |
|||
| intact newborn P1, P4, P10 rats | 10 60-s bouts of 5% O2 with 2-min of reoxygenation, separated by 5-min normoxic intervals | No frequency plasticity. Long-lasting (> 120 min) increases in ventilation and tidal volume are observed only in P10 rats | Julien et al. (2008) |
| brainstem-spinal cord preparations from P2 mice | 3 3-min bouts of bath-applied hypoxic solution (95% N2/5% CO2; PO2 = ~15kPa) with 5-min intervals of 95% O2/5% CO2) | 34% increase in motor burst frequency after 40 min of washout with normoxic solution (95% O2/5% CO2) | Berner et al. (2007) |
| rhythmically-active medullary slices from P0–P7 mice | intermittent bath application of hypoxic solution (95% N2/5% CO2) | 40-65% increase in motor burst frequency when 3 3-min hypoxic solution exposures are separated by 5-min intervals compared to only a 10–30% increase with 10-min intervals | Blitz and Ramirez (2002) |
However, the in vitro hypoxia-induced frequency plasticity experiments are difficult to interpret with respect to underlying mechanisms. Within the central nervous system (CNS), hypoxia induces a variety of changes at the molecular, cellular, synaptic, neuronal, and network levels (Pena and Ramirez, 2005) in addition to altering neuronal-glial interactions (Funk et al., 2015), all of which are likely to contribute to frequency plasticity induction. Hypoxia-induced CNS alterations contribute to the overall hypoxic ventilatory response, although the precise role that they play in intact animals is still under investigation (Powell et al., 2009; Solomon, 2000; Solomon et al., 2000; Solomon, 2004, 2005).
1.1.4. Potential role of SubP for frequency plasticity induction
SubP was originally proposed as a candidate mediator of frequency plasticity induction in adult rats (Baker-Herman and Mitchell, 2008). When intermittent bath-application of hypoxic solution was tested on murine P2 brainstem-spinal cord preparations from mice lacking SubP and neurokinin-A peptides (Tac1 gene deleted), no frequency plasticity was observed (Berner et al., 2007). While these data are consistent with the hypothesis that SubP is required for frequency plasticity induction in neonates in vitro, the caveat is whether the result is due to a lack of SubP itself, or an alteration of some other feature of the respiratory control system as a result of SubP and neurokinin-A absence during fetal development. Nevertheless, SubP is a candidate neurotransmitter for respiratory frequency plasticity induction because: (a) SubP is co-localized with serotonin in raphe neurons (Kachidian et al., 1991); (b) caudal raphe neuron activity is increased by carotid chemoafferent nerve activation (Morris et al., 1996); and (c) SubP (and serotonin) are released following raphe obscurus stimulation in medullary slices (Ptak et al., 2009). Thus, SubP may be released intermittently during bouts of intermittent hypoxia, and thereby contribute to frequency plasticity induction. In addition, from a comparative perspective, SubP is also a powerful inducer of frequency plasticity in non-respiratory motor networks. In isolated lamprey spinal cord, bath-applied SubP for 10 min induced a 250% increase in locomotor-related motor bursts that lasted for > 24 h (Parker et al., 1998), suggesting that SubP is an evolutionarily conserved neurotransmitter involved in frequency plasticity of vertebrate rhythmic motor networks.
1.1.5. SubP effects on respiratory motor control in vitro: relation to frequency plasticity
SubP increased respiratory-related burst frequency in vitro in isolated rat and mouse brainstem-spinal cords, with increases in burst regularity (Murakoshi et al., 1985; Yamamoto et al., 1992; Ptak and Hilaire, 1999). Bath-application of high SubP concentrations (1000 nM) decreased respiratory burst frequency in neonatal rat brainstem-spinal cord preparations due to excessive depolarization of respiratory neurons (Sharev et al., 2002). Short duration (< 6 min) and low concentration (0.1–100 nM) SubP applications increased burst frequency, but the effects were readily reversible during the washout period (Yamamoto et al., 1992; Monteau et al., 1996), suggesting that SubP application at these durations, concentrations, and timing are not sufficient to induce frequency plasticity. Another relevant observation is that SubP-induced increases in respiratory burst frequency were larger in brainstem-spinal cord preparations with a lower baseline frequency (Monteau et al., 1996; Ptak and Hilaire, 1999), suggesting that in vitro SubP effects may be related to the status of the respiratory control system prior to SubP applications.
The pharmacology of SubP-dependent effects on respiratory burst frequency are complex, involving interactions with neurokinin-1 (NK1), neurokinin-2 (NK2), and neurokinin-3 (NK3) receptors within the tachykinin receptor family. Increases in respiratory burst frequency were observed with bath-applied selective agonists for NK1, NK2, and NK3 receptors, with selective NK3 receptor agonists increasing respiratory burst frequency as much as SubP in neonatal rat brainstem-spinal cords (Monteau et al., 1996). This suggests that activation of any one of the three neurokinin receptor family members are sufficient to increase burst frequency. In neonatal brainstem-spinal cords from mutant mice lacking NK1 receptors, SubP had no effect on respiratory burst frequency, nor did SubP depolarize phrenic motoneurons (Ptak et al., 2002). Taken together, these data suggest that functional NK1 receptors are necessary for increases in SubP-induced respiratory frequency, but the neurokinin receptors necessary or sufficient for SubP-induced frequency plasticity are not yet known.
1.1.6. Key questions to be addressed experimentally in young neonatal rodents
Intermittent hypoxia-induced frequency plasticity is observed in adult rats and rats as young as P10, but interestingly, not in younger neonatal rodents. Further, frequency plasticity expressed in in vitro brainstem-spinal cords from P2 mice exposed to intermittent hypoxic solutions appear to require endogenous SubP or neurokinin A because brainstem-spinal cords from mutant mice lacking these peptides do not express frequency plasticity (Berner et al., 2007). However, it is not known whether SubP application by itself is sufficient to induce frequency plasticity in neonatal rat isolated brainstem-spinal cord preparations, and if so, which SubP concentration is optimal for inducing frequency plasticity. Likewise, given that continuous hypoxic exposures do not induce frequency plasticity (Tadjalli et al., 2007), does SubP application pattern (intermittent or sustained) matter for frequency plasticity induction? Since the magnitude of the acute SubP-dependent increase in respiratory burst frequency is larger in in vitro brainstem-spinal cords with a lower baseline burst frequency (Monteau et al., 1996; Ptak and Hilaire, 1999), is the magnitude of SubP-induced frequency plasticity also dependent on baseline frequency? Finally, there is growing interest in sex-dependent differences in respiratory neuroplasticity (Bavis and MacFarlane, 2017; Behan and Kinkead, 2011), but there are no studies (to our knowledge) that have investigated sex-dependent differences in respiratory neuroplasticity in newborn rats. Only one study has, so far, tested for potential sex differences in intermittent hypoxia-induced neuroplasticity (tidal volume increase), and none were observed (Julien et al., 2008). Thus, lastly, are there sex-dependent differences in SubP-induced frequency plasticity in vitro?
To address these questions, isolated brainstem-spinal cords from neonatal (P0-P3) rats were used because these in vitro preparations allow precise temporal control of drug application. Specifically, we tested whether: (1) SubP is sufficient to induce frequency plasticity in pons-attached (pons attached) or medullary (pons removed) brainstem-spinal cord preparations; (2) the magnitude of frequency plasticity depends on the pattern of SubP application (i.e., intermittent or sustained); (3) SubP-induced frequency plasticity is differentially expressed in preparations from male and female neonatal rats.
2. Methods
2.1. Electrophysiology experiments
All experimental procedures followed NIH guidelines and this study was approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Neonatal (P0-P3) Sprague-Dawley rats (Charles River, Wilmington, MA, USA) were visually inspected and the genitourinary distance was used to determine the sex of the rat. Neonatal rats (n = 178) were then anesthetized with 5% isoflurane (O2 balance) before being decerebrated. The remaining tissue was placed in ice-cold artificial cerebrospinal fluid (aCSF), composed of (in mM): 120 NaCl, 26 NaHCO3, 20 glucose, 2 MgSO4, 1 CaCl2, 3 KCl, and 1.25 Na2HPO4. Two types of brainstem-spinal cord preparations were tested: medullary brainstem-spinal cords (intact from pontomedullary border to spinal segment C8 (Fig. 1A) and pontine brainstem-spinal cords (intact from collicular-pontine border to spinal segment C8; Fig. 4A). Each brainstem-spinal cord was removed in ice-cold aCSF and pinned down ventral side up in a standard recording chamber (volume = ~0.75 ml).
Fig. 1. SubP-induced changes in respiratory motor bursts produced by medullary neonatal rat brainstem-spinal cords.
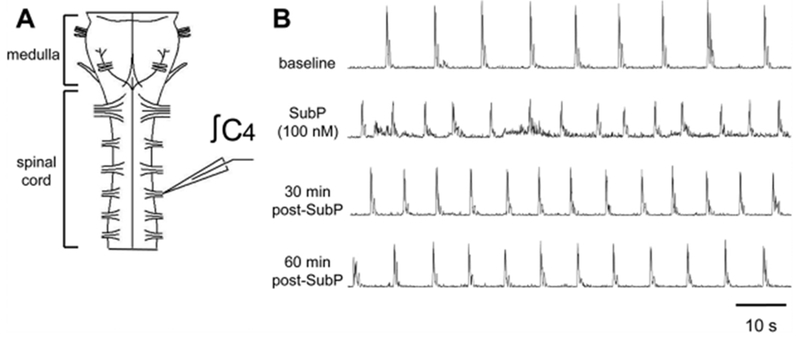
(A) Drawing of isolated neonatal rat medullary brainstem-spinal cord with a suction electrode attached to the cervical spinal C4 ventral root. (B) Voltage traces of respiratory-related motor output are shown at baseline (top trace), during intermittent 100 nM SubP application (2nd trace), and at 30 and 60 min post-drug application (3rd and 4th traces, respectively). Intermittent 100 nM SubP application induced a long-lasting increase in respiratory burst frequency.
Fig. 4. SubP-induced changes in respiratory motor bursts produced by pontine neonatal rat brainstem-spinal cords.
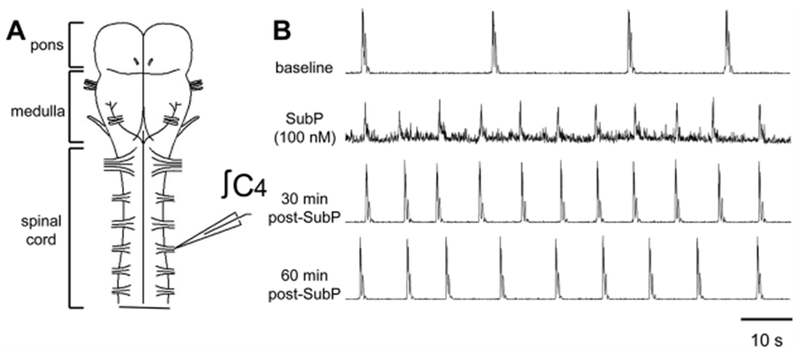
(A) Drawing of isolated neonatal rat pontine brainstem-spinal cord with a suction electrode attached to the cervical spinal C4 ventral root. (B) Voltage traces of respiratory-related motor output are shown at baseline (top trace), during intermittent 100 nM SubP application (2nd trace), and at 30 and 60 min post-drug application (3rd and 4th traces, respectively). Intermittent 100 nM SubP application induced a long-lasting increase in respiratory burst frequency.
Isolated brainstem-spinal cords were continuously bathed with oxygenated aCSF solution (26–27 °C, aerated with 5% CO2 and 95% O2, pH = ~7.4) at a flow rate of 6–8 ml/min. The bath volume and flow rate were designed to facilitate rapid solution turnover in the bath to enable testing intermittent patterns of drug application. Brainstem-spinal cords were allowed to equilibrate for 40–65 min before recording baseline data and initiating an experimental protocol. Spontaneously produced respiratory motor output was recorded by attaching glass suction electrodes (1.0 mm glass tube pulled on an electrode puller with the tip broken off and then fire polished) to ventral cervical (C4-C5; phrenic motoneurons) nerve roots. Signals were acquired at 50 Hz, amplified (1000–10,000 ×), and band-pass filtered (0.1–500 Hz) using a differential AC amplifier (model 1700, A-M Systems, Everett, WA, USA) before being rectified and integrated (time constant = 50 ms) using a moving averager (MA-821/RSP, CWE, Inc., Ardmore, PA, USA; Fig. 1B). Data were collected using Axoscope hardware and software (Molecular Devices, Sunnyvale, CA, USA). SubP (1.0, 10, 100 nM) was obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA), and the neurokinin-1 receptor antagonist L760735 (5-[[(2R,3S)-2-[(1R)-1-[3,5-Bis(tri-fluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl-N,N-dimethyl-1H-1,2,3-triazole-4-methanamine hydrochloride; 10 μM) was obtained from R&D Systems (Minneapolis, MN, USA), dissolved in water and frozen in aliquots (SubP = 1.0 ml; L760735 = 0.15 ml).
2.2. Data analysis
Voltage traces of spinal respiratory motor output or “bursts” were analyzed using Clampfit software (Molecular Devices, Sunnyvale, CA, USA). Respiratory motor burst amplitude was measured at the peak of integrated nerve discharge and normalized to baseline. Data were averaged into 5-min bins and reported as mean ± SEM. Since our goal was to examine long-lasting effects of drug application, statistical comparisons were only performed on pooled data at 30 and 60 min after drug application. Accordingly, a two-way repeated measures ANOVA was performed with post-hoc comparisons using the Student-Newman-Keuls test in Sigma Stat software (Jandel Scientific Software, San Rafael, CA, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. SubP-induced frequency plasticity in medullary brainstem-spinal cords
To quantify time-dependent changes in respiratory burst variables, medullary brainstem-spinal cord preparations (n = 22; 10 male, 12 female) were placed in the recording chamber and allowed to equilibrate. After recording baseline data (5-min time point), the recording was maintained for another 85 min. Burst frequency started at 10.9 ± 0.4 bursts/min (baseline) and decreased slowly to 16 ± 2% below baseline at the 90-min time point (p < 0.001; Fig. 2A). The percent change in burst frequency at the 90-min time point for time controls was larger for males than females (p = 0.036; Fig. 2B). Normalized burst amplitude was unchanged at 1.0 ± 0.1 at the 90-min time point (p = 0.82; Fig. 2C).
Fig. 2. Long-lasting effects of intermittent SubP application on burst frequency and amplitude in medullary brainstem-spinal cords.
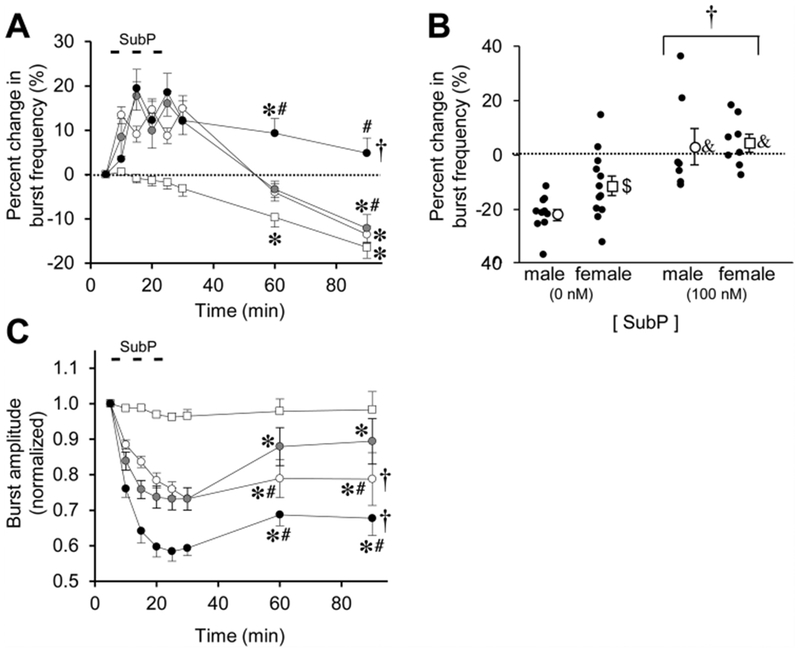
(A) The time course of burst frequency effects are shown for intermittent SubP application (3-min application/7-min washout, ×3) at 1 nM (white circles), 10 nM (gray circles), and 100 nM (black circles). Data from time control experiments are shown (white squares). Intermittent SubP applications induces frequency plasticity at 100 nM, but not at 1 or 10 nM. (B) Mean percent change in burst frequency at the 90-min time point is shown for males (white circles) and females (white squares) following 0 nM SubP (time controls) and 100 nM SubP intermittent applications. Individual data points in each group are shown as black circles. There were no sex-dependent differences in frequency plasticity, but the percent change in burst frequency was decreased in males compared to females in the time controls. (C) The time course of burst amplitude effects are shown for intermittent SubP applications. There were long-lasting decreases in burst amplitude following intermittent 100 and 1 nM SubP applications. For panels (A) and (B), statistics symbols are as follows: † = significant drug-dependent effect; * = different from baseline; # = different from time controls at that time point. For panel (B), † = significant drug-dependent effect with males and females combined; & = different from the time control for that sex; $ = difference between males and females for that [SubP].
For both intermittent and sustained SubP applications, SubP (1 or 10 nM) acutely increased burst frequency and increased tonic motoneuron discharge. Respiratory bursts were disrupted briefly during several 10 and 100 nM SubP applications due to tonic motoneuron depolarization, but the tonic depolarization stopped within 1–2 min or washout with normal aCSF solution. To test for pattern-sensitivity of SubP applications, SubP was first applied intermittently (3-min application/7-min washout, ×3) at 1 nM (n = 9; 5 male, 4 female), 10 nM (n = 12; 8 male, 4 female), or 100 nM (n = 15; 7 male, 8 female). Intermittent SubP application at 1 or 10 nM increased burst frequency above baseline by 10–20% (11.3 ± 0.5 and 11.5 ± 0.5 bursts/min, respectively), but only during the application; burst frequency quickly decreased to levels similar to those in time controls (Fig. 2A), indicating a lack of frequency plasticity. Intermittent SubP applications at 100 nM increased burst frequency by 10–20% above baseline (12.2 ± 0.7 bursts/min) during the application, and it remained elevated 9 ± 3% and 5 ± 3% above baseline respectively, at the 60-min and 90-min time points (p < 0.001 for drug effect; Fig. 2A). Thus, frequency plasticity was only observed following the intermittent 100 nM SubP applications. When the percent change in burst frequency data at the 90-min time point for the intermittent 100 nM SubP applications were pooled and separated by sex, both males and females exhibited a drug-dependent effect greater than time controls (p < 0.001 for drug effect; p < 0.001 for males; p = 0.007 for females; Fig. 2B), suggesting an absence of sex-dependent differences in SubP-induced frequency plasticity. Respiratory motor burst amplitude decreased during SubP application at all three concentrations, with drug-dependent decreases being maintained at the 90-min time point for the 1 nM and 100 nM SubP applications (p < 0.001; Fig. 2C). There were no long-term changes in burst duration or shape after intermittent SubP applications (data not shown).
For sustained SubP applications (one 9-min application), SubP was applied at 1 nM (n = 11; 6 male, 5 female), 10 nM (n = 9; 5 male, 4 female), and 100 nM (n = 13; 6 male, 7 female). Sustained SubP applications at 1 or 10 nM had similar effects in that burst frequency was increased by 10–20% above baseline (11.7 ± 0.2 and 12.4 ± 0.9 bursts/min, respectively) during the application, but then decreased back to levels similar to those in time controls (Fig. 3A). Sustained SubP applications (100 nM) increased burst frequency by 10–20% above baseline (10.1 ± 0.6 bursts/min) during the application, but burst frequency returned to baseline frequency at the 60- and 90-min time points (p = 0.365 for drug effect; Fig. 3A). When the percent change in burst frequency data at the 90-min time point were pooled and separated by sex, both males and females exhibited a drug-dependent effect (p = 0.001 for males and females pooled together; p = 0.015 for males only; p = 0.018 for females only; Fig. 3B). Frequency plasticity was not very robust with sustained SubP applications since burst frequency at the 90-min was near baseline levels. The frequency plasticity for sustained SubP applications was not due to an increase in burst frequency compared to baseline, but rather due to being compared with the decreased percent change in burst frequency for the time control experiments. Respiratory motor burst amplitude was decreased during SubP application at all three concentrations, but the mean amplitude was only significant at the 60-min time point at the 10 nM SubP dose (p = 0.022; Fig. 3C). There were no long-term changes in burst duration or shape after intermittent SubP applications (data not shown).
Fig. 3. Long-lasting effects of sustained SubP applications in medullary brainstem-spinal cords.
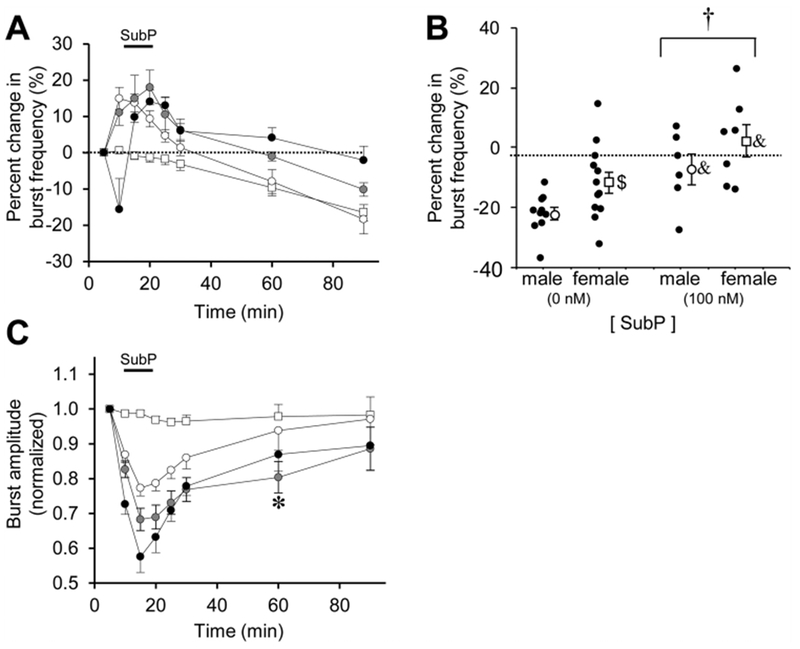
(A) The time course of burst frequency effects are shown for a sustained SubP application (one 9-min application) at 1 nM (white circles), 10 nM (gray circles), and 100 nM (black circles). Data from time control experiments are shown (white squares). There was no frequency plasticity observed following sustained SubP applications. (B) Mean percent change in burst frequency at the 90-min time point is shown for males (white circles) and females (white squares) following 0 nM SubP (time controls) and 100 nM SubP sustained applications. Individual data points in each group are shown as black circles. When comparing data only at the 90-min time point, frequency plasticity was expressed and there was a significant drug-dependent effect. There were no sex-dependent differences in frequency plasticity, but the percent change in burst frequency was decreased in males compared to females for time controls (as shown in Fig. 2B). (C) The time course of burst amplitude effects are shown for intermittent SubP applications. There were no long-lasting decreases in burst amplitude with sustained SubP applications. Statistics symbols as in Fig. 2 legend.
3.2. SubP-induced frequency plasticity in pontine brainstem-spinal cords
To quantify time-dependent changes in respiratory burst variables, pontine brainstem-spinal cord preparations (n = 18; 9 male, 9 female) were placed in the recording chamber and allowed to equilibrate (Fig. 4). Burst frequency started at 6.0 ± 0.6 bursts/min (baseline) and was unchanged at 15 ± 7% below baseline at the 90-min time point (p = 0.758; Fig. 5A). The percent change in frequency at the 90-min time point was similar for males and females (p = 0.811; Fig. 5B). Normalized burst amplitude was decreased at 0.8 ± 0.1 at the 90-min time point (p = 0.001; Fig. 5C).
Fig. 5. Long-lasting effects of intermittent SubP application on burst frequency and amplitude in pontine brainstem-spinal cords.
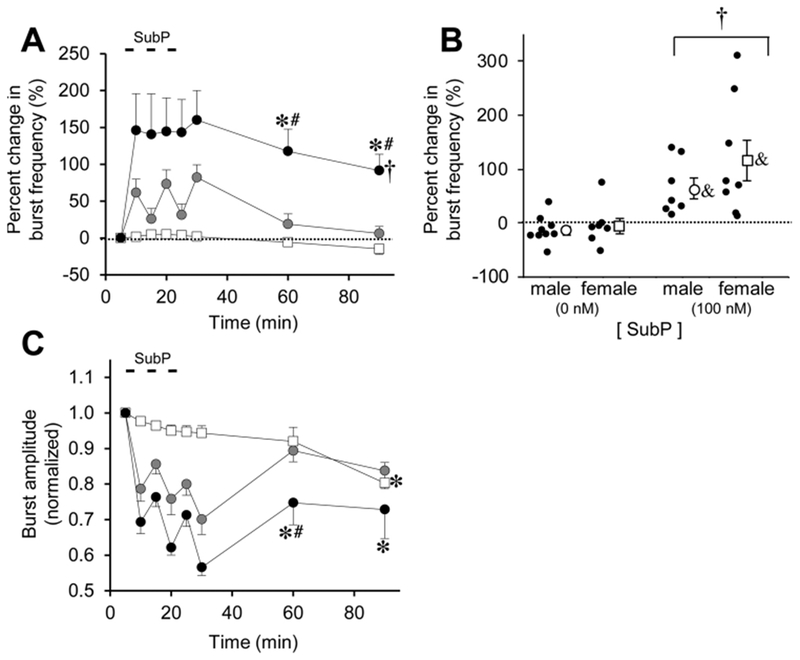
(A) The time course of burst frequency effects are shown for intermittent SubP application (3-min application/7-min washout, ×3) at 10 nM (gray circles) and 100 nM (black circles). Data from time control experiments are shown (white squares). Intermittent SubP applications induced frequency plasticity at 100 nM, but not at 10 nM. (B) Mean percent change in burst frequency at the 90-min time point is shown for males (white circles) and females (white squares) following 0 nM SubP (time controls) and 100 nM SubP intermittent applications. Individual data points in each group are shown as black circles. There were no sex-dependent differences in frequency plasticity. (C) The time course of burst amplitude effects are shown for intermittent SubP applications. There were no long-lasting decreases in burst amplitude following intermittent 100 and 10 nM SubP applications compared to time controls. Statistics symbols as in Fig. 2 legend.
Similar to the SubP applications for medullary brainstem-spinal cords, SubP (10 or 100 nM) acutely increased burst frequency and increased tonic motoneuron discharge in pontine brainstem-spinal cords (Fig. 4B). To test for pattern-sensitivity, SubP was applied intermittently (3-min application/7-min washout, ×3) at 10 nM (n = 11; 5 male, 6 female) and 100 nM (n = 16; 8 male, 8 female). Intermittent SubP applications at 10 nM increased burst frequency by 25–85% above baseline (from 5.3 ± 0.7 to 8.3 ± 0.6 bursts/min) during the application, but frequency returned to near baseline levels at the 60- and 90-min time points (p > 0.675; Fig. 5A). Intermittent SubP application (100 nM) increased burst frequency by ~150% above baseline (from 4.0 ± 0.6 to 7.8 ± 0.6 bursts/min) during the application, an effect that persisted at 118 ± 30% and 92 ± 22% above baseline at the 60-min and 90-min time points (6.8 ± 0.5 and 6.1 ± 0.5 bursts/min, respectively; p < 0.001 at each time point; p < 0.001 for drug effect; Fig. 5A). When the percent change in burst frequency data at the 90-min time point were pooled and separated by sex, there was a drug-dependent increase in males (p = 0.024) and females (p = 0.001) compared to their controls (Fig. 5B). Thus, frequency plasticity was robust following intermittent 100 nM SubP applications, but not intermittent 10 nM SubP applications. Respiratory motor burst amplitude decreased during SubP application at both concentrations, but only the 60-min time point was decreased compared to time controls (p = 0.032; Fig. 5C). There were no long-term changes in burst duration or shape after intermittent SubP applications (data not shown).
For sustained SubP applications (one 9-min application, 100 nM) in pontine brainstem-spinal cords, the acute and long-term SubP-induced effects were diminished compared to the results from intermittent SubP applications. Sustained SubP applications (n = 14; 7 male, 7 female) increased burst frequency by ~50% above baseline (from 5.5 ± 0.8 to 7.3 ± 0.5 bursts/min) during the application, and then frequency remained 37 ± 6% and 27 ± 6% above baseline at the 60- and 90-min time points (7.3 ± 1.0 6.7 ± 0.9 bursts/min, respectively; p < 0.001 at the time points; p < 0.001 for drug effect; Fig. 6A). Thus, for pontine medullary brainstem-spinal cords, frequency plasticity was expressed following either intermittent or sustained 100 nM SubP applications, but was larger following intermittent applications. When the percent change in burst frequency data at the 90-min time point were pooled and separated by sex, there was a drug-dependent increase for the pooled males and females (p = 0.002), and for males (p = 0.007) and females (p = 0.043) individually compared to their controls (Fig. 6B). Respiratory motor burst amplitude decreased during the sustained 100 nM SubP application and remained decreased at the 60- and 90-min time points (p < 0.001; Fig. 6C). There were no long-term changes in burst duration or shape after intermittent SubP applications (data not shown).
Fig. 6. Long-lasting effects of sustained SubP applications in pontine brainstem-spinal cords.
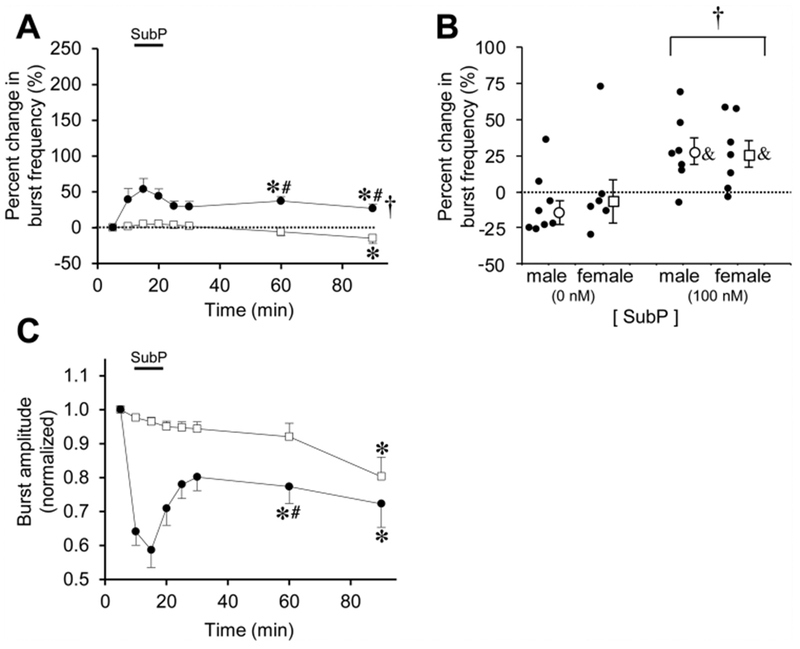
(A) The time course of burst frequency effects are shown for a sustained SubP application (one 9-min application) at 100 nM (black circles). Data from time control experiments are shown (white squares). Frequency plasticity was observed following sustained SubP applications, but a much lower amount compared to intermittent 100 nM SubP applications. (B) Mean percent change in burst frequency at the 90-min time point is shown for males (white circles) and females (white squares) following 0 nM SubP (time controls) and 100 nM SubP sustained applications. Individual data points in each group are shown as black circles. When comparing data only at the 90-min time point, frequency plasticity was expressed and there was a significant drug-dependent effect. There were no sex-dependent differences in frequency plasticity. (C) There were no long-lasting decreases in burst amplitude with sustained SubP applications compared to time controls. Statistics symbols as in Fig. 2 legend.
All medullary brainstem-spinal cords produced respiratory motor bursts after equilibration in the recording chamber. However, a subset of pontine brainstem-spinal cords were silent during the equilibration period, and remained silent for > 120 min (n = 4; 1 male, 3 female). Each of these pontine brainstem-spinal cords produced respiratory motor bursts in response to a brief SubP (100 nM) application after 120 min of equilibration in the recording chamber (data not shown). Two groups of silent pontine brainstem-spinal cords were exposed to intermittent SubP applications (100 nM; 3-min application/7-min washout, ×3; n = 6; 2 male; 4 female) or a sustained SubP application (100 nM; one 9-min application; n = 8; 3 male, 5 female). All of these silent pontine brainstem-spinal cords produced respiratory motor bursts when SubP was applied, and kept producing motor bursts for > 60 min after SubP application (Fig. 7A, B). The burst frequency at the 60- and 90-min time points was larger for the silent preparations exposed to intermittent SubP applications compared to those exposed to a single sustained SubP application (p = 0.052 and p = 0.060; Fig. 7C), suggesting that intermittent SubP applications were more effective at inducing frequency plasticity compared to sustained SubP applications.
Fig. 7. SubP induced respiratory motor in silent pontine neonatal rat brainstem-spinal cords.
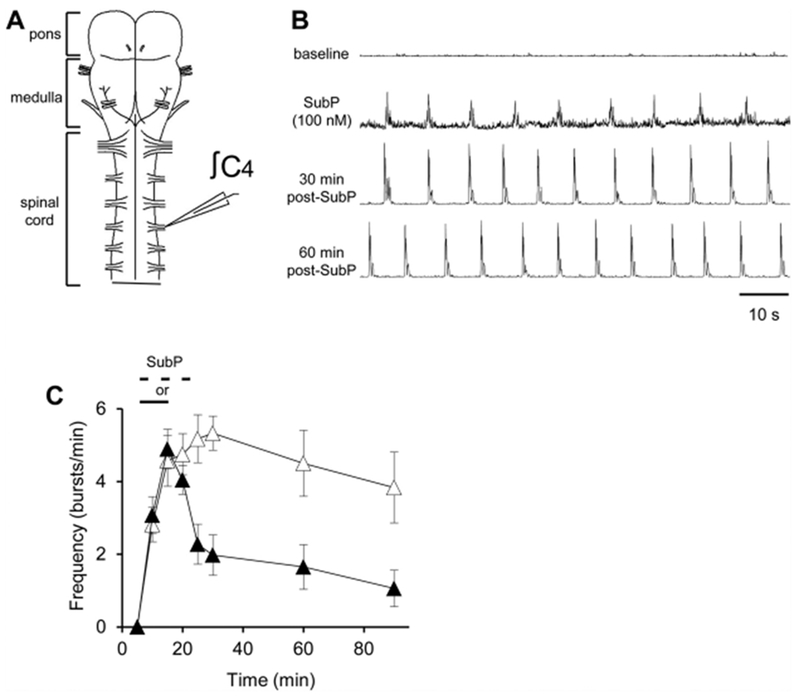
(A) Drawing of isolated neonatal rat pontine brainstem-spinal cord with a suction electrode attached to the cervical spinal C4 ventral root. (B) Voltage traces of respiratory-related motor output are shown at baseline that was silent (top trace). During intermittent 100 nM SubP application (2nd trace), respiratory motor bursts were induced, and the preparation continued to produce motor bursts for the next 30 and 60 min post-drug application (3rd and 4th traces, respectively). (C) Burst frequency in these preparations following intermittent 100 nM SubP applications (white triangles) and sustained 100 nM SubP applications (black triangles). Intermittent SubP applications to silent pontine brainstem-spinal cords tended to induce respiratory motor bursts at a higher frequency for 60 min post-drug application.
3.3. Baseline burst frequency and SubP-induced frequency plasticity
A meta-analysis of intermittent hypoxia-induced plasticity in adult rats indicated an indirect correlation between the magnitude of frequency plasticity and baseline frequency such that greater frequency is observed in preparations with lower baseline frequencies (Baker-Herman and Mitchell, 2008). Interestingly, we found that for neonatal medullary brainstem-spinal cords following intermittent 100 nM SubP applications, there was no relationship with between the percent change in burst frequency at the 90-min time point and baseline frequency (r2 = 0.0561, p = 0.395; Fig. 8A). In contrast, for neonatal pontine medullary brainstem-spinal cords, the percent change in burst frequency at the 90-min time point was indirectly correlated with baseline frequency (r2 = 0.621, p < 0.001; Fig. 8B), suggesting an important role for the pons in the effects of SubP on frequency plasticity.
Fig. 8. The magnitude of SubP-induced frequency plasticity is indirectly correlated with baseline burst frequency in pontine (B), but not medullary brainstem-spinal cords (A).
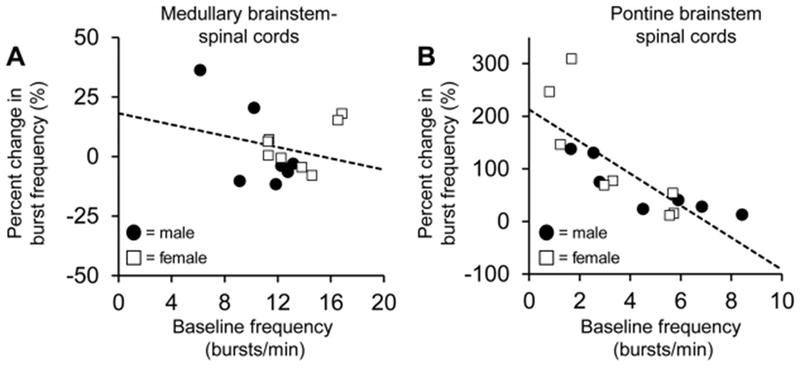
Individual data points are shown for males (black circles) and females (white squares). Dotted lines indicate the line of regression calculated for each data set.
3.4. SubP-induced frequency plasticity requires neurokinin-1 (NK1) receptor activation
SubP is the endogenous ligand for NK1 receptors and frequency plasticity is abolished in brainstem-spinal cords from mutant mice lacking SubP and neurokinin-A peptides (Berner et al., 2007). Thus, we hypothesized that SubP-induced frequency plasticity requires NK1 receptor activation. To test this hypothesis, baseline recordings were obtained from pontine brainstem-spinal cords (these preparations express the most robust frequency plasticity) before bath-applying intermittent SubP application (100 nM; 3-min application/7-min washout, ×3) in the presence of 10 μM L760735 (10 μM; NK1 receptor antagonist; bath-applied 10 min before, during, and 10 min after the SubP applications; (n = 5; 2 male, 3 female). Time control experiments involved bath-application of L760735 (10 μM) for 43 min (n = 5, 3 male, 2 female). Bath-application of L760735 alone increased burst frequency by ~30% during the application, but it returned to baseline and was −8 ± 9% below baseline at the 100-min time point (Fig. 9A). Thus, L760735 alone had no long-lasting, time-dependent effects on burst frequency. However, the acute increase in burst frequency and frequency plasticity induced by SubP were nearly abolished in the presence of L760735 (Fig. 9A). At the 70- and 100-min time points (representing approximately 30 and 60 post-SubP application), the percent change in burst frequency was only 6 ± 14% and 1 ± 17% above baseline (p > 0.86; Fig. 9A). The SubP-dependent acute decrease in burst amplitude was still expressed, but the long-lasting effects on burst amplitude were similar to time controls at the 70- and 100-min time points (p > 0.87; Fig. 9B).
Fig. 9. SubP-induced frequency plasticity in pontine brainstem-spinal cords is abolished by a neurokinin-1 (NK1) receptor antagonist drug (L760735, 10 μM).
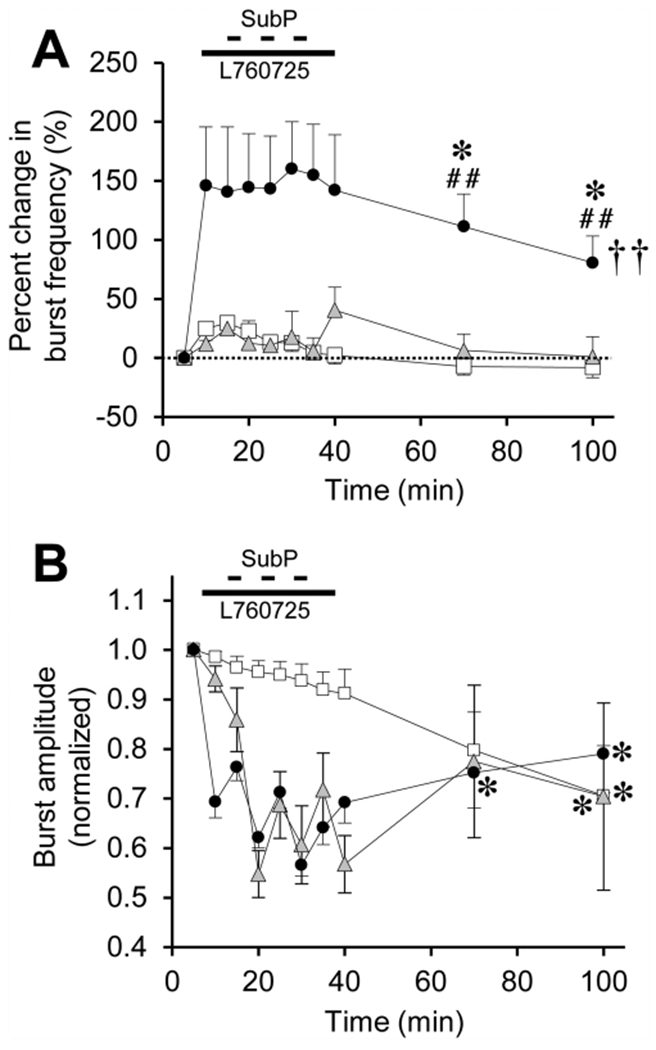
(A) The time course of burst frequency effects are shown for intermittent SubP applications alone (3-min application/7-min washout, ×3) at 100 nM (black circles; same data as in Fig. 5A), and intermittent SubP applications with L760735 pretreatment (gray triangles). Data from time control experiments are shown (white squares; same data as in Fig. 5A). No frequency plasticity was observed with L760735 treatment. (B) L760735 did not block the acute SubP-induced decrease in burst amplitude, and did not alter burst amplitude with respect to intermittent SubP applications only, or time control experiments. Statistics symbols as in Fig. 2 legend.
4. Discussion
This the first study to examine the long-lasting effects of SubP on isolated neonatal brainstem-spinal cords, and the main finding is that the neonatal rat respiratory control system has the capacity to express SubP-induced frequency plasticity. Bath-applied SubP is sufficient to induce frequency plasticity, and SubP is more effective when applied intermittently, rather than in a single sustained pattern. The magnitude of SubP-induced frequency plasticity was much larger when using pontine brainstem-spinal cords that had lower baseline frequencies. Similar to adult rodents, there was an indirect correlation between baseline frequency and the magnitude of SubP-induced frequency plasticity for neonatal pontine brainstem-spinal cords. Lastly, we found that SubP-induced frequency plasticity required NK1 receptor activation. Previously, respiratory frequency plasticity in perinatal rodent in vitro preparations required severe intermittent anoxia for induction. The ability to express frequency plasticity with low concentrations of intermittently or sustained SubP application suggests that this form of respiratory plasticity may be induced pharmacologically without confounding effects of severe hypoxia.
4.1. SubP-dependent modulation of neonatal respiratory control system
SubP is one of several neurotransmitters that modulate respiratory motor output (Doi and Ramirez, 2008). NK1 receptors, the primary high affinity receptor for SubP, are abundantly expressed in respiratory-related neurons in the brainstem and spinal cord (Gray et al., 1999; Hayes and Del Negro, 2007; Morgado-Valle and Feldman, 2004; Ptak et al., 2000, 2009). Since SubP is colocalized with serotonin in raphe neurons (Kachidian et al., 1991) and chemoafferent nerve stimulation increases raphe neuron activity (Morris et al., 1996), SubP is likely released onto rhythm-generating neurons in the brainstem and spinal respiratory neurons during hypoxia. Consistent with this hypothesis, raphe obscurus neuron activation in rhythmically-active neonatal rat medullary slices increases respiratory burst frequency by a mechanism that requires 5-HT and NK1 receptor activation (Ptak et al., 2009). Likewise, mutant mice lacking NK1 receptors have attenuated hypoxic ventilatory responses both as neonates (Berner et al., 2012) and adults (Ptak et al., 2002).
In isolated brainstem-spinal cords of neonatal rodents, bath-applied SubP acutely increases respiratory burst frequency in a concentration-dependent manner (Monteau et al., 1996; Murakoshi et al., 1985; Yamamoto et al., 1992), although decreased burst frequency is observed at relatively high SubP concentrations (50–1000 nM) due to large depolarization of respiratory neurons (Shvarev et al., 2002). Prior to the present study, the long-term effects of SubP application on respiratory burst frequency were not known, nor was the pattern sensitivity (intermittent versus sustained) of bath-applied SubP. Our results show that acute SubP application can increase burst frequency at all concentrations and patterns, but sustained 100 nM SubP application produces transient decreases in burst frequency both during and immediately following the SubP application (Fig. 3A). With respect to long-lasting changes, intermittent bath-applied SubP at 100 nM induced frequency plasticity in both medullary and pontine brainstem-spinal cords, with much larger frequency plasticity occurring in pontine brainstem-spinal cords. Similar to what is observed in adult rodents (Baker-Herman and Mitchell, 2008), the magnitude of frequency plasticity is indirectly correlated with baseline frequency, such that larger amounts of frequency plasticity are observed in pontine, but not medullary brainstem-spinal cord preparations with lower baseline frequency. The fact that the magnitude of frequency plasticity is indirectly correlated with the starting baseline frequency suggests that frequency plasticity may be more clinically significant or impactful in situations in which infants have low respiratory drive, such as with apnea of prematurity, or pathologically depressed breathing (e.g., due to maternal opioid drug abuse).
SubP-induced frequency plasticity appears to be pattern-sensitive to a certain extent because sustained SubP applications more weakly induced frequency plasticity. Sustained SubP applications induced a small, but statistically significant, frequency plasticity in medullary brainstem-spinal cords, but only when comparing burst frequencies at the 90-min time point (Fig. 3A). In pontine brainstem-spinal cords, sustained SubP applications also induced frequency plasticity (Fig. 6A, B), but the magnitude was much smaller compared to intermittent SubP applications at the same concentration (Fig. 5A, B). Also, intermittent SubP application to pontine brainstem-spinal cords that were silent during the baseline period tended to induce a more robust response compared to a sustained SubP application at the same concentration. Thus, sustained SubP applications can induce frequency plasticity, but to a much smaller extent than intermittent SubP applications. Our results are consistent with the finding that frequency plasticity in vitro is induced only by intermittent (and not sustained) bath-applied hypoxic solution (Berner et al., 2007; Blitz and Ramirez, 2002). In contrast, in adult rodents, frequency plasticity is weakly induced by both intermittent and sustained hypoxia (Baker-Herman and Mitchell, 2008). However, developing an in vitro model of SubP-induced frequency plasticity that does not involve the non-specific effects of hypoxic conditions (e.g., free radical generation, ATP and ion gradient disturbances, lower pH) is useful for testing mechanisms underlying frequency plasticity.
In this study, respiratory burst amplitude was always decreased during intermittent or sustained SubP applications and often returned to levels similar to time controls at the 90- or 100-min time points. Persistent decreases in burst amplitude were observed in medullary brainstem-spinal cords for intermittent 1.0 and 100 nM SubP applications, similar to the long-term depression of phrenic motor output observed with intermittent hypercapnic exposures (Bach and Mitchell, 1998; Valic et al., 2016). Thus, with respect to plasticity for the 100 nM SubP application, the increase in burst frequency may be offset by the decrease in burst amplitude and overall minute activity may not be altered (e.g., mean minute activity at 90-min time points are similar for time control and intermittent 100 nM SubP data; data not shown). However, for other examples of frequency plasticity in pontine brainstem-spinal cords, there was no significant decrease in burst amplitude to offset the increased burst frequency (Figs. 5 and 6). Thus, it’s possible to induce frequency plasticity without long-term depression of burst amplitude. In an intact animal, the effect of frequency plasticity induction on breathing and blood-gas homeostasis is not known. Increased ventilation due to frequency plasticity could alter blood gasses (e.g., decrease arterial PCO2) and initiate compensatory mechanisms to restore breathing back to normal limits. However, if breathing were depressed due to development, disease, or other conditions, frequency plasticity induction could improve ventilation and increase tissue oxygenation.
The SubP-dependent decrease in cervical spinal motor burst amplitude is likely due to activation of neurons in the brainstem since SubP applied to the brainstem in a split-bath preparation decreased burst amplitude, whereas SubP applied to the spinal cord only increased spinal burst amplitude (Monteau et al., 1996). SubP-dependent depolarization of neonatal rat phrenic motoneurons is primarily due to NK1 receptor activation, although smaller depolarizations were also observed with NK2 and NK3 receptor agonists (Ptak et al., 2000). Since SubP-dependent phrenic motoneuron depolarization in vitro is blocked by pretreatment with an NMDA receptor antagonist drug, it is hypothesized that SubP-dependent effects are due to potentiation of NMDA-dependent currents in phrenic motoneurons (Ptak et al., 2000). Respiratory frequency plasticity is likely to be influenced by mechanosensory input that is carried by the vagus nerve. In other forms of respiratory plasticity, such as long-term facilitation, vagal afferent input inhibits long-term facilitation expression in cranial nerve motor output (Mateika and Fregosi, 1997; Golder and Martinez, 2008), but enhances long-term facilitation in phrenic nerve motor output (Golder and Martinez, 2008). In contrast, it is not known whether vagal afferent input modulates frequency plasticity. In the review of intermittent hypoxia-induced respiratory neuroplasticity in adult rats (Baker-Herman and Mitchell, 2008), frequency plasticity was expressed more in unanesthetized compared to anesthetized rats, but the distinction may also be the presence (intact, unanesthetized) and absence of vagal input (anesthetized, vagotomized). In either rodent preparation, frequency plasticity was expressed at about 7-11% above baseline frequency. With the vagus nerve intact, frequency plasticity is expressed in adult rats (McGuire and Ling, 2005), young P14-P25 rats (Tadjalli et al. 2007; Reid and Solomon, 2014), and neonatal rats (Julien et al., 2008). To our knowledge, frequency plasticity expression had not been studied in vagotomized, but otherwise intact, rodent preparations. The use of in vitro neonatal rodent preparations, such as this study and others (Berner et al., 2007; Blitz and Ramirez, 2002), precludes the opportunity to test this question since the vagus nerve is absent in these preparations.
4.2. Pharmacology and locus of SubP-induced frequency plasticity
SubP binds to tachykinin NK1, NK2, and NK3 receptors, but has the highest affinity for NK1 receptors (Garcia-Recio and Gascon, 2015). SubP binding to NK1 receptors is hypothesized to elevate intracellular calcium levels, stimulate phosphoinositol turnover, mobilize arachidonic acid, and increase cAMP levels, as well as modulate Na+, Ca2+ and K+ channel activity (Garcia-Recio and Gascon, 2015; Quartara and Maggi, 1997). Several of these NKl-dependent responses could induce long-lasting changes (> 60 min) in the electrophysiological properties of respiratory-related neurons, which then lead to the frequency plasticity induction. In this study, SubP-induced frequency plasticity was nearly completely abolished by an NK1 antagonist (Fig. 9A), suggesting that NK1 receptors are necessary. Although other tachykinin receptors may also contribute to frequency plasticity since SubP is an agonist for the NK2 and NK3 receptors as well, NK1 receptor activation appears to be required. The NK1 antagonist used in this study, L-760735, blocked frequency plasticity and the acute SubP-induced frequency increase, but it did not block the depolarization of phrenic motoneurons. As discussed above, SubP may depolarize neonatal rat phrenic motoneurons during NK1 receptor blockade by activating NK2 or NK3 receptors.
The anatomic locus of SubP-induced frequency plasticity is not known. Since inspiratory motor activity in isolated brainstem-spinal cord preparations originates in the pre-Bötzinger complex (pre-BötC; Smith et al., 1991), it is reasonable to speculate that frequency plasticity may be due to long-lasting changes in pre-BötC neuron properties. NK1 receptors are a marker for pre-BötC neurons (Gray et al., 1999; Guyenet and Wang, 2001) and preBötC neurons expressing NK1 receptors are required for normal breathing in adult rats (Gray et al., 2001). Accordingly in pre-BötC neurons, SubP application induces an inward current and augments intrinsic bursting properties in cadmium-insensitive pacemaker neurons via a transient receptor protein channel (Ben-Mabrouk and Tryba, 2010; Hayes and Del Negro, 2007; Pena and Ramirez, 2004). The mechanisms by which NK1 receptor activation induces frequency plasticity are difficult to speculate. In the robust model of frequency plasticity in the lamprey locomotor network, SubP enhances glutamatergic synaptic transmission via pre- and postsynaptic mechanisms, particularly enhancing NMDA-dependent synaptic transmission without altering synaptic inhibition (Parker and Grillner, 1998). Thus, inducing a long-lasting enhancement of glutamatergic synaptic transmission within the preBötC could be a mechanism for SubP-dependent frequency plasticity. Since SubP may enhance NMDA currents in phrenic motoneurons, a reasonable hypothesis is that intermittent SubP application enhances NMDA currents in preBötC neurons that subsequently induces frequency plasticity. A logical future direction would be to test whether SubP induces frequency plasticity in rhythmically-active, neonatal rat medullary slices that contain only the pre-BötC (Smith et al., 1991) to investigate the potential role of NMDA receptors in frequency plasticity.
On the other hand, and not mutually exclusive, respiratory-related neurons located elsewhere in the brainstem could respond to SubP and contribute to SubP-induced frequency plasticity. For example, pre-I neurons in the parafacial respiratory group within the caudal pons are depolarized by SubP (Yamamoto et al., 1992; Sharev et al., 2002). Respiratory-related parafacial neurons are likely found in both the medullary and pontine brainstem-spinal cords used in this study. Alternatively, NK1 receptors are expressed on neurons throughout the ventrolateral medulla and particularly on glutamatergic neurons (Wang et al., 2001; Guyenet et al., 2002). Glutamatergic NKl-expressing neurons projecting to the preBötC could also contribute to SubP-induced frequency plasticity.
4.3. Sex-dependent differences in respiratory control and newoplasdcity in neonates
Few studies have addressed the potential for sex-dependent differences in respiratory control or neuroplasticity in neonates. For example, post-hypoxic depression is observed in all male mice, but in only 3/11 female mice at postnatal age P10-P13 (Garcia et al., 2013). Rhythmically active medullary slices from neonatal female mice recover more quickly from severely hypoxic conditions compared to slices from male mice, suggesting that male/female differences are manifested at the inspiratory rhythm generator, the pre-BötC (Garcia et al., 2013). Similarly, there are sex-dependent differences in the effects of caffeine on minute ventilation and hypercapnic responses in P12, but not P1, neonatal rats, and female P12 rats have a lower spontaneous apnea frequency during hypoxia (Kouchi et al., 2017). Thus, sex-dependent differences in respiratory motor control in newborn rodents can be demonstrated. In newborn humans, apnea of prematurity and caffeine treatment show sex differences, with slightly more females being diagnosed with apnea of prematurity compared to males, but caffeine treatment is often stopped earlier in females than males, suggesting that the female respiratory control system develops faster than males (Bairam et al., 2018). In this study, there was only one sex-dependent difference observed: the percent change in burst frequency in time control experiments in medullary brainstem-spinal cords was larger in females than in males (Figs. 2B and 3 B). Together with the other two rodent studies described above, it is possible that sex-dependent differences in respiratory control are not manifested until ~P10, but additional studies are necessary to confirm this.
4.4. Significance and conclusions
In this study, SubP applications induced frequency plasticity, thereby demonstrating the capacity of the isolated brainstem-spinal cord to express frequency plasticity. Respiratory neuroplasticity typically requires the activation of complex signaling pathways to produce long-lasting changes in the frequency and amplitude of respiratory motor output (e.g., Dale et al., 2014). Disruption of these signaling pathways may attenuate or prevent the expression of respiratory neuroplasticity. The final expression of respiratory neuroplasticity is likely to depend on the integrated outcome of competing factors that act to reduce or enhance respiratory motor burst frequency. Plasticity may be difficult to express in young neonates (P0–P5) except in in vitro conditions. Physiological “brakes” on plasticity are also likely to be important during development to allow normal maturation to occur. Understanding factors that induce or prevent neuroplasticity will be important for developing novel therapies for treating respiratory diseases in neonates.
Although in the present study we found that SubP induces frequency plasticity, administration of an NK1 receptor agonist is not a viable candidate for treating hypoventilation-related diseases in human newborns (e.g., apnea of prematurity, congenital hypoventilation) given the role of SubP in a wide range of physiological processes (including pain). Instead, these data illustrate the capacity of the isolated neonatal rodent respiratory control system to undergo frequency plasticity without requiring severe anoxia for induction, and suggests that recruitment of receptors with similar signal transduction pathways may be useful therapeutic targets. The identification and development of alternative approaches or targets to induce long-lasting increases in breathing frequency and regularity are needed, and should be an area of future research.
Acknowledgements
Funding: This work was supported by the National Institutes of Health (NS085226, 2T35OD011078-06), and the Department of Comparative Biosciences in the School of Veterinary Medicine at the University of Wisconsin-Madison.
References
- Bach KB, Mitchell GS, 1998. Hypercapnia-induced long-term depression of respiratory activity requires alpha2-adrenergic receptors. J. Appl. Physiol 84, 2099–2105. [DOI] [PubMed] [Google Scholar]
- Bairam A, Laflamme N, Drolet C, Piedboeuf B, Shah PS, Kinkead R, 2018. Sex-based differences in apnoea of prematurity: a retrospective cohort study. Exp. Physiol 103, 1403–1411. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS, 2000. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J. Physiol 529, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS, 2008. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir. Physiol. Neurobiol 162, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, MacFarlane PM, 2017. Developmental plasticity in the neural control of breathing. Exp. Neurol 287, 176–191. [DOI] [PubMed] [Google Scholar]
- Behan M, Kinkead R, 2011. Neuronal control of breathing: sex and stress hormones. Compr. Physiol 1, 2101–2139. [DOI] [PubMed] [Google Scholar]
- Ben-Mabrouk F, Tryba AK, 2010. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur. J. Neurosci 31, 1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner J, Shvarev Y, Lagercrantz H, Bilkei-Gorzo A, Hökfelt T, Wickström R, 2007. Altered respiratory pattern and hypoxic response in transgenic newborn mice lacking the tachykinin-1 gene. J. Appl. Physiol 103, 552–559. [DOI] [PubMed] [Google Scholar]
- Berner J, Shvarev Y, Zimmer A, Wickstrom R, 2012. Hypoxic ventilatory response in Tac1−/− neonatal mice following exposure to opioids. J. Appl. Physiol 113, 1718–1726. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Ramirez JM, 2002. Long-term modulation of respiratory network activity following anoxia in vitro. J. Neurophysiol 87, 2964–2971. [DOI] [PubMed] [Google Scholar]
- Chan GJ, Lee AC, Baqui AH, Tan J, Black RE, 2015. Prevalence of early-onset neonatal infection among newborns of mothers with bacterial infection or colonization: a systematic review and meta-analysis. BMC Infect. Dis 15, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS, 2014. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology 29, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS, 2013. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann. N. Y. Acad. Sci 1279, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson NR, Patel RM, 2016. The role of caffeine in noninvasive respiratory support. Clin. Perinatol 43, 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson NR, Patel RM, Smith PB, Kuehn DR, Clark J, Vyas-Read S, Herring A, Laughon MM, Carlton D, Hunt CE, 2014. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J. Pediatr 164, 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM, 2008. Neuromodulation and the orchestration of the respiratory rhythm. Respir. Physiol. Neurobiol 164, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erenberg A, Leff RD, Haack DG, Mosdell KW, Hicks GM, Wynne BA, 2000. Caffeine citrate for the treatment of apnea of prematurity: a double-blind,placebo-controlled study. Pharmacotherapy 20, 644–652. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Mitchell GS, 2017. Respiratory neuroplasticity – overview, significance and future directions. Exp. Neurol 287, 144–152. [DOI] [PubMed] [Google Scholar]
- Funk GD, Rajani V, Alvares TS, Revill AL, Zhang Y, Chu NY, Biancardi V, Linhares-Taxini C, Katzell A, Reklow R, 2015. Neuroglia and their roles in central respiratory control; an overview. Comp. Biochem. Physiol. A: Mol. Integr. Physiol 186, 83–95. [DOI] [PubMed] [Google Scholar]
- Gallacher DJ, Hart K, Kotecha S, 2016. Common respiratory conditions of the newborn. Breathe 12, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ, Rotem-Kohavi N, Doi A, Ramirez JM, 2013. Post-hypoxic recovery of respiratory rhythm generation is gender dependent. PLoS One 8, e60695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Recio S, Gascón P, 2015. Biological and pharmacological aspects of the NK1-receptor. BioMed Res. Int. 1–10 Article ID: 495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Martinez SD, 2008. Bilateral vagotomy differentially alters the magnitude of hypoglossal and phrenic long-term facilitation in anesthetized mechanically ventilated rats. Neurosci. Lett 442, 213–218. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015. Intermittent hypoxia and neurorehabilitation. J. Appl. Physiol 119, 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL, 1999. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286, 1566–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL, 2001. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat. Neurosci 4, 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, 2012. Control of breathing activity in the fetus and newborn. Compr. Physiol 2, 1873–1888. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Wang H, 2001. Pre-Bötzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J. Neurophysiol 86, 438–446. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL, 2002. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J. Neurosci 22, 3806–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JA, Del Negro CA, 2007. Neurokinin receptor-expressing pre-Bötzinger complex neurons in neonatal mice studied in vitro. J. Neurophysiol 97, 4215–4224. [DOI] [PubMed] [Google Scholar]
- Hofstetter AO, Legnevall L, Herlenius E, Katz-Salamon M, 2008. Cardiorespiratory development in extremely preterm infants: vulnerability to infection and persistence of events beyond term-equivalent age. Acta Paediatr 97, 285–292. [DOI] [PubMed] [Google Scholar]
- Julien C, Bairam A, Joseph V, 2008. Chronic intermittent hypoxia reduces ventilatory long-term facilitation and enhances apnea frequency in newborn rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 294, R1356–R1366. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Poulat P, Marker L, Privat A, 1991. Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. J. Neurosci. Res 30, 521–530. [DOI] [PubMed] [Google Scholar]
- Keegan J, Parva M, Finnegan M, Gerson A, Belden M, 2010. Addiction in pregnancy. J. Addict. Dis 29, 175–191. [DOI] [PubMed] [Google Scholar]
- Kouchi H, Uppari N, Joseph V, Bairam A, 2017. Sex-specific respiratory effects of acute and chronic caffeine administration in newborn rats. Respir. Physiol. Neurobiol 240, 8–16. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF, 1997. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J. Appl. Physiol 82, 419–525. [DOI] [PubMed] [Google Scholar]
- McGuire M, Ling L, 2005. Ventilatory long-term facilitation is greater in 1- vs. 2-mo-old awake rats. J. Appl. Physiol 98, 1195–1201. [DOI] [PubMed] [Google Scholar]
- McPherson C, Neil JJ, Tjoeng TH, Pineda R, Inder TE, 2015. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr. Res 78, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, 1980a. Prolonged stimulation of respiration by endogenous central serotonin. Respir. Physiol. Neurobiol 42, 171–188. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, 1980b. Prolonged stimulation of respiration by a new central neural mechanism. Respir. Physiol. Neurobiol 41, 87–103. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM, 2003. Neuroplasticity in respiratory motor control. J. Appl. Physiol 94, 358–374. [DOI] [PubMed] [Google Scholar]
- Monteau R, Ptak K, Broquère N,, Hilaire G, 1996. Tachykinins and central respiratory activity: an in vitro study on the newborn rat. Eur. J. Pharmacol 314, 41–50. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Feldman JL, 2004. Depletion of substance P and glutamate by capsaicin blocks respiratory rhythm in neonatal rat in vitro. J. Physiol 555, 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Arata A, Shannon R, Lindsey BG, 1996. Inspiratory drive and phase duration during carotid chemoreceptor stimulation in the cat: medullary neurone correlations. J. Physiol 491, 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi T, Suzue T, Tamai S, 1985. A pharmacological study on respiratory rhythm in the isolated brainstem-spinal cord preparation of the newborn rat. Br. J. Pharmacol 86, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Grillner S, 1998. Cellular and synaptic modulation underlying substance P-mediated plasticity of the lamprey locomotor network. J. Neurosci 18, 8095–8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Zhang W, Grillner S, 1998. Substance P modulates NMDA responses and causes long-term protein synthesis-dependent modulation of the lamprey locomotor network. J. Neurosci 18, 4800–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RM, 2016. Short- and long-term outcomes for extremely preterm infants. Am. J. Perinatol 33, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM, 2004. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J. Neurosci 24, 7549–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM, 2005. Hypoxia-induced changes in neuronal network properties. Mol. Neurobiol 32, 251–283. [DOI] [PubMed] [Google Scholar]
- Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, Bairam A, Moddemann D, Peliowski A, Rabi Y, Solimano A, Nelson H, 2015. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. J. Am. Med. Assoc 314, 595–603. [DOI] [PubMed] [Google Scholar]
- Powell FL, Kim BC, Johnson SR, Fu Z, 2009. Oxygen sensing in the brain—invited article. Adv. Exp. Med. Biol 648, 369–376. [DOI] [PubMed] [Google Scholar]
- Ptak K, Hilaire G, 1999. Central respiratory effects of substance P in neonatal mice: an in vitro study. Neurosci. Lett 266, 189–192. [DOI] [PubMed] [Google Scholar]
- Ptak K, Konrad M, Di Pasquale E, Tell F, Hilaire G, Monteau R, 2000. Cellular and synaptic effect of substance P on neonatal phrenic motoneurons. Eur. J. Neurosci 12, 126–138. [DOI] [PubMed] [Google Scholar]
- Ptak K, Burnet H, Blanchi B, Sieweke M, De Felipe C, Hunt SP, Monteau R, Hilaire G, 2002. The murine neurokinin NK1 receptor gene contributes to the adult hypoxic facilitation of ventilation. Eur. J. Neurosci 16, 2245–2252. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC, 2009. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J. Neurosci 29, 3720–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartara L, Maggi CA, 1997. The tachykinin NK1 receptor. Part I: ligands and mechanisms of cellular activation. Neuropeptides 31, 537–563. [DOI] [PubMed] [Google Scholar]
- Reid IM, Solomon IC, 2014. Intermittent hypoxia-induced respiratory long-term facilitation is dominated by enhanced burst frequency, not amplitude, in spontaneously breathing urethane-anesthetized neonatal rats. Prog. Brain Res 212, 221–235. [DOI] [PubMed] [Google Scholar]
- Savran O, Ulrik CS, 2018. Early life insults as determinants of chronic obstructive pulmonary disease in adult life. Int. J. Chron. Obstruct. Pulmon. Dis 13, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharev YN, Lagercrantz H, Yamamoto Y, 2002. Biphasic effects of substance P on respiratory activity and respiration-related neurones in ventrolateral medulla in the neonatal rat brainstem in vitro. Acta Physiol. Scand 174, 67–84. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL, 1991. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, 2000. Excitation of phrenic and sympathetic output during acute hypoxia: contribution of medullary oxygen detectors. Respir. Physiol 121, 101–117. [DOI] [PubMed] [Google Scholar]
- Solomon IC, 2004. Ionotropic excitatory amino acid receptors in pre-Bötzinger complex play a modulatory role in hypoxia-induced gasping in vivo. J. Appl. Physiol 96, 1643–1650. [DOI] [PubMed] [Google Scholar]
- Solomon IC, 2005. Glutamate neurotransmission is not required for, but may modulate, hypoxic sensitivity of pre-Bötzinger complex in vivo. J. Neurophysiol 93, 1278–1284. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA, 2000. Pre-Bötzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J. Neurophysiol 83, 2854–2868. [DOI] [PubMed] [Google Scholar]
- Tadjalli A, Dufifin J, Li YM, Hong H, Peever J, 2007. Inspiratory activation is not required for episodic hypoxia-induced respiratory long-term facilitation in postnatal rats. J. Physiol 585, 593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, Streeter KA, Greer J, Mitchell GS, Fuller DD, 2018. Pharmacological modulation of hypoxia-induced respiratory neuroplasticity. Respir. Physiol. Neurobiol 256, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valic M, Pecotic R, Pavlinac Dodig I, Valic Z, Stipica I, Dogas Z, 2016. Intermittent hypercapnia-induced phrenic long-term depression is revealed after serotonin receptor blockade with methysergide in anaesthetized rats. Exp. Physiol 101, 319–331. [DOI] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG, 2001. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J. Comp. Neurol 434, 128–146. [DOI] [PubMed] [Google Scholar]
- Xing T, Fong AY, Bautista TG, Pilowsky PM, 2013. Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin. Exp. Pharmacol. Physiol 40, 602–609. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Onimaru H, Homma I, 1992. Effect of substance P on respiratory rhythm and pre-inspiratory neurons in the ventrolateral structure of rostral medulla oblongata: an in vitro study. Brain Res 599, 272–276. [DOI] [PubMed] [Google Scholar]


