The clinical picture and genetic basis of PH
Pulmonary Hypertension (PH) is a rare but progressive and devastating clinical problem, associated with 6.5 in 100,000 deaths and 131 per 100,000 hospitalizations in the United States of America in 20101,2. PH can arise from a diverse range of etiologies, all of which lead to increased pulmonary arterial pressure. This elevated arterial pressure consequently increases right ventricular afterload and wall stress, which results in maladaptive cardiac remodeling, and ultimately right heart failure. Additional symptoms include dyspnea, fatigue, heart palpitations and lower limb edema. PH is classified into 5 groups by The World Health Organization3. Mechanistically precise sub-classification has been provided by continuing advances in clinical genetics, with mutations of multiple genes implicated in PH. This is most clearly evident for Group 1 disease, also often referred to as Pulmonary Arterial Hypertension (PAH), which can arise from mutations in multiple genes, amongst other causes. The most commonly associated gene, BMPR2, which encodes bone morphogenic protein receptor type 2 (a member of the TGFβ super family of receptors) is mutated in ~70% of patients with hereditary PAH4–7. Mutations are also found in other TGFβ super-family genes including ALK1 and ENG8, in addition to the gene encoding Smad99, a downstream effector of BMPR2 signalling, GDF210 – a BMPR2 ligand, and CAV111 which codes for caveolin-1 - a scaffolding protein capable of regulating TGFβ-SMAD signalling12. These genetic pathways present new potential therapeutic targets.
Decreased potassium channel activity has long been recognized as a potential pathological substrate for PH13–16, and recent genetic evidence for decreased potassium (K+) channel function in discrete subsets of PAH patients has been provided by the identification of loss-of-function (LoF) mutations in KCNK3, which encodes TASK-117,18, and in ABCC819, a regulatory subunit of ATP-sensitive (KATP) potassium channels. Curiously, pulmonary hypertension is also a common feature of the rare genetic disorder Cantu Syndrome20–24, which arises from gain-of-function (GoF) mutations in the KATP channel subunit genes KCNJ8 and ABCC9. This begs the question, how can both decreased and increased potassium channel activity in the cardiovascular system result in the same clinical endpoint?
K+ channel loss of function in PAH
PAH is characterized by progressive vascular remodeling, involving endothelial proliferation and medial hyperplasia, which together result in narrowing of medium-to-small pulmonary arterioles and the formation of plexiform lesions in the most severe cases25. Potassium channel activity controls the membrane potential of vascular smooth muscle cells. Decreased K+ conductance will result in membrane depolarization and activation of L-type voltage-gated calcium channels (LTCCs), calcium influx, cellular contraction, and ultimately vasoconstriction. In addition to this canonical role in regulating vascular tone, K+ channel activity can also influence the balance of proliferation and apoptosis. For example, pharmacological activation or overexpression of voltage-gated potassium channels in pulmonary artery smooth muscle cells (PASMCs) increases apoptosis, whilst K+ channel downregulation has the opposite effect26–28. Furthermore, mechanical forces in PASMCs during vasoconstriction also promote proliferation13,29,30. Therefore, K+ channel activity can play distinct roles in determining vessel diameters, both by regulating vascular contractility and cellular growth.
Extensive studies have identified voltage-gated K+ channels (most notably Kv1.5) as key regulators of PASMC excitability, and multiple stimuli associated with PAH, including hypoxia, anorexigenic drugs, serotonin (5-HT) and thromboxane A2 (TXA2) decrease PASMC Kv currents13,16. In addition, LoF mutations in the 2-pore domain potassium (K2P) channel TASK-1 (KCNK3), which is expressed in the lungs and regulates PASMC resting membrane potential, have recently been identified as causal factors in familial and idiopathic PAH pathogenesis17,18,31–34. This demonstrates that decreased K+ currents are not a mere epiphenomenon during PAH development, and support the general hypotheses that (1) decreased K+ conductance in PASMC can cause PAH and (2) that this could potentially arise from downregulation of multiple molecularly diverse K+ channels.
LoF mutations in the KATP channel ABCC8 gene in PAH
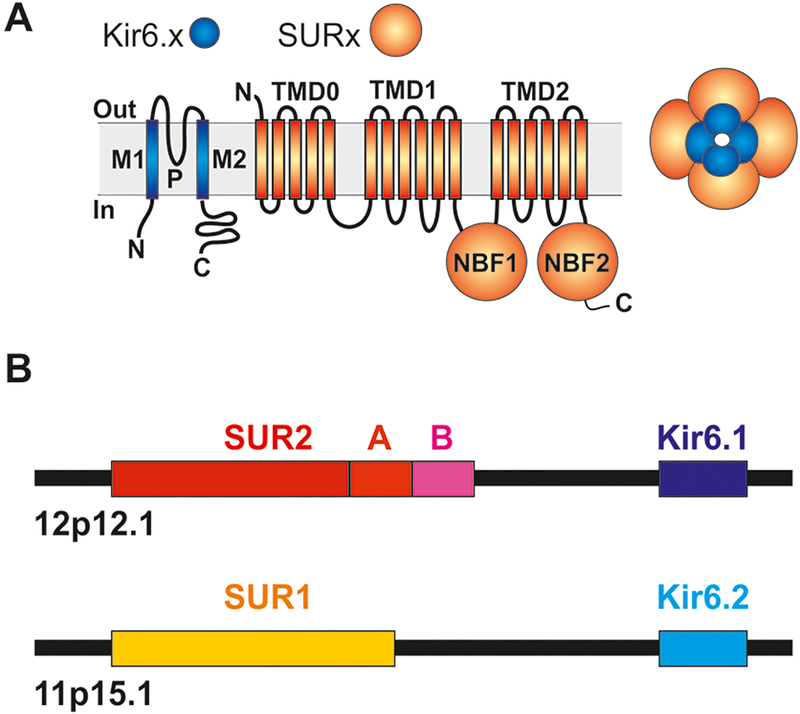
ATP-senstive potassium (KATP) channels represent a sub-family of potassium channels that link metabolic state to electrical activity in tissues throughout the body. KATP channel activity in vascular tissues controls vascular tone and regulates systemic blood pressure35,36. Uniquely, KATP channels are assembled as octameric complexes of pore-forming (Kir6.1 or Kir6.2) subunits associated with regulatory SUR1 or SUR2 subunits (Fig. 1). SUR1 is encoded by ABCC8, located on human chromosome 11, immediately preceding the gene encoding the Kir6.2 subunit (KCNJ11), whilst the paralogous ABCC9 (SUR2) and KCNJ8 (Kir6.1) gene pair are immediately adjacent to each other on chromosome 12. Several LoF mutations in ABCC8 were recently identified in two cohorts of pediatric- and adult-onset PAH patients19.This association is initially surprising for a number of reasons: First, Kir6.2/SUR1 channels are critical regulators of pancreatic β-cell excitability and LoF mutations in ABCC8 are an established cause of congenital hyperinsulinism (CHI)37, yet ABCC8-variant PAH patients do not exhibit, or report any history of, hyperinsulinism19. Second, extensive molecular characterization of KATP channels in smooth muscle and endothelial cells of various tissues in multiple species demonstrates a predominance of ABCC9 (SUR2) expression, not ABCC8 (SUR1) which is instead highly expressed in the pancreas and neurons35,38–44.
Fig. 1. Molecular basis of KATP channel activity.
(A) KATP channels are generated as octamers of 4 pore-forming Kir6.x (Kir6.1 or Kir6.2) and 4 regulatory SURx (SUR1 or SUR2) subunits. (B) Two pairs of genes located on human chromosome 12 (ABCC9, KCNJ8) and chromosome 11 (ABCC8, KCNJ11) encode SUR2 (C-terminally spliced to SUR2A or SUR2B) and Kir6.1, or SUR1 and Kir6.2 subunits, respectively.
Remarkably, four functionally-confirmed missense LoF mutations have been identified both in patients with either PAH or CHI, yet no patients have been reported with any clinical overlap between these pathologies19. Limited penetrance of disease-associated mutations is commonly observed in heritable PAH. This suggests that causal gene variants may only predispose patients to disease which requires a “second-hit” genetic, developmental, or environmental insult to fully manifest, which might then explain why CHI patients with such variants do not exhibit PAH7,19,45. Three of the variants associated with both PAH and CHI are found in homozygous or compound heterozygous CHI patients (G111R, L135V and D1472N)19,46–48, whilst D1472N is also observed as a heterozygous variant in focal CHI (where the imprinting of the maternal allele in specific pancreatic regions unmasks paternally inherited KATP LoF mutations)49,50. The fourth (D813N) was reported in a heterozygous patient with the variant inherited from the father51. Interestingly, imprinting of the maternal allele of chromosome 11p15, near the ABCC8 locus, has been reported in focal CHI, which may explain how paternally inherited variants have effects in specific cases52.
Intriguingly, whilst SUR2 (ABCC9) is likely the predominant SUR isoform expressed in human lung tissues42,53, ABCC8 expression was reported to be upregulated in lung tissue samples from PAH patients carrying BMPR2 mutations19. Furthermore, antibody staining identified SUR1 expression in proximal pulmonary arteries and, prominently, in alveolar macrophages19. These data may point to currently unknown roles for SUR1-containing KATP channels in the lung, and the possibility that SUR1-dependent KATP function is somehow necessary to counter PAH triggers. Detailed studies of recombinant channels show that SUR1 and SUR2 can co-assemble in functional KATP channels in vitro54–56, and both genes are expressed in certain smooth muscle tissues42,57. It is therefore also conceivable that SUR1 may be functionally expressed together with SUR2 in various cells in the human lung, and that ABCC8/SUR1 expression may be upregulated in PAH, perhaps as a protective response. Consistent with such lability, SUR1 upregulation has been documented in response to hypoxia in cerebral vascular endothelial cells via hypoxia-inducible factor 1 α (HIF1)58, a transcription factor which is also highly activated in cultured PASMCs from human PAH patients59. If SUR1 is expressed in PASMC, either in normal physiology or in disease states, then LoF variants - resulting in decreased KATP activity - would be predicted to have a depolarizing effect on the membrane potential and thus to functionally converge with the effects of LoF mutations in KCNK3.
SUR1 has also been reported to co-assemble with TRPM4 non-selective pore-forming subunits to form SUR1-TRPM4 (Sur1-NCCa-ATP) complexes, in cerebral microvessels, neurons, and microglia60–62. TRPM4 is also expressed in vascular smooth muscle63, pulmonary smooth muscle64 and rat airway smooth muscle63 and thus it is possible that SUR1-TRPM4 co-assembly may occur in the lung. However, the validity of the TRPM4-SUR1 association has been questioned65, no study to date has reported SUR1-NCCa-ATP channels in the lung, and the function of any such channel in PAH pathophysiology is unknown. Importantly, TRPM4 channels are non-selective cation channels and thus would underlie depolarizing conductances. How the loss of a SUR1-dependent TRPM4 mediated depolarizing current would result in PAH is thus not clear at this time.
In vitro, SUR1 expression can also mediate apoptosis induced by the SUR ligands glibenclamide, resveratrol, and 17β-estradiol66–68. This effect does not require KATP channel function. Therefore, it is possible that PAH-associated ABCC8 mutations may reduce a KATP-independent effect of SUR1 on induction of apoptosis, which could promote the medial hyperplasia or intimal overgrowth observed in PAH.
Gain-of-function mutations in ABCC9 and KCNJ8 cause PH
Autosomal dominant mutations in ABCC9 (SUR2) and KCNJ8 (Kir6.1) cause the complex heritable disorder, Cantu Syndrome (CS)69–74, characterized by hypertrichosis, distinct facies, and multiple cardiovascular abnormalities, including cardiomegaly, dilated and tortuous vasculature, pericardial effusion, and edema. Since mutations in both genes converge in a common pathophysiology, the underlying defect is gain-of-function (GoF) mutations in SUR2/Kir6.1-dependent KATP channels, and the primary cellular dysfunction is likely to be in a tissue in which both Kir6.1 and SUR2 are expressed. We have recently demonstrated that KATP channel activity in vascular SMC is markedly increased by CS-associated mutations in both KCNJ8 and ABCC9 in two novel CRISPR/Cas9 engineered mouse models which recapitulate the low systemic blood pressures and cardiac hypertrophy observed clinically75. As both Kir6.1 and SUR2 are also expressed in the pulmonary vasculature40–42 it is a simple prediction that CS patients will exhibit pulmonary vasodilation and hence lower pulmonary blood pressures – as is observed in the systemic circulation21,75,76. However, CS patients present with the opposite effect, frequently demonstrating elevated pulmonary artery pressures and potentially fatal PH22–24.
How could PH arise from KATP channel over-activity?
A clue as to how potassium channel over-activity in CS may paradoxically cause PH is provided by studies of the effects of vasodilatory KATP channel openers (KCOs), including diazoxide and minoxidil. Adverse effects of these drugs overlap strikingly with the clinical features observed in CS - edema, pericardial effusion, hypertrichosis, reopening of the ductus arteriosus, and PH all being reported both as side effects of KCO treatment and common in CS77–82. It has been proposed that KCOs will trigger compensatory feedback mechanisms in response to their potent systemic blood pressure lowering effect in patients83. Such feedback includes elevated sympathetic activity, and upregulation of renin-angiotensin-aldosterone axis signalling (RAAS) due to decreased renal perfusion. Activation of RAAS leads to elevated salt and water retention, and blood plasma volume expansion (hypervolemia) which helps to normalize systemic blood pressure. However, it is also recognized that elevated RAAS can contribute to the development of PH, and that inhibition of this action via angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers can reverse hypoxia- and monocrotaline-induced PH in rats84–87. In animal models, chronic minoxidil treatment results in blood volume expansion and cardiac hypertrophy in spontaneously hypertensive88 and in normotensive rats89. Left and right ventricular hypertrophy are observed in parallel with increased plasma renin activity90, and prevented by administration of the angiotensin-receptor blocker losartan. This suggests that RAAS is a critical factor in the cardiac hypertrophy induced by chronic KCO administration. We therefore hypothesize that KCO-induced PH arises due to volume-overload of the pulmonary circulation, downstream of RAAS upregulation. Notably, diazoxide, which is used to treat hyperinsulinism by activating pancreatic KATP channels, also promotes vasodilation via vascular KATP activation and has been reported to cause PH in patients78,79. For this reason, diazoxide is often co-administered with diuretic drugs to counteract adverse effects associated with fluid retention91–93.
Congenital defects in CS may contribute to PH.
Left heart disease can lead to Group 2 pulmonary hypertension. Chronic left ventricular systolic dysfunction, left sided valvular disease, or congenital heart defects, can all cause PH, and it is possible that in certain CS patients various structural cardiovascular abnormalities may contribute to PH. Reported CS abnormalities include patent ductus arteriosus (PDA) and persistence of other fetal circulation, aorto-pulmonary collaterals, aortic root dilation, and aortic valve defects, stenosis and regurgitation21,22,72. Constitutive dilation of the aortic root is observed in both CS patients and ‘Cantu mice’, which results in aortic regurgitation and aortic valve defects which can cause Group 2 PH75. Additionally, if not corrected in a timely manner, PDA can lead to PH and progress irreversibly to Eisenmenger’s Syndrome94–96.
Many CS patients are born prematurely21. Extreme prematurity may also lead to bronchopulmonary dysplasia and cause Group 3 hypoxia-induced PH23,97. Pulmonary venous occlusion (PVO) was reported in a single CS case22. The highly tortuous vasculature in CS is suggestive of abnormal development, which may result in malformed vessels such as the PVO reported by Kobayashi and colleagues22. Furthermore, CS patients frequently present with “high-output” hypertrophic hearts69. As discussed above, we hypothesize that these structural and functional changes arise secondary to KATP GoF–induced vasodilation to compensate for lowered systemic blood pressure75. Elevation of stroke volume results in increased pulmonary artery pressures, as is observed in healthy individuals in exercise98,99, and may therefore also contribute to the chronically elevated pulmonary pressures in CS patients.
Thus there are multiple potential structural, hemodynamic, and neurohumoral factors which may contribute to PH in CS patients, all of which may be secondary to GoF of KATP channels and consequent vasodilation in the systemic vasculature.
KATP dysfunction and PH: smooth muscle and endothelial contributions?
There are key roles for both smooth muscle and endothelial KATP channels in regulating vascular function. Unlike in excitable vascular smooth muscle cells, where KATP channel activation results in decreased calcium influx via voltage-gated calcium channels, , KATP activation causes hyperpolarization in non-excitable endothelial cells, increasing the driving force for Ca2+ influx through receptor- and store-operated channels, and thereby increases intracellular calcium100. As intracellular calcium critically regulates endothelial function, including mediator release, KATP activity could clearly affect endothelial physiology and the vasodilatory effects of sheer stress, adenosine, and hypo-osmolarity have been attributed in part to activation of endothelial KATP channels101–103.
KATP expression has been demonstrated in pulmonary artery endothelial cells, where it is regulated by sheer stress104,105, but endothelial KATP function has mostly been studied outside of the pulmonary vasculature. Interestingly, coronary vasospasm has been reported in both Kir6.1 and SUR2 null mice106,107. This phenotype reportedly persists in SUR2 KO mice even when SUR2B is transgenically overexpressed specifically in smooth muscle – suggesting that vasospasm may arise from non-smooth muscle dysfunction108. Consistent with this, endothelial specific expression of dominant-negative KATP subunits results in increased coronary perfusion pressure due to increased endothelin-1 secretion from ECs109. This was not observed following conditional deletion of Kir6.1 in endothelial cells (where Kir6.2 expression may remain) which did however impair hypoxia-induced vasorelaxation in the coronary circulation43.
If these features are conserved in the pulmonary vasculature, it is conceivable that decreased pulmonary endothelial KATP activity could promote pulmonary vasoconstriction. In addition, the KCO nicorandil has recently been shown to reduce lipopolysaccharide-induced inflammation (via decreased reactive oxygen species generation), and monocrotaline-induced damage in pulmonary artery endothelial cells, pointing to a protective role of KATP activity in the pulmonary endothelium110,111. However, as Kir6.2, Kir6.1 and SUR2B are the major subunits expressed in vascular endothelial cells112, there is no simple rationale for why either endothelial SUR1 LoF or SUR2 GoF mutations should be associated with PH. Studies of the effects of GoF in pulmonary endothelium (and vascular endothelium in general) are lacking and could provide telling novel insights.
Linking KATP channel dysfunction to PAH and CS-associated PH
Clearly much remains to be elucidated about how LoF mutations in ABCC8 result in PAH whereas GoF mutations in KCNJ8 and ABCC9 cause PH in CS. In the case of ABCC8 LoF, insights may be gleaned from studies of knockout mice113. ABCC8 null and LoF transgenic mice exhibit abnormalities in insulin secretion and glucose intolerance113,114, but to date there is little insight to cardiovascular dysfunction or remodelling. There are many examples of murine models providing novel insights into PAH pathophysiology (reviewed in115–117), but in some cases, species specific differences in pulmonary physiology can result in failures of mouse models to recapitulate human disease. For example KCNK3 knockout mice do not exhibit RV hypertrophy or pulmonary vasculature remodelling despite strong genetic evidence for the role of KCNK3 LoF in human disease7,17,32,33,118,119. Recently, we demonstrated that knock-in of CS-causing mutations into the endogenous KCNJ8 and ABCC9 loci in mice results in vasodilation, decreased systemic blood pressure, and pronounced cardiac hypertrophy – mirroring clinical observations75. These “Cantu mice” therefore provide a faithful model of key cardiovascular abnormalities in patients and allow for investigation of pathophysiological mechanisms. The effects of KATP GoF on pulmonary vascular physiology in mice remain to be established. Based on the studies of KATP channel activating drugs in rodents, we hypothesize that KATP GoF will trigger RAAS activation and blood volume expansion, which may precipitate volume-overload of the pulmonary circulation in Cantu mice.
In addition to global knockout mice, insights to the role of KATP in cardiovascular system have been provided by mouse models either expressing dominant-negative KATP channel subunit transgenes or floxed alleles, which allow for inducible and tissue-specific downregulation of KATP channel activity35,36,106,107,109,120. Meanwhile, overexpression of GoF Kir6.1 mutant subunits in both vascular smooth muscle and cardiomyocytes has been shown to recapitulate certain features of Cantu Syndrome36,121. Most recently, we demonstrated that the introduction of Cantu Syndrome-associated point mutations into the endogenous KCNJ8 and ABCC9 mouse genes recapitulates the decreased systemic vascular resistance and high-output hypertrophic hearts observed in CS75.
While the hypertensive effect of KATP knockdown in the systemic vasculature is well described36,106,107, we are unaware of parallel in vivo studies in pulmonary vessels. The different mouse models described above provide valuable tools for future experiments to define the role of KATP dysfunction in various tissues in PH. Global SUR2 and Kir6.1 knockout mice would be expected to exhibit increased pulmonary vasoconstriction via loss of either smooth muscle or endothelial KATP function. Knockdown of over-active KATP channels in the Cantu mice in smooth muscle or endothelial cells using dominant-negative or floxed Kir6.1 alleles would establish the tissue in which KATP GoF causes PH in CS. The effect of loss of SUR1 on pulmonary physiology could be tested in global or tissue specific SUR1 knockout122. Together, such experiments have the potential to provide mechanistic explanations for how both loss- and gain-of-function of KATP channels can ultimately result in PH.
KATP channels as therapeutic targets in PH
Because KATP activators cause vascular smooth muscle hyperpolarization resulting in vasodilation, they present an interesting potential target for PAH therapy83,123–125. However, as noted above, classical KCOs effectively dilate pulmonary vessels but their powerful systemic vasodilatory effects may provoke counter-productive hypervolemia and exacerbate PH with long-term administration. Thus an important feature of an ideal vasodilatory drug for PH would be specific targeting of the pulmonary vasculature to avoid secondary compensation for associated systemic vasodilation. Vasodilators with more pulmonary-specific action, such as endothelin receptor antagonists or PDE5 inhibitors may be preferable to currently available KCOs. Intriguingly, one newer KATP channel activator, iptakalim, has been reported to be a selective vasodilator acting specifically on resistance vessels in hypertensive patients, without effects on normotensive patients126. Iptakalim has been reported to reduce hypoxia- or endothelin-induced proliferation in PASMCs127,128 and to protect endothelial function in rats129, but whether these properties can be translated to benefit PAH patients without activation of potentially confounding secondary consequences in the long-term has not been established.
Why KCOs can cause PH yet other vasodilatory drugs do not is intriguing. KCOs have long been recognized as particularly powerful vasodilatory agents, capable of reducing systemic blood pressure in patients in which other therapies have been ineffective82,83,130,131. Therefore, perhaps the magnitude of the reflex sympathetic/RAAS activation is greater for KCOs than for other vasodilatory agents. Additionally, diazoxide and minoxidil have been shown to produce only weak venodilation (in contrast to many other vasodilatory drugs)131–133. Marked arteriolar dilation coupled with minimal venodilation, together with sympathetic/RAAS activation results in increased venous return and cardiac output, leading to increased cardiopulmonary blood volume and pulmonary arterial pressures134. Notably, this vasoactivity profile is shared by hydralazine, another vasodilator with weak venodilatory effects that can also induce increased pulmonary pressures134,135.
Whether the specific dysfunction of SUR1 can be precisely targeted in the ABCC8-variant PAH patient population remains to be seen. Diazoxide activates SUR1-containing KATP channels, but exerts its vasodilatory effects via activation of SUR2B-dependent VSMC KATP channels126, which would have undesirable systemic effects. A SUR1-specific activator might therefore be desirable, to avoid systemic vasodilation. A recently identified novel SUR1-selective activator, reported by Raphemot and colleagues, may prove a useful experimental tool for dissecting SUR1-specific dysfunction, without targeting SUR2-containing channels136. Importantly, however, any SUR1-selective activator would also activate pancreatic KATP channels, potentially decreasing β-cell excitability and insulin secretion, and thus hyperglycemic effects would have to be carefully monitored.
Conversely, directly targeting KATP GoF using inhibitors represents a potential strategy for treating cardiovascular pathologies in CS or related pathologies. The potent second-generation sulfonylureas (SUs), including glibenclamide, and glinidies, such as repaglinide, inhibit both pancreatic and cardiovascular KATP channels137. As their name suggests, the SUR domain contains the binding site for SUs, which was recently resolved in cryo-EM structures of Kir6.2/SUR1 channels bound by glibenclamide138. The sensitivity of pancreatic/neuronal channels (Kir6.2/SUR1) for SU inhibition is significantly higher than SUR2-containing channels in vitro137,139,140. Thus, it would be expected that higher doses than are used in the treatment of diabetes might be required to effectively target CV KATP channels in CS. Furthermore, certain disease-causing GoF mutations in KATP channels can reduce SU sensitivity, and thus the drugs may not be efficacious in specific patients141,142. However, significant sensitivity is retained for multiple other CS mutations and thus KATP inhibitors may serve as effective therapies for many CS patients143.
As detailed above, the complex cardiopulmonary abnormalities in CS seem to arise from a primary dysfunction of vascular smooth muscle channels which could therefore represent the ideal target for a pharmacotherapy75. We predict that inhibiting VSMC KATP GoF in the systemic circulation will attenuate the primary systemic vasodilation, which will in turn inhibit the secondary RAAS activation, predicted hypervolemia and PH. ‘Cantu mice’ represent a key tool for investigating the pathophysiological mechanisms underlying PH in CS and for determining the pre-clinical efficacy of potential therapies.
Summary
Recent advances in medical genetics have demonstrated that both loss-of-function and gain-of-function mutations in genes encoding KATP channel subunits can result in pulmonary hypertension. Just how loss-of-function mutations in ABCC8 result in PAH is currently poorly understood but, in general, decreased K+ channel activity is associated with vasoconstriction and proliferation of pulmonary artery smooth muscle and endothelial cells. Conversely, gain-of-function mutations in ABCC9 and KCNJ8 cause Cantu Syndrome, which is associated with multiple cardiovascular abnormalities including PH, which potentially arises as a secondary consequence of systemic vasodilation. While there are currently no directed therapies for these pathologies, mechanistic insights to the precise consequences of KATP channel dysfunction will be provided by appropriate animal models, and novel insights to channel-dependent PH pathophysiology will then facilitate targeted therapies for distinct patient subsets.
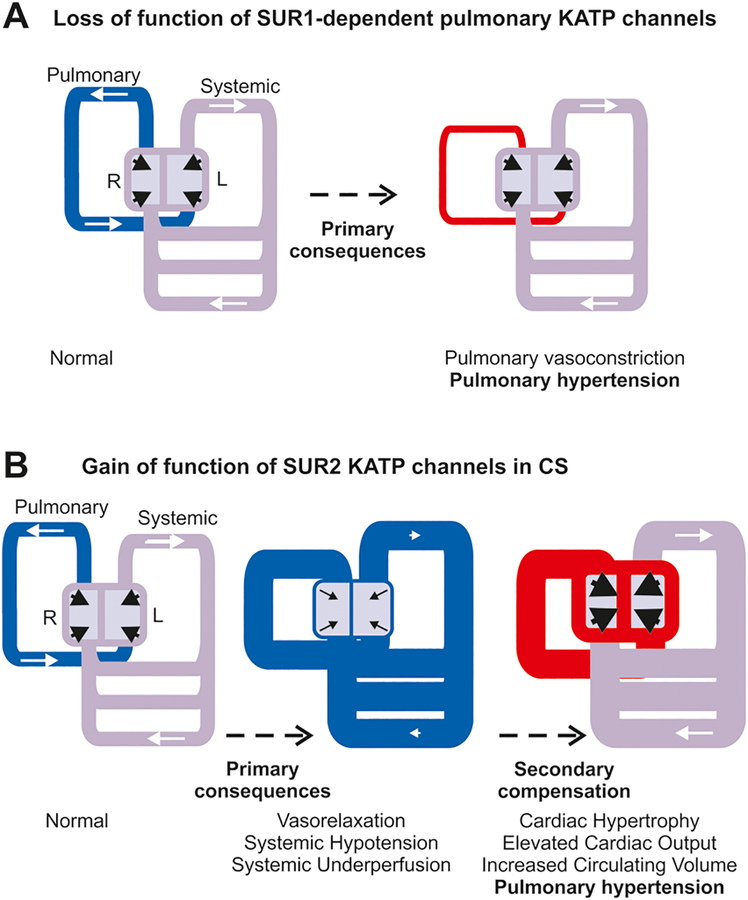
Fig. 2. Hypothesized mechanisms of KATP induced PH.
(A) Schematic of cardiovascular system indicates normal pressures (grey) in systemic circulation and low pressures (blue) in pulmonary circulation resulting from normal pumping from the left (L) and right (R) heart, respectively. Loss-of-function of SUR1-dependent KATP (or other K) channels in pulmonary circulation may directly result in inappropriate pulmonary vasoconstriction and hypertension (red). (B) Gain-of-function of SUR2-dependent KATP (or other K) channels results primarily in inappropriate vasorelaxation and systemic hypotension (blue). Secondary compensatory mechanisms drive enlarged, hypercontractile hearts, raising pressures in the systemic circulation (to normal, grey) and in the pulmonary circulation (to hypertension, red).
Sources of Funding
Our own experimental work has been supported by National Institutes of Health grant (HL140024 to CGN), and Children’s Discovery Institute grant CH-MD-II-2015–488 (to CGN). CMcC is supported by AHA Fellowship (19POST34380407).
Footnotes
The authors have declared that no conflict of interest exists.
Disclosures
None.
References
- 1.George MG, Schieb LJ, Ayala C, Talwalkar A & Levant S Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest 146, 476–495, doi: 10.1378/chest.14-0527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43, 5S–12S, doi: 10.1016/j.jacc.2004.02.037 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, D34–41, doi: 10.1016/j.jacc.2013.10.029 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Lane KB et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 26, 81–84, doi: 10.1038/79226 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Deng Z et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67, 737–744, doi: 10.1086/303059 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machado RD et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat 27, 121–132, doi: 10.1002/humu.20285 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Ma L & Chung WK The role of genetics in pulmonary arterial hypertension. J Pathol 241, 273–280, doi: 10.1002/path.4833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison RE et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet 40, 865–871 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shintani M, Yagi H, Nakayama T, Saji T & Matsuoka R A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genet 46, 331–337, doi: 10.1136/jmg.2008.062703 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Graf S et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 9, 1416, doi: 10.1038/s41467-018-03672-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin ED et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet 5, 336–343, doi: 10.1161/CIRCGENETICS.111.961888 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razani B et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem 276, 6727–6738, doi: 10.1074/jbc.M008340200 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Mandegar M & Yuan JX Role of K+ channels in pulmonary hypertension. Vascul Pharmacol 38, 25–33 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Yuan JX et al. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation 98, 1400–1406 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Yuan XJ, Wang J, Juhaszova M, Gaine SP & Rubin LJ Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet 351, 726–727, doi: 10.1016/S0140-6736(05)78495-6 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Boucherat O et al. Potassium channels in pulmonary arterial hypertension. Eur Respir J 46, 1167–1177, doi: 10.1183/13993003.00798-2015 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Ma L et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med 369, 351–361, doi: 10.1056/NEJMoa1211097 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navas Tejedor P et al. An homozygous mutation in KCNK3 is associated with an aggressive form of hereditary pulmonary arterial hypertension. Clin Genet 91, 453–457, doi: 10.1111/cge.12869 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Bohnen MS et al. Loss-of-Function ABCC8 Mutations in Pulmonary Arterial Hypertension. Circ Genom Precis Med 11, e002087, doi: 10.1161/CIRCGEN.118.002087 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantu JM, Garcia-Cruz D, Sanchez-Corona J, Hernandez A & Nazara Z A distinct osteochondrodysplasia with hypertrichosis- Individualization of a probable autosomal recessive entity. Hum Genet 60, 36–41 (1982). [DOI] [PubMed] [Google Scholar]
- 21.Grange DK, Nichols CG & Singh GK in GeneReviews((R)) (eds Adam MP et al. ) (2014).
- 22.Kobayashi D, Cook AL & Williams DA Pulmonary hypertension secondary to partial pulmonary venous obstruction in a child with Cantu syndrome. Pediatr Pulmonol 45, 727–729, doi: 10.1002/ppul.21215 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Park JY, Koo SH, Jung YJ, Lim YJ & Chung ML A patient with Cantu syndrome associated with fatal bronchopulmonary dysplasia and pulmonary hypertension. Am J Med Genet A 164A, 2118–2120, doi: 10.1002/ajmg.a.36563 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Scurr I et al. Cantu syndrome: report of nine new cases and expansion of the clinical phenotype. Am J Med Genet A 155A, 508–518, doi: 10.1002/ajmg.a.33885 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Rabinovitch M Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122, 4306–4313, doi: 10.1172/JCI60658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krick S, Platoshyn O, McDaniel SS, Rubin LJ & Yuan JX Augmented K(+) currents and mitochondrial membrane depolarization in pulmonary artery myocyte apoptosis. Am J Physiol Lung Cell Mol Physiol 281, L887–894, doi: 10.1152/ajplung.2001.281.4.L887 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Krick S, Platoshyn O, Sweeney M, Kim H & Yuan JX Activation of K+ channels induces apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol 280, C970–979, doi: 10.1152/ajpcell.2001.280.4.C970 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Brevnova EE, Platoshyn O, Zhang S & Yuan JX Overexpression of human KCNA5 increases IK V and enhances apoptosis. Am J Physiol Cell Physiol 287, C715–722, doi: 10.1152/ajpcell.00050.2004 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Hishikawa K et al. Pressure promotes DNA synthesis in rat cultured vascular smooth muscle cells. J Clin Invest 93, 1975–1980, doi: 10.1172/JCI117189 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolpakov V, Rekhter MD, Gordon D, Wang WH & Kulik TJ Effect of mechanical forces on growth and matrix protein synthesis in the in vitro pulmonary artery. Analysis of the role of individual cell types. Circ Res 77, 823–831 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Antigny F et al. Potassium Channel Subfamily K Member 3 (KCNK3) Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 133, 1371–1385, doi: 10.1161/CIRCULATIONAHA.115.020951 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Girerd B, Perros F, Antigny F, Humbert M & Montani D KCNK3: new gene target for pulmonary hypertension? Expert Rev Respir Med 8, 385–387, doi: 10.1586/17476348.2014.909731 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Olschewski A et al. TASK-1 (KCNK3) channels in the lung: from cell biology to clinical implications. Eur Respir J 50, doi: 10.1183/13993003.00754-2017 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Duprat F et al. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J 16, 5464–5471, doi: 10.1093/emboj/16.17.5464 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aziz Q et al. The ATP-sensitive potassium channel subunit, Kir6.1, in vascular smooth muscle plays a major role in blood pressure control. Hypertension 64, 523–529, doi: 10.1161/HYPERTENSIONAHA.114.03116 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Li A et al. Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J Am Heart Assoc 2, e000365, doi: 10.1161/JAHA.113.000365 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Green A & Lindley KJ Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev 84, 239–275, doi: 10.1152/physrev.00022.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Nichols CG KATP channels as molecular sensors of cellular metabolism. Nature 440, 470–476, doi: 10.1038/nature04711 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Foster MN & Coetzee WA KATP Channels in the Cardiovascular System. Physiol Rev 96, 177–252, doi: 10.1152/physrev.00003.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clapp LH, Davey R & Gurney AM ATP-sensitive K+ channels mediate vasodilation produced by lemakalim in rabbit pulmonary artery. Am J Physiol 264, H1907–1915, doi: 10.1152/ajpheart.1993.264.6.H1907 (1993). [DOI] [PubMed] [Google Scholar]
- 41.Clapp LH & Gurney AM ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol 262, H916–920, doi: 10.1152/ajpheart.1992.262.3.H916 (1992). [DOI] [PubMed] [Google Scholar]
- 42.Cui Y, Tran S, Tinker A & Clapp LH The molecular composition of K(ATP) channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol 26, 135–143, doi: 10.1165/ajrcmb.26.1.4622 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Aziz Q et al. Molecular and functional characterization of the endothelial ATP-sensitive potassium channel. J Biol Chem 292, 17587–17597, doi: 10.1074/jbc.M117.810325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tinker A, Aziz Q, Li Y & Specterman M ATP-Sensitive Potassium Channels and Their Physiological and Pathophysiological Roles. Compr Physiol 8, 1463–1511, doi: 10.1002/cphy.c170048 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Austin ED & Loyd JE The genetics of pulmonary arterial hypertension. Circ Res 115, 189–202, doi: 10.1161/CIRCRESAHA.115.303404 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tornovsky S et al. Hyperinsulinism of infancy: novel ABCC8 and KCNJ11 mutations and evidence for additional locus heterogeneity. J Clin Endocrinol Metab 89, 6224–6234, doi: 10.1210/jc.2004-1233 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Muzyamba M et al. Complex ABCC8 DNA variations in congenital hyperinsulinism: lessons from functional studies. Clin Endocrinol (Oxf) 67, 115–124, doi: 10.1111/j.1365-2265.2007.02847.x (2007). [DOI] [PubMed] [Google Scholar]
- 48.Henwood MJ et al. Genotype-phenotype correlations in children with congenital hyperinsulinism due to recessive mutations of the adenosine triphosphate-sensitive potassium channel genes. J Clin Endocrinol Metab 90, 789–794, doi: 10.1210/jc.2004-1604 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Greer RM et al. Genotype-phenotype associations in patients with severe hyperinsulinism of infancy. Pediatr Dev Pathol 10, 25–34, doi: 10.2350/06-04-0083.1 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Ismail D et al. Familial focal congenital hyperinsulinism. J Clin Endocrinol Metab 96, 24–28, doi: 10.1210/jc.2010-1524 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SE et al. Characterization of ABCC8 and KCNJ11 gene mutations and phenotypes in Korean patients with congenital hyperinsulinism. Eur J Endocrinol 164, 919–926, doi: 10.1530/EJE-11-0160 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Suchi M et al. Molecular and immunohistochemical analyses of the focal form of congenital hyperinsulinism. Mod Pathol 19, 122–129, doi:3800497 [pii] 10.1038/modpathol.3800497 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Fagerberg L et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 13, 397–406, doi: 10.1074/mcp.M113.035600 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng WW, Tong A, Flagg TP & Nichols CG Random assembly of SUR subunits in K(ATP) channel complexes. Channels (Austin) 2, 34–38, doi:6046 [pii] (2008). [DOI] [PubMed] [Google Scholar]
- 55.Chan KW, Wheeler A & Csanady L Sulfonylurea receptors type 1 and 2A randomly assemble to form heteromeric KATP channels of mixed subunit composition. J Gen Physiol 131, 43–58, doi:jgp.200709894[pii] 10.1085/jgp.200709894 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheeler A et al. Coassembly of different sulfonylurea receptor subtypes extends the phenotypic diversity of ATP-sensitive potassium (KATP) channels. Mol Pharmacol 74, 1333–1344, doi:mol.108.048355[pii] 10.1124/mol.108.048355 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buckner SA et al. Pharmacological and molecular analysis of ATP-sensitive K(+) channels in the pig and human detrusor. Eur J Pharmacol 400, 287–295 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Woo SK et al. Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab 32, 525–536, doi: 10.1038/jcbfm.2011.159 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonnet S et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113, 2630–2641, doi: 10.1161/CIRCULATIONAHA.105.609008 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Woo SK, Kwon MS, Ivanov A, Gerzanich V & Simard JM The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem 288, 3655–3667, doi: 10.1074/jbc.M112.428219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tosun C et al. Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 44, 3522–3528, doi: 10.1161/STROKEAHA.113.002904 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurland DB et al. The Sur1-Trpm4 channel regulates NOS2 transcription in TLR4-activated microglia. J Neuroinflammation 13, 130, doi: 10.1186/s12974-016-0599-2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Earley S TRPM4 channels in smooth muscle function. Pflugers Arch 465, 1223–1231, doi: 10.1007/s00424-013-1250-z (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang XR, Lin MJ, McIntosh LS & Sham JS Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290, L1267–1276, doi: 10.1152/ajplung.00515.2005 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Sala-Rabanal M, Wang S & Nichols CG On potential interactions between non-selective cation channel TRPM4 and sulfonylurea receptor SUR1. The Journal of biological chemistry 287, 8746–8756, doi: 10.1074/jbc.M111.336131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ackermann S et al. 17beta-Estradiol modulates apoptosis in pancreatic beta-cells by specific involvement of the sulfonylurea receptor (SUR) isoform SUR1. J Biol Chem 284, 4905–4913, doi: 10.1074/jbc.M807638200 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Hambrock A et al. Resveratrol binds to the sulfonylurea receptor (SUR) and induces apoptosis in a SUR subtype-specific manner. J Biol Chem 282, 3347–3356, doi: 10.1074/jbc.M608216200 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Hambrock A, de Oliveira Franz CB, Hiller S & Osswald H Glibenclamide-induced apoptosis is specifically enhanced by expression of the sulfonylurea receptor isoform SUR1 but not by expression of SUR2B or the mutant SUR1(M1289T). J Pharmacol Exp Ther 316, 1031–1037, doi: 10.1124/jpet.105.097501 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Grange DK, Lorch SM, Cole PL & Singh GK Cantu syndrome in a woman and her two daughters: Further confirmation of autosomal dominant inheritance and review of the cardiac manifestations. Am J Med Genet A 140, 1673–1680, doi: 10.1002/ajmg.a.31348 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Harakalova M et al. Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat Genet 44, 793–796, doi: 10.1038/ng.2324 (2012). [DOI] [PubMed] [Google Scholar]
- 71.van Bon BW et al. Cantu syndrome is caused by mutations in ABCC9. Am J Hum Genet 90, 1094–1101, doi: 10.1016/j.ajhg.2012.04.014 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brownstein CA et al. Mutation of KCNJ8 in a patient with Cantu syndrome with unique vascular abnormalities - support for the role of K(ATP) channels in this condition. Eur J Med Genet 56, 678–682, doi: 10.1016/j.ejmg.2013.09.009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooper PE, McClenaghan C, Chen X, Stary-Weinzinger A & Nichols CG Conserved functional consequences of disease-associated mutations in the slide helix of Kir6.1 and Kir6.2 subunits of the ATP-sensitive potassium channel. J Biol Chem 292, 17387–17398, doi: 10.1074/jbc.M117.804971 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooper PE et al. Cantu syndrome resulting from activating mutation in the KCNJ8 gene. Hum Mutat 35, 809–813, doi: 10.1002/humu.22555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y et al. Cardiovascular consequences of KATP overactivity in Cantu syndrome. JCI Insight 3, doi: 10.1172/jci.insight.121153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leon Guerrero CR et al. Neurologic and neuroimaging manifestations of Cantu syndrome: A case series. Neurology 87, 270–276, doi: 10.1212/WNL.0000000000002861 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen KH & Marks JG Jr. Pseudoacromegaly induced by the long-term use of minoxidil. J Am Acad Dermatol 48, 962–965, doi: 10.1067/mjd.2003.325 (2003). [DOI] [PubMed] [Google Scholar]
- 78.Timlin MR, Black AB, Delaney HM, Matos RI & Percival CS Development of Pulmonary Hypertension During Treatment with Diazoxide: A Case Series and Literature Review. Pediatr Cardiol 38, 1247–1250, doi: 10.1007/s00246-017-1652-3 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Demirel F, Unal S, Cetin II, Esen I & Arasli A Pulmonary hypertension and reopening of the ductus arteriosus in an infant treated with diazoxide. J Pediatr Endocrinol Metab 24, 603–605 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Cilingiroglu M, Akkus N, Sethi S & Modi KA Large pericardial effusion induced by minoxidil. Turk Kardiyol Dern Ars 40, 255–258, doi: 10.5543/tkda.2012.63904 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Nautiyal A, Wong T, Kumar S, Mukherjee JT & Schick EC Very large incidental pericardial effusion attributable to minoxidil: resolution without drainage. Journal of Cardiovascular Medicine 12, 186–188, doi: 10.2459/JCM.0b013e328334fb07 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Sica DA Minoxidil: an underused vasodilator for resistant or severe hypertension. J Clin Hypertens (Greenwich) 6, 283–287 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jahangir A & Terzic AK (ATP) channel therapeutics at the bedside. J Mol Cell Cardiol 39, 99–112, doi: 10.1016/j.yjmcc.2005.04.006 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morrell NW, Atochina EN, Morris KG, Danilov SM & Stenmark KR Angiotensin converting enzyme expression is increased in small pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. J Clin Invest 96, 1823–1833, doi: 10.1172/JCI118228 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrell NW, Morris KG & Stenmark KR Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol 269, H1186–1194, doi: 10.1152/ajpheart.1995.269.4.H1186 (1995). [DOI] [PubMed] [Google Scholar]
- 86.Orte C, Polak JM, Haworth SG, Yacoub MH & Morrell NW Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary plexiform pulmonary hypertension. J Pathol 192, 379–384, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 87.de Man FS et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med 186, 780–789, doi: 10.1164/rccm.201203-0411OC (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsoporis J & Leenen FH Effects of arterial vasodilators on cardiac hypertrophy and sympathetic activity in rats. Hypertension 11, 376–386 (1988). [DOI] [PubMed] [Google Scholar]
- 89.Tsoporis J, Yuan BX & Leenen FH Arterial vasodilators, cardiac volume load, and cardiac hypertrophy in normotensive rats. Am J Physiol 256, H876–880, doi: 10.1152/ajpheart.1989.256.3.H876 (1989). [DOI] [PubMed] [Google Scholar]
- 90.Ruzicka M & Leenen FH Renin-angiotensin system and minoxidil-induced cardiac hypertrophy in rats. Am J Physiol 265, H1551–1556, doi: 10.1152/ajpheart.1993.265.5.H1551 (1993). [DOI] [PubMed] [Google Scholar]
- 91.Welters A et al. Long-term medical treatment in congenital hyperinsulinism: a descriptive analysis in a large cohort of patients from different clinical centers. Orphanet J Rare Dis 10, 150, doi: 10.1186/s13023-015-0367-x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gillies DR Complications of diazoxide in the treatment of nesidioblastosis. Arch Dis Child 60, 500–501 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGraw ME & Price DA Complications of diazoxide in the treatment of nesidioblastosis. Arch Dis Child 60, 62–64 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diller GP et al. Current therapy and outcome of Eisenmenger syndrome: data of the German National Register for congenital heart defects. Eur Heart J 37, 1449–1455, doi: 10.1093/eurheartj/ehv743 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dimopoulos K Eisenmenger syndrome in an adult patient with a large patent ductus arteriosus. Eur Respir Rev 22, 558–564, doi: 10.1183/09059180.00007013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryan JJ, Suksaranjit P, Hatton N, Bull DA & Wilson BD Eisenmenger syndrome with unrepaired patent ductus arteriosus. Circulation 131, e409–411, doi: 10.1161/CIRCULATIONAHA.114.013810 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Kim GB Pulmonary hypertension in infants with bronchopulmonary dysplasia. Korean J Pediatr 53, 688–693, doi: 10.3345/kjp.2010.53.6.688 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kovacs G, Berghold A, Scheidl S & Olschewski H Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 34, 888–894, doi: 10.1183/09031936.00145608 (2009). [DOI] [PubMed] [Google Scholar]
- 99.La Gerche A, Roberts T & Claessen G The response of the pulmonary circulation and right ventricle to exercise: exercise-induced right ventricular dysfunction and structural remodeling in endurance athletes (2013 Grover Conference series). Pulm Circ 4, 407–416, doi: 10.1086/677355 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aziz Q, Li Y & Tinker A Endothelial biology and ATP-sensitive potassium channels. Channels (Austin) 12, 45–46, doi: 10.1080/19336950.2017.1412151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hutcheson IR & Griffith TM Heterogeneous populations of K+ channels mediate EDRF release to flow but not agonists in rabbit aorta. Am J Physiol 266, H590–596, doi: 10.1152/ajpheart.1994.266.2.H590 (1994). [DOI] [PubMed] [Google Scholar]
- 102.Ishizaka H & Kuo L Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity. Am J Physiol 273, H104–112, doi: 10.1152/ajpheart.1997.273.1.H104 (1997). [DOI] [PubMed] [Google Scholar]
- 103.Kuo L & Chancellor JD Adenosine potentiates flow-induced dilation of coronary arterioles by activating KATP channels in endothelium. Am J Physiol 269, H541–549, doi: 10.1152/ajpheart.1995.269.2.H541 (1995). [DOI] [PubMed] [Google Scholar]
- 104.Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T & Fisher AB Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol 285, C959–967, doi: 10.1152/ajpcell.00511.2002 (2003). [DOI] [PubMed] [Google Scholar]
- 105.Chatterjee S, Levitan I, Wei Z & Fisher AB KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation 13, 633–644, doi: 10.1080/10739680600930255 (2006). [DOI] [PubMed] [Google Scholar]
- 106.Miki T et al. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med 8, 466–472, doi: 10.1038/nm0502-466 (2002). [DOI] [PubMed] [Google Scholar]
- 107.Chutkow WA et al. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. Journal of Clinical Investigation 110, 203–208, doi: 10.1172/jci0215672 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kakkar R et al. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res 98, 682–689, doi: 10.1161/01.RES.0000207498.40005.e7 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Malester B et al. Transgenic expression of a dominant negative K(ATP) channel subunit in the mouse endothelium: effects on coronary flow and endothelin-1 secretion. FASEB J 21, 2162–2172, doi: 10.1096/fj.06-7821com (2007). [DOI] [PubMed] [Google Scholar]
- 110.He M et al. Nicorandil Attenuates LPS-Induced Acute Lung Injury by Pulmonary Endothelial Cell Protection via NF-κB and MAPK Pathways. Oxidative Medicine and Cellular Longevity 2019, 1–13, doi: 10.1155/2019/4957646 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sahara M, Sata M, Morita T, Hirata Y & Nagai R Nicorandil attenuates monocrotaline-induced vascular endothelial damage and pulmonary arterial hypertension. PLoS One 7, e33367, doi: 10.1371/journal.pone.0033367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoshida H et al. K ATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol 37, 857–869, doi: 10.1016/j.yjmcc.2004.05.022 (2004). [DOI] [PubMed] [Google Scholar]
- 113.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L & Bryan J Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem 275, 9270–9277 (2000). [DOI] [PubMed] [Google Scholar]
- 114.Shimomura K et al. A mouse model of human hyperinsulinism produced by the E1506K mutation in the sulphonylurea receptor SUR1. Diabetes 62, 3797–3806, doi: 10.2337/db12-1611 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gomez-Arroyo J et al. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol 302, L977–991, doi: 10.1152/ajplung.00362.2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Das M, Fessel J, Tang H & West J A process-based review of mouse models of pulmonary hypertension. Pulm Circ 2, 415–433, doi: 10.4103/2045-8932.105030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Colvin KL & Yeager ME Animal Models of Pulmonary Hypertension: Matching Disease Mechanisms to Etiology of the Human Disease. J Pulm Respir Med 4, doi: 10.4172/2161-105X.1000198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manoury B, Lamalle C, Oliveira R, Reid J & Gurney AM Contractile and electrophysiological properties of pulmonary artery smooth muscle are not altered in TASK-1 knockout mice. J Physiol 589, 3231–3246, doi: 10.1113/jphysiol.2011.206748 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kitagawa MG, Reynolds JO, Wehrens XHT, Bryan RM Jr. & Pandit LM Hemodynamic and Pathologic Characterization of the TASK-1(−/−) Mouse Does Not Demonstrate Pulmonary Hypertension. Front Med (Lausanne) 4, 177, doi: 10.3389/fmed.2017.00177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aziz Q et al. Molecular and functional characterization of the endothelial ATP-sensitive potassium channel. J Biol Chem, doi: 10.1074/jbc.M117.810325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levin MD et al. K(ATP) channel gain-of-function leads to increased myocardial L-type Ca(2+) current and contractility in Cantu syndrome. Proc Natl Acad Sci U S A 113, 6773–6778, doi: 10.1073/pnas.1606465113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakamura Y & Bryan J Targeting SUR1/Abcc8-type neuroendocrine KATP channels in pancreatic islet cells. PLoS One 9, e91525, doi: 10.1371/journal.pone.0091525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wanstall JC The pulmonary vasodilator properties of potassium channel opening drugs. Gen Pharmacol 27, 599–605 (1996). [DOI] [PubMed] [Google Scholar]
- 124.Jiang L, Zhou T & Liu H Combined effects of the ATP-sensitive potassium channel opener pinacidil and simvastatin on pulmonary vascular remodeling in rats with monocrotaline-induced pulmonary arterial hypertension. Pharmazie 67, 547–552 (2012). [PubMed] [Google Scholar]
- 125.Hicks PE, Martin D, Dumez D, Zazzi-Sudriez E & Armstrong JM Pulmonary vascular and renal effects of cromakalim in anaesthetized dogs. Pflugers Arch 414 Suppl 1, S192–193 (1989). [DOI] [PubMed] [Google Scholar]
- 126.Pan Z et al. Targeting hypertension with a new adenosine triphosphate-sensitive potassium channel opener iptakalim. J Cardiovasc Pharmacol 56, 215–228, doi: 10.1097/FJC.0b013e3181e23e2b (2010). [DOI] [PubMed] [Google Scholar]
- 127.Zuo X et al. Iptakalim, a novel ATP-sensitive potassium channel opener, inhibits pulmonary arterial smooth muscle cell proliferation by downregulation of PKC-alpha. J Biomed Res 25, 392–401, doi: 10.1016/S1674-8301(11)60052-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu Y et al. Iptakalim inhibited endothelin-1-induced proliferation of human pulmonary arterial smooth muscle cells through the activation of K(ATP) channel. Vascul Pharmacol 48, 92–99, doi: 10.1016/j.vph.2008.01.001 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Wang SY, Cui WY & Wang H The new antihypertensive drug iptakalim activates ATP-sensitive potassium channels in the endothelium of resistance blood vessels. Acta Pharmacol Sin 36, 1444–1450, doi: 10.1038/aps.2015.97 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitchell HC & Pettinger WA Long-term treatment of refractory hypertensive patients with minoxidil. JAMA 239, 2131–2138 (1978). [PubMed] [Google Scholar]
- 131.Pettinger WA & Mitchell HC Side effects of vasodilator therapy. Hypertension 11, II34–36 (1988). [DOI] [PubMed] [Google Scholar]
- 132.Thirwell MP & Zsoter TT The effect of diazoxide on the veins. Am Heart J 83, 512–517 (1972). [DOI] [PubMed] [Google Scholar]
- 133.Moulds RF, Jauernig RA & Shaw J A comparison of the effects of hydrallazine, diazoxide, sodium nitrite and sodium nitroprusside on human isolated arteries and veins. Br J Clin Pharmacol 11, 57–61 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tarazi RC, Dustan HP, Bravo EL & Niarchos AP Vasodilating drugs: contrasting haemodynamic effects. Clin Sci Mol Med Suppl 3, 575s–578s (1976). [DOI] [PubMed] [Google Scholar]
- 135.Cohn JN, McInnes GT & Shepherd AM Direct-acting vasodilators. J Clin Hypertens (Greenwich) 13, 690–692, doi: 10.1111/j.1751-7176.2011.00507.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raphemot R et al. Direct activation of beta-cell KATP channels with a novel xanthine derivative. Mol Pharmacol 85, 858–865, doi: 10.1124/mol.114.091884 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gribble FM & Reimann F Sulphonylurea action revisited: the post-cloning era. Diabetologia 46, 875–891, doi: 10.1007/s00125-003-1143-3 (2003). [DOI] [PubMed] [Google Scholar]
- 138.Martin GM, Kandasamy B, DiMaio F, Yoshioka C & Shyng SL Anti-diabetic drug binding site in a mammalian KATP channel revealed by Cryo-EM. Elife 6, doi: 10.7554/eLife.31054 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dorschner H, Brekardin E, Uhde I, Schwanstecher C & Schwanstecher M Stoichiometry of sulfonylurea-induced ATP-sensitive potassium channel closure. Mol Pharmacol 55, 1060–1066 (1999). [DOI] [PubMed] [Google Scholar]
- 140.Stephan D, Winkler M, Kuhner P, Russ U & Quast U Selectivity of repaglinide and glibenclamide for the pancreatic over the cardiovascular K(ATP) channels. Diabetologia 49, 2039–2048, doi: 10.1007/s00125-006-0307-3 (2006). [DOI] [PubMed] [Google Scholar]
- 141.Koster JC, Remedi MS, Dao C & Nichols CG ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes 54, 2645–2654 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Ashcroft FM, Puljung MC & Vedovato N Neonatal Diabetes and the KATP Channel: From Mutation to Therapy. Trends Endocrinol Metab 28, 377–387, doi: 10.1016/j.tem.2017.02.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McClenaghan C et al. Cantu syndrome-associated SUR2 (ABCC9) mutations in distinct structural domains result in KATP channel gain-of-function by differential mechanisms. J Biol Chem 293, 2041–2052, doi: 10.1074/jbc.RA117.000351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]