Abstract
The “neurotrophic hypothesis of depression” posits that low levels of brain-derived neurotrophic factor (BDNF) are associated with Major Depressive Disorder (MDD). Low levels of BDNF have also been found in individuals with suicide attempts, in MDD or other disorders, suggesting that low BDNF may also be associated with suicidality. We assessed serum BDNF in 68 physically healthy and unmedicated (for at least 6 weeks) MDD subjects, who expressed no suicidal ideation (NSI; N=40) or endorsed suicidal ideation (SI; N=28), but were not actively suicidal, and in healthy controls (HC; N=76). Serum BDNF levels were significantly lower in MDD with SI compared to NSI MDD but were not significantly correlated with total Hamilton Depression Rating Scale (HDRS-17) severity or severity on any HDRS subscale. Covarying for age, sex, body mass index, platelets, perceived stress, smoking and physical activity did not alter the significant association between BDNF and SI. SI status was not significantly different between HC and MDD. Our findings show an association between low serum BDNF and SI in individuals with less than severe and non-active suicidal intent, suggesting that the individual symptom of suicidality may extend the neurotrophic hypothesis of depression to include suicidal ideation within MDD.
Keywords: BDNF, suicide, depression
1. Introduction
Major Depressive Disorder (MDD) affects 300 million people globally. It is a leading cause of disability, and is associated with increased risk of suicidal ideation (SI), suicide attempts and completed suicide (World Health Organization, 2017a). An estimated 8.3% of the general population have suicidal thoughts in a given 12-month period (Crosby, Cheltenham, & Sacks, 1999), and an estimated 50 percent of people who commit suicide also have MDD (Center for Disease Control and Prevention, 2015; Schimelpfening, 2017).
The “neurotrophic hypothesis of depression” seeks to understand depression through regulatory proteins such as brain-derived neurotrophic factor (BDNF) that promote neuroplasticity and adult neurogenesis (Duman & Monteggia, 2006). Generally, low levels are reported in blood in unmedicated individuals with MDD (Castren & Kojima, 2017; Kishi, Yoshimura, Ikuta, & Iwata, 2018; Lee & Kim, 2010; Molendijk et al., 2014), although there are some mixed results showing no significant association between BDNF and MDD (Ihara et al.,2016), or even contradictory results, and the neurotrophic hypothesis may need to be reassessed to “provide a more valid account of the complex relationship between growth factors, mood disorders and their treatment” (Groves, 2007). It is uncertain whether low BDNF levels typify MDD in general or relate more specifically to certain symptoms. It is possible, although inadequately investigated, that low BDNF levels relate to suicidality rather than to MDD specifically (Ahrens & Linden, 1996; Lee & Kim, 2011). Previous postmortem studies found lower levels of BDNF in the prefrontal cortex and hippocampus of patients who committed suicide, regardless of psychiatric diagnosis (Dwivedi et al., 2003; Karege, Vaudan, Schwald, Perroud, & La Harpe, 2005). Moreover, low levels of plasma BDNF have been reported in MDD subjects who attempted suicide when compared to non-suicidal MDD and healthy controls (Kim et al., 2007).
While these studies investigated actively suicidal MDD, the present study aims to assess the relationship between BDNF and mild-moderate SI in MDD. We quantified serum BDNF levels in MDD subjects with or without SI to examine the relationship between low serum BDNF to SI vs general depressive symptomatology. We also sought to isolate BDNF’s relationship with SI by also assessing its relationship with other aspects of depression, such as anxiety, overall severity of depression, appetite disturbance, physical activity, smoking and stress. While several studies have examined serum BDNF levels in actively suicidal patients with MDD or other psychiatric diagnosis, to our knowledge there are no studies that have examined serum BDNF levels in MDD patients with milder forms of suicidal ideation (i.e., non-lethal ideation in the absence of attempts or completed suicide). Investigation of the neurobiology of mild-to-moderate SI, not just active or completed suicides, is important in order to understand and identify an effective solution for suicide prevention. In this study, we sought to explore whether serum BDNF in MDD subjects with mild-to-moderate, sub-lethal suicidal ideation was lower than in MDD subjects with no suicidal ideation.
2. Methods
2.1. Subjects
The study was approved by the University of California, San Francisco (UCSF) Committee on Human Research and all subjects gave written informed consent to participate. Data are from the NIMH-funded “Cell Aging in Major Depression” study (R01MH083784). All subjects enrolled in this study through 12/24/2014, and who had BDNF data available, were included. We enrolled 68 unipolar MDD subjects who were all outpatients and were recruited by clinical referrals, newspaper advertisement, flyers, bulletin board notices, and Craigslist postings.
Healthy Controls (HC) were recruited by the same means and matched for age, gender, and ethnicity; 76 control subjects were enrolled. All psychiatric diagnoses, including MDD, were determined through Structural Clinical Interview for the DSM-IV (SCID-IV) (First, Spitzer, & Williams, 1997) and were confirmed through an additional clinical interview with a board- certified psychiatrist. Control subjects had no history of any DSM-IV-TR axis disorder, which was also confirmed by SCID-IV interview and MDD subjects must have scored a minimum of 17 on the 17-item Hamilton Depression Rating Scale (HDRS-17; Hamilton, 1960). All subjects must have been free of any psychotropic medication for at least six weeks, with the exception of prn short-acting sedative hypnotics, which were allowed in the MDD group for treating insomnia, up to a maximum of three times per week, but none within one week prior to enrollment and venipuncture for BDNF. Subjects were also free from any steroid-containing contraceptives, exogenous steroids, hormone supplements or vitamin supplements above the United States Recommended Daily Allowances, and any other potentially confounding medications for six weeks prior to enrollment. Demographics including group size, age, sex, body mass index (BMI), tobacco consumption, and platelet count are presented in Table 1. Data relating serum BDNF levels to SSRI treatment response were previously reported in a subsample of the present sample (Wolkowitz et al., 2011).
Table 1:
Sample demographics and clinical characteristics
| HC (N) |
MDD with No Suicidal Ideation (N) |
MDD with Suicidal Ideation (N) |
MDD NSI vs. MDD SI p-value |
Three Group Comparison p-value |
|
|---|---|---|---|---|---|
| Sex (% female) | 61.84 | 60.00 | 57.14 | 0.817 | 0.910 |
| BMI (kg/m2; M±SD) | 24.44±4.58 (76) |
26.21±4.95 (40) |
25.08±3.80 (28) | 0.290 | 0.140 |
| Age (years; M±SD) | 37.16±13.29 (76) |
38.33±14.75 (40) |
40.04±12.54(28) | 0.619 | 0.625 |
| Years of Education (M±SD) | 16.74±1.81 (76) |
16.13±2.21 (40) |
16.25±2.3 (28) | 0.822 | 0.250 |
| Smoking status (% smoking) | 7 (9.2) | 9 (22.5) | 8 (27.6) | 0.628 | 0.038* |
| Platelet count (M±SD) | 225.24±46.9 3 (71) | 215.79±54.57 (39) |
204.44±60.75 (27) |
0.431 | 0.200 |
| PSS score (M±SD) | 8.88±4.68 (76) |
22.89±6.98 (38) |
26.60±7.44 (25) | 0.049* | <0.001* |
| HDRS-17 score (M+SD) | - | 19.13±2.27 (40) | 21.36±3.99 (28) | 0.005* | - |
| Lifetime Depression Chronicity (months; adjusted; M±SD) | - | 106.95±102.94 (40) |
140.13±130.04 (27) |
0.249 | - |
| BDNF (z-scores) | −.08+.97 (75) |
.34±1.07 (40) | −.26±.82 (28) | .016 | .029 |
| YPAS (frequency x duration) | 4.86±3.14 (71) |
3.21±3.74 (38) |
2.90±3.23 (29) |
0.719 | 0.008* |
Significant at the p<0.05 level.
BMI: Body Mass Index, PSS: Perceived Stress Score, HDRS-17: 17 item Hamilton Depressive Rating Scale, YPAS: Yale Physical Activities Scale
Subjects were excluded if they were actively suicidal through clinician assessment and HDRS-17 ratings or had current psychosis or a history of bipolar disorder or psychotic symptoms that existed outside a major depressive episode. Subjects were also excluded if they had an eating disorder or post-traumatic stress disorder (PTSD) within one month of entering the study, or alcohol or substance dependence or abuse within six months of entering the study. Comorbid anxiety disorder diagnoses were allowed if the MDD diagnosis was judged to be primary.
Medical conditions were assessed with medical history, physical examination and routine blood screenings (chemistry panel, complete blood count, thyroid stimulating hormone, liver enzymes, cholesterol and lipids). Subjects were excluded if they had acute illnesses, infections, neurological disorders, chronic inflammatory disorders, or other major medical conditions that could potentially be confounding or if they had vaccinations within two months of the study.
2.2. Procedures
Subjects fasted since 22:00 h the night before, except water, and were admitted as outpatients to the UCSF Clinical and Translational Science Institute between 08:00 and 11:00 h. Once admitted, subjects had to first test negative on a urine toxicology screening and, if applicable, a urine pregnancy test. A blood draw was started after 25–45 minutes of relaxation. Blood for BDNF was collected into serum separator tubes (Vacutainer; BD, Franklin Lakes, NJ). After sitting at room temperature for one hour to allow clotting, blood was centrifuged at 2000 × g for 20 min, serum was stored at –80° C until assay.
After the blood draw, depression symptoms (including SI) were rated using the HDRS-17. The presence and the extent of suicidality was assessed through clinical interview and response to HDRS-17 item #3. In the event that SI was endorsed, study staff and clinicians followed risk-assessment protocols to determine the extent to which subjects may be a risk to themselves. Individuals who were determined by a clinician as having current active suicidal intent were excluded from the present study, due to its outpatient nature. Because of this, no subjects had HDRS-17 ratings of “4” on item #3 (see below for definitions of severity scores on this item), as this would indicate that the subjects had attempted suicide in the past week or were of imminent danger to themselves.
2.3. Assay for BDNF
Serum for BDNF was assayed in three batches due to different recruitment dates; the batches did not significantly differ in the proportion of SI vs non-SI subjects. To account for possible batch effects, BDNF data within each batch were standardized to z-scores, which were then combined across the batches. Serum BDNF values, therefore, are expressed in z-scores.
Serum was assayed for BDNF in duplicate, using a commercial BDNF ELISA assay kit (R&D Systems, Minneapolis, MN, USA, catalog # DBD00). Sera were diluted 1:60 with diluent supplied by the kit manufacturer, to obtain BDNF concentrations within the linear range of the standard curve. To evaluate inter-assay variability, an internal control consisting of serum obtained from a single individual, frozen in multiple aliquots, was run on each plate processed. BDNF concentrations of this control sample were measured on several different days and multiple 96-well plates. The R&D Systems Human BDNF Quantikine ELISA Kit was found to have an acceptable 8–14% inter-assay variability of this control sample, when measured on each plate run with MDD samples. Intra-assay CV was <10%, or samples were re-assayed.
2.4. Measures
2.4.1. Depression, Suicidal Ideation and Psychopathological Dimensions
The total HDRS-17 score was used to measure overall depression severity during the past week. The suicide item #3 was used as the rating for suicidal ideation, which has been studied as a valid measure for suicidal ideation (Desseilles et al., 2012). Subjects’ SI ratings ranged from 0–3, with scores of 3 indicating a higher degree of current suicidal plans or gestures (i.e., ideas or plans related to suicide, without current intent), scores of 2 representing a moderate degree of SI (i.e., wishes they were dead), scores of 1 representing a low degree of SI (i.e., feelings like life isn’t worth living), and scores of 0 indicating the absence of any SI. Subjects with ratings of 0 were classified as MDD with NSI, while those with ratings of 1–3 were classified as MDD with SI. As mentioned above, subjects with scores of 4 (attempts at suicide) were not admitted to the study.
The HDRS-17 can be divided into four psychopathological dimensions or subscales: “Somatic Anxiety/Somatization,” “Psychic Anxiety,” “Pure Depressive,” and “Anorexia” (Pancheri, Picardi, Pasquini, Gaetano, & Biondi, 2002). Past research found that these four subscales are a reliable factor structure found in the HDRS and are prominent in depression (Konstantakopoulos, Masdrakis, Markianos, & Oulis, 2013).
2.4.2. Stress
Stress was measured using the Perceived Stress Scale (PSS) questionnaire, a widely used psychological instrument for measuring the perception of stress (Cohen, Kamarck, & Mermelstein, 1983). The questionnaire consists of 10-items and asks participants the level of stress they experienced in the past month. Each item was rated from 0 (never) to 4 (very often), with higher scores reflecting greater perceived stress.
2.4.3. Physical Activity
Physical activity was measured using a modified Yale Physical Activity Survey (YPAS; Dipietro, Caspersen, Ostfeld, & Nadel, 1993). Subjects self-reported frequency and duration of vigorous physical activity in the past month. This modified YPAS “vigorous activity” measure was calculated by multiplying the subject’s frequency of vigorous exercise by the duration of activity.
2.5. Data Analysis
Statistical analyses were performed with IBM SPSS Statistics, version 22. Between-group comparisons of demographic and clinical characteristics were performed using t-test and Chi-Square tests as appropriate. BDNF levels were standardized to account for inter-assay batch variability and were shown to be normally distributed. Comparisons of BDNF between SI and NSI MDD subjects were conducted using independent sample t-test. Analysis of Covariance (ANCOVA) was used to additionally account for effects of potential confounds (age, sex, BMI, platelet count, and PSS) since these variables may be associated with BDNF (Duman & Monteggia, 2006; Lee, Kim, Park, & Kim, 2007). Differences in serum BDNF levels by suicidality status were assessed by between-group comparisons rather than my linear regression, due to the small number of subjects with the highest scores on the single HDRS suicidality item and due to the narrow range of values associated with a single item. These constraints did not apply to assessing the relationships between serum BDNF levels and total HDRS ratings and HDRS subscale ratings. Therefore, the latter were assessed by regression analysis, specifically, standard linear regressions were conducted to explore any associations between BDNF, depression severity and the four HDRS-17 subscales of depression identified by Pancheri and colleagues (2002). All tests were 2-tailed with an alpha=0.05.
3. Results
3.1. Sample Demographics
Subjects are grouped into Healthy Controls (HC), MDD with No Suicidal Ideation (MDD NSI) and MDD with Suicidal Ideation (MDD SI). Demographic and clinical characteristics of each group are included in Table 1. We found no significant between-group differences with regards to sex, ethnicity, BMI, age, years of education, or platelet count. PSS scores were significantly different between all three groups (p<0.001), with the HC group scoring the lower than both MDD groups. PSS was significantly different between SI and NSI groups, (p=0.049) with SI groups scoring higher, although this was not significantly related to BDNF levels (p=0.884). YPAS scores were significantly different between all three groups (p=0.008), but not significantly different between SI and NSI groups. Smoking status were also significantly differently between all three groups (p=0.038), but also not significantly different between SI and NSI groups.
3.2. Comparisons with Healthy Controls
BDNF concentrations were not significantly different between healthy controls and all MDD subjects combined (df=141, p= 0.292). When comparing MDD (N=68) subjects to HC (N=76) based on their SI status (NSI and SI) we found that there was a significant difference between HC, NSI and SI (df=2, F=3.641, p=0.029). Post-hoc analysis revealed only significant differences between MDD with SI and MDD with NSI (p=0.038), while the differences between HC and MDD SI (p=0.074) and HC and MDD NSI (p= 0.70) were not significant.
3.3. BDNF and Suicidal Ideation
Serum BDNF levels were significantly lower in MDD with SI (N=28) compared to MDD with NSI and the effect size of the two-group comparison was moderate (N=40; t=2.468, p=0.016, d=0.622). Further, this group difference remained significant when controlling for potential confounds (age, sex, BMI, platelet count and Perceived Stress Score, YPAS and smoking; df=1, F=4.442, p< 0.05). The following variables had no significant relationship with serum BDNF levels as follows: age (df=1, F=2.666, p=0.108), sex (df=1, F=1.039, p=0.313), BMI (df=1, F= 0.090, p=0.765), platelet count (df=1, F=0.053, p=0.818), and PSS (df=1, F= 0.021, p=0.884).
3.4. BDNF and Other Depressive Symptomatology
HRDS-17 total scores were significantly higher in the SI group compared to the NSI group (p=0.005). However, when we excluded the HDRS-17 suicide item from the total score, the two groups’ scores did not remain significantly different (p= 0.465). Serum BDNF levels were not significantly associated with overall depression severity (total HDRS-17 score; df=1, β=−0.098, p=0.424). Further, BDNF was not significantly associated with any HDRS-17 subscales that were assessed as exploratory independent variables (Pancheri et al., 2002). These results were as follows: somatic anxiety (df=1, β=0.78, p=0.525), psychic anxiety (df=1, β=−0.160, p=0.192), pure depressive dimension (df=1, β=−0.077, p=0.531), anorexia (df=1, β=−0.083, p=0.501).
4. Discussion
We found that serum BDNF levels in unmedicated subjects with MDD were significantly lower in those who expressed mild to moderate SI than those with NSI. To the best of our knowledge, this is the first study to find significantly lower BDNF levels in unmedicated MDD subjects with SI (in the absence of active attempts or recent attempts) compared to MDD with NSI. Our results are consistent with previous studies linking lower BDNF with suicidal behavior (Sher, 2011), and extend these findings by including MDD subjects with only mild to moderate suicidal ideation.
A small number of previous studies examined peripheral BDNF levels in suicidal attempters in MDD subjects (Kim et al., 2007; Lee et al., 2007), and one study was found to examine only suicide risk (Dawood et al., 2007). Dawood et al. (2007) found that there is a significantly lower jugular venous/peripheral arterial BDNF plasma concentration gradient (nominally reflecting lower brain production of BDNF) in MDD with medium to high risk for suicide as compared to MDD with low risk for suicide, indicating that there is a negative correlation between suicide risk and veno-arterial BDNF plasma levels (Dawood et al., 2007). Kim et al. (2007) found that suicide attempters with MDD had significantly lower plasma BDNF levels than non-suicide attempters with MDD and healthy controls (Kim et al., 2007a). Lee et al. (2007) also found that plasma BDNF was significantly lower in suicidal MDD with a suicide attempt when compared to non-suicidal MDD patients (Lee et al., 2007). Apart from MDD, lower serum BDNF has also been found in individuals that made suicide attempts and had clinical diagnosis of personality disorder and adjustment disorder compared to healthy controls (Grah et al., 2014). To our knowledge, no studies have compared BDNF levels in individuals with mild-to-moderate suicidal ideation, vs. those with active suicidality or suicide attempts; therefore, it is not known whether these exist on a continuum, with even lower levels in the actively suicidal individuals.
Fewer studies have examined BDNF levels in post-mortem brain samples from subjects who committed suicide (Castren & Kojima, 2017). Pandey et al. (2008) found low BDNF levels and tyrosine kinase B receptor signaling in brains of teenage suicide victims (Pandey et al., 2008). Dwivedi et al. (2003) found lower levels of BDNF in post-mortem brain samples in suicide completers compared to healthy controls (Dwivedi et al., 2003). Lower BDNF levels in brain tissue have also been found in subjects who completed suicide but were diagnosed with other psychiatric conditions other than MDD when compared with non-psychiatric healthy controls. These diagnosis include alcohol abuse, drug abuse, bipolar disorder schizoaffective disorder (Banerjee, Ghosh, Ghosh, Bhattacharyya, & Mondal, 2013; Dwivedi et al., 2003; Karege et al., 2005).
Although a number of previous studies have reported an association between low BDNF and suicidality, there are some inconsistencies in the literature. In one study, no significant association was found between BDNF levels and attempted suicide in subjects who completed suicide across different psychiatric diagnoses including MDD, anxiety disorder, alcohol abuse, substance abuse, eating disorder, and more, when compared to non-suicidal psychiatric controls and healthy controls (Eisen et al., 2016). However, in this study 40% of the suicidal subjects and 32% of the psychiatric controls were using antidepressants including SSRIs. This is relevant since antidepressants have been found to increase BDNF levels (Castrén & Kojima, 2017; Sen, Duman, & Sanacora, 2008; Wolkowitz et al., 2011), and could account for the non-significant relationship between groups. In another study, Huang and Lee (2006) found that there was no significant difference in BDNF levels between schizophrenia patients with a lifetime history of a suicide attempt and schizophrenia patients who had never attempted suicide (Huang & Lee, 2006). However, the amount of time between suicide attempt and blood collection is unknown and BDNF levels may vary over time as a response to external stimuli such as drinking, smoking, diet and endurance training (Eisen et al., 2015). Therefore, mixed results found in other studies may be a result of differences in study design, such as subjects’ use of medication and blood collection times.
We further examined this relationship between BDNF and suicidality in MDD by accounting for possible confounds, some of which have been previously studied in relation to MDD and suicidality. None of the possible confounds (age, sex, BMI and platelet count), additional variables that can affect BDNF levels (physical activity and smoking), or alternative ways of assessing depressive symptoms: overall depression severity, perceived stress, and the four HDRS subscales were found to be significantly associated with BDNF levels. Previous studies have found that sex may play a role in BDNF mRNA expression levels in suicidal patients. Specifically, male suicide victims had lower levels of BDNF mRNA than females (Kozicz, Tilburg-Ouwens, Faludi, Palkovits, & Roubos, 2008). However, we did not find that adjusting for sex altered the significant relationship between SI and BDNF. Severity of depression has been studied previously as a possible correlate of lower BDNF in MDD subjects, and studies have found no relation between depression severity and BDNF (Caldieraro et al., 2017; Wolkowitz et al., 2011). Additionally, in a study of active suicidal behavior, it was found that there were no significant correlations between plasma BDNF and depression severity scores in suicide attempters (Ambrus, Sunnqvist, Ekman, Traskman-Bendz, & Westrin, 2016). Other studies have found that depression severity is negatively associated with serum BDNF (Varambally et al., 2013). While the overall results are mixed, our findings agree with the studies that found no relationship between BDNF and depression severity. Another variable that has been examined is the relationship between decreased BDNF levels and exposure to stress. Animal studies found that after exposure to acute and chronic stress, mice and rats had reduced levels of BDNF (Duman & Monteggia, 2006; Smith, Makino, Kvetnansky, & Post, 1995). We found that perceived stress was significantly different between the SI and NSI groups, however, the perceived stress levels were not significantly related to BDNF. Physical activity, particularly endurance exercise, has been found to facilitate the production of proteins that regulate BDNF expression (Phillips, 2017), however we found no significant difference between SI and NSI groups.
Finally, our study did not find a significant difference in serum BDNF levels in unmedicated subjects with MDD (whether with SI or NSI) vs. healthy controls, although our power was limited to detect significant differences (we could only detect effect sizes of 0.47 or greater with 0.80 power, given our sample size). Previous studies examining the relationship between HC and MDD generally found relatively lower serum BDNF levels, without accounting for SI, in the MDD subjects compared to HC (Molendijk et al., 2014; Phillips, 2017). However, our study found results similar to Kim et al. (2007) who found that actively suicidal patients had the lowest levels of BDNF, and no significant difference between non-suicidal MDD and HC. While our subjects were not actively suicidal, the lowest levels of BDNF were found in those with SI, suggesting that suicidal ideation may be more specifically associated with lower levels of BDNF. While our overall between-group findings differ from the original neurotrophic hypothesis of depression, the generalizability of this hypothesis has been questioned, with suggestions that, “now is a critical time to reassess the original BDNF hypothesis of depression, and look towards the formation of new models that can provide a more valid account of the complex relationships between growth factors, mood disorders and their treatment” (Groves, 2007).
According to the neurotrophic hypothesis, the altered expression of BDNF as seen in MDD subjects can lead to increased neuronal atrophy or decreased neurogenesis in key limbic brain regions including the hippocampus and prefrontal cortex (Duman & Monteggia, 2006). This has been further extended by studies of actively suicidal MDD, which suggest that since BDNF plays a role in neuronal survival and function, the decreased BDNF expression in the hippocampus may play a role in generating a suicide risk. Although the mechanisms are unclear, one proposal is that this reduces neuronal plasticity, impairing an individual’s ability to adapt to stressful or crisis situations (Dawood et al., 2007; Dwivedi, 2012; Lee & Kim, 2011). Related to this, corticosteroids may decrease BDNF levels (Issa, Wilson, Terry, & Pillai, 2010; Schaaf, De Kloet, & Vreugdenhil, 2000), providing another mechanism by which stress may decrease BDNF levels, although cortisol levels are not routinely elevated in individuals with suicide attempt histories (O’Connor, Ferguson, Green, O’Carroll, & O’Connor, 2016). Other studies of actively suicidal subjects imply that addressing low BDNF levels with antidepressant treatment and therapy could strengthen neural integrity and aid in the recovery from mental disorders and also prevent suicide attempts (Schmidt & Duman, 2010). Our study was limited to an examination of serum BDNF levels, which bear an uncertain relationship to brain or CSF levels of BDNF, although it has been found that blood and plasma BDNF reflect brain tissue BDNF levels across several animal species (Klein et al., 2011).
Strengths of our study include the study of well-phenotyped, medically healthy MDD and control subjects who were unmedicated for at least six weeks prior to enrollment. Subjects with co-morbid psychiatric or medical illnesses that could potentially interfere with blood biomarkers were excluded. Further, we adjusted for several variables thought to be important in determining serum BDNF levels, and we found that none of these altered our findings. In addition, we assessed a more isolated relationship between SI and BDNF by assessing BDNF relationships with global severity and with severity of specific depressive subscales. Finally, our study adds to the literature by demonstrating a relationship with even mild-to-moderate SI, whereas the majority of prior studies have looked at severe SI through suicide attempts and completions.
Our study has several limitations. We assessed serum BDNF, and peripheral BDNF has an uncertain relationship with brain levels of BDNF (Kim et al., 2007; Salas-Magaña et al.,2017). The origins of serum BDNF levels are still uncertain and it is suggested to be derived from platelet stores and vascular endothelial cells as well as from neurons and glial cells in the brain if it crosses the blood-brain barrier (Guo et al., 2008; Kim et al., 2007). In addition, BDNF levels in human serum are found to be higher than in human plasma and according to some studies the relationship between plasma BDNF and depression varies (Bocchio-Chiavetto et al., 2010; Karege et al., 2005). Also, our findings are based on a single time point of BDNF measures, and our sample size was relatively small and therefore a replication of this study with a bigger cohort and more serum BDNF assessments would strengthen these findings. Further, since this was a cross-sectional study, we cannot assess whether BDNF associations with SI are “state” or “trait” markers in this population. Lastly, there are many other relevant factors that affect BDNF levels, and we did not assess other factors such as epigenetics or genetic polymorphisms.
5. Conclusion
Our finding presents a novel extension of the neurotrophic hypothesis of depression to include BDNF levels as a peripheral marker associated with mild to moderate SI within MDD. While some studies have examined BDNF levels in suicidal attempts and completion, our assessment of SI and its relationship to BDNF levels provides some insight into the neurobiology of depression and suicidality and should be further explored to understand the mechanisms by which SI develops, although our data do not specifically address causal relationships.
Figure 1:
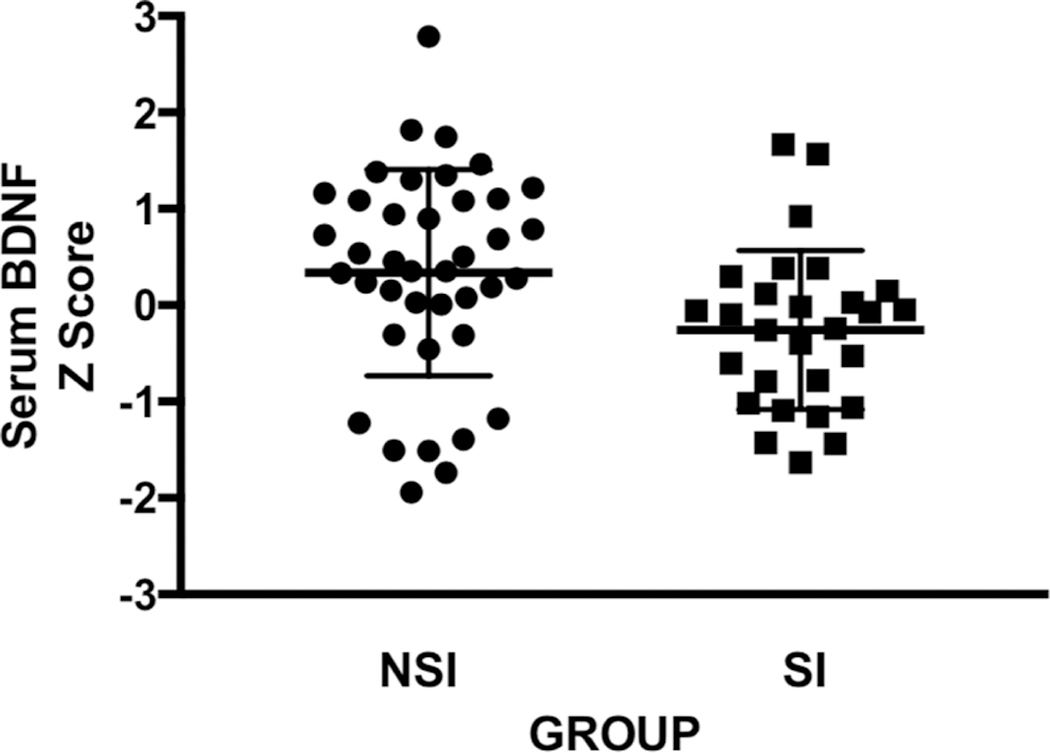
Scatter plot of standardized serum BDNF concentrations for MDD with no suicidal ideation (MDD NSI N=40) and MDD with suicidal ideation (MDD SI N=28).
Highlights.
BDNF levels are lower in MDD with SI than MDD with NSI (p=0.016)
BDNF differed in MDD with mild-to-moderate suicidal ideation without active intent
Findings extend neurotrophic hypothesis of depression to include suicidal ideation in MDD
We found no significant difference between HC and MDD based on their SI status.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Phuong Hoang and the nursing and other staff of UCSF CTSI Clinical Research Center, and all past and present UCSF PNE Lab volunteer research assistants, as well as all of the participants in this research.
Funding
This study was funded by grants from the National Institute of Mental Health (NIMH; Grant Number R01-MH083784), the O’Shaughnessy Foundation, the Tinberg family, and grants from the UCSF Academic Senate, the UCSF Research Evaluation and Allocation Committee (REAC). This project was also supported by National Institutes of Health/National Center for Research Resources (NIH/NCRR) and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Daniel Lindqvist was supported by the Swedish Research Council (registration number 2015–00387), Marie Sklodowska Curie Actions, Cofund (Project INCA 600398), the Swedish Society of Medicine, the Söderström-Königska Foundation, the Sjöbring Foundation, OM Persson Foundation and the province of Scania (Sweden) state grants (ALF). Christina Hough is supported by the Graduate Division of the University of California, Los Angeles, and the National Science Foundation Graduate Research Fellowship Program (NSF Grant Number DGE-1650604). None of the granting or funding agencies had a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript. The Co-Principal Investigators, Owen Wolkowitz, MD, and Synthia Mellon, PhD, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrens B, & Linden M (1996). Is there a suicidality syndrome independent of specific major psychiatric disorder? Results of a split half multiple regression analysis. Acta Psychiatrica Scandinavica, 94(2), 79–86. 10.1111/j.1600-0447.1996.tb09829.x [DOI] [PubMed] [Google Scholar]
- Ambrus L, Sunnqvist C, Ekman R, Träskman-Bendz L, & Westrin Å (2016). Plasma Brain- Derived Neurotrophic Factor and Psychopathology in Attempted Suicide. Neuropsychobiology, 73(4), 241–248. 10.1159/000446286 [DOI] [PubMed] [Google Scholar]
- Banerjee R, Ghosh AK, Ghosh B, Bhattacharyya S, & Mondal AC (2013). Decreased mRNA and Protein Expression of BDNF, NGF, and their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clinical Medicine Insights: Pathology, 6, CPath.S12530. 10.4137/CPath.S12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Gabriela Nielsen M, Placentino A, ... Gennarelli M. (2010). Serum and plasma BDNF levels in major depression: A replication study and meta-analyses. The World Journal of Biological Psychiatry, 11(6), 763–773. 10.3109/15622971003611319 [DOI] [PubMed] [Google Scholar]
- Caldieraro MA, Vares EA, Souza LH, Spanemberg L, Guerra TA, Wollenhaupt-Aguiar B, ... Fleck MP. (2017). Illness severity and biomarkers in depression: Using a unidimensional rating scale to examine BDNF. Comprehensive Psychiatry, 75, 46–52. 10.1016/j.comppsych.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Castrén E, & Kojima M (2017). Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiology of Disease, 97, 119–126. https://doi.org/10.1016Zj.nbd.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2015). Suicide claims more lives than war, murder, and natural disasters combined. Retrieved from https://afsp.donordrive.com/index.cfm?fuseaction=cms.page&id=1226&cmsContentSetID=D5C4DC12-C299-258B-B0B6FCF9EF015CE0
- Cohen S, Kamarck T, & Mermelstein R (1983). A Global Measure of Perceived Stress. Journal of Health and Social Behavior, 24(4), 385 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Crosby AE, Cheltenham MP, & Sacks JJ (1999). Incidence of Suicidal Ideation and Behavior in the United States, 1994. Suicide and Life-Threatening Behavior, 29(2), 131–140. [PubMed] [Google Scholar]
- Dawood T, Anderson J, Barton D, Lambert E, Esler M, Hotchkin E, ... Lambert G. (2007). Reduced overflow of BDNF from the brain is linked with suicide risk in depressive illness. Molecular Psychiatry, 12(11), 981–983. 10.1038/sj.mp.4002059 [DOI] [PubMed] [Google Scholar]
- Desseilles M, Perroud N, Guillaume S, Jaussent I, Genty C, Malafosse A, & Courtet P (2012). Is it valid to measure suicidal ideation by depression rating scales? Journal of Affective Disorders, 136(3), 398–404. 10.1016/jjad.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Dipietro L, Caspersen CJ, Ostfeld AM, & Nadel ER (1993). A survey for assessing physical activity among older adults. Medicine and Science in Sports and Exercise, 25(5), 628–642. [PubMed] [Google Scholar]
- Duman RS, & Monteggia LM (2006). A Neurotrophic Model for Stress-Related Mood Disorders. Biological Psychiatry, 59(12), 1116–1127. 10.1016/j.biopsych.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Dwivedi Y (Ed.). (2012). The neurobiological basis of suicide. Boca Raton, FL: Taylor & Francis/CRC Press. [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, & Pandey GN (2003). Altered Gene Expression of Brain-Derived Neurotrophic Factor and Receptor Tyrosine Kinase B in Postmortem Brain of Suicide Subjects. Archives of General Psychiatry, 60(8), 804 10.1001/archpsyc.60.8.804 [DOI] [PubMed] [Google Scholar]
- Eisen RB, Perera S, Banfield L, Anglin R, Minuzzi L, & Samaan Z (2015). Association between BDNF levels and suicidal behaviour: a systematic review and meta-analysis. Systematic Reviews, 4(1). 10.1186/s13643-015-0179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RB, Perera S, Bawor M, Dennis BB, El-Sheikh W, DeJesus J, ... Samaan Z. (2016). Exploring the Association between Serum BDNF and Attempted Suicide. Scientific Reports, 6(1). 10.1038/srep25229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, & Williams JB (1997). User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version. American Psychiatric Pub. [Google Scholar]
- Grah M, Mihanovic M, Ruljancic N, Restek-Petrovic B, Molnar S, & Jelavic S (2014). Brain-derived neurotrophic factor as a suicide factor in mental disorders. Acta Neuropsychiatrica, 26(06), 356–363. 10.1017/neu.2014.27 [DOI] [PubMed] [Google Scholar]
- Groves JO (2007). Is it time to reassess the BDNF hypothesis of depression? Molecular Psychiatry, 12(12), 1079–1088. 10.1038/sj.mp.4002075 [DOI] [PubMed] [Google Scholar]
- Guo S, Kim WJ, Lok J, Lee S-R, Besancon E, Luo B-H, ... Lo EH. (2008). Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proceedings of the National Academy of Sciences, 105(21), 7582–7587. 10.1073/pnas.0801105105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, & Lee C (2006). Associations between serum brain-derived neurotrophic factor levels and clinical phenotypes in schizophrenia patients. Journal of Psychiatric Research, 40(7), 664–668. 10.1016/jjpsychires.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Ihara K, Yoshida H, Jones PB, Hashizume M, Suzuki Y, Ishijima H, ... Hachisu M. (2016). Serum BDNF levels before and after the development of mood disorders: a case–control study in a population cohort. Translational Psychiatry, 6(4), e782–e782. 10.1038/tp.2016.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa G, Wilson C, Terry AV, & Pillai A (2010). An inverse relationship between cortisol and BDNF levels in schizophrenia: Data from human postmortem and animal studies. Neurobiology of Disease, 39(3), 327–333. https://doi.org/10.1016Zj.nbd.2010.04.017 [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, & La Harpe R (2005). Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Molecular Brain Research, 136(1–2), 29–37. 10.1016/j.molbrainres.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Kim Y-K, Lee H-P, Won S-D, Park E-Y, Lee H-Y, Lee B-H, ... Choi S-H. (2007a). Low plasma BDNF is associated with suicidal behavior in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 31(1), 78–85. 10.1016/j.pnpbp.2006.06.024 [DOI] [PubMed] [Google Scholar]
- Kim Y-K, Lee H-P, Won S-D, Park E-Y, Lee H-Y, Lee B-H, ... Choi S-H. (2007b). Low plasma BDNF is associated with suicidal behavior in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 31(1), 78–85. 10.1016/j.pnpbp.2006.06.024 [DOI] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Ikuta T, & Iwata N (2018). Brain-Derived Neurotrophic Factor and Major Depressive Disorder: Evidence from Meta-Analyses. Frontiers in Psychiatry, 8 10.3389/fpsyt.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, ... Aznar S. (2011). Blood BDNF concentrations reflect brain-tissue BDNF levels across species. The International Journal of Neuropsychopharmacology, 14(03), 347–353. 10.1017/S1461145710000738 [DOI] [PubMed] [Google Scholar]
- Konstantakopoulos G, Masdrakis VG, Markianos M, & Oulis P (2013). On the Differential Diagnosis of Anxious from Nonanxious Major Depression by means of the Hamilton Scales. The Scientific World Journal, 2013, 1–4. 10.1155/2013/294516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, & Roubos E (2008). Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience, 152(4), 1015–1023. 10.1016/j.neuroscience.2007.12.050 [DOI] [PubMed] [Google Scholar]
- Lee B-H, Kim H, Park S-H, & Kim Y-K (2007). Decreased plasma BDNF level in depressive patients. Journal of Affective Disorders, 101(1–3), 239–244. 10.1016/j.jad.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Lee B-H, & Kim Y-K (2010). The Roles of BDNF in the Pathophysiology of Major Depression and in Antidepressant Treatment. Psychiatry Investigation, 7(4), 231 10.4306/pi.2010.7A231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-H, & Kim Y-K (2011). Potential peripheral biological predictors of suicidal behavior in major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(4), 842–847. https://doi.org/10.1016Zj.pnpbp.2010.08.001 [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Spinhoven P, Polak M, Bus BAA, Penninx BWJH, & Elzinga BM (2014). Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Molecular Psychiatry, 19(7), 791–800. 10.1038/mp.2013.105 [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Ferguson E, Green JA, O’Carroll RE, & O’Connor RC (2016). Cortisol levels and suicidal behavior: A meta-analysis. Psychoneuroendocrinology, 63, 370–379. 10.1016/j.psyneuen.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Pancheri P, Picardi A, Pasquini M, Gaetano P, & Biondi M (2002). Psychopathological dimensions of depression: a factor study of the 17-item Hamilton depression rating scale in unipolar depressed outpatients. Journal of Affective Disorders, 68(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, & Dwivedi Y (2008). Brain- derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. The International Journal of Neuropsychopharmacology, 11(08), 1047 10.1017/S1461145708009000 [DOI] [PubMed] [Google Scholar]
- Phillips C (2017). Brain-Derived Neurotrophic Factor, Depression, and Physical Activity: Making the Neuroplastic Connection. Neural Plasticity, 2017, 1–17. 10.1155/2017/7260130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Magaña M, Tovilla-Zárate CA, González-Castro TB, Juárez-Rojop IE, López- Narváez ML, Rodríguez-Pérez JM, & Ramírez Bello J (2017). Decrease in brain-derived neurotrophic factor at plasma level but not in serum concentrations in suicide behavior: A systematic review and meta-analysis. Brain and Behavior, 7(6), e00706 10.1002/brb3.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, De Kloet ER, & Vreugdenhil E (2000). Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress (Amsterdam, Netherlands), 3(3), 201–208. [DOI] [PubMed] [Google Scholar]
- Schimelpfening N (2017, March 16). Tips for Coping with Suicidal Thoughts. Retrieved from https://www.verywell.com/tips-for-coping-with-suicidal-thoughts-1067530
- Schmidt HD, & Duman RS (2010). Peripheral BDNF Produces Antidepressant-Like Effects in Cellular and Behavioral Models. Neuropsychopharmacology, 35(12), 2378–2391. 10.1038/npp.2010.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Duman R, & Sanacora G (2008). Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biological Psychiatry, 64(6), 527–532. https://doi.org/10.1016Zj.biopsych.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L (2011). Brain-derived neurotrophic factor and suicidal behavior. QJM, 104(5), 455–458. 10.1093/qjmed/hcq207 [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, & Post RM (1995). Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 15(3 Pt 1), 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S, Naveen G, Rao M, Thirthalli J, Sharma R, Christopher R, & Gangadhar B (2013). Low serum brain derived neurotrophic factor in non-suicidal out-patients with depression: Relation to depression scores. Indian Journal of Psychiatry, 55(7), 397 10.4103/0019-5545.116311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Wolf J, Shelly W, Rosser R, Burke HM, Lerner GK, ... Mellon SH. (2011). Serum BDNF levels before treatment predict SSRI response in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(7), 1623–1630. 10.1016/j.pnpbp.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2017, February). Depression. Retrieved from http://www.who.int/mediacentre/factsheets/fs369/en/


