Abstract
Cardiotonic steroids (CTS) are Na+/K+-ATPase α−1 (NKA α−1) ligands which are increased in volume expanded states and associated with cardiac and renal diseases. While initiation and resolution of inflammation is an important component of cellular injury and repair in renal disease, it is unknown whether CTS activation of NKA α−1 signaling in this setting regulates this inflammatory response. On this background, we hypothesized that CTS signaling through the NKA α−1-Src kinase complex promotes a pro-inflammatory response in renal epithelial and immune cells. First, we observed that the CTS telocinobufagin (TCB) activated multiple pro-inflammatory cyto/chemokines in renal epithelial cells, and these effects were attenuated after either NKA α−1 knock-down or with a specific inhibitor of the NKA α−1-Src kinase complex (pNaKtide). Similar findings were observed in immune cells, where we demonstrated that while TCB induced both oxidative burst and enhanced NF-KB activation in macrophages (p<0.05), the effects were abolished in NKA α−1+/− macrophages or by pretreatment with pNaKtide or the Src inhibitor PP2 (p<0.01). In a series of in vivo studies we found that 5/6th nephrectomy (PNx) induced significantly less oxidative stress in the remnant kidney of NKA α−1+/− vs wild type mice. Similarly, PNx yielded decreased levels of the urinary oxidative stress marker 8-Oxo-2’-deoxyguanosine in NKA α−1+/− vs wild type mice. Finally, we found that in vivo inhibition of the NKA α−1-Src kinase complex with pNaKtide significantly inhibited renal pro-inflammatory gene expression after PNx. These findings suggest that the NKA α−1-Src kinase complex plays a central role in regulating the renal inflammatory response induced by elevated CTS both in vitro and in vivo.
Keywords: Na+/K+-ATPase, cardiotonic steroids, renal inflammation, macrophages
Introduction
Cardiotonic steroids (CTS) are Na+/K+-ATPase α−1 (NKA α−1) ligands which are increased in volume expanded states and implicated in cardiac and renal diseases both clinically and experimentally (reviewed in1 and2). We have demonstrated that CTS signaling through the NKA α−1 contributes to cardiac and renal fibrosis through enhanced collagen production in several cardiac3, and renal4, 5 cell types. After initial stimulation of the NKA–Src–EGFR signaling cascade, this process involves activation of protein kinase C δ (PKC δ) and degradation of friend leukemia integration 1 (Fli-1) pathway3, 6 as well as suppression of microRNA (miR)-29b-3p (miR29b)7, 8 and amplification of a feed forward oxidant signaling loop9. Interestingly, we and others have also demonstrated a role for macrophage NKA α−1 in mediating early atherosclerotic events including activation of NF-κB leading to pro-inflammatory cytokine production through a signaling complex including scavenger receptor CD36, toll-like receptor-4 (TLR4), and NKA α−110, 11.
It is well known that initiation and resolution of inflammation is an important component of cellular injury and repair in diseases such as CKD. Inflammation is a nontraditional risk factor that mediates the onset and progression of renal injury in CKD12,13. Despite advances in management strategies, patients with CKD often suffer from persistent oxidative stress and inflammation which underlies their disease14. Thus discovering molecular links for inflammation in CKD is of clinical and therapeutic importance. While inflammation plays a crucial role in the pathogenesis and long-term complications of CKD15, 16, the molecular basis underlying these inflammatory events is not well understood. Recent studies suggest CTS may promote inflammation, although these studies used much higher doses of CTS than are likely to exist endogenously in vivo17. CTS have been implicated in mediating inflammation and oxidative stress in settings including heart and lung tissue17, 18. We and others have demonstrated that CTS are capable of inducing renal dysfunction, injury, and fibrosis through activation of the NKA α−1-Src kinase signaling cascade4, 19, 20. Since inflammation precedes these conditions we sought to determine if activation of the NKA α−1-Src kinase pathway by CTS was capable of regulating the inflammatory response of cell types involved in renal inflammation including macrophages and renal epithelial cells. Thus, we tested the hypothesis that CTS signaling through the NKA α−1-Src kinase complex promotes a pro-inflammatory response in renal epithelial and immune cells.
While there is some variability in measured concentrations of CTS based on the specific CTS and assay method, in general CTS have been shown to circulate in the mid-picomolar range for normal healthy subjects (physiologic levels) and up to the mid-nanomolar range for patients with volume expanded states (pathophysiologic levels) (reviewed in2). Importantly, nanomolar concentrations of CTS have been shown to bind and activate a non-pumping, signaling population of the NKA α−121–23 independent of its well-known ion transporting function24. Hence, the following studies were performed to test the hypothesis that pathophysiologically relevant doses of CTS such as telocinobufagin (TCB) are capable of initiating an inflammatory response of both renal epithelial and immune cells, via a NKA α−1- Src kinase signaling pathway.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Reagents
Telocinobufagin and marinobufagenin (both >98% by HPLC) were purchased from Herbest Bio-Tech (Baoji City, Shannxi Provience, China). Ouabain was purchased from Enzo Life Sciences (Ann Arbor, MI, USA). Digoxin was purchased from Sigma (St. Louis, MO, USA). pNaKtide was synthesized and purchased from Ohio Peptide (Powell, OH, USA). Materials for SDS-Page were purchased from Bio-Rad Laboratories. Dulbecco’s Modified Eagle Medium (DMEM) with L-Glutamine, 4.5g/L Glucose and Sodium Pyruvate was purchased from Fisher Scientific (Hampton, NH, USA). Fetal Bovine Serum was purchased from Atlanta Biologicals (Flowery Branch, GA, USA). Trypsin-EDTA (0.25%) and Penicillin-Streptomycin (10,000 U/mL) and all other tissue culture media and supplements were from Life Technologies (ThermoFisher Scientific,) (Waltham, MA, USA). All other chemicals and reagents, were from Sigma (St. Louis, MO, USA).
Cell Culture
Peritoneal macrophages were obtained by lavage after 72 hours of thioglycollate injection and adherent cells maintained in culture as we have previously described11. The human HK-2 and porcine LLC-PK1 renal proximal tubule cell lines were obtained from American Tissue Type Culture Collection (ATCC, Manassas, VA, USA). Sublines of LLCPK1 cells expressing NKA α−1 small interfering RNA to knock down expression by 90% (PY-17 cells), or control transfected cells (P-11) were cultured in the same manner as the parent cells11, 25.
HK2 cells were purchased from American Type Culture Collection (ATCC) (CRL-21900) (Manassas, VA, USA), murine macrophage Raw Blue cells were purchased from Invivogen (San Diego, CA, USA). For TCB treatment of adherent cells, upon reaching 80–90% confluence cells were serum starved for 16 hrs. For experiments utilizing NKA α−1 /Src signaling antagonist, pNaKtide, 1 μM pNaKtide was added to the cells for 30 minutes prior to any TCB treatments. Cell lysate or conditioned media were collected as we have previously published11, 25. Because of the known differences in NKA sensitivity to CTS26–29, in general, experiments performed in rodent derived cell types were treated with 10 times higher concentrations of CTS.
Reactive Oxygen Species measurement
The Fc OxyBURST® Green fluorometric assay reagent was purchased from Thermo Fisher Scientific (Waltham, MA, USA) and used for detection of reactive oxygen species in macrophages. Macrophages (2×106 cells/mL) were incubated with 10 ug/mL OxyBurst H2HFF Green BSA in a fluorescence cuvette for 2 minutes at 37 C and then treated. Oxidative burst was measured by monitoring change in fluorescence intensity excited at 488 nm and detected at 530 nm. Fluorescence intensity was measured kinetically in a multimode plate reader (Cytation 5, BioTek, Winooski, VT, USA). Fold change was calculated by dividing the data from the treated group by the data from the control group.
For detection of reactive oxygen species in renal epithelial cells the Cellular ROS Assay Kit (Red) was used (Abcam, Cambridge, MA, USA). Cells were plated in growth media at 4×104 cells/ 100 μL per well overnight. The next day, cells were incubated with working solution for 1 hour and then treated. After treatment cells were incubated in 37C/5% CO2 incubator for 1 hour. Fluorescence increase was monitored at excitation/emission of 520/605 nm. Fold change was calculated by dividing the data from the treated group by the data from the control group.
Cytokine measurement
Cytokine arrays were purchased from RayBiotech (Norcross, GA, USA). Human Cytokine Array C3 and Porcine Cytokine Array q1 were used to measure cytokines in culture supernatant. In summary, cells were serum starved for 16 hrs followed by 24 hrs of the indicated treatment. Conditioned media was collected and centrifuged at 200 xg for 10 min and cytokines involved in known inflammatory cascades in renal epithelial cells were profiled according to the manufacturer’s instructions. For these experiments, arrays were run with conditioned media from n = 3 pooled samples per array and n = 3 arrays per group for the human cytokine array and n=4 arrays per group for the porcine cytokine array. Signal intensity was measured for each spot and local background was subtracted for each array. Signal intensity on each array was normalized using the internal controls provided prior to calculating the mean values of the respective groups. Fold change was calculated by dividing the data from the treated group by the data from the control group.
NFκB reporter assay
Murine macrophage RAW-blue cells, with a secreted embryonic alkaline phosphatase (SEAP) reporter system (InvivoGen) (San Diego, CA, USA) were used to assess NFκB activity according to the manufacturer’s instructions. Briefly, after rinsing twice with PBS and detachment of cells with a cell scraper, cells were resuspended in test media (containing 10% heat-inactivated FBS) at 5×105 cells/mL. Then 180 μL of cell suspension were seeded in 96 well plates. Cells were treated and incubated at 37’ C in a 5% CO2 incubator for 18–24 hrs, followed by addition of 5 mM ATP for 2 hours. Twenty microliters of cell supernatant was then obtained after centrifugation at 200 xg for 10 min. SEAP levels (indicator of NF-κB activity) were measured by incubating the supernatant with 180 μL of Quanti-Blue (InvivoGen, San Diego, CA, USA) substrate for 2h and absorption was measured with a multimode plate reader at 620 nm (Cytation 5, BioTek, Winooski, VT, USA).
Intracellular Sodium Measurement
Intracellular sodium was assayed using Molecular Probe™ Sodium Green™ Tetraacetate, cell permeant reagent purchased from Thermo Fisher Scientific (Waltham, MA, USA) according to the manufacturer’s instructions. Cells were incubated with sodium green tetraacetate for 1 hr at room temperature. After 1 hr cells were washed to remove excess probe. After indicated treatments intracellular sodium was monitored by measuring fluorescence monitored at excitation/emission of 507/532 nm.
Animals
All animal studies were performed in accordance to the National Institutes of Health’s Guide for the Care and approved by the Institutional Animal Care and Use Committee at the University of Toledo. Na+/K+-ATPase α−1 subunit heterozygous null mice (NKA α−1+/−) and their wild type littermate controls (Wild Type NKA α−1+/+) were produced as explained30. Mice were received as a generous gift from Dr. Jerry Lingrel’s laboratory (University of Cincinnati) and maintained at the University of Toledo animal facility. For this study, 2–3 month old, male mice weighing 25–27 g were used. Wild type and NKA α−1+/− mice were each randomly divided into two groups based on surgical intervention: one group underwent sham operation to serve as controls while the second group was subjected to 5/6th partial nephrectomy (PNx) surgery as previously described8, 31. After the surgery, wild type mice were further randomly divided into two subgroups: one group received pNaKtide 25 mg/kg body weight via intraperitoneal injection every other week for a total of 3 injections from the 12th to 16th week, and the second group, which served as the control group, received a similar volume of saline.
Histology
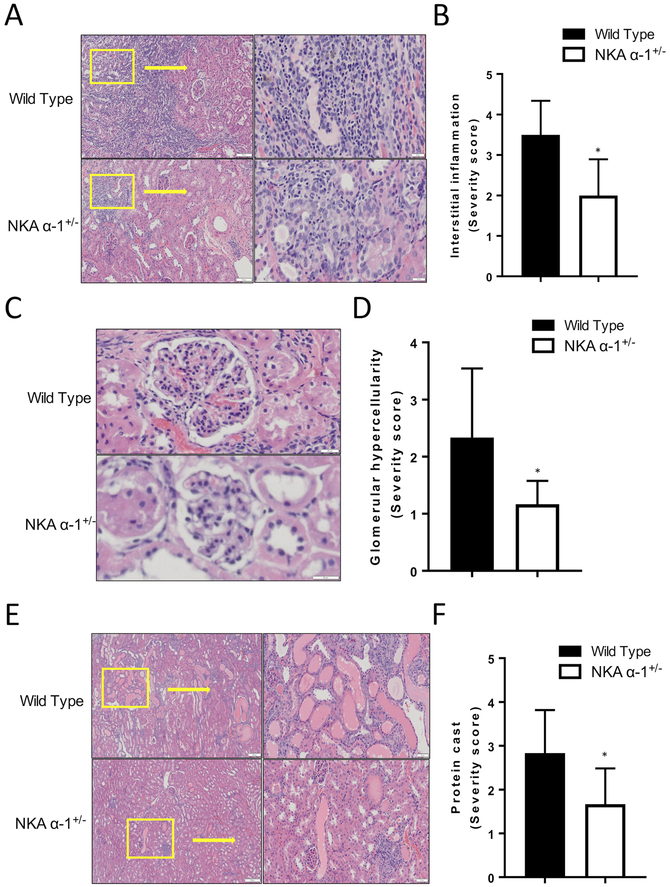
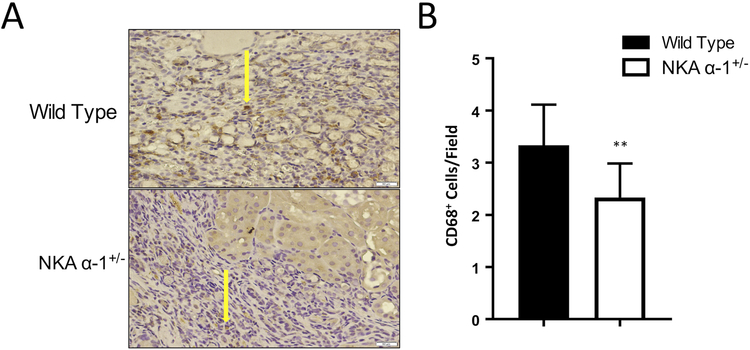
Kidneys were fixed in 4% formaldehyde (pH 7.2) paraffin embedded and cut into 4 μm sections. The tissue sections were deparaffinized with xylene and rehydrated by sequential incubations in ethanol and water. 8-Oxo-2’-deoxyguanosine and CD68 antibodies were purchased from Abcam (Cambridge, MA, USA), and H&E staining for kidney was conducted on the 4 μm kidney tissue sections. Vectastain Elite-ABC kit (Vector Labs) (Burlingame, CA, USA) was used following manufacturer’s protocol. For each section, 10 images were randomly taken with a bright-field microscope with a 20X lens and quantitative morphometric analysis was performed using automated and customized algorithms/scripts for batch analysis (ImageIQ Inc., Cleveland, OH, USA) written for Image Pro Plus 7.0, as we have described in detail32. Renal histology was graded in a blinded fashion by a pathologist (A.G.) and scored on a scale of 0–4 for interstitial inflammation, glomerular hyper cellularity, and protein casts.
RNA Isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA extraction, cDNA preparation, and RT-qPCR were all performed utilizing the QIAGEN (Germantown, MD, USA) automated workflow system which utilizes the QIAcube HT and QIAgility liquid handling robots. RNA from kidney tissue was isolated utilizing QIAzol/Chloroform extraction methodology via automated liquid handling equipment (QIAcube HT). Approximately 500 ng of extracted RNA was used to synthesize cDNA (QIAGEN’s RT2 First Strand Kit cat #330404). RT-PCR was performed utilizing QIAGEN’s Rotor-Gene Q thermocycler. Calculation of gene expression was conducted by comparing the relative change in cycle threshold value (ΔCt). Fold change in expression was calculated using the 2-ΔΔCt equation as previously described19.
8-Oxo-2’-deoxyguanosine measurement in urine
8-Oxo-2’-deoxyguanosine (8-OHdG) in 24 hour urine samples was measured by ELISA was purchased from Biovision (Milpitas, CA, USA) and performed according to the manufacturers’ protocol.
Statistical analysis
Data presented are the mean ± standard error of the mean of at least 3 independent experiments. Student’s Unpaired T-test was used to assess statistically significant differences between two groups. One-way ANOVA and post-hoc multiple comparisons tests were used when comparing more than two groups. Statistical significance was accepted as p<0.05. All statistical analysis were performed using GraphPad Prism 6 software.
RESULTS
Cardiotonic Steroids Signaling Through Na+/K+-ATPase α−1 and Src KinaseEnhance Renal Epithelial Cells Pro-inflammatory Response.
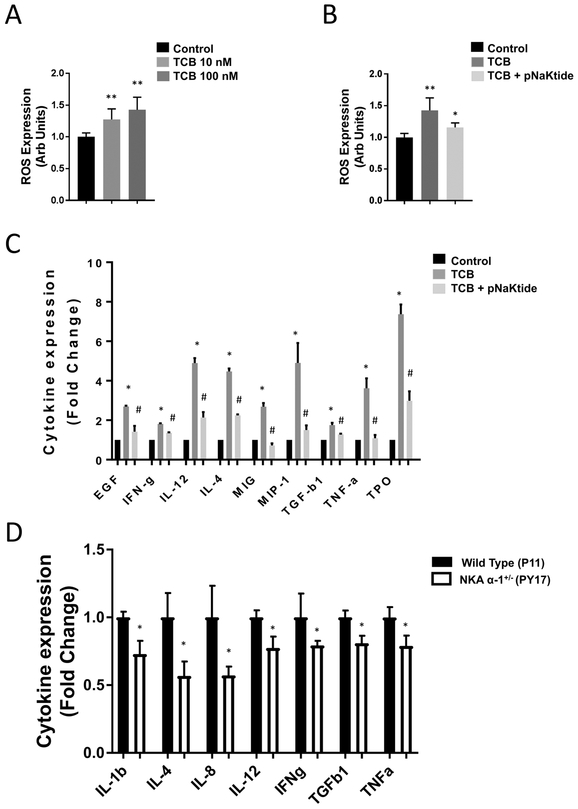
In order to test the pro-inflammatory role of CTS signaling on renal epithelium we treated HK2 cells, a human kidney epithelial cell line, for 1 hour with 10 nM and 100 nM TCB. We observed a dose dependent increase in reactive oxygen species (ROS) generation with TCB treatment when compared to the control group (Figure 1A). Similar increases in ROS were also noted with other CTS including ouabain, digoxin, and marinobufagenin (Figure S1).
Figure 1.
Telocinobufagin (TCB) induced inflammatory response in renal proximal tubule cells is attenuated in the Na+/K+-ATPase α−1 (NKA α−1) knock-down cell line and after treating the wild type cell line with pNaKtide. (A) Dose dependent effect of TCB (10nM and 100nM) on ROS expression levels (n=8). (B) Blocking Src kinase using pNaKtide 1μM attenuates TCB (100 nM) induced ROS expression (n=8). (C) TCB (10 nM) induced inflammatory cytokine expression in renal epithelial cells is attenuated via pretreatment with Src kinase inhibitor pNaKtide 1μM (n=3). The full list of all measured cytokines is shown in Supplementary Table S1. (D) Reduction of Na+/K+-ATPase α−1 in the NKA α−1+/− renal epithelial cells attenuates TCB (10 nM) induced inflammatory cytokines expression compared to WT renal epithelial cells (n=4). The full list of all measured cytokines is shown in Supplementary Table S2. *p<0.05 vs. control, **p<0.01 vs. control, #p<0.05 vs. pNaKtide, ##p<0.01 vs. pNaKtide.
Next, we tested the involvement of Src kinase signaling in TCB induced ROS expression using biochemical approaches including the use of a specific peptide inhibitor of NKA α−1 related Src signaling (pNaKtide)33. Pretreatment with pNaKtide (1 μM, 30 minutes) attenuated TCB induced increase in ROS expression (Figure 1B p<0.01) as well as ROS generation induced by other CTS including ouabain, digoxin, and marinobufagenin (Figure S2). Next, we investigated the effect of CTS on inflammatory cytokine expression in renal epithelium and profiled major cytokines involved in known inflammatory cascades. As shown in Figure 1C and Supplementary Table S1, TCB treatment (10 nM, 24 hrs) of HK2 cells resulted in significant increase in expression of a number of key pro-inflammatory cytokines related to monocyte/macrophage recruitment compared to the vehicle treated control group. Similar to ROS generation, when we tested the involvement of NKA α−1 related Src signaling in TCB induced cytokine expression, we found that TCB induced expression of these key pro-inflammatory cytokines was diminished with pretreatment of the NKA α−1 specific Src kinase inhibitor pNaKtide (1 μM, 30 minutes) (Figure 1C). Further, to assess whether TCB induced pro-inflammatory signaling is specific to the α1 isoform of NKA, we used genetic approaches to modulate NKA α−1 expression in the porcine LLC-PK1 proximal tubular cell line using both wild type parent (P11 cells) and NKA α−1 knock-down (PY-17 cells, 90% reduction in NKA α−1) cell lines11, 25. Here we found that TCB (10nM TCB, 24 hours) induced increases in expression of multiple pro-inflammatory cyto/chemokines was reduced in PY-17 NKA α−1 knock-down cells compared to the wild type parent P11 control cells (Figure 1D). Similarly, we found that as TCB and other CTS including ouabain, digoxin, and marinobufagenin induced ROS expression in P11 control cells (Figure S3), knockdown of the NKA α−1 isoform significantly attenuated CTS induced ROS expression in PY-17 cells (Figure S4).
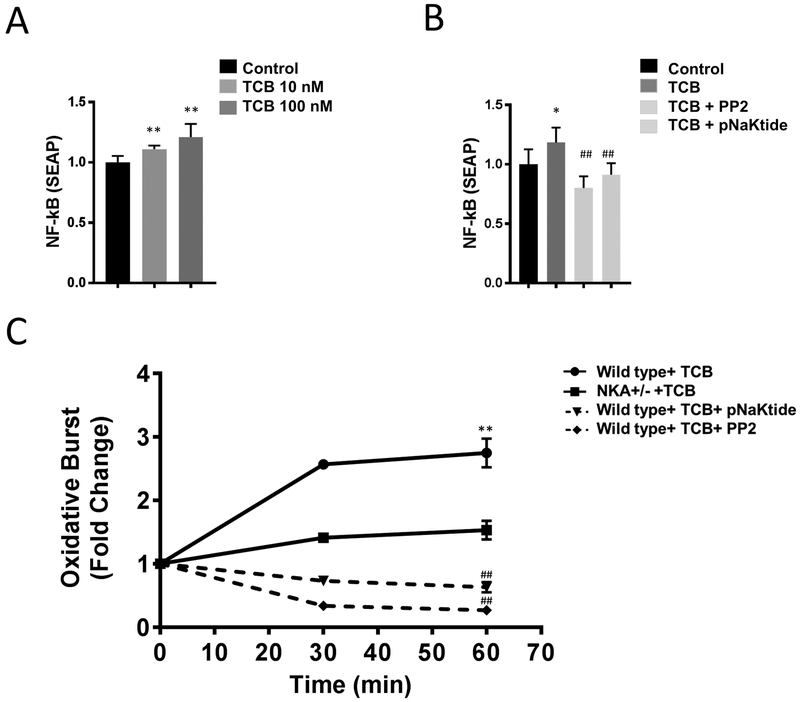
Next, we examined the effects of CTS signaling on pro-inflammatory activation of macrophages. First, we tested whether TCB enhances NF-κB activation in macrophages, we assayed NF-κB activity in RAW-blue cells. We found that TCB (10 and 100 nM, 24 hours) significantly enhanced NF-κB activation in a dose dependent manner (Figure 2A). Next, we examined the involvement of Src kinase signaling in TCB induced NF-κB activation using Src kinase inhibitors. We found that pretreatment with Src kinase inhibitors PP2 or pNaKtide (both 30 min 1μM) suppressed TCB induced NF-κB activation (Figure 2B). Additionally, other CTS including ouabain, digoxin, and marinobufagenin also demonstrated enhanced NF-κB activation which was attenuated by pre-treatment with pNaKtide (Figures S5, S6).
Figure 2.
Telocinobufagin (TCB) induced inflammatory response in macrophages is attenuated after NKA α−1 knock-down or treatment with Src kinase inhibitors pNaKtide and PP2. (A) Dose dependent effect of TCB (10 nM and 100 nM) on NF-κB activity in RAW-blue cells (n=8). (B) Blocking Src kinase using pNaKtide and/or PP2 (both at 1μM) attenuates TCB (100 nM) induced NF-κB activity in RAW-blue cells (n=8). (C) TCB (100 nM) induced oxidative burst in macrophages isolated from wild type mice is attenuated via pretreatment with Src kinase inhibitors pNaKtide and PP2 (both at 1μM) and in macrophages isolated from NKA α−1+/− mice compared to macrophages isolated from wild type (n=8). *p<0.05 vs. control, **p<0.01 vs. control, #p<0.05 vs. pNaKtide, ##p<0.01 vs. pNaKtide.
Further, we noted that TCB (100 nM, 24 hours) resulted in a significant increase in macrophage ROS generation compared to vehicle treated controls (Figure 2C). We next examined the involvement of NKA α−1 Src signaling in TCB induced oxidative burst, using genetic and biochemical approaches. Pharmacological inhibition of Src significantly suppressed TCB induced oxidative burst in macrophages (Figure 2C) as did reduction of macrophage NKA α−1.
Next, in order to understand the biological significance of these changes in relation to other known pro-inflammatory stimuli, we treated both HK2 cells and RAW-blue cells with lipopolysaccharide (LPS, 0.1 ug/mL). These experiments indicate that while TCB treatment yielded comparable levels of ROS (Figures S1–S4), LPS induced more potent activation of NFkB (Figure S6). Finally, in order to assess the effect of TCB treatment on ion transportation function of the NKA, we measured intracellular Na+ in response to different doses of TCB treatment. Our data demonstrated that TCB up to 100 nM (both 1 hour and 24 hour) had no effect on intracellular Na+ in both renal epithelial cells and macrophages (Figure S7, S8).
PNx Induced Renal inflammation and Oxidative Stress Depends on Na+/K+-ATPase α−1 Signaling in Vivo.
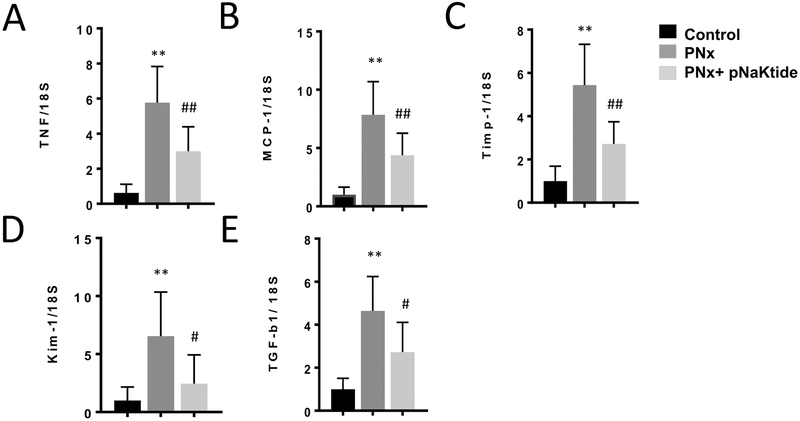
Our group has previously shown that 5/6th partial nephrectomy (PNx) is a volume expanded model of CKD and elevated CTS8, 32, 34, 35. Using this model to examine the role of the NKA α−1 in renal inflammation in vivo, we performed the following studies. First, 12 weeks after either PNx or sham surgery in wild type mice, we administered pNaKtide (25 mg/kg bodyweight) or saline vehicle by intraperitoneal injection every other week for a total of 3 injections. As shown in (Figure 3 A– E), while PNx induced expression of key inflammatory genes in renal tissue, this effect was attenuated by inhibition of the NKA α−1-Src signaling pathway via pNaKtide.
Figure 3.
pNaKtide attenuates 5/6th Partial Nephrectomy (PNx) induced increase in key inflammatory genes associated with renal oxidative stress and injury including (A) TNF, (B) MCP-1, (C) Timp-1, (D) KIM-1, (E) TGF-β. Quantitative PCR from wild type mice kidneys after 12 weeks of PNx surgery and intraperitoneal injection of pNaKtide at 25 mg/kg body weight every other week until the 16th week for a total of 3 injections (n=6). *p<0.05 vs. control, **p<0.01 vs. control, #p<0.05 vs. pNaKtide, ##p<0.01 vs. pNaKtide.
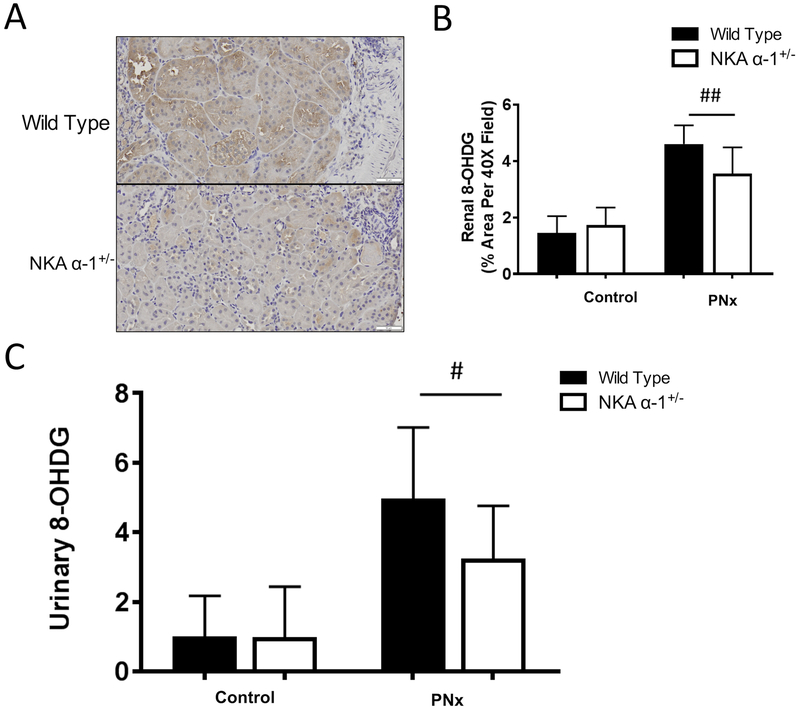
In order to further examine the role of the NKA α−1 in mediating PNx induced renal inflammation and oxidative stress, we performed PNx or sham surgery on both wild type and NKA α−1+/− mice. Sixteen weeks post PNx surgery, kidneys of mice were sectioned and stained for 8-Oxo-2’-deoxyguanosine (8-OHdG) in order to examine evidence of renal oxidative stress. Kidneys from NKA α−1+/− mice demonstrated significantly lower levels of DNA oxidation compared to wild type controls (Figure 4 A, B). Similarly, twenty-four hour urine collected from the NKA α−1+/− mice showed significantly lower excretion levels of 8-OHdG compared to urine collected from wild type controls 16 weeks of PNx surgery (Figure 4 C). Histological examination demonstrated that NKA α−1+/− mice had significantly lower inflammation levels represented by less interstitial immune cell accumulation, glomerular hypercellularity, and glomerular immune cell infiltration compared to kidneys from wild type mice (Figure 5 A–F). Additionally, kidneys from NKA α−1+/− mice showed less protein cast formation compared to the kidneys from wild type mice. Next, we examined recruitment of CD68 positive immune cells (macrophage) within the kidney. Consistent with the other histology, kidneys from NKA α−1+/− mice showed less macrophage infiltration compared to kidneys from the wild type controls (Figure 6 A, B).
Figure 4.
Na+/K+-ATPase α−1 (NKA α−1) knock-down attenuates 5/6th Partial Nephrectomy (PNx) induced oxidative stress in the kidney. Representative 8-Oxo-2’-deoxyguanosine (8-OHdG) histology (A) and quantification (B) from wild type and NKA α−1+/− mouse kidneys after PNx and sham surgeries (n=6). *p<0.05, **p<0.01. (C) Urinary 8-OHdG quantification from wild type and NKA α−1+/− mouse urine after PNx and sham surgeries (n=8). # p<0.05, ##p<0.01.
Figure 5.
Na+/K+-ATPase α−1 (NKA α−1) knock-down attenuates 5/6th Partial Nephrectomy (PNx) induced increases in renal interstitial inflammation (A, B), glomerular hypercellularity (C, D), and protein casts (E, F). Representative H&E histology (Right) and quantification (Left) from wild type and NKA α−1+/− mouse kidneys after PNx surgery (n=6). *p<0.05, **p<0.01.
Figure 6.
Na+/K+-ATPase α−1 (NKA α−1) knock-down attenuates 5/6th Partial Nephrectomy (PNx) induced increases of macrophage infiltration in the kidney (CD68-positive). Representative histologic images (A) and quantification (B) from wild type and NKA α−1+/− mouse kidneys after PNx surgery (n=6). *p<0.05, **p<0.01.
Discussion
In the current study, we identified the NKA α−1-Src signaling complex as a central component of CTS mediated pro-inflammatory response and oxidative stress in both renal epithelial and immune cells. This study provides in vitro and in vivo evidence demonstrating that the NKA α−1-Src kinase signaling mediate CTS induced inflammation and oxidative stress in these cell types. We show that in vitro, while TCB induces cytokine and ROS expression in renal epithelial cells, genetic reduction of the NKA α−1 or pharmacological inhibition of the NKA α−1-Src signaling complex reduces TCB induced pro-inflammatory effects. Similarly, in immune cells disruption of the NKA α−1-Src signaling pathway reduces TCB induced oxidative burst and NFkB activity in macrophages. Together these results indicate that the NKA α−1-Src kinase signaling pathway is an important mediator of CTS induced pro-inflammatory effects in both renal epithelial and immune cells.
This study has important implications in mechanistically understanding the link between elevated endogenous CTS and progression of CKD. Inflammation and oxidant stress play an essential role in the onset and progression of CKD and chronic elevations of CTS are associated with renal injury in CKD2, 5, 19. The in vitro findings of the current study suggest that CTS are capable of regulating the inflammatory response associated with CKD in key cell types including renal epithelium and macrophages. Furthermore, we investigated the role of elevated endogenous CTS in mediating renal inflammation and oxidative stress in an in vivo model of CKD. As the NKA α−1 is the receptor for CTS, we examined whether disrupting the NKA α−1-Src signaling complex would reduce inflammation and oxidative stress in PNx model, a well-established CKD model of elevated CTS20, 34, 35. In fact, we found that in the PNx model, genetic reduction of the NKA α−1 significantly reduced renal inflammation and oxidative stress compared to that in wild type mice. Similarly, pharmacological inhibition of the NKA α−1- Src signaling complex with pNaKtide significantly reduced renal inflammation and oxidative stress in this model. The potential role of NKA α−1-Src signaling in driving CTS mediated inflammation and fibrosis is becoming more appreciated2, 36, 37. Our group has previously shown that both active and passive immunization against the CTS marinobufagenin significantly improves cardiac and renal renal function and markedly reduces cardiac and renal fibrosis following PNx5, 32. Additionally, many studies have linked the association of high levels of CTS to renal pathology (reviewed in32, 34, 35). Our lab and others has reported that patients with CKD have higher plasma CTS levels compared to healthy individuals19, 20, 38. We have also shown that TCB infusion causes renal fibrosis along with renal dysfunction noted by increased proteinuria and cystatin C which is attenuated by knock-down of the NKA α−119. This further highlights the potential role of CTS induced NKA α−1 signaling in mediating key events in the progression of CKD. In fact, administration of the NKA α−1-Src signaling complex inhibitor, pNaKtide, in murine models of CKD has been shown to reduce pro-fibrotic effects by interrupting amplification of the oxidant signaling loop39 and restoring miR-29b-3p expression31 in this setting. This anti-fibrotic effect was also achieved by genetic reduction of NKA α−1 expression31.
Further studies have also shown that targeting NKA α−1 mediated signaling can effectively attenuate unilateral ureteral obstruction (UUO)-induced renal fibrogenesis37. Taken together these studies highlight the role of CTS in potentiating some pathophysiologic features associated with activation of NKA α−1-Src signaling in renal disease. Our study confirms these findings and demonstrates that NKA α−1-Src signaling is involved and has important implications for enhancing pro-inflammatory events in immune cells and renal epithelium both in vitro and in vivo.
While our study focused on investigating the proinflammatory effects of CTS in renal epithelial and immune cells, other studies have demonstrated that various CTS are also capable of exhibiting anti-inflammatory and anti-fibrotic effects. For instance, Zhakeer and coworkers demonstrated that in a mouse asthma model, bufalin treated mice had a significant reduction in total inflammatory cells in the lung as well as reduction in inflammatory markers such as IL-4, IL-5, and IL-1340. Additional studies have shown that the CTS ouabain inhibits fibrosis triggered by TGF-b. Here, ouabain inhibition of the ion pumping function of NKA increased intracellular [Na+]-to-[K+] ratio which induces COX-2 expression and attenuated myofibroblast differentiation in response to TGF-β41.
These observations suggest that CTS may play a role in mediating both adaptive and maladaptive immune responses and that the effects of these hormones may also be affected by tissue specific actions in different disease settings.
Previous studies that investigated the role of CTS in pro-inflammatory settings used much higher doses of CTS than are likely to exist endogenously in vivo17. The CTS ouabain has been shown to induce NF-κB-transcriptional activity and promote pro-inflammatory cytokine production in macrophages42. Additional studies have demonstrated that elevated CTS levels cause sharp elevation of Prostaglandin-endoperoxide synthase2 (PTGS2) and Interleukin 6 (IL-6) expression in rat vascular smooth muscle cells, human endothelial cells, as well as in HeLa cells43. High levels of ouabain also induce macrophage infiltration and IL-1β expression in cardiac tissue17. These studies cumulatively provide several lines of evidence that CTS are capable of promoting an inflammatory response. Importantly, the current study extends these finding by addressing the molecular mechanism through which CTS induces its effect in these settings. Furthermore we demonstrate that pathophysiological doses of CTS can initiate these inflammatory responses in key cell types both in vitro and in vivo.
In this study we examined pathophysiological doses of a biologically relevant CTS, TCB that has been previously reported in volume expanded states such as CKD1, 38, 44, 45 and heart failure32. In order to characterize the biological significance of the inflammatory response initiated by CTS, we evaluted the effects in the context of lipopolysacharide (LPS) treatment as a positive control. Here we noted that while CTS activation of NFkB was lower than that of LPS, the generation of oxidant stress was nearly equivalent. These results suggest that long-term elevation of CTS not only activates pro-inflammatory signaling cascades, but that the deleterious effects of elevated CTS are likely more pronounced in chronic settings. In the current study we tested the central hypothesis that CTS signaling through NKA α−1- Src complex is mechanistically linked to renal inflammation and oxidative stress accompanying diseases such as CKD. Our findings suggest that modulation of CTS levels or activity may present a novel therapeutic target in settings such as CKD or heart failure where elevated levels of CTS have been linked to cardiovascular and renal pathology. This study also opens up the potential that the CTS- NKA α−1-Src-pathway may be an important therapeutic target in order to limit the pathological effects of inflammation which potentiates renal injury.
Limitations
There are several limitations which should be noted in our study. In many of our cell culture experiments, while there was a general trend toward dose dependent effects for end-points such as ROS and NFkB, dose-dependent effects were not always statistically significant. Our experience has been that clear CTS induced dose-response relationships are more clearly seen in some cell types (e.g. dermal fibroblasts and SYF+cSrc cells) than others (e.g. cardiac fibroblasts and renal epithelial cells) and these differences seem to track with CTS induced Src phosphorylation6, 46. Furthermore, although the focus of the current study was the assessment of CTS-NKA α−1-Src-pathway on renal inflammation and oxidative stress, the study is limited by the fact that we did not fully interrogate measures of renal function. We have previously reported that pNaKtide did significantly reduce plasma creatinine and BUN at 5 weeks after PNx surgery39. Given the complexity of CKD and the fact that many factors influence the onset and progression of this condition47, we believe that additional studies will be needed to examine the effects of modulating the CTS-NKA α−1-Src-pathway so that appropriate renal protective therapeutic applications can be assessed.
Perspectives
Our study provides several lines of evidence highlighting the role of CTS as important mediators of renal inflammation and oxidative stress associated with CKD. Furthermore, our study demonstrates that CTS mediate pro-inflammatory effects through the NKA α−1-Src signaling complex in both renal epithelial and immune cells. This knowledge, coupled with our understanding of the key role of inflammation and oxidative stress in the pathogenesis of CKD development and progression, suggests that CTS may be an important diagnostic and therapeutic target for attenuating inflammation mediated renal injury in patients with CKD. As CTS are elevated in other volume expanded states which experience significant inflammation mediated organ injury, these findings may have therapeutic relevance beyond CKD as well.
Supplementary Material
Novelty and Significance:
1). What Is New?
Cardiotonic steroids are capable of activating pro-inflammatory pathways in both kidney epithelial cells and immune cells.
This process appears to involve a signaling pathway mediated by the Na+/K+-ATPase α−1 and Src kinase.
2). What Is Relevant?
While cardiotonic steroids have been show to participate in an adaptive natriuretic response in volume expanded settings like chronic kidney disease, the current study supports the notion that long term elevations of these hormones may also contribute to a maladaptive trade-off and contribute to kidney inflammation and injury.
3). Summary
Cardiotonic steroids can promote renal inflammation and oxidative stress through the Na+/K+-ATPase α−1 and Src kinase signaling complex in both renal epithelial and immune cells. As these hormones are elevated in other volume expanded states which experience significant inflammation mediated organ injury, these findings may have therapeutic relevance beyond chronic kidney disease.
Acknowledgements
Some of these data were presented in abstract form at the Clinical and Translational Research and the Midwestern Section of the American Federation for Medical Research 2017 Combined Annual Meeting and the American Heart Association Scientific Sessions 2017.
Sources of Funding
This work was supported by the National Institutes of Health (HL-137004 and HL-105649), the National Affiliate of the American Heart Association (14SDG18650010), the American Society of Nephrology (Pre-doctoral Fellowship to FKK) the David and Helen Boone Foundation Research Fund, an Early Career Development Award from the Central Society for Clinical and Translational Research, the University of Toledo Women and Philanthropy Genetic Analysis Instrumentation Center, and the University of Toledo Medical Research Society.
Footnotes
Disclosures
NONE
References
- 1.Bagrov AY, Shapiro JI and Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalaf F, Dube P, Mohamed A, Tian J, Malhotra D, Haller S and Kennedy D. Cardiotonic Steroids and the Sodium Trade Balance: New Insights into Trade-Off Mechanisms Mediated by the Na+/K+-ATPase. International journal of molecular sciences. 2018;19:2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, Kim EG, Smaili S, Periyasamy SM, Hariri IM and Fedorova L. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49:215–224. [DOI] [PubMed] [Google Scholar]
- 4.Fedorova LV, Raju V, El-Okdi N, Shidyak A, Kennedy DJ, Vetteth S, Giovannucci DR, Bagrov AY, Fedorova OV, Shapiro JI and Malhotra D. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. Am J Physiol Renal Physiol. 2009;296:F922–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller ST, Drummond CA, Yan Y, Liu J, Tian J, Malhotra D and Shapiro JI. Passive immunization against marinobufagenin attenuates renal fibrosis and improves renal function in experimental renal disease. American journal of hypertension. 2013;27:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkareh J, Periyasamy SM, Shidyak A, Vetteth S, Schroeder J, Raju V, Hariri IM, El-Okdi N, Gupta S and Fedorova L. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. American Journal of Physiology-Renal Physiology. 2009;296:F1219–F1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond C, Hill M, Cooper C, Shapiro J and Tian J. MicroRNA 29b and Cardiotonic Steroid-Induced Cardiac Fibrosis in Adult Cardiac Fibroblasts. The FASEB Journal. 2015;29:814.5. [Google Scholar]
- 8.Drummond CA, Hill MC, Shi H, Fan X, Xie JX, Haller ST, Kennedy DJ, Liu J, Garrett MR and Xie Z. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiological genomics. 2015;48:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro M and Joseph I. Na/K-ATPase amplification of oxidant stress; a universal but unrecognized clinical target? Marshall Journal of Medicine. 2016;2:8. [Google Scholar]
- 10.Chen Y, Kennedy DJ, Ramakrishnan DP, Yang M, Huang W, Li Z, Xie Z, Chadwick AC, Sahoo D and Silverstein RL. Oxidized LDL–bound CD36 recruits an Na+/K+-ATPase–Lyn complex in macrophages that promotes atherosclerosis. Sci Signal. 2015;8:ra91–ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy DJ, Chen Y, Huang W, Viterna J, Liu J, Westfall K, Tian J, Bartlett DJ, Tang WW and Xie Z. CD36 and Na/K-ATPase-α1 form a proinflammatory signaling loop in kidney. Hypertension. 2012:HYPERTENSIONAHA. 112198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidt DG. Inflammation in renal disease. The American journal of cardiology. 2006;97:20–27. [DOI] [PubMed] [Google Scholar]
- 13.Wang AY-M, Wang M, Woo J, Lam CW-K, Lui S-F, Li PK-T and Sanderson JE. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. Journal of the American Society of Nephrology. 2004;15:2186–2194. [DOI] [PubMed] [Google Scholar]
- 14.Cachofeiro V, Goicochea M, De Vinuesa SG, Oubiña P, Lahera V and Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: New strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int J1 - ki. 2008;74:S4–S9. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatric nephrology. 2009;24:1445–1452. [DOI] [PubMed] [Google Scholar]
- 16.Dai L, Golembiewska E, Lindholm B and Stenvinkel P. End-stage renal disease, inflammation and cardiovascular outcomes Expanded Hemodialysis: Karger Publishers; 2017(191): 32–43. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Usui-Kawanishi F, Karasawa T, Kimura H, Watanabe S, Mise N, Kayama F, Kasahara T, Hasebe N and Takahashi M. The cardiac glycoside ouabain activates NLRP3 inflammasomes and promotes cardiac inflammation and dysfunction. PloS one. 2017;12:e0176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves-de-Albuquerque CF, Burth P, Silva AR, de Moraes IMM, de Jesus Oliveira FM, Santelli RE, Freire AS, de Lima GS, da Silva ED and da Silva CI. Murine lung injury caused by Leptospira interrogans glycolipoprotein, a specific Na/K-ATPase inhibitor. Respiratory research. 2014;15:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy D, Khalaf F, Sheehy B, Weber M, Agatisa-Boyle B, Conic J, Hauser K, Medert C, Westfall K and Bucur P. Telocinobufagin, a Novel Cardiotonic Steroid, Promotes Renal Fibrosis via Na+/K+-ATPase Profibrotic Signaling Pathways. International journal of molecular sciences. 2018;19:2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolmakova EV, Haller ST, Kennedy DJ, Isachkina AN, Budny GV, Frolova EV, Piecha G, Nikitina ER, Malhotra D, Fedorova OV, Shapiro JI and Bagrov AY. Endogenous cardiotonic steroids in chronic renal failure. Nephrol Dial Transplant. 2011;26:2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J and Xie Z-J. Identification of a pool of non-pumping Na/K-ATPase. Journal of Biological Chemistry. 2007;282:10585–10593. [DOI] [PubMed] [Google Scholar]
- 22.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang X-Y and Xie Z-J. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Molecular biology of the cell. 2006;17:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Tian J, Haas M, Shapiro JI, Askari A and Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. Journal of Biological Chemistry. 2000;275:27838–27844. [DOI] [PubMed] [Google Scholar]
- 24.Tverskoi A, Sidorenko S, Klimanova E, Akimova O, Smolyaninova L, Lopina O and Orlov S. Effects of ouabain on proliferation of human endothelial cells correlate with Na+, K+-ATPase activity and intracellular ratio of Na+ and K+. Biochemistry (Moscow). 2016;81:876–883. [DOI] [PubMed] [Google Scholar]
- 25.Xie JX, Zhang S, Cui X, Zhang J, Yu H, Khalaf FK, Malhotra D, Kennedy DJ, Shapiro JI and Tian J. Na/K-ATPase/src complex mediates regulation of CD40 in renal parenchyma. Nephrology Dialysis Transplantation. 2017;33(7):1138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedorova OV, Agalakova NI, Morrell CH, Lakatta EG and Bagrov AY. ANP differentially modulates marinobufagenin-induced sodium pump inhibition in kidney and aorta. Hypertension. 2006;48:1160–1168. [DOI] [PubMed] [Google Scholar]
- 27.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG and Bagrov AY. Coordinated shifts in Na/K-ATPase isoforms and their endogenous ligands during cardiac hypertrophy and failure in NaCl-sensitive hypertension. Journal of Hypertension. 2004;22:389–97. [DOI] [PubMed] [Google Scholar]
- 28.Fedorova OV, Kashkin VA, Zakharova IO, Lakatta EG and Bagrov AY. Age-associated increase in salt sensitivity is accompanied by a shift in the atrial natriuretic peptide modulation of the effect of marinobufagenin on renal and vascular sodium pump. Journal of hypertension. 2012;30:1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedorova OV, Simbirtsev AS, Kolodkin NI, Kotov AY, Agalakova NI, Kashkin VA, Tapilskaya NI, Bzhelyansky A, Reznik VA and Frolova EV. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. Journal of hypertension. 2008;26:2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA and Lingrel JB. Identification of a specific role for the Na, K-ATPase α2 isoform as a regulator of calcium in the heart. Molecular cell. 1999;3:555–563. [DOI] [PubMed] [Google Scholar]
- 31.Drummond CA, Fan X, Haller ST, Kennedy DJ, Liu J and Tian J. Na/K-ATPase signaling mediates miR-29b-3p regulation and cardiac fibrosis formation in mice with chronic kidney disease. PloS one. 2018;13:e0197688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy DJ, Shrestha K, Sheehy B, Li XS, Guggilam A, Wu Y, Finucan M, Gabi A, Medert CM and Westfall K. Elevated plasma marinobufagenin, an endogenous cardiotonic steroid, is associated with right ventricular dysfunction and nitrative stress in heart failure. Circulation: Heart Failure. 2015:CIRCHEARTFAILURE. 114001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Zhang Z, Xie JX, Li X, Tian J, Cai T, Cui H, Ding H, Shapiro JI and Xie Z. Na/K-ATPase mimetic pNaKtide peptide inhibits the growth of human cancer cells. Journal of Biological Chemistry. 2011;286:32394–32403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D and Kolodkin NI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. [DOI] [PubMed] [Google Scholar]
- 35.Fedorova LV, Raju V, El-Okdi N, Shidyak A, Kennedy DJ, Vetteth S, Giovannucci DR, Bagrov AY, Fedorova OV and Shapiro JI. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. American Journal of Physiology-Renal Physiology. 2009;296:F922–F934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Huang W, Yang M, Xin G, Cui W, Xie Z and Silverstein RL. Cardiotonic Steroids Stimulate Macrophage Inflammatory Responses Through a Pathway Involving CD36, TLR4, and Na/K-ATPase. Arteriosclerosis, thrombosis, and vascular biology. 2017:ATVBAHA. 117309444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng X, Song Y and Wang Y. pNaKtide ameliorates renal interstitial fibrosis through inhibition of sodium-potassium adenosine triphosphatase-mediated signaling pathways in unilateral ureteral obstruction mice. Nephrology Dialysis Transplantation. 2018;34(2):242–52 [DOI] [PubMed] [Google Scholar]
- 38.Komiyama Y, Dong XH, Nishimura N, Masaki H, Yoshika M, Masuda M and Takahashi H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clinical Biochemistry. 2005;38:36–45. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Tian J, Chaudhry M, Maxwell K, Yan Y, Wang X, Shah PT, Khawaja AA, Martin R and Robinette TJ. Attenuation of Na/K-ATPase mediated oxidant amplification with pNaKtide ameliorates experimental uremic cardiomyopathy. Scientific reports. 2016;6:34592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhakeer Z, Hadeer M, Tuerxun Z and Tuerxun K. Bufalin inhibits the inflammatory effects in asthmatic mice through the suppression of nuclear factor-kappa B activity. Pharmacology. 2017;99:179–187. [DOI] [PubMed] [Google Scholar]
- 41.La J, Reed EB, Koltsova SV, Akimova O, Hamanaka RB, Mutlu GM, Orlov SN and Dulin NO. Regulation of myofibroblast differentiation by cardiac glycosides. American Journal of Physiology-Heart and Circulatory Physiology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Huang W, Yang M, Xin G, Cui W, Xie Z and Silverstein RL. Cardiotonic steroids stimulate macrophage inflammatory responses through a pathway involving CD36, TLR4, and Na/K-ATPase. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:1462–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koltsova SV, Trushina Y, Haloui M, Akimova OA, Tremblay J, Hamet P and Orlov SN. Ubiquitous [Na+] i/[K+] i-sensitive transcriptome in mammalian cells: evidence for Ca2+ i-independent excitation-transcription coupling. PLoS One. 2012;7:e38032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagrov YY, Manusova NB, Egorova IA, Fedorova OV and Bagrov AY. Endogenous digitalis-like ligands and Na/K-ATPase inhibition in experimental diabetes mellitus. Frontiers in Bioscience. 2005;10:2257–62. [DOI] [PubMed] [Google Scholar]
- 45.Bagrov YY, Manusova NB, Egorova IA, Frolova EV, Fedorova OV and Bagrov AY. Marinobufagenin, an endogenous inhibitor of alpha-1 Na/K-ATPase, is a novel factor in pathogenesis of diabetes mellitus. Dokl Biol Sci. 2005;404:333–7. [DOI] [PubMed] [Google Scholar]
- 46.El-Okdi N, Smaili S, Raju V, Shidyak A, Gupta S, Fedorova L, Elkareh J, Periyasamy S, Shapiro AP, Kahaleh MB, Malhotra D, Xie Z, Chin KV and Shapiro JI. Effects of cardiotonic steroids on dermal collagen synthesis and wound healing. J Appl Physiol. 2008;105:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Group KDIGOC-MW. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney international Supplement. 2009:S1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.