Abstract
Preeclampsia is a hypertensive disorder of pregnancy associated with vascular dysfunction and cardiovascular risk to offspring. We hypothesize that endothelial PPARγ provides cardiovascular protection in offspring from pregnancies complicated by hypertension. C57BL/6J dams were bred with E-V290M sires, which express a dominant negative allele of PPARγ selectively in the endothelium. Arginine vasopressin was infused throughout gestation. Vasopressin elevated maternal blood pressure at gestational day 14–15 and urinary protein at day 17 consistent. Systolic blood pressure and vasodilation responses to acetylcholine were similar in vasopressin-exposed offspring compared to offspring from control pregnancies. We treated offspring with a sub-pressor dose of angiotensin-II to test if hypertension during pregnancy predisposes offspring to hypertension. Male and female angiotensin-II-treated E-V290M offspring from vasopressin-exposed but not control pregnancy exhibited significant impairment in acetylcholine-induced relaxation in carotid artery. Endothelial dysfunction in angiotensin-II-treated E-V290M vasopressin-exposed offspring was attenuated by tempol, an effect which was more prominent in male offspring. Nrf2 protein levels was significantly elevated in aorta from male E-V290M offspring, but not female offspring compared to controls. Blockade of Rho-kinase (ROCK) signaling and incubation with a ROCK2 specific inhibitor improved endothelial function in both male and female E-V290M offspring from vasopressin-exposed pregnancy. Our data suggest that interference with endothelial PPARγ in offspring from vasopressin-exposed pregnancies increases the risk for endothelial dysfunction upon exposure to a cardiovascular stressor in adulthood. This implies that endothelial PPARγ provides protection to cardiovascular stressors in offspring of a pregnancy complicated by hypertension and perhaps in preeclampsia.
Keywords: Preeclampsia, PPARγ, endothelial dysfunction, cardiovascular, offspring, angiotensin II
Summary
Genetic interference with PPARγ specifically in the vascular endothelium augments Ang-II-induced endothelial dysfunction in adult offspring born from AVP-infused pregnancies. This impairment in endothelial function was attenuated by scavengers of ROS, an effect more prominent in male offspring than female offspring. Further, the effects of ROCK inhibitors in improving endothelial function suggest that ROCK2 isoform plays a more prominent role in mediating the effects of Ang-II in the vasculature. Thus, loss of the beneficial effects of PPARγ in the endothelium increases the susceptibility of adult offspring born to mothers with preeclampsia to endothelial dysfunction.
Introduction
Preeclampsia (PE) is a life-threatening syndrome often characterized by the onset of hypertension and proteinuria during the second half of pregnancy. PE is a systemic vascular disorder that may also affect the liver, kidney, and brain.1 Risk factors for PE include both genetics and preexisting cardiovascular risk factors such as insulin resistance, obesity, diabetes mellitus, systemic low grade inflammation and renal disease.2–5 Clinical manifestations of PE can occur as early as mid-gestation and progress until the medically directed delivery of the fetus and placenta.6 Women with a history of PE have an elevated risk for developing cardiovascular diseases later in life.7,8 Moreover, maternal PE and consequent early fetal delivery results in lifelong cardiovascular risks in offspring, including elevated blood pressure, endothelial dysfunction, arterial stiffness and higher risk for stroke.9 Hence, PE is not only an important women’s health issue, but also represents a significant health risk for both male and female offspring.
Endothelial dysfunction is believed to be an underlying cause of the syndromic manifestations of PE. Early vascular dysfunction detected by uterine artery doppler has been observed in PE.10,11 Vascular damage sustained during PE persists post-partum and contributes to increased angiotensin-II (Ang-II) sensitivity and impaired nitric oxide-dependent vascular dilatation.12 Recent evidence suggests that PE-associated complications affect the vascular health of the offspring into adulthood.13 Despite this, the cause of lifelong health risks in children born from PE pregnancies remains unknown.
Our lab and others have demonstrated that copeptin, a stable marker of arginine vasopressin (AVP) secretion, is elevated as early as the sixth week of gestation in women who eventually develop PE.1,6,14,15 Importantly, we also demonstrated that low-dose AVP infusion in mice throughout gestation is sufficient to induce various phenotypes of PE, including elevations in systolic blood pressure (SBP), proteinuria, renal glomerular endotheliosis, immune dysfunction and fetal growth restriction, further providing experimental evidence for a causal role for AVP in the development of PE. Herein, we employed this AVP-infusion model to induce pregnancy-specific hypertension in dams, and studied the cardiovascular effects of in utero exposure to elevated AVP in adult male and female offspring.1,6,16
Peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand activated transcription factor known to regulate anti-inflammatory and anti-oxidant signaling, and has been implicated in the pathogenesis of PE.17–20 PPARγ mutations cause hypertension and synthetic agonists of PPARγ have been shown to reduce blood pressure, improve insulin sensitivity, and exert vascular protection.21,22 Inhibition of PPARγ during gestation was sufficient to induce various pathological features associated with PE in animal models, and agonists of PPARγ have been shown to attenuate phenotypes of PE in the reduced uterine perfusion pressure (RUPP) model.18–20 This suggests PPARγ plays a protective role in PE.
We generated mice expressing a PPARγ dominant-negative (DN) mutation selectively in the endothelium (termed E-V290M) to study the role of endothelial PPARγ. E-V290M mice exhibit impaired vasodilation in response to cardiovascular stressors such as high fat diet, renin-angiotensin system activation and aging.23–25 In light of this increased sensitivity to endothelial impairment, we tested the hypothesis that loss of endothelial PPARγ activity in pups born from a pregnancy complicated by hypertension enhances the risk for cardiovascular dysfunction compared to their non-transgenic (NT) littermates. Herein, we provide evidence that genetic interference with PPARγ in the endothelium predisposes adult male and female offspring born from AVP-infused pregnancies to endothelial dysfunction. The potential mediators of this endothelial impairment were observed to be partially sex-dependent, involve imbalances in redox homeostasis and a critical role for Rho/Rho-kinase signaling pathway.
Methods
Details of the experimental procedures for blood pressure measurements, vascular function studies using myograph systems, use of inhibitors, and methods for Western blotting are presented in the expanded Methods section of the online-only data supplement.
The data and study materials from this study will be made available from the corresponding author on reasonable request.
Animals:
All protocols were approved by the University of Iowa and Medical College of Wisconsin Animal Care and Use Committees. Care of the mice used in this study met the standards set forth by the National Institutes of Health (NIH) guidelines. Adult male and female transgenic mice expressing a dominant-negative mutation in human PPARγ under the control of an endothelial-specific vascular cadherin promoter (E-V290M) were used as experimental models as described below.23,25–27 Animals were maintained in conventional housing on a standard 12-hour light cycle with ambient temperature of 25°C. Mice were fed standard chow and were allowed drinking water ad libitum.
C57BL/6J dams were bred with heterozygous E-V290M sires, and symptoms of PE were induced by infusion of vasopressin (AVP, 24 ng/hr sc) throughout gestation.6 E-V290M mice were maintained by serial backcross to C57BL/6 for the past 10 years. Offspring from these pregnancies were stratified by sex, genotype and in utero exposure to AVP vs saline, but the litters were not culled to a specific number. Because the dams were subjected to two different treatment (saline vs AVP) it was not possible for all groups to be derived from a single litter. Whenever possible, age-and sex-matched non-transgenic (NT) littermate offspring from the same pregnancies were used as controls. Offspring could either be NT or heterozygous for the E-V290M transgene. The genotypes of the offspring were blinded during the vascular function experiments. A subset of adult offspring was administered a sub-pressor dose of angiotensin II (Ang-II, 120ng/kg/min sc) for 2 weeks using osmotic mini-pumps (Alzet 1002).
The investigator and operators were blinded to the genotype and group assignments of the dams subjected to blood pressure and proteinuria and to the genotype of the offspring used in the vascular function studies.
Statistical Analysis:
Samples sizes were determined a priori using α=0.05, β=0.2, and effect sizes and variance estimated from our previous studies. There were no exclusions in this study. Results are expressed as mean±SEM. Statistical evaluation of the data was performed using GraphPad Prism. Data were analyzed by ANOVA or 4-parameter regression modeling as previously,28 followed by the multiple-comparisons procedure noted in individual figure legends. P value less than 0.05 was considered statistically significant. For transparency, individual data points are plotted in dot/whisker plots where appropriate.
Results
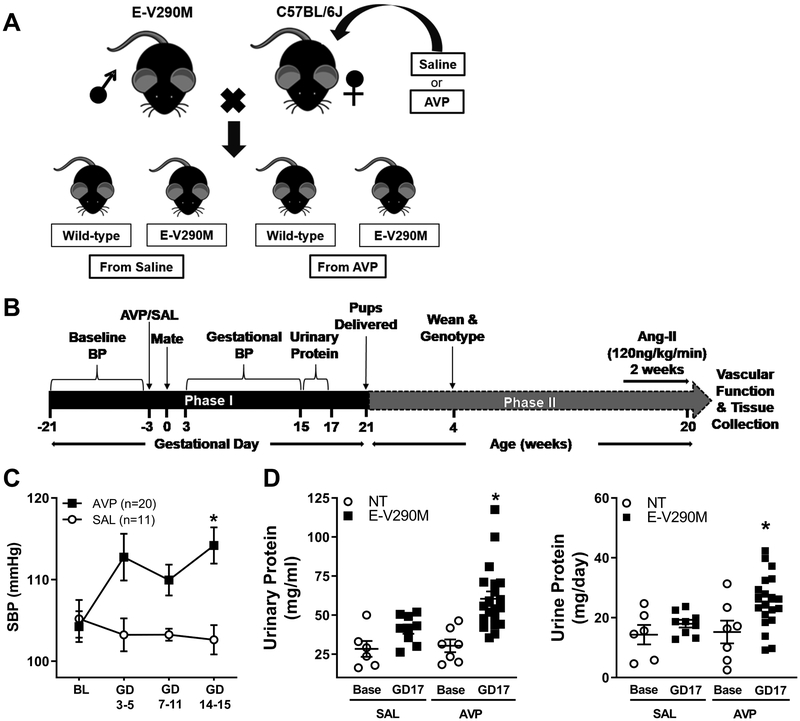
Female C57BL/6J mice were infused with saline vehicle (SAL) or AVP and bred with E-V290M sires (Figure 1A). We first assessed cardiovascular phenotypes of PE in pregnant mice and then studied offspring born from these pregnancies later in adulthood. This multi-generational study was divided into two phases: Phase I – maternal phenotypes during pregnancy, and Phase II – offspring phenotypes into adulthood (Figure 1B). To confirm the cardiovascular phenotypes of the AVP-infusion model, we monitored blood pressure in pregnant C57BL/6J mice throughout gestation. Pre-pregnancy SBP was similar (P=0.75) in dams receiving either AVP (104.2±1.8) or SAL (105.2±2.3). Dams infused with SAL did not exhibit any changes in SBP during gestation. In contrast, dams chronically infused with AVP exhibited significantly elevated gestational SBP (Figure 1C). Urinary protein levels were similar in all dams before pregnancy. Robust elevations in urinary protein (measured as mg/mL or mg/day) were observed in the pregnant dams infused with AVP (Figure 1D). These data confirm that infusion of AVP throughout gestation was sufficient to induce some of the cardinal phenotypes of PE in the pregnant C57BL/6J dams and thus this confirms a model of pregnancy complicated by hypertension.
Figure 1. Experimental Design and Preeclamptic Phenotypes in the Mother.
A) Schematic of the experimental design. Heterozygous E-V290M sires were bred with NT dams infused with either SAL (vehicle) or AVP. Offspring could either be wild-type (NT) or E-V290M. B) Longitudinal timeline of the study. Phase I is the gestational phase (in number of days) and Phase II is the life of offspring (in weeks). C-D) Preeclamptic phenotypes in the mother. C) Systolic blood pressure (SBP) was measured using tail-cuff plethysmography in SAL and AVP infused pregnant mice until gestational day (GD) 15. Data are presented as mean±SEM and analyzed by two-way repeated measures ANOVA, *P<0.05 vs. SAL pregnant. D) Urinary protein levels expressed as mg/mL (left) or mg/day (right) measured at baseline (base) and after infusion (GD17) of SAL or AVP in pregnant mice (Before SAL, n=6; GD17 SAL, n=9; before AVP, n=7; GD 17 AVP, n=20). Data are presented as mean±SEM and analyzed by two-way ANOVA. *P<0.05 vs. baseline.
Endothelial dysfunction is believed to be a major cause of the syndromic and clinical manifestations of PE.29 Evidence suggests that PE-associated complications affect the vascular health of offspring, both postnatally and as adults.30 Therefore, we assessed SBP and vascular function in adult NT and heterozygous E-V290M offspring born from AVP-infused pregnancies. SBP was not altered in NT or E-V290M mice exposed to elevated AVP vs SAL in utero (Figure S1A). There was also no difference in body weight 5 weeks or 21 weeks of age in the offspring (Table S1). KCl-induced vasoconstriction was also similar in the adult offspring irrespective of their genotype (Figure S1B). Similarly, we did not observe any changes in vasodilation to the endothelium-dependent vasodilator acetylcholine (ACh) or the nitric oxide donor sodium nitroprusside (SNP) in NT and E-V290M adult offspring born from either SAL- or AVP-infused pregnancies (Figure S1C–D). These data suggest that exposure to elevated AVP in utero itself is not sufficient to induce cardiovascular dysfunction in adult offspring (at least at 20 weeks of age), even in the presence of an impaired endothelial PPARγ activity.
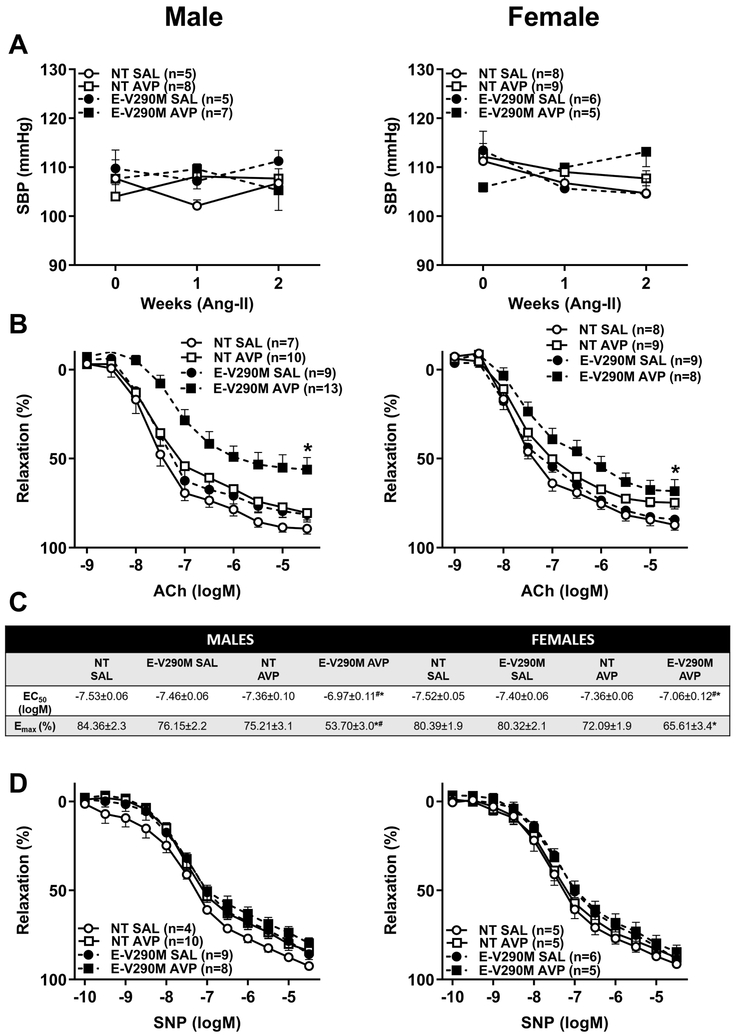
Preeclampsia is associated with a hyperreactivity to Ang-II which is believed to persist post-partum.12,31 Therefore, we tested if increased sensitivity to Ang-II could be a contributing factor to the future vascular disease in adult offspring born from AVP-infused pregnancies. A subpressor dose of Ang-II (120 ng/kg/min) was employed because we previously showed that E-V290M exhibit increased susceptibility and sensitivity to stressors including high fat diet, Ang-II and aging.23,25,27 Administration of a sub-pressor dose of Ang-II did not affect SBP irrespective of genotype or pregnancy in either male or female offspring (Figure 2A). Endothelium-dependent relaxation was not affected in Ang-II-treated male nor female NT offspring born from AVP-infused pregnancies (Figure 2B). However, we observed that ACh-mediated vasodilation was significantly impaired in male and female E-V290M mice that were exposed to elevated AVP in utero when compared to both NT mice born from AVP-infused pregnancies and E-V290M born from control pregnancies. The EC50 and Emax were significantly altered in vessels from male and female offspring from AVP-infused pregnancies (Figure 2C). SNP-induced vasorelaxation responses were normal in all groups (Figure 2D). Receptor-independent vasoconstriction responses to KCl were not altered in any groups of adult male (Figure S2A) and female (Figure S2B) offspring in response to Ang-II infusion.
Figure 2. Ang-II Sensitivity in Adult Offspring.
A) Systolic blood pressure measured using tail-cuff plethysmography in Ang-II-treated adult male (left) and female (right) NT and E-V290M offspring born from SAL or AVP-infused pregnancies. B) Cumulative concentration response curves for ACh in carotid arteries from Ang-II-treated adult male (left) and female (right) NT and E-V290M offspring born from SAL or AVP-infused pregnancies. C) 4-parameter non-linear curve fitting was used to calculate EC50 and maximum responses for all relaxation curves. Baseline values were constrained to zero. All data are presented as mean±SEM analyzed by 2-way RM ANOVA with Tukey’s multiple comparison test. *P<0.05 vs E-V290M SAL, #P<0.05 vs NT AVP. D) Cumulative concentration response curves for SNP in carotid arteries from Ang-II-treated adult male (left) and female (right) NT and E-V290M offspring born from SAL or AVP-infused pregnancies. Data are presented as mean±SEM analyzed by two-way ANOVA with repeated measures.
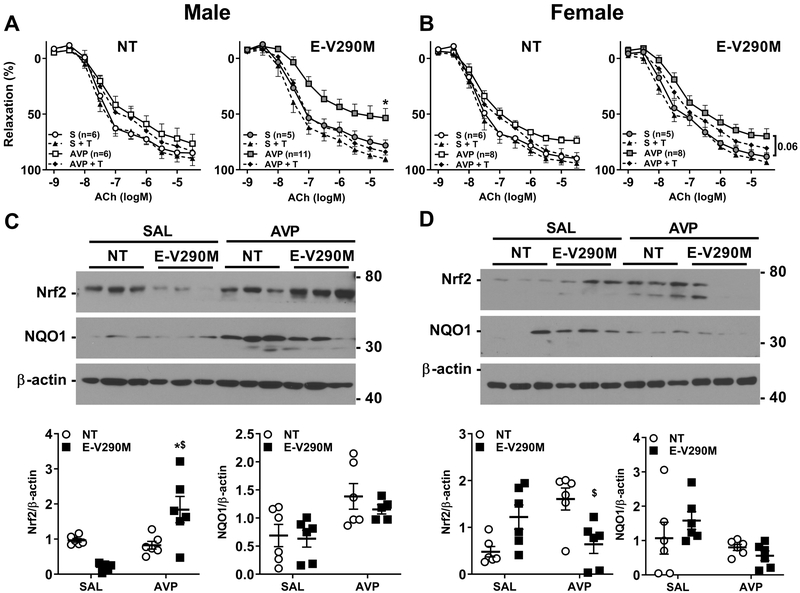
Because PPARγ has antioxidant properties in the endothelium, we investigated if the endothelial dysfunction observed in Ang-II-treated offspring is mediated by reactive oxygen species (ROS).26,32 Incubation with tempol, a scavenger of superoxide, did not modify relaxation responses in male or female NT offspring irrespective of their treatment with saline or AVP (Figure 3A–B, Table S2). In male E-V290M offspring of AVP-infused dams, ACh-mediated vasodilatation was significantly improved by Tempol suggesting a contribution by ROS (Figure 3A, Table S2). In this cohort of female offspring from AVP-infused dams, there was a trend toward endothelial dysfunction (P=0.06), which was modestly improved with Tempol (Figure 3B). Indeed, there was no significant difference between the Tempol-treated AVP-group compared with either saline-treated group. Thus the effects of Tempol appear more pronounced in males than females. Similar results were obtained when experiments were repeated with apocynin (Figure S3, Table S3).
Figure 3. Endothelial Function in Adult Offspring: Role of ROS.
A-B) Cumulative concentration response curves for ACh in carotid arteries with and without tempol (1mmol/L, 30 mins) in male (A) and female (B) offspring from the indicated pregnancy. S, saline; T, Tempol. Data are presented as mean±SEM analyzed by two-way ANOVA with repeated measures. *P<0.05 vs. E-V290M AVP + Tempol and Saline + Tempol. C-D) Representative western blots showing expression levels of Nrf2 and NQO1 in the aorta and its quantification from Ang-II-treated adult male (C) and female (D) NT and E-V290M offspring born from SAL or AVP-infused pregnancies (n=5–6 per group). Actual size markers transferred from the blots are shown. Data from the entire experiment are shown in the graphs. Data are presented as mean±SEM analyzed by two-way ANOVA. *P<0.05 vs. E-V290M SAL, $P<0.05 vs. NT AVP.
The nuclear factor-E2-related factor (Nrf2)/Kelch-like ECH-associated protein 1 (Keap1) signaling pathway is a prominent pathway involved in redox homeostasis and provides antioxidant protection.33 Consistent with the results using antioxidants, we observed a significant increase in the expression of Nrf2 protein in aorta from male Ang-II-treated E-V290M offspring born from AVP-infused pregnancies (Figure 3C). Although it appears that there was a modest reduction in Nrf2 expression in E-V290M mice from normal pregnancies, this did not reach significance (P=0.062); and in NT offspring there was no change in Nrf2 levels between saline vs AVP-treated pregnancies. Interestingly, the increase in Nrf2 protein observed in Ang-II-treated male offspring was not observed in females. Instead, there was a modest reduction in Nrf2 expression in female E-V290M offspring exposed to Ang-II (Figure 3D). We also measured the expression of a Nrf2-Keap1 target gene, NADPH dehydrogenase (quinone) 1 (NQO1) in the aorta from adult male and female offspring. Despite the increase in Nrf2, there was no difference in expression of the Nrf2-target gene NQO1 in male or female E-V290M offspring born from AVP-pregnancies.
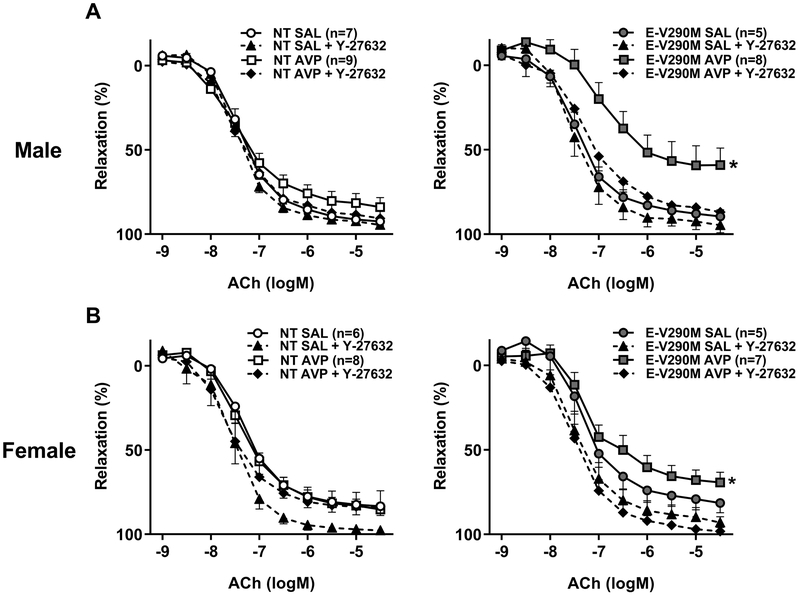
The RhoA/Rho-kinase (ROCK) pathway is a key regulator of vascular function in health and disease.34 To understand if the impaired endothelial function was mediated by activation of ROCK signaling, we assessed endothelium-dependent relaxation in carotid arteries from male and female offspring in response to Y-27632, which inhibits both ROCK1 and ROCK2 equally. Inhibition of ROCK activity using Y-27632 had no effect on ACh-mediated relaxation in Ang-II-treated NT groups, but restored endothelial function in adult male (Figure 4A) and female (Figure 4B) Ang-II-treated E-V290M mice born from AVP-infused pregnancies. Both EC50 and Emax were improved by ROCK blockade (Table S4).
Figure 4. Endothelial Function in Adult Offspring: Role of Rho-kinase.
Cumulative concentration response curves for ACh in carotid arteries from adult male (A) and female (B) with and without ROCK inhibitor, Y-27632 (1 μmol/L, 30 min). Data are presented as mean±SEM analyzed by two-way ANOVA with repeated measures. *P<0.05 vs. E-V290M AVP + Y-27632.
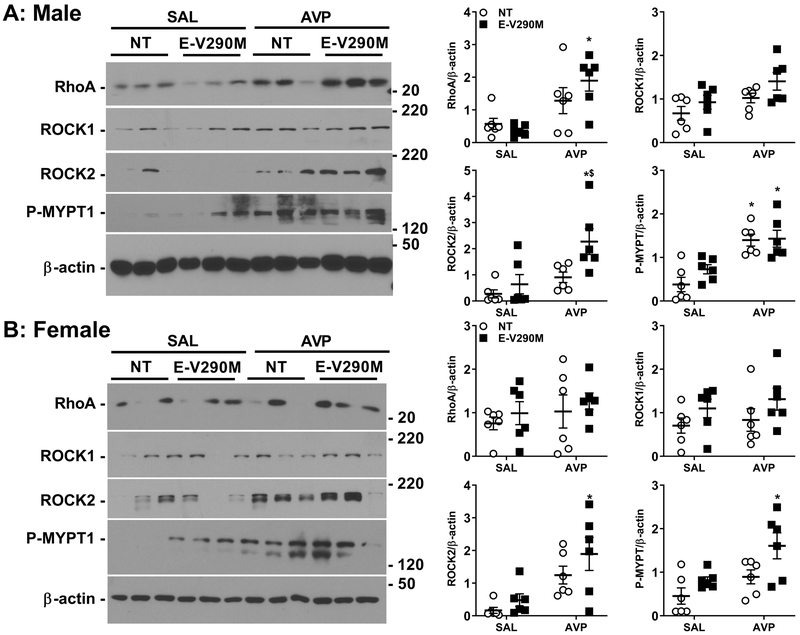
We next measured Rho/Rho-kinase pathway components RhoA, ROCK1, ROCK2 and its downstream target, phosphorylated myosin phosphatase targeting protein (P-MYPT), in aorta from Ang-II-treated male and female offspring. In male E-V290M offspring exposed to elevated AVP in utero, the level of RhoA protein expression was significantly increased compared to either E-V290M born from control pregnancies and NT mice born to AVP-infused dams (Figure 5A). We also observed a significant increase in ROCK2 but not ROCK1 expression, indicating that ROCK2 isoform might be playing a prominent role in mediating the endothelial impairment observed in these adult male E-V290M offspring. Consistently, P-MYPT levels were elevated in male offspring from AVP-treated pregnancies. There was no difference in the levels of RhoA or ROCK1 in female offspring, but the increase in ROCK2 was preserved (Figure 5B). Adult female E-V290M offspring also showed increased expression of P-MYPT compared to their SAL-exposed counterparts.
Figure 5. Protein expression RhoA-Rho kinase signaling pathway.
Representative western blots showing expression levels of total RhoA, ROCK1, ROCK2 and P-MYPT in the aorta and its quantification from Ang-II-treated adult male (A) and female (B) NT and E-V290M offspring born from SAL or AVP-infused pregnancies (n=6 per group). Actual size markers transferred from the blots are shown. Data are presented as mean±SEM analyzed by two-way ANOVA. *P<0.05 vs. E-V290M SAL, $P<0.05 vs. NT AVP.
In light of our protein expression data indicating elevated ROCK2 levels, we assessed endothelial function in response to a ROCK2-specific inhibitor, SLX-2119. Inhibition of ROCK2 did not affect relaxation responses to ACh in NT groups but significantly improved endothelial function in both male (Figure 6A) and female (Figure 6B) E-V290M mice that were exposed to elevated AVP in utero (Table S5). These data suggest that ROCK2 played a prominent functional role in mediating the endothelial impairment observed in these Ang II-treated adult offspring born from pregnancies complicated by hypertension.
Figure 6. Endothelial Function in Adult Offspring: Attenuation by ROCK2-specific inhibitor.
Cumulative concentration response curves for ACh in carotid arteries from adult male (A) and female (B) with and without ROCK2-specific inhibitor, SLX-2119. Data are presented as mean±SEM analyzed by two-way ANOVA with repeated measures. *P<0.05 vs. E-V290M AVP + SLX-2119.
Discussion
The salient findings of the present study are that 1) genetic interference with PPARγ specifically in the endothelium in adult offspring born from AVP-infused pregnancies did not affect endothelial function at baseline, but augmented the effects of a sub-pressor dose of Ang-II causing endothelial impairment, 2) the effects of PPARγ interference on endothelial function in response to Ang-II exposure was more prominent in male than female offspring, 3) endothelial dysfunction in carotid artery of E-V290M offspring was attenuated by scavengers of ROS and was more prominent in males, 4) Ang-II-induced impairment in endothelial function in both male and female E-V290M offspring was dependent on RhoA/Rho-kinase pathway activation, and 5) ROCK2 plays a role in the mechanism of Ang-II-induced endothelial dysfunction in adult offspring. Together, these findings highlight the effects of in utero exposure to elevated AVP in adult offspring and provide insights into the mechanisms by which PPARγ in the endothelium protects against endothelial dysfunction in response to exposure to a cardiovascular stressor in adult life.
We employed a model in which AVP administered throughout gestation in mice results in phenotypic similarities to PE including proteinuria, fetal growth restriction and pathognomonic renal glomerular endotheliosis.1,6 In this study we recapitulated previous findings by showing that AVP-infused dams exhibited proteinuria and increased blood pressure. We measured total urinary protein as it is considered a cardinal feature of preeclampsia. Whereas total urinary protein and urinary albumin are closely correlated in humans including those suffering from diabetes, hypertension, obesity and renal impairment, there are some patients with proteinuria who excreted normal amounts of albumin.35 Blood pressure was measured in the dams and adult offspring by tail cuff plethysmography. Although measuring blood pressure by radiotelemetry is the current gold standard, we made a conscious choice in the experimental design to use tail cuff in the dams because we did not want to decrease the success rate of the pregnancies nor alter the physiology of the offspring. Female mice were trained for 3 weeks on the plethysmograph before mating to minimize the effects of stress on the pregnancy. Moreover, in previous studies, we showed using radiotelemetry that the magnitude of the AVP-induced increase in arterial pressure was similar to what was observed in this study.1,6 Further, we sought to replicate the pregnancy specific increase in blood pressure due to AVP which was originally observed using tail cuff plethysmography.6 For the adult offspring, we cannot formally rule out the possibility that a subtle effect of fetal programming on the blood pressure was masked because of the use of tail cuff. However, it should be noted that at baseline, there was no change in the ACh response in carotid artery from NT offspring from AVP-infused pregnancy which is consistent with the blood pressure data. In previous studies, arterial pressure, measured by radiotelemetry, was not different between adult non-transgenic and E-V290M mice even in response to various stressors such a high fat diet and subpressor Ang-II.23,27
While the etiology of PE is largely unknown, PE is believed to initiate in the placenta and have a detrimental effect on the maternal endothelium. After PE, women continue to have an increased cardiovascular risk several years postpartum.5,7,36 Furthermore, prenatal and early-life stress act to program the vasculature toward higher susceptibility to the development of endothelial impairment. Offspring of mothers with PE were shown to have systemic vascular dysfunction, and are at increased life-time risk for hypertension and other cardiovascular diseases; however, the mechanisms mediating these phenotypes are not clear.37 Our data indicate that endothelial PPARγ plays a critical protective role against endothelial dysfunction in adult offspring that are born to mothers exposed to elevated AVP during gestation, a model of pregnancy complicated by hypertension. The fact that this phenotype of endothelial impairment in E-V290M mice is unmasked only upon exposure to a cardiovascular stressor later in adult life suggests the significance of genetic, epigenetic and environmental factors in mediating cardiovascular diseases in offspring born to complicated pregnancies. We recognize that it might have been attractive to use an experimental design where for each litter we would study one male NT, one male E-V290M, one female NT, and one female E-V290M as might be done in other developmental programming studies in order to cull litters to control for nutrient access for offspring.38 That offspring were derived from both AVP-infused and saline-infused dams made this impossible. Factors which made this challenging was: 1) need for offspring to be derived from separate AVP-infused and saline-infused pregnancies, 2) blinding of the experimental groups to enhance rigor, 3) uneven distribution of offspring by genotype and sex in each litter, 4) the need to stagger pregnancies in order to complete the studies in offspring in a timely way, and 5) the number and complexity of experiments dissecting the mechanisms of endothelial dysfunction.
Activation of PPARγ lowers blood pressure in humans, whereas autosomal dominant mutations in PPARγ cause impairment in PPARγ function and result in hypertension.21 Experimental evidence implicates a role for PPARγ in the pathogenesis of PE. PPARγ inhibition throughout gestation induced various pathological features associated with PE whereas activation of PPARγ has been shown to attenuate phenotypes of PE in animal models.18–20 This clearly establishes the consequences on cardiovascular events when PPARγ function is impaired.
Previously, our lab and others have demonstrated a protective role for PPARγ in the endothelium and vascular smooth muscle.23,39 Endothelial PPARγ exhibits anti-oxidant and anti-inflammatory properties.26 Ang-II is well known to activate pro-oxidant signaling and to contribute to endothelial dysfunction.40,41 It is well established that the pro-oxidant effects of Ang-II in promoting vascular dysfunction are independent of its effects on blood pressure.24 The existence of sex differences in mechanisms of vascular dysfunction induced by stressors during early life and adulthood has been reported.42 Maternal separation which acts as a psychosocial stressor has been shown to have distinct sex-dependent effects on the physiological responses to Ang-II.43 Here we show that Ang-II exposure in adulthood causes endothelial dysfunction in both male and female E-V290M born to AVP-infused pregnancies but the effects appear to be more prominent in male offspring. This finding underscores the fact that interference with PPARγ in endothelial cells is sufficient to substantially increase vascular sensitivity to cardiovascular stressors in offspring exposed to intrauterine environment complicated by hypertension. We further show that Ang II-induced endothelial dysfunction in adult offspring is attenuated by scavengers of ROS, an effect much more prominent in male offspring. Considering this, the robust activation of Nrf2 expression in adult male E-V290M offspring and downregulation of Nrf2 expression observed in female E-V290M offspring remains puzzling. Perhaps this might be a compensatory effect in response to Ang-II exposure in males, and the absence of this compensation in females indicate that the endothelial dysfunction observed in female E-V290M offspring may not be as dependent on redox mechanisms. Indeed, the effects of tempol and apocynin were much more modest in female offspring. It is puzzling that we did not observe significant differences in NQO1 expression in male or female E-V290M offspring exposed to elevated AVP. NQO1 is one of several antioxidant target genes of the Nrf2-Keap1 signaling system, the other major players being heme oxygenase 1 (HO-1), glutathione peroxidase (GPX), glutathione S-transferase (GST) and glutamate-cysteine ligase modifier subunit (GCLM).44 Although NQO1 is a target of the Nrf2 signaling cascade, it is plausible that the endothelial dysfunction observed in male offspring is mediated through the activation of other Nrf2/Keap1 target enzymes and is not NQO1-dependent. Our data highlight the need to further investigate the underlying mechanisms of sex differences on endothelial impairment in adult offspring born from PE pregnancies. For example, hormonal differences have been reported in male and female offspring from preeclamptic pregnancies.45 Consistently, preliminary meta-analyses of sex-based differences on the effects of PE upon adult blood pressure in humans hint that male sex may indeed confer increased sensitivity to the programming effects of PE.46 It is notable that differential placental-regulated pathways may be sex-specific which may correlate with increased programmed disease outcomes in males.47
In addition to oxidative stress, ROCK has emerged as a significant contributor to the development of vascular diseases. ROCK is a serine-threonine kinase member of a family of protein kinases with two distinct isoforms - ROCK1 and ROCK2.48 Although ROCK1 and ROCK2 have highly similar structures with almost 65% amino acid homology, several functional differences have been reported between these isoforms.49 For example, genetic studies have shown that ROCK1 is required for the formation of stress fibers, whereas ROCK2 is necessary for cellular contraction.49 Previous studies have demonstrated the importance of ROCK signaling by using pharmacological inhibitors such as Y-27632 and fasudil which target both ROCK isoforms.50 The findings in our study that Y-27632 had similar effects in improving endothelial function in male and female E-V290M exposed to elevated AVP in utero suggests that the endothelial dysfunction is, at least in part, mediated through ROCK activation. We also observed significantly elevated expression levels of ROCK2 in aorta from adult offspring irrespective of their sex, further indicating a possible key role for ROCK signaling. That no difference was found in ROCK1 protein expression was further indicative of the functional importance of ROCK2 in mediating these effects on the endothelium. Our experiments testing vasorelaxation responses in the presence of SLX-2119, a ROCK2-specific inhibitor, further corroborated our finding that the endothelial dysfunction observed in male and female E-V290M offspring was dependent on ROCK2 activation. In keeping with the functional differences mentioned earlier for ROCK isoforms, there have been previous reports showing a preferential binding for P-MYPT1 to ROCK2 compared with ROCK1.51 Consistent with this, we observed that the downstream target of Rho/ROCK signaling, P-MYPT1, was elevated in the vasculature of male and female E-V290M mice. Another difference observed between AVP-exposed male and female E-V290M offspring was in the expression levels of aortic RhoA protein. While RhoA was elevated in males in response to Ang-II treatment, its expression was unaffected in the female counterparts. Although this remains unexplained, it is possible that the activation of ROCK in females could have been mediated by other members of the Rho family of proteins. Thus, we recognize that different members of the Rho family might be mediating these effects on the endothelium in males and females, and this topic warrants further study. These findings provide the first line of evidence for interactions between PPARγ and ROCK2 signaling in mediating endothelial dysfunction in adult offspring born from complicated pregnancies. Of note, this interaction was sex-independent.
Perspectives
Even though the initiating cause of PE is unclear, several other disorders such as diabetes mellitus, obesity and hypertension have all been shown to be significant risk factors for the development of PE.52 However, PE still remains a major health problem due to the high prevalence of maternal and fetal mortality and morbidity. Notably, young offspring of mothers with PE have been shown to have impaired systemic and pulmonary endothelium.5 This endothelial impairment is believed to contribute the development of premature cardiovascular diseases later in adulthood. Previous reports also indicate elevated Ang-II sensitivity as a contributing factor for the vascular complications associated with PE.53 Herein, our data support the concept that interference with PPARγ specifically in the endothelium sensitizes adult offspring born from complicated pregnancies to the effects of Ang-II, thereby causing endothelial dysfunction.
Further, this study demonstrates that the potential mechanisms involved in Ang-II-mediated endothelial dysfunction might be distinct in male and females. Although our data is specific to offspring born from AVP-infused model of PE, it is indeed promising and warrants further experimental and clinical studies aimed at better elucidating the mechanisms underlying these phenotypes. Finally, since human studies have previously reported that exposure to PE compromises the cardiometabolic health of the offspring, our data from this study identify endothelial PPARγ as a relevant therapeutic target in adults born from preeclamptic pregnancies.54,55
Supplementary Material
Novelty and Significance:
What Is New?
Infusion of AVP throughout gestation induces phenotypes of preeclampsia (PE), such as elevated blood pressure and increased urinary protein levels, in pregnant mice.
PPARγ in the endothelium exerts protection against endothelial dysfunction induced by Ang-II in adult male and female offspring born from AVP-infused pregnancies.
Incubation with scavengers of ROS significantly improved endothelial function in adult male E-V290M mice exposed to elevated AVP in utero, a response which was greater in males than females.
Ang-II-induced endothelial dysfunction was mediated via activation of ROCK signaling with a prominent contribution by ROCK2.
What Is Relevant?
Although Ang-II-sensitivity has been attributed to the development of cardiovascular diseases in offspring exposed to PE, cell-specific mechanisms involved in this phenomenon are poorly understood.
Impaired endothelial PPARγ may predispose offspring born from complicated pregnancies to endothelial dysfunction upon exposure to cardiovascular stressors in adult life.
This work is pertinent to our understanding of the sex-specific mechanisms of endothelial dysfunction in adult offspring born to mothers during a preeclamptic pregnancy.
Acknowledgements
The authors thank William Paradee, Norma Sinclair, JoAnne Schwarting, and Patricia Yarolem from the University of Iowa for genotyping mice. Transgenic mice were generated at the University of Iowa Genome Editing Facility supported in part by grants from the National Institutes of Health (NIH) and from the Roy J. and Lucille A. Carver College of Medicine.
Funding Source
This work was supported through research grants from the National Institutes of Health (NIH) to C.D.S. (HL084207, HL125603, HL131689), and J.L.G (HL134850), grants from the American Heart Association to C.D.S. (15SFRN23480000), J.L.G. (18EIA33890055), J.W. (17POST33660685), and J.A.S. (16PRE30980043), and the University of Iowa (UI). The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official view of the UI. The authors gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Footnotes
Disclosures
Authors M.K.S. and J.L.G. have submitted patents describing the utility of measuring and manipulating the AVP system for the diagnosis and treatment of PE.
References
- 1.Sandgren JA, Deng G, Linggonegoro DW, Scroggins SM, Perschbacher KJ, Nair AR, Nishimura TE, Zhang SY, Agbor LN, Wu J, Keen HL, Naber MC, Pearson NA, Zimmerman KA, Weiss RM, Bowdler NC, Usachev YM, Santillan DA, Potthoff MJ, Pierce GL, Gibson-Corley KN, Sigmund CD, Santillan MK and Grobe JL. Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsnes IV, Vatten LJ, Fraser A, Bjorngaard JH, Rich-Edwards J, Romundstad PR and Asvold BO. Hypertension in Pregnancy and Offspring Cardiovascular Risk in Young Adulthood: Prospective and Sibling Studies in the HUNT Study (Nord-Trondelag Health Study) in Norway. Hypertension. 2017;69:591–598. [DOI] [PubMed] [Google Scholar]
- 3.Galaviz-Hernandez C, Sosa-Macias M, Teran E, Garcia-Ortiz JE and Lazalde-Ramos BP. Paternal Determinants in Preeclampsia. Front Physiol. 2018;9:1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J and Varner MW. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344:867–872. [DOI] [PubMed] [Google Scholar]
- 5.Powe CE, Levine RJ and Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN and Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension. 2014;64:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellamy L, Casas JP, Hingorani AD and Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. British Medical Journal. 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K and Platt RW. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation. 2019;139:1069–1079. [DOI] [PubMed] [Google Scholar]
- 9.Davis EF, Newton L, Lewandowski AJ, Lazdam M, Kelly BA, Kyriakou T and Leeson P. Pre-eclampsia and offspring cardiovascular health: mechanistic insights from experimental studies. Clin Sci (Lond). 2012;123:53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poon LC and Nicolaides KH. Early prediction of preeclampsia. Obstet Gynecol Int. 2014;2014:297397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poon LC, Staboulidou I, Maiz N, Plasencia W and Nicolaides KH. Hypertensive disorders in pregnancy: screening by uterine artery Doppler at 11–13 weeks. Ultrasound Obstet Gynecol. 2009;34:142–148. [DOI] [PubMed] [Google Scholar]
- 12.Stanhewicz AE, Jandu S, Santhanam L and Alexander LM. Increased Angiotensin II Sensitivity Contributes to Microvascular Dysfunction in Women Who Have Had Preeclampsia. Hypertension. 2017;70:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang AM and Karumanchi SA. Offspring Cardiovascular Disease in Preeclampsia: Nature Versus Nurture? Hypertension. 2017;69:589–590. [DOI] [PubMed] [Google Scholar]
- 14.Zulfikaroglu E, Islimye M, Tonguc EA, Payasli A, Isman F, Var T and Danisman N. Circulating levels of copeptin, a novel biomarker in pre-eclampsia. J Obstet Gynaecol Res. 2011;37:1198–1202. [DOI] [PubMed] [Google Scholar]
- 15.Jadli A, Ghosh K, Satoskar P, Damania K, Bansal V and Shetty S. Combination of copeptin, placental growth factor and total annexin V microparticles for prediction of preeclampsia at 10–14 weeks of gestation. Placenta. 2017;58:67–73. [DOI] [PubMed] [Google Scholar]
- 16.Scroggins SM, Santillan DA, Lund JM, Sandgren JA, Krotz LK, Hamilton WS, Devor EJ, Davis HA, Pierce GL, Gibson-Corley KN, Sigmund CD, Grobe JL and Santillan MK. Elevated vasopressin in pregnant mice induces T-helper subset alterations consistent with human preeclampsia. Clin Sci (Lond). 2018;132:419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigmund CD. Endothelial and vascular muscle PPARgamma in arterial pressure regulation: lessons from genetic interference and deficiency. Hypertension. 2010;55:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gokina NI, Chan S-L, Chapman AC, Oppenheimer K, Jetton TL and Cipolla MJ. Inhibition of PPARγ during rat pregnancy causes intrauterine growth restriction and attenuation of uterine vasodilation. Frontiers in Physiology. 2013;4:184–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC and Walsh SK. Evidence implicating peroxisome proliferator-activated receptor-gamma in the pathogenesis of preeclampsia. Hypertension. 2011;58:882–887. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK and Kenny LC. Peroxisome proliferator-activated receptor-gamma as a potential therapeutic target in the treatment of preeclampsia. Hypertension. 2011;58:280–286. [DOI] [PubMed] [Google Scholar]
- 21.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK and O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. [DOI] [PubMed] [Google Scholar]
- 22.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U and Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. [DOI] [PubMed] [Google Scholar]
- 23.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM and Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103:654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair AR, Agbor LN, Mukohda M, Liu X, Hu C, Wu J and Sigmund CD. Interference With Endothelial PPAR (Peroxisome Proliferator-Activated Receptor)-gamma Causes Accelerated Cerebral Vascular Dysfunction in Response to Endogenous Renin-Angiotensin System Activation. Hypertension. 2018;72:1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Silva TM, Li Y, Kinzenbaw DA, Sigmund CD and Faraci FM. Endothelial PPARgamma (Peroxisome Proliferator-Activated Receptor-gamma) Is Essential for Preventing Endothelial Dysfunction With Aging. Hypertension. 2018;72:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu C, Keen HL, Lu KT, Liu X, Wu J, Davis DR, Ibeawuchi SC, Vogel S, Quelle FW and Sigmund CD. Retinol-binding protein 7 is an endothelium-specific PPARgamma cofactor mediating an antioxidant response through adiponectin. JCI Insight. 2017;2:e91738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Lu KT, Mukohda M, Davis DR, Faraci FM and Sigmund CD. Interference with PPARgamma in endothelium accelerates angiotensin II-induced endothelial dysfunction. Physiol Genomics. 2016;48:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littlejohn NK, Siel RB Jr., Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD and Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol. 2013;304:R818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M and Kooner JS. Association of Maternal Endothelial Dysfunction With Preeclampsia. JAMA. 2001;285:1607–1612. [DOI] [PubMed] [Google Scholar]
- 30.Palinski W Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease. Circulation. 2014;129:2066–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usselman CW and Stachenfeld NS. Contribution of Increased Angiotensin II Sensitivity to Microvascular Dysfunction in Women With a History of Preeclampsia. Hypertension. 2017;70:245–246. [DOI] [PubMed] [Google Scholar]
- 32.Woll AW, Quelle FW and Sigmund CD. PPARgamma and retinol binding protein 7 form a regulatory hub promoting antioxidant properties of the endothelium. Physiol Genomics. 2017;49:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen B, Lu Y, Chen Y and Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol. 2015;225:R83–99. [DOI] [PubMed] [Google Scholar]
- 34.Shimokawa H, Sunamura S and Satoh K. RhoA/Rho-Kinase in the Cardiovascular System. Circ Res. 2016;118:352–366. [DOI] [PubMed] [Google Scholar]
- 35.Atkins RC, Briganti EM, Zimmet PZ and Chadban SJ. Association between albuminuria and proteinuria in the general population: the AusDiab Study. Nephrol Dial Transplant. 2003;18:2170–2174. [DOI] [PubMed] [Google Scholar]
- 36.Mongraw-Chaffin ML, Cirillo PM and Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palinski W Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease. Circulation. 2014;129:2066–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojeda NB, Grigore D, Robertson EB and Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM and Sigmund CD. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen Dinh Cat A, Montezano AC, Burger D and Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxidants & Redox Signaling. 2013;19:1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniyama Y and Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. [DOI] [PubMed] [Google Scholar]
- 42.Chrissobolis S and Faraci FM. Sex differences in protection against angiotensin II-induced endothelial dysfunction by manganese superoxide dismutase in the cerebral circulation. Hypertension. 2010;55:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho DH, Burch ML, Musall B, Musall JB, Hyndman KA and Pollock JS. Early life stress in male mice induces superoxide production and endothelial dysfunction in adulthood. Am J Physiol Heart Circ Physiol. 2016;310:H1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JM, Calkins MJ, Chan K, Kan YW and Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. [DOI] [PubMed] [Google Scholar]
- 45.Alsnes IV, Janszky I, Asvold BO, Okland I, Forman MR and Vatten LJ. Maternal Preeclampsia and Androgens in the Offspring around Puberty: A Follow-Up Study. PLoS One. 2016;11:e0167714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C and Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–1561. [DOI] [PubMed] [Google Scholar]
- 47.Cheong JN, Wlodek ME, Moritz KM and Cuffe JS. Programming of maternal and offspring disease: impact of growth restriction, fetal sex and transmission across generations. J Physiol. 2016;594:4727–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartmann S, Ridley AJ and Lutz S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Frontiers in Pharmacology. 2015;6:276–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao L, Romero MJ, Toque HA, Yang G, Caldwell RB and Caldwell RW. The role of RhoA/Rho kinase pathway in endothelial dysfunction. Journal of Cardiovascular Disease Research. 2010;1:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Silva TM, Kinzenbaw DA, Modrick ML, Reinhardt LD and Faraci FM. Heterogeneous Impact of ROCK2 on Carotid and Cerebrovascular Function. Hypertension. 2016;68:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X and Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong TY, Groen H, Faas MM and van Pampus MG. Clinical risk factors for gestational hypertensive disorders in pregnant women at high risk for developing preeclampsia. Pregnancy Hypertens. 2013;3:248–253. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham MW Jr., Williams, Amaral, Usry, Wallukat, Dechend and LaMarca. Agonistic Autoantibodies to the Angiotensin II Type 1 Receptor Enhance Angiotensin II-Induced Renal Vascular Sensitivity and Reduce Renal Function During Pregnancy. Hypertension (Dallas, Tex : 1979). 2016;68:1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice MM, Landon MB, Varner MW, Casey BM, Reddy UM, Wapner RJ, Rouse DJ, Tita ATN, Thorp JM Jr., Chien EK, Saade G , Peaceman AM and Blackwell SC. Pregnancy-Associated Hypertension and Offspring Cardiometabolic Health. Obstet Gynecol. 2018;131:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraser A, Nelson SM, Macdonald-Wallis C, Sattar N and Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.