Abstract
The introduction of immune checkpoint blockade (ICB) therapy has transformed the management of advanced bladder cancer (BC). Despite its limitations, PD-L1 immunohistochemistry may serve as a predictive biomarker of anti-PD-L1/PD1 therapy. While urothelial carcinoma (UC) patients with predominant or pure variant histology (UCV) account for up to one third of advanced cases, to date, most ICB bladder cancer studies have excluded patients with such histologies. To assess the potential utility of ICB in patients with UCV, we analyzed PD-L1 expression in UCV and compared three commonly used and commercially available PD-L1 antibodies. Full sections from 84 UCV cases were stained with clones SP263, 22C3 and SP142, all of which are considered predictive assays to identify UC patients who are more likely to respond to anti-PD-1/PD-L1 inhibitors durvalumab, pembrolizumab and atezolizumab, respectively. Expression on tumor cells (TC) and tumor infiltrating immune cells (IC) was assessed. Staining extent and characteristics were evaluated and concordance among the three clones was determined at various cutoff points as used in previous studies in BC. We found that PD-L1 was expressed in a significant percentage of UCV at different cutoff points (cutoff 1% TC: 37%−54%, cutoff 5% TC: 23%−37%) with the highest expression in UC with squamous differentiation. These figures are equal to or higher than those for classic/pure UC (4%−30%). The results suggest that patients with UCV may benefit from anti PD-1/PD-L1 therapy and argue against exclusion of UC with predominant or pure variant histology from clinical ICB studies. The highest expression in both TC and IC was observed with clone SP263, followed by 22C3 and SP142 and all clones showed strong agreement in pairwise comparison both in TC and IC (R-values: 0.780–0.901) which indicates that all three clones are potentially useful in the evaluation of PD-L1 expression in UCV.
Keywords: Urothelial Carcinoma, Variant Histology, PD-L1, Immunohistochemistry, concordance, Immune therapy
Introduction
Bladder cancer (BC) is the fifth most common cancer in the United States and the ninth most frequent worldwide.(1) In the US, it is estimated that there will be more than 81,000 new BC cases and more than 17,000 deaths in 2018.(2) Although the majority are detected as non-muscle-invasive BC (NMIBC, i.e. in stages pTa/pT1), 90% of reported deaths are associated with advanced or metastatic disease. Cisplatin-based combination chemotherapy has been the gold standard for the treatment of advanced or metastatic BC for the last 30 years.(3) However, platinum-based regimens are associated with serious toxicities and complete and/or durable remissions are rare. In addition, approximately 50% of patients are cisplatin-ineligible and such patients typically receive less effective chemotherapeutic agents. Recently, immune checkpoint inhibitors have been shown to induce durable responses in a subset of patients with progressive disease following chemotherapy and in those who are cisplatin-ineligible.(4) The durability and tolerability of immune checkpoint blockade (ICB) therapy resulted in FDA approval of five immune checkpoint inhibitors targeting either the programmed cell death protein 1 (PD-1, CD279) or programmed death-ligand 1 (PD-L1, CD274). As only 20–25% of treated patients with advanced or metastatic BC respond to ICB, predictive biomarkers of response are clearly needed to select those patients most likely to derive clinical benefit. (4)
Markers that have been associated with ICB response include PD-L1 expression as assessed by immunohistochemistry (IHC), tumor mutational burden (TMB), molecular subtyping as defined by The Cancer Genome Atlas (TCGA) classification, and immune cell profiling. Although each of these assessments correlate with response in a subset of patients, none alone is a robust predictive biomarker.(4) Despite these limitations, PD-L1 IHC offers a practical and rapid assay that may help to guide therapeutic decision making. Specifically, higher PD-L1 expression is enriched in responders to ICB in most BC-related studies.(5–8) In the multiple studies that led to FDA approval, however, different PD-L1 IHC clones were utilized, each with a unique epitope and with no standardized evaluation method or cutoff level employed.(5–11) Apart from these methodological differences, the inherent morphologic and genomic heterogeneity observed in BC may have confounded efforts by investigators to understand the different PD-L1 expression rates and ICB response rates observed in different trials.
At the histomorphological level, the heterogeneity of BC is reflected by the presence of divergent differentiation which is found in up to one third of invasive UC and has been associated with worse prognosis compared to conventional UC.(12, 13) However, few studies have explored PD-L1 expression in UC with predominant or pure variant histology.(14–16) While Pichler et al. found a higher expression rate in UC with variant histology (UCV) compared to pure UC (16) and Tretiakova et al. reported a high concordance of four commonly used PD-L1 antibodies used in UC employing different techniques, detailed annotation of UCV cases was unavailable.(15) Furthermore, despite its prevalence, UCV has been largely excluded from most ICB studies. With evidence suggesting higher TMB in certain subtypes (e.g. plasmacytoid and small cell carcinoma) (17, 18), some patients with variant histologies might benefit from ICB. Furthermore, assessing PD-L1 expression in UCV may become more relevant following the recent announcement by the FDA restricting first line ICB therapy for cisplatin-ineligible patients only to those whose tumors express PD-L1 as evaluated by clones 22C3 and SP142 for treatment with pembrolizumab and atezolizumab, respectively.(19)
In this study, we therefore evaluated PD-L1 expression of both tumor cells (TC) and immune cells (IC) in a cohort of UCV and compared the results of three different PD-L1 antibodies commonly used in BC.
Material and Methods
Cohort
The study was approved by the MSKCC institutional Review Board. A total of 84 cases of UCV were retrospectively selected from the archives of the Department of Pathology at the Memorial Sloan Kettering Cancer Center and included the following variant histologies: micropapillary UC (n=19), UC with squamous differentiation (n=16), nested UC (n=14), plasmacytoid UC (n=14), small cell carcinoma (n=12), and UC with glandular differentiation (n=9). A region of classic UC component (not-otherwise specified, NOS) was present in 17 of 84 cases on the same slide (10 micropapillary UC, 4 UC with squamous differentiation, 2 UC with glandular differentiation, 1 small cell carcinoma).
Immunohistochemistry (IHC)
PD-L1 IHC was performed using 4μm thick full sections. All staining was carried out on a Benchmark Ultra System (Ventana Medical Systems, Tucson, AZ, USA) with antibody visualization using the OptiView DAB IHC Detection Kit (Ventana Medical Systems) according to the manufacturer’s instructions. Three different PD-L1 antibodies were applied in every case: clone SP263 (Ventana Medical Systems; retrieval: CC1 40’; incubation: 32’; ready to use [RTU] dilution); clone 22C3 (Dako Agilent Technologies, Santa Clara, CA, USA; retrieval: CC1 40’; incubation: 40’; RTU dilution); clone SP142 (Ventana Medical Systems; retrieval: CC1 48’; incubation: 16’; RTU dilution). Positive controls were included in every run (placenta and/or tonsil tissue).
All slides were manually and independently reviewed by two genitourinary pathologists (HAA, HR) and consensus was achieved in cases of initial discrepancy. The entire tumor regions of whole slide sections were evaluated. Assessment of PD-L1 expression on TC and IC was performed. In sections containing NOS UC components in addition to the variant histology, both areas were evaluated and scored separately to investigate the presence of intra-tumoral heterogeneity of PD-L1 expression.
The percentage of PD-L1 positive TC of the total number of TC over the entire tumor region was evaluated. In addition, an H-Score was calculated as the sum of the percentage of strong (x3), moderate (x2) and weak (x1) immunoreactivity of all TC resulting in a value range of 0–300.(20) PD-L1 expression in tumor infiltrating IC was assessed as the percentage of PD-L1 expressing leucocytes and/or macrophages within the tumor area.
Statistics
Statistical analyses were done using SPSS (v.23; IBM, Armonk, NY, USA). Non-parametrical Spearman rank correlation analyses were performed. As most studies that led to FDA approval of anti-PD-1/PD-L1 agents in BC used cutoff values of 1% or 5%, we also used these values in further analyses.(6, 8, 11) In addition, further cutoff criteria adapted from studies of atezolizumab (IC0, IC1 and IC2/3 if <1%, 1%-<5% or ≥5% immune cells express PD-L1, respectively)(5, 9), durvalumab (TC ≥ 25% or IC ≥ 25%) (7, 21) and pembrolizumab (a combined positive score of TC+IC ≥ 10%)(8, 10) were also evaluated. These adapted score criteria were used to maximize comparability of the data across different studies from one basic data collection without the need of re-evaluation for each scoring system and thus minimizing data scattering effects.
Results
PD-L1 is expressed in UCV
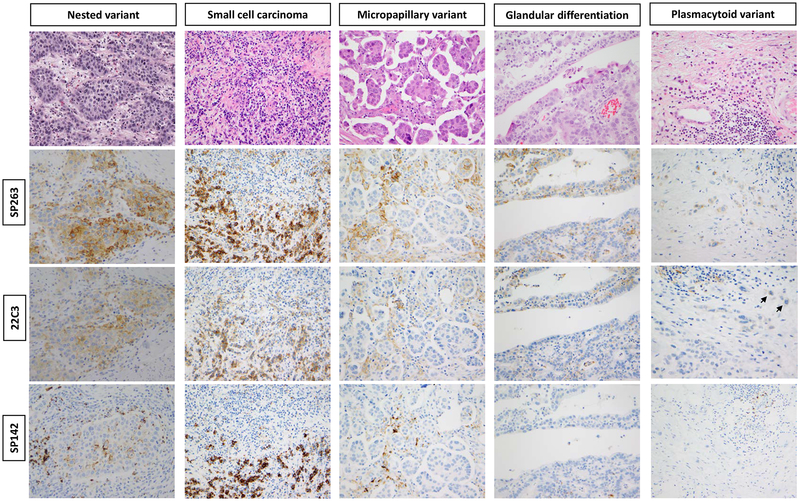
When the cutoff was set to 1% TC reactivity to define positivity, a total of 45 cases (54%) were positive for PD-L1 with the SP263 clone followed by 36 cases (43%) with the 22C3 clone and 31 cases (37%) with the SP142 clone (detailed results in Table 1a). With all three clones, the highest number of positive cases and the highest mean value of positive TC was found in UC with squamous differentiation. Other UCV entities exhibited lower percentages of positive cases and lower mean numbers of positive TC per case. The mean numbers of IC were higher compared to TC reactivity in some (micropapillary UC, nested UC, and UC with glandular differentiation) variant histologies (Figure 1 and Table 1a).
Table 1.
Rates of PD-L1 expression in UCV using cutoff values to define positivity as 1% of TC (Table 1a) or 5% (Table 1b)
| Table 1a | SP263 | SP142 | 22C3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histologic differentiation (n) | Tumors pos.* (n, %) | TC (%; mean) | TC (H-score, mean) | IC (%; mean | Tumors pos.* (n, %) | TC (%; mean) | TC (H-score, mean) | IC (%; mean | Tumors pos.* (n, %) | TC (%; mean) | TC (H-score, mean) | IC (%; mean |
| Micropapillary (19) | 13 (69%) | 5 | 7 | 12 | 6 (32%) | 2 | 4 | 5 | 7 (37%) | 2 | 4 | 8 |
| Squamous differentiation (16) | 14 (88%) | 42 | 86 | 12 | 14 (88%) | 20 | 43 | 9 | 15 (94%) | 30 | 50 | 14 |
| Nested (14) | 5 (36%) | 4 | 7 | 10 | 1 (7%) | 3 | 8 | 4 | 4 (29%) | 4 | 7 | 8 |
| Plasmacytoid (14) | 5 (36%) | 5 | 8 | 9 | 3 (21%) | 1 | 1 | 3 | 2 (14%) | 8 | 3 | 4 |
| Small cell carcinoma (12) | 2 (17%) | 9 | 24 | 7 | 2 (17%) | 7 | 19 | 3 | 2 (17%) | 3 | 18 | 6 |
| Glandular differentiation (9) | 6 (67%) | 6 | 9 | 14 | 5 (56%) | 2 | 2 | 5 | 6 (67%) | 9 | 4 | 10 |
| Total (84) | 45 (54%) | 13 | 25 | 11 | 31 (37%) | 6 | 13 | 5 | 36 (43%) | 9 | 15 | 8 |
| Table 1b | SP263 | SP142 | 22C3 | |||||||||
| Histologic differentiation (n) | Tumors pos.* (n, %) | TC (%; mean) | TC (H-score, mean) | IC (%; mean | Tumors pos.* (n, %) | TC (%; mean) | TC (H-score, mean) | IC (%; mean | Tumors pos.* (n, %) | TC (%; mean) | TC (H-score, mean) | IC (%; mean |
| Micropapillary (19) | 6 (32%) | 5 | 7 | 12 | 2 (11%) | 2 | 4 | 5 | 3 (16%) | 2 | 4 | 8 |
| Squamous differentiation (16) | 14 (88%) | 42 | 86 | 12 | 13 (81%) | 20 | 43 | 9 | 15 (94%) | 30 | 50 | 14 |
| Nested (14) | 1 (7%) | 4 | 7 | 10 | 1 (7%) | 3 | 8 | 4 | 1 (7%) | 4 | 7 | 8 |
| Plasmacytoid (14) | 3 (21%) | 5 | 8 | 9 | 0 (0%) | 1 | 1 | 3 | 2 (14%) | 8 | 3 | 4 |
| Small cell carcinoma (12) | 2 (17%) | 9 | 24 | 7 | 2 (17%) | 7 | 19 | 3 | 2 (17%) | 3 | 18 | 6 |
| Glandular differentiation (9) | 5 (56%) | 6 | 9 | 14 | 1 (11%) | 2 | 2 | 5 | 2 (22%) | 9 | 4 | 10 |
| Total (84) | 31 (37%) | 13 | 25 | 11 | 19 (23%) | 6 | 13 | 5 | 25 (30%) | 9 | 15 | 8 |
TC: Tumor cells, IC: immune cells
Figure 1. PD-L1 immunoreactivity in UC variants.
An example of UC nest variant with diffuse PD-L1 expression on TC and IC. The extent of expression is highest with clone SP263 followed by 22C3 and least by SP142. Similar findings in an example of small cell carcinoma but less obvious expression in immune cells. In examples of micropapillary UC and UC with glandular differentiation, weak PD-L1 expression in TC but more prominent on IC that also decreased from SP263 to 22C3 to SP142. An example of plasmacytoid UC with weak expression on TC by clone SP263, nearly absent expression with 22C3 (short arrows) and no expression with SP142.
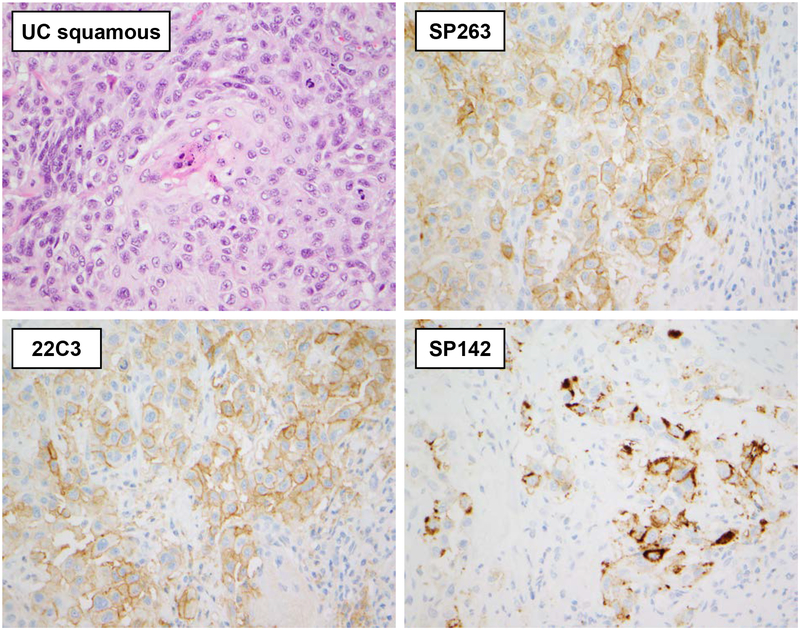
When the cutoff was set to 5% TC reactivity, the results remained comparable albeit with a drop in the mean number of positive cases (Table 1b). The number of positive cases was 31 (37%) with the SP263 clone, 25 (30%) with the 22C3 clone, and 19 (23%) with the SP142 clone (Table 1b). The positivity rate in UC with squamous differentiation, however, remained high due to the high mean number of positive TC per case (Figure 2). In addition, the positivity rate in small cell UC remained stable in analyses with all 3 antibodies with only two of 12 cases being positive (17%).
Figure 2. Staining characteristics of three PD-L1 clones in UC with squamous differentiation.
In this example of UC with squamous differentiation, membranous and focally circumferential staining of the SP263 and 22C3 PD-L1 clones in TC is evident. Clone SP142 shows a coarser and more granular TC immunoreactivity which in some cases was difficult to discriminate from IC reactivity. All 400×
The H-score characteristics followed the trends of the percentage of positive TC with the highest mean H-score observed with the SP263 clone (25), followed by the 22C3 clone (15), and the SP142 clone (13). The same distribution was detected when the (adapted) criteria for positivity from the clinical studies in BC were applied (Table 2).
Table 2.
Rates of positive UCV cases using (adapted) criteria of clinical atezolizumab, pembrolizumab and durvalumab trials.
| Table 2 | Atezolizumab criteria* | Pembrolizumab criteria* | Durvalumab criteria | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Histologic differentiation (n) | IC2/3 (SP263) (n, %) | IC2/3 (SP142) (n, %) | IC2/3 (22C3) (n, %) | TC+IC ≥10% (SP263) (n, %) | TC+IC ≥10% (SP142) (n, %) | TC+IC ≥10% (22C3) (n, %) | TC or IC ≥25% (SP263) (n, %) | TC or IC ≥25% (SP142) (n, %) | TC or IC ≥25% (22C3) (n, %) |
| Micropapillary (19) | 13 (68%) | 6 (32%) | 8 (42%) | 11 (58%) | 6 (32%) | 7 (37%) | 6 (32%) | 1 (5%) | 3 (16%) |
| Squamous differentiation (16) | 14 (88%) | 12 (75%) | 14 (88%) | 14 (88%) | 12 (75%) | 14 (88%) | 1 (6%) | 1 (6%) | 2 (13%) |
| Nested (14) | 8 (57%) | 3 (21%) | 4 (29%) | 5 (36%) | 2 (14%) | 3 (21%) | 4 (29%) | 1 (7%) | 2 (14%) |
| Plasmacytoid (14) | 12 (86%) | 3 (21%) | 7 (50%) | 7 (50%) | 1 (7%) | 2 (14%) | 2 (14%) | 0 (0%) | 0 (0%) |
| Small cell carcinoma (12) | 6 (50%) | 2 (17%) | 5 (42%) | 4 (33%) | 2 (17%) | 3 (25%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Glandular differentiation (9) | 8 (89%) | 5 (56%) | 8 (89%) | 7 (78%) | 2 (22%) | 4 (44%) | 2 (22%) | 2 (22%) | 1 (11%) |
| Total (84) | 61 (73%) | 31 (37%) | 46 (55%) | 48 (57%) | 25 (30%) | 33 (39%) | 15 (18%) | 5 (6%) | 8 (10%) |
IC2/3: immune cell reactivity in ≥5% of tumor-associated IC (*adapted IC score), TC+IC ≥10%: combined positive score as a sum of tumor cell and immune cell reactivity in ≥10% (*adapted positive score), TC or IC ≥25%: immunoreactivity in tumor cells or immune cells in ≥25%.
Staining and assay differences between the SP263, 22C3 and SP142 clones
The staining characteristics of the SP263 and 22C3 clones were comparable (Figures 1 and 2). Both showed finely dispersed membranous and circumferential expression in TC as well as clearly identifiable IC reactivity in all different histologies. The SP142 clone showed a coarse and clumpy pattern of expression in TC and IC with mostly strong intensity. The staining characteristics were identical in the NOS and variant histology components. However, in UC with squamous differentiation, stronger areas of PD-L1 expression in TC were noted at the periphery/invasive front of tumor. PD-L1 immunostaining was mostly weaker and more focal in the remaining entities.
Any percentage of strong PD-L1 immunostaining was observed in 22 of 84 cases (26%) with the SP263 clone, in 14 cases (17%) with the SP142 clone, and in 11 cases (13%) with the 22C3 clone. Inter-assay concordance (i.e. a case was classified as positive or negative with all three clones) was achieved in only 66 of 84 cases (79%) when a 1% TC positivity threshold was applied to define a case as positive.
Different PD-L1 clones show strong agreement in UCV but moderate agreement in UCV and UC NOS in the same cases
In UCV, the three PD-L1 antibodies that were tested exhibited strong agreement in pairwise comparison for both TC and IC analyses (R-values: 0.780–0.901) (Table 3).
Table 3.
Pairwise correlation analyses of the different PD-L1 antibody clones.
| Pairwise comparison in UCV | Pairwise comparison UCV/NOS same case | ||||
|---|---|---|---|---|---|
| TC | R | TC | R | ||
| SP263 | SP142 | 0.886 | SP263 UCV | SP263 NOS | 0.631 |
| SP263 | 22C3 | 0.886 | SP142 UCV | SP142 NOS | 0.538 |
| SP142 | 22C3 | 0.898 | 22C3 UCV | 22C3 NOS | 0.650 |
| TC H-score | TC H-Score | ||||
| SP263 | SP142 | 0.887 | SP263 UCV | SP263 NOS | 0.632 |
| SP263 | 22C3 | 0.886 | SP142 UCV | SP142 NOS | 0.491 |
| SP142 | 22C3 | 0.901 | 22C3 UCV | 22C3 NOS | 0.654 |
| IC | IC | ||||
| SP263 | SP142 | 0.780 | SP263 UCV | SP263 NOS | 0.876 |
| SP263 | 22C3 | 0.809 | SP142 UCV | SP142 NOS | 0.888 |
| SP142 | 22C3 | 0.855 | 22C3 UCV | 22C3 NOS | 0.919 |
UCV: urothelial carcinoma with divergent differentiation, NOS: pure urothelial carcinoma, TC: tumor cell analyses, IC: immune cell analyses.
In cases of UCV and UC NOS present on the same slides, agreement figures were moderate in TC analyses (R-values: 0.491–0.654) (Table 3). The lowest concordance was observed with the SP142 clone (R-values: 0.538 (TC), 0.491 (TC H-score)). In IC analyses, agreement was strong (R-values: 0.876–0.919) with no outliers (Table 3). However, the number of cases with mixed histology (i.e., NOS as well as a variant histology component on the same slide) was too small to draw definite conclusions.
Discussion
PD-L1 expression on tumor cells (TC) and tumor infiltrating immune cells (IC) are established tumor and host factors associated with improved response to immune checkpoint inhibitor therapy in UC and other tumor types. Other factors associated with immunotherapy response include TMB and neoantigen load.(9, 22) Variant differentiation in invasive UC is a common finding (12, 13), but the presence of predominant variant differentiation or pure non-urothelial histology has been used as an exclusion criterion for most BC ICB trials. (6) Whether UCV patients respond to ICB is thus poorly studied. To assess the likelihood that ICB could be active in UCV, we investigated the intensity and pattern of PD-L1 staining in a cohort of UC tumors with a variety of variant histology (UCV).
The results of PD-L1 testing by IHC can be confounded by various factors such as lack of standardized evaluation methods (TC versus IC or both), different cutoff levels for positive cases and differences in assay design including the use of different antibody clones, reagents and staining platforms, and the potential for spatial and temporal heterogeneity of PD-L1 expression. However, as predictive biomarkers are needed to complement the rapidly expanding number of ICB-based therapeutic approaches, PD-L1 IHC offers the advantage of a rapid and cost-effective test that could be widely available across many clinical laboratories. Moreover, PD-L1 testing by IHC is becoming more crucial in selecting cisplatin-ineligible patients for first-line ICB treatment.(19) To reduce the variability of PD-L1-IHC tests, studies directly comparing the performance of different PD-L1 clones are needed. We therefore analyzed three PD-L1 antibody clones approved by the FDA as diagnostic tests for use in BC (SP263: durvalumab, 22C3: pembrolizumab, SP142: atezolizumab). In addition, all PD-L1-IHC labeling was performed using the same Ventana platform to minimize inter-assay variability.
In this study, we found that a significant proportion of UCV expresses PD-L1 as assessed by different antibodies, at different cutoff levels and methods of evaluation (TC and/or IC). Irrespective of these parameters, UCV exhibited equal or higher PD-L1 expression on TC compared to that reported in the literature for classic/pure UC (4–30%).(14–16, 23–29) This was also the case when (adapted) selection criteria from clinical ICB studies were applied. For example, between 73% (with SP263 clone) and 37% (with SP142 clone) of the UCV cases were classified as adapted IC2/3 (IC staining in ≥5% of tumor associated IC). In contrast, in the clinical trials that led to FDA approval of atezolizumab, only 32% of UC cases were classified as IC2/3 in the 2nd line and 27% in the 1st line setting using the SP142 clone.(5, 9) Similar findings were observed when adapted pembrolizumab positivity criteria were applied. The clinical trials in the 1st and 2nd line settings identified a rate of 30% of PD-L1 positive cases using the 22C3 clone and a TC+IC composite score ≥10 (CPS) to define positivity.(8, 10) In our study, the same value was found in UCV with the SP142 clone using the adapted positivity score, while rates were higher using the 22C3 and SP263 clones (39% and 57%, respectively). These higher rates of PD-L1 immunopositivity in UCV were, however, not detected when the durvalumab trial criteria were applied. These criteria define a case as positive if either TC or IC PD-L1 reactivity was detected in ≥25% with the SP263 clone.(7, 21) However, the criteria for patient enrollment in these studies were variable and changed during the course of the studies which resulted in higher rates of positive cases compared to our UCV cohort (Table 2).
In this study, UC with squamous differentiation which exhibited significantly higher mean TC reactivity rates compared to the remaining UCV entities (Table 1). These results are consistent with those of a recent report on high rates of PD-L1 expression detected in pure squamous cell carcinoma of the bladder. (30) The findings in UC with squamous differentiation in our cohort are also supported by results from the 2nd line atezolizumab study (IMvigor210) that found PD-L1 expression on TC predominantly in tumors of the basal subtype according to the TCGA classification.(5) The basal molecular subtype of BC is enriched with squamous differentiation by histological evaluation.(31) In addition to UC with squamous differentiation, we report PD-L1 expression in other aggressive variant histologies such as plasmacytoid, micropapillary and small cell carcinoma, tumor types that are associated with higher TMB compared to pure UC (17, 18), further supporting their inclusion in ICB clinical trials. The results of clinical trials to evaluate immune therapy in patients with metastatic bladder cancer with variant (and non-urothelial) histologies, that are currently underway (32), will help address this hypothesis.
As in previous reports (15, 33), we found that staining characteristics of the SP142 clone are unique and less comparable. This is a consistent finding in different cancers and also in our UCV analyses as there was a lower mean number of positive TC detected using this clone.(15, 33) In addition, SP142 expression pattern is coarsely granular “clumpy” compared to other PD-L1 clones which can make the discrimination between TC and IC challenging and might impede PD-L1 assessment. These difficulties might also be a cause for the inferior concordance reported with the SP142 clone as compared to other PD-L1 antibodies. (15, 34) Another potential reason might be due to different epitope binding sites of these clones. Although both the SP263 and SP142 clones were shown to bind to intracellular and extracellular PD-L1 epitopes, the SP142 clone identifies epitopes involving amino acids 19–132 of the extracellular domain that have been reported as absent in isoform 2 of PD-L1, which might be predominant in some cases. (35)
Our results showed strong agreement (R: 0.780–0.901) among all three clones that were tested (Table 3). These observations are similar to findings from other cancers such as non-small cell lung carcinoma (33) and malignant melanoma (36) as well as other reports in bladder cancer.(15, 34) Our findings are consistent with these earlier studies and indicate that potentially useful expression details can be gleaned from any of the three PD-L1 antibody clones used in the evaluation of UCV.
The present study had some limitations. We retrospectively selected cases with variant histology without taking further clinicopathological data into account such as TMB or the presence of other genes commonly mutated in bladder cancers like FGFR3, TP53 and others. We have also not tested all available PD-L1 clones, though we believe that we have tested those clones that are most relevant in BC.
In summary, we report PD-L1 expression in a high percentage of UCV and showed comparable results using three different and readily available clones. This may provide rationale and further support to include UCV in clinical anti-PD-1/PD-L1 trials.
Acknowledgement
Data from this manuscript was presented at the 107th Annual Meeting of the United States and Canadian Academy of Pathology (USCAP) in Vancouver, BC in March 2018.
Conflicts of Interest and Source of Funding
This study was supported by the Parker Institute for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center, Sloan Kettering Institute for Cancer Research Cancer Center Support
Grant P30CA008748 and by SPORE in Bladder Cancer P50CA221745.
Dr. Al-Ahmadie is a member of the Parker Institute for Cancer Immunotherapy.
Dr. Reis received honoraria from Roche Pharma and is currently receiving research grants from Bristol-Myers Squibb (CA224–063, TM224PA10).
Dr. Funt has consulted for AstraZeneca/MedImmune, received research funding from Genentech and AstraZeneca/MedImmune, and owns stocks in Urogen Pharma, Allogene Therapeutics, Neogene Therapeutics and Kite Pharma
Dr. Rosenberg has consulted for Lilly, Merck, Agensys, Roche/Genentech, AstraZeneca/MedImmune, Sanofi, Bristol-Myers Squibb, EMD Serono, Seattle Genetics, Bayer, Inovio Pharmaceuticals, BioClin Therapeutics, QED Therapeutics, Adicet Bio, Sensei Biotherapeutics, Fortress Biotech, Pharmacyclics and Western Oncolytics. He received research funding from Genentech, Oncogenex, Agensys, Mirati Therapeutics, Novartis, Viralytics, Genentech/Roche, Incyte, Seattle Genetics, Bayer, AstraZeneca and he owns stocks in Merck and Illumina.
Dr. Bajorin has consulted for Esai, EMD Serono, Merck, Genentech, Pfizer, Eli Lilly, Hoffman La Roche, Urogen, Fidia Farmaceutici; and received research funding from Merck and Novartis
Dr. Solit has consulted for Pfizer, Loxo Oncology, Illumina and Vivideon Therapeutics.
Dr. Al-Ahmadie has consulted for AstraZeneca/MedImmune, Bristol-Myers Squibb, EMD Serono and Roche/Genentech.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: The future is now. Cancer Treat Rev. 2017;54:58–67. [DOI] [PubMed] [Google Scholar]
- 4.Aggen DH, Drake CG. Biomarkers for immunotherapy in bladder cancer: a moving target. J Immunother Cancer. 2017;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. [DOI] [PubMed] [Google Scholar]
- 7.Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34:3119–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 9.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol. 2017;35:2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder BJ, Boorjian SA, Cheville JC, et al. The impact of histological reclassification during pathology re-review--evidence of a Will Rogers effect in bladder cancer? J Urol. 2013;190:1692–1696. [DOI] [PubMed] [Google Scholar]
- 13.Dalbagni G, Genega E, Hashibe M, et al. Cystectomy for bladder cancer: a contemporary series. J Urol. 2001;165:1111–1116. [PubMed] [Google Scholar]
- 14.Faraj SF, Munari E, Guner G, et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology. 2015;85:703.e701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tretiakova M, Fulton R, Kocherginsky M, et al. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: comparison of four commonly used antibodies and RNA expression. Mod Pathol. 2017;31:623–632. [DOI] [PubMed] [Google Scholar]
- 16.Pichler R, Heidegger I, Fritz J, et al. PD-L1 expression in bladder cancer and metastasis and its influence on oncologic outcome after cystectomy. Oncotarget. 2017;8:66849–66864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Ahmadie HA, Iyer G, Lee BH, et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat Genet. 2016;48:356–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang MT, Penson AV, Desai NB, et al. Small cell carcinomas of the bladder and lung are characterized by a convergent but distinct pathogenesis. Clin Cancer Res. 2018;24:1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA updates prescribing information for Keytruda and Tecentriq. 2018. Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm617378.htm?utm_campaign=Oncology%208%2F20%2F2018%20Keytruda&utm_medium=email&utm_source=Eloqua&elqTrackId=ee4a03050bef42a6a9f124ad548374f0&elq=898039304a9041c4a12d120c3d7b73b4&elqaid=4703&elqat=1&elqCampaignId=3741. Accessed 08.31.2018.
- 20.McClelland RA, Finlay P, Walker KJ, et al. Automated Quantitation of Immunocytochemically Localized Estrogen Receptors in Human Breast Cancer. Cancer Research. 1990;50:3545–3550. [PubMed] [Google Scholar]
- 21.Powles T, O’Donnell PH, Massard C, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase ½ Open-label Study. JAMA Oncol. 2017;3:e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. [DOI] [PubMed] [Google Scholar]
- 23.Wankowicz SAM, Werner L, Orsola A, et al. Differential Expression of PD-L1 in High Grade T1 vs Muscle Invasive Bladder Carcinoma and its Prognostic Implications. J Urol. 2017;198:817–823. [DOI] [PubMed] [Google Scholar]
- 24.Erlmeier F, Seitz AK, Hatzichristodoulou G, et al. The Role of PD-L1 Expression and Intratumoral Lymphocytes in Response to Perioperative Chemotherapy for Urothelial Carcinoma. Bladder Cancer. 2016;2:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. [DOI] [PubMed] [Google Scholar]
- 26.Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26:812–817. [DOI] [PubMed] [Google Scholar]
- 27.Mukherji D, Jabbour MN, Saroufim M, et al. Programmed Death-Ligand 1 Expression in Muscle-Invasive Bladder Cancer Cystectomy Specimens and Lymph Node Metastasis: A Reliable Treatment Selection Biomarker? Clin Genitourin Cancer. 2016;14:183–187. [DOI] [PubMed] [Google Scholar]
- 28.Mullane SA, Werner L, Rosenberg J, et al. Correlation of Apobec Mrna Expression with overall Survival and pd-l1 Expression in Urothelial Carcinoma. Sci Rep. 2016;6:27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baras AS, Drake C, Liu JJ, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology. 2016;5:e1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udager AM, McDaniel AS, Hovelson DH, et al. Frequent PD-L1 Protein Expression and Molecular Correlates in Urinary Bladder Squamous Cell Carcinoma. Eur Urol. 2018;74:529–531. [DOI] [PubMed] [Google Scholar]
- 31.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017;171:540–556.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinicaltrials.gov. Evaluating Immune Therapy, Durvalumab (MEDI4736) With Tremelimumab for Metastatic, Non-transitional Cell Carcinoma of the Urinary Tract [Clinical trial database]. 2018. Available at: https://clinicaltrials.gov/ct2/show/study/NCT03430895. Accessed 12-02-2018, 2018. [Google Scholar]
- 33.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–222. [DOI] [PubMed] [Google Scholar]
- 34.Hodgson A, Slodkowska E, Jungbluth A, et al. PD-L1 Immunohistochemistry Assay Concordance in Urothelial Carcinoma of the Bladder and Hypopharyngeal Squamous Cell Carcinoma. Am J Surg Pathol. 2018;42:1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schats K, Vre EAV, Schrijvers D, et al. Epitope mapping of PD-L1 primary antibodies (28–8, SP142, SP263, E1L3N). Journal of Clinical Oncology. 2017;35:3028–3028. [Google Scholar]
- 36.Sunshine JC, Nguyen PL, Kaunitz GJ, et al. PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison. Clin Cancer Res. 2017;23:4938–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]