Abstract
We report the development of engineered myoglobin biocatalysts for executing asymmetric intramolecular cyclopropanations resulting in cyclopropane-fused γ-lactones, which are key motifs found in many bioactive molecules. Using this strategy, a broad range of allyl diazoacetate substrates were efficiently cyclized in high yields with up to 99% enantiomeric excess. Upon remodeling of the active site via protein engineering, myoglobin variants with stereodivergent selectivity were also obtained. In combination with whole-cell transformations, these biocatalysts enabled the gram-scale assembly of a key intermediate useful for the synthesis of the insecticide permethrin and other natural products. The enzymatically produced cyclopropyl-γ-lactones can be further elaborated to furnish a variety of enantiopure trisubstituted cyclopropanes. This work introduces a first example of biocatalytic intramolecular cyclopropanation and provides an attractive strategy for the stereodivergent preparation of fused cyclopropyl-γ-lactones of high value for medicinal chemistry and the synthesis of natural products.
Graphical Abstract
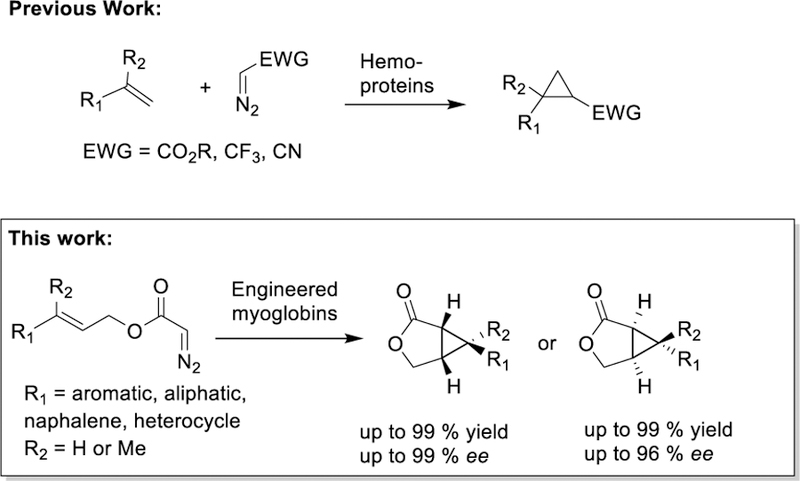
Fused cyclopropyl-lactones are structural motifs found in many biologically active natural products (e.g., blepharolides, cedkathryn, laevinoids, sterelactones)1, 2 and synthetic compounds.3 In addition, they constitute versatile intermediates for the total synthesis of a diverse range of medicinally important compounds, including basiliolide B, ambruticin S4–6 and others.7, 8 Because of their high synthetic value, significant efforts have been devoted to developing methods for the preparation of these molecular scaffolds, in particular through transition metal-catalyzed intramolecular cyclopropanations.9–18 Despite this progress, the development of catalytic protocols for asymmetric intramolecular cyclopropanations involving an earth-abundant, inexpensive, and non-toxic metal like iron has been difficult, with success being reported thus far only with donor-acceptor diazo compounds.19
Engineered hemoproteins have recently emerged as promising biocatalytic platforms for carbene transfer reactions.20–37 Recently, our group have demonstrated that engineered myoglobins (Mb) are capable of catalyzing the stereoselective intermolecular cyclopropanation of vinylarenes in the presence of diazo compounds with varied α-electron withdrawing groups (-COOR, -CF3, -CN), thus providing access to enantioenriched cyclopropanes (Scheme 1).21, 22, 24, 25, 38 Engineered P450s20, 23, 26, 27 as well as artificial metalloenzymes32, 39–46 have also proven useful for promoting intermolecular cyclopropanation reactions. Despite this progress, biocatalytic strategies for intramolecular cyclopropanations have thus far been elusive. In contrast to synthetic catalysts featuring an ‘open active site’, achieving this task using an enzyme is challenged by the need of orchestrating the intramolecular cyclopropanation reaction within the confined environment of a protein’s active site. Furthermore, the development of stereodivergent biocatalysts for a desired transformation remains an important yet challenging endeavor.47, 48 Here, we report the successful development of engineered myoglobin-based catalysts capable of promoting the intramolecular cyclopropanation of allyl α-diazoacetate derivatives with high stereocontrol and complementary enantioselectivity. This method provides efficient access to a variety of bicyclic cyclopropane-fused γ-lactones, in both enantiomeric forms and at a synthetically useful scale, for use as pharmacophores or as key intermediates for the synthesis of enantioenriched cyclopropane-containing molecules.
Scheme 1.
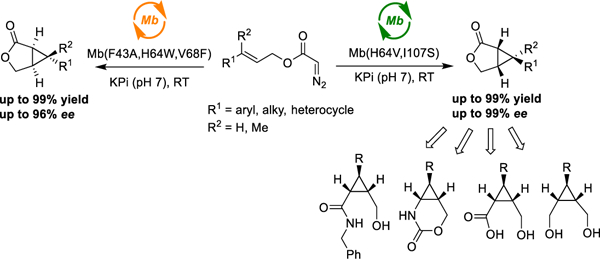
Biocatalytic methods for olefin cyclopropanation
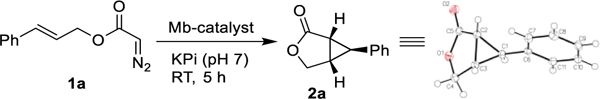
In initial studies, we discovered that wild-type sperm whale myoglobin (Mb) is able to catalyze the cyclization of trans-cinnamyl-2-diazoacetate (1a) to give 2a (Table 1). Despite its modest activity, which is comparable to that of free hemin (Table S1), Mb exhibits good enantioselectivity toward formation of the (1R,5S,6S)-configured intramolecular cyclopropanation product as determined by single crystal X-ray diffractometry (80% ee; Table 1, Entry 1). Compared to Mb, other hemoproteins including P450BM3, cytochrome c, and catalase show negligible activity (0–2%) as well as lower enantioselectivity in this reaction (6–22% ee) (Table S1). To develop a more efficient and selective biocatalyst for this transformation, we screened a panel of Mb variants (~40) featuring one to four mutations within the distal pocket of this protein (i.e., at positions Leu29, Phe43, His64, Val68, Ile107; Figure S1 and Table S2). These tests revealed the beneficial effect of large-to-small substitutions at the level of Leu29 and Val68 toward improving both enantioselectivity (80→90–93% ee) and catalytic activity (32→163 TON; Entries 2–3) (Table 1; Entries 2–3). Mb(H64V,V68A), a previously optimized Mb variant for intermolecular cyclopropanation,21 showed higher activity (32→82 TON) but similar enantioselectivity compared to Mb (82% ee).
Table 1.
Intramolecular cyclopropanation of cinnamyl 2-diazoacetate (1a) with Mb and variants thereof.a
 | |||||
|---|---|---|---|---|---|
| Entry | catalyst | OD600 | Yieldb | TON | e.e. |
| 1 | Mb | - | 13% | 32 | 80% |
| 2 | Mb(L29A) | - | 65% | 163 | 93% |
| 3 | Mb(V68A) | - | 65% | 162 | 90% |
| 4 | Mb(H64V,V68A) | - | 33% | 82 | 81% |
| 5 | Mb(L29A,H64V,V68A) | - | 99% | 250 | 96% |
| 6c | Mb(L29A,H64V,V68A) | 40 | 74% | 185 | 96% |
| 7d | Mb(L29A,H64V,V68A) | 40 | 99% | 90 | 96% |
| 8 | Mb(H64V,I107S) | - | 78% | 195 | 97% |
| 9d | Mb(H64V,I107S) | 40 | 99% (83%)e |
90 | >99% |
Reaction conditions: 5 mM cinnamyl 2-diazoacetate (1a), 20 μM Mb variant (or C41(DE3) E. coli cells at indicated OD600) in KPi buffer (50 mM, pH 7), 10 mM Na2S2O4 (protein only), r.t., 5 hours in anaerobic chamber.
GC yield.
15 min reaction time.
Using 2.5 mM 1a.
Isolated yield.
Based on this information, the beneficial L29A mutation was introduced into Mb(H64V,V68A) to give the triple variant Mb(L29A,H64V,V68A). Gratifyingly, this variant enabled the quantitative conversion of 1a into the (1R,5S,6S)-configured cyclopropane-fused γ-lactone 2a with high enantioselectivity (96% ee) (Table 1, Entry 5). Moreover, about 74% product conversion was reached in merely 15 minutes (Table 1, Entry 6 and Figure S2). Following an alternative strategy for catalyst optimization, we also screened an ‘active-site mutational landscape’ library which samples all possible 19 amino acid substitutions at the active-site positions Leu29, Phe43, Val68, and Ile107 (Figure S1) in the Mb(H64V) background.22 From this library, Mb(H64V,I107S) was identified as another efficient and highly enantioselective catalyst (97% ee) for the synthesis of 2a from 1a (Entry 8). Using either catalyst, the intramolecular cyclopropanation reaction can be conveniently carried out in whole cells using E. coli cells expressing these Mb variants (Entries 7 and 9). Notably, in addition to quantitative conversion, the whole-cell reactions with Mb(H64V,I107S)-containing E. coli cells yielded 2a in high enantiopurity (>99% ee; Entry 9). A cell density (OD600) of 40–60 was determined to be optimal for granting high conversion and high enantioselectivity in these biotransformations (Table S3). The whole-cell reaction with Mb(H64V,I107S) could be readily scaled up to enable the isolation of 180 mg of enantiopure 2a (>99% ee) in 83% isolated yield (Entry 9), thus demonstrating the scalability of the biocatalytic transformation.
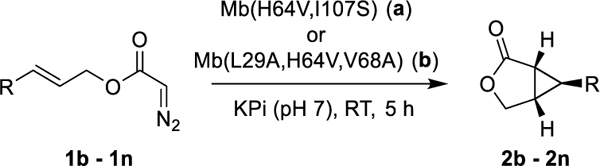
To assess the substrate scope of these biocatalysts, a diverse panel of allylic diazoacetate derivatives were then subjected to Mb(H64V,I107S)- and Mb(L29A,H64V,V68A)-catalyzed cyclization in whole-cell reactions on a semi-preparative scale (0.1 mmol) (Table 2). Substrates carrying para, meta, and ortho substituents on the phenyl group (1b–1i) were all efficiently processed by Mb(H64V,I107S), leading to the corresponding bicyclic products 2b-2i in good to quantitative yields (62–99%). Both electron withdrawing groups and electron donating groups were well tolerated, with high enantioselectivity being maintained across all of these substrates (90–99% ee; Entries 1–8). This include the ortho-substituted product 2i, indicating a good tolerance of this catalyst to steric hindrance in proximity to the olefinic bond. Good conversion and enantioselectivity (97% ee) was also achieved for the intramolecular cyclopropanation of the sterically demanding naphtyl-based substrate 1j (Entry 9). Compared to Mb(H64V,I107S), Mb(L29A,H64V,V68A) displays a similarly broad substrate scope, albeit with overall reduced activity and/or enantioselectivity toward this set of substrates. Notably, both Mb variants exhibit a consistent (1R,5S,6S) stereoselectivity across this diverse panel of substrates, as evinced from crystallographic analysis of 2a, 2c and 2d (Figures S4-6) and the similar chromatographic behavior of the other products in chiral SFC or GC.
Table 2.
Substrate scope of Mb(H64V,I107S) and Mb(L29A,H64V,V68A).a
 | ||||
|---|---|---|---|---|
| Entry | Product | Catalyst | Yieldb | e.e. |
| 1 |  |
a b |
99% 95% |
99% 96% |
| 2 |  |
a b |
83% 66% |
97% 89% |
| 3 |  |
a b |
99% 85% |
94% 80% |
| 4 |  |
a b |
62% 45% |
95% 68% |
| 5 |  |
a b |
90% 55% |
96% 70% |
| 6 |  |
a b |
77% 82% |
90% 39% |
| 7 |  |
a b |
95% 70% |
99% 74% |
| 8 |  |
a b |
99% 62% |
99% 68% |
| 9 |
a b |
65% 23% |
97% 81% |
|
| 10 |  |
a b |
71% 95% |
79% 97% |
| 11 |  |
a b |
99% 99% |
10% 52% |
| 12 |  |
a b |
87% 89% |
5% 38% |
| 13 |
a b |
43% 68% |
74% 51% |
|
Reaction conditions: 2.5 mM diazoacetate, Mb-expressing E. coli (OD600 = 40) in KPi buffer (50 mM, pH 7), 40 mL-scale, r.t., 3–5 hours.
GC or SFC yield. See Table S5 for additional data.
These results prompted us to investigate challenging substrates such as diazoacetates equipped with unactivated olefins (1l, 1m) or multiple olefinic groups (1k, 1m, 1n). Notably, all these substrates were efficiently cyclized by either Mb variant to generate 2k-n in good to quantitative yields (68–99%; Entries 10–13). Interestingly, Mb(L29A,H64V,V68A) offered significantly higher enantioselectivity in the transformation of diazoacetates with aliphatic substituents compared to Mb(H64V,I107S), thus complementing its scope across this group of substrates. The results with 2n and the nerol derivative 2m also demonstrated the high regioselectivity of the biocatalysts toward formation of the cyclopropane-γ-lactones in the presence of competing olefinic groups. In addition to 2m, other (Z)-allylic diazoacetates (1o-p) could be efficiently cyclized with good enantioselectivity (88–89% ee), but modest diastereoselectivity (Scheme S1). Methyl-substituted cinnamyl 2-diazoacetates and homoallylic diazoacetates could not be processed by the current biocatalysts (Scheme S2), defining targets for future catalyst development.
While challenging to obtain,47, 48 stereocomplementary biocatalysts are key assets for the synthesis of drugs and complex molecules.22, 49–55 To develop a stereodivergent biocatalyst for this reaction, wild-type Mb was subjected to iterative rounds of site-saturation mutagenesis (a.k.a. ISM)56 directed to the active site residues Leu29, Phe43, His64, Val68, and Ile107 (Figure S1). The resulting libraries were screened in whole cells using cinnamyl-2-diazoacetate (1a) as the substrate. Partial inversion of enantioselectivity was initially achieved via a Val→Phe mutation at position 68 (80% → −10%; Figure 1). Progressive improvement of the desired (1S,5R,6R)-selectivity was then obtained through optimi zation of position 43 and 64 via two additional rounds of mutagenesis and screening. The resulting variant, Mb(F43A,H64W,V68F), catalyzes the intramolecular cyclopropanation of 1a to give 3a in 89% ee and quantitative yield (Table S4). To assess its substrate scope, this biocatalyst was then challenged with the panel of diazoacetate substrates described in Table 2. To our delight, all these substrates were converted by Mb(F43A/H64W/V68F) to give enantioenriched 3a-n in up to 96% ee and 41–99% yields (Scheme 2). In each case, Mb(F43A,H64W,V68F) exhibits opposite enantioselectivity compared to the (1R,5S,6S)-selective variants (Table 2), thus furnishing a stereodivergent catalyst for this reaction.
Figure 1.
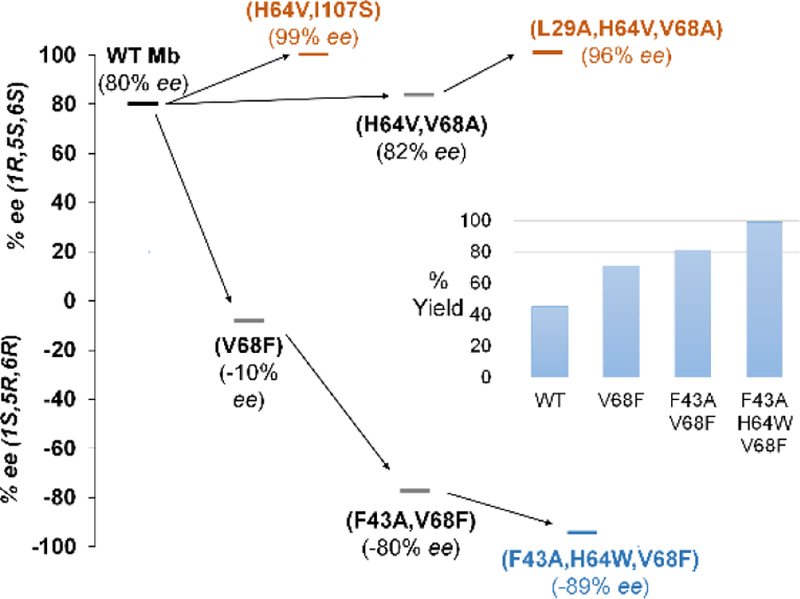
Evolutionary paths to stereodivergent intramolecular cyclopropanation biocatalysts.
Scheme 2.
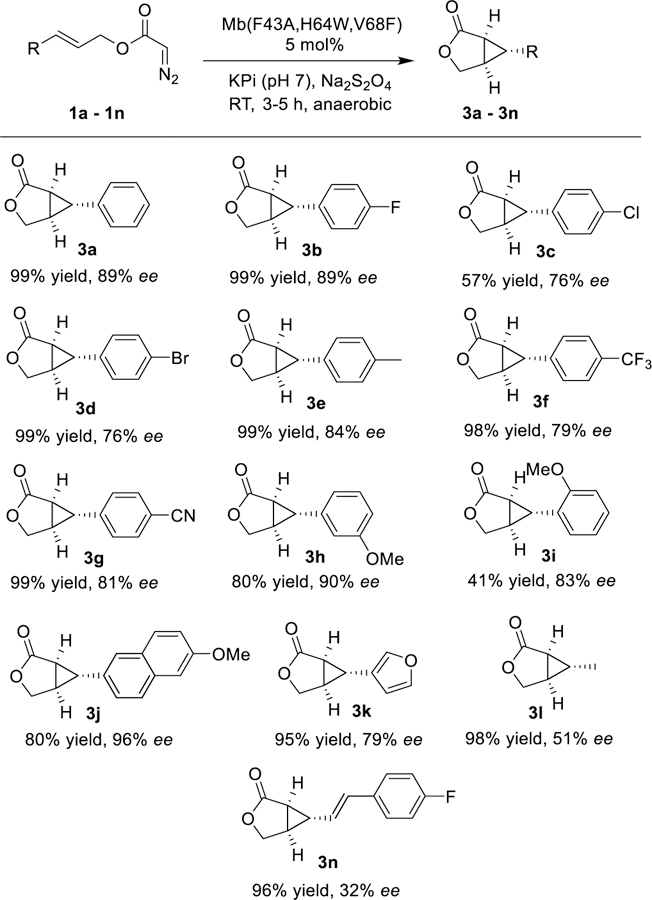
Substrate scope of (1S,5R,6R)-selective Mb(F43A,H64W,V68F).
Upon mapping their mutations onto Mb structure (Figure S1), the stereocomplementary Mb variants clearly feature a distinct active site configuration. The mutations in Mb(L29A/H64V/V68A) expand the distal cavity in correspondence to the upper side of the pocket (Leu29→Ala; His64→Trp) and the ring A/D side of the heme (Val68→Ala). In contrast, Mb(F43A/H64W/V68F) features significantly increased steric occlusion at these positions (Leu29; His64→Ala; Val68→Phe), but an enlarged cavity at the level of the opposite side of the cofactor (i.e., ring B/D via Phe43→Ala). Based on these considerations, we propose a stereochemical model for the Mb(L29A/H64V/V68A)-catalyzed reaction whereby intramolecular attack to the re face of the carbene is favored by accommodating the ester group and phenyl group into the cavities created by V68A and L29A/H64V, respectively (Figure 2). This mode of attack is likely disfavored in the case of the (1S,5R,6R)-selective variant Mb(F43A/H64W/V68F) due to steric hindrance provided by the bulky Trp/Phe residues at positions 64/68, whereas attack to the si face of the carbene may be further facilitated by accommodating the phenyl group into the cavity created by the F43A mutation (Figure 2). While further computational and structural studies are warranted to probe these stereochemical models,38 it is instructive to observe how complete remodeling of Mb active site was not only required for achieving stereodivergent selectivity in the intramolecular cyclopropanation reaction, but also with respect to enantioselective Mb-based biocatalysts previously developed for the intermolecular version22 of this transformation (Table S4).
Figure 2.
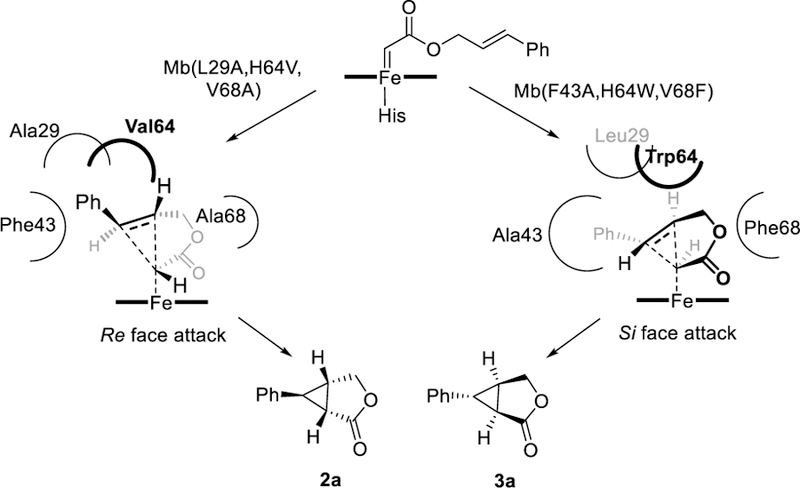
Stereochemical model for intramolecular cyclopropanation catalyzed by the stereodivergent Mb variants.
Gem-dimethyl substituted cyclopropanes are found in several bioactive natural products, including the insecticide permethrin. To further demonstrate the synthetic utility of the present strategy, a large scale biotransformation with Mb(L29A,H64V,V68A)-expressing E. coli cells was carried out in the presence of 1.5 g 3-methylbut-2-en-1-yl 2-diazoacetate (4). This reaction enabled the stereoselective synthesis of dimethyl cyclopropane 3-oxabicyclo[3.1.0]hexan-2-one 5 in 81% ee and 83% isolated yield (Scheme 3A). Further elaboration of this key intermediate via known methods57, 58 can furnish the pyrethroid natural products chrysanthemic acid, permethrin and phenothrin.
Scheme 3.
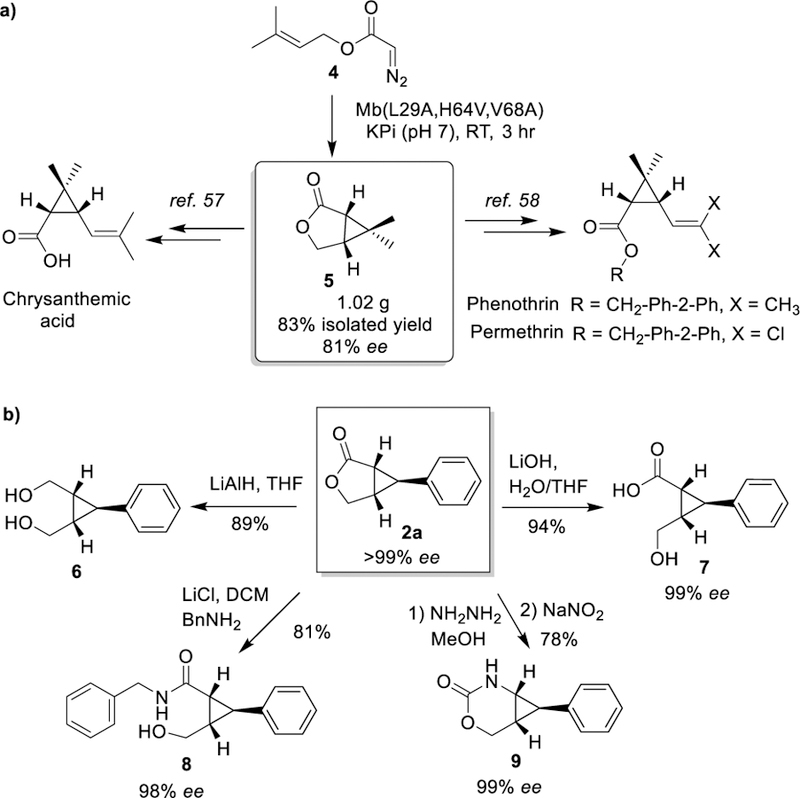
Formal total synthesis of pyrethroid natural products (A) and chemoenzymatic synthesis of trisubstituted cyclopropanes (B).
The bicyclic lactones accessible through the present method also constitute versatile intermediates for affording chiral trisubstituted cyclopropanes, which are highly valuable synthons for medicinal chemistry and total synthesis.59 Illustrating this point, enantiopure 2a produced with Mb(H64V,I107S) was reduced with LiAlH4 to give the cis-hydroxymethyl-substituted cyclopropane 6 in 89% yield in a single step (Scheme 3). On the other hand, alkaline hydrolysis of 2a or its treatment with benzyl amine in the presence of LiCl afforded the trisubstituted cyclopropanes 7 and 8 in 94% and 81% yield, respectively. Finally, hydrazinolysis of 2a followed by treatment with nitrous acid furnish the cyclopropane-fused urethane 9 in 78% yield. In all cases, these transformations occur with minimal (8; 98% ee) to no erosion (7, 9; 99% ee) of enantiopurity (Scheme 3b).
In summary, the first example of biocatalytic intramolecular olefin cyclopropanation was accomplished through the engineering of myoglobin-based catalysts capable of offering high enantioselectivity as well as stereodivergent selectivity for the asymmetric construction of bicyclic cyclopropane-γ-lactones from allyl diazoacetates. These biocatalytic transformations can be performed in whole cells, at a gram scale, and they can be applied to gain stereoselective access to key intermediates for the synthesis of cyclopropane-containing natural products and a variety of highly valuable trisubstituted cyclopropane synthons for medicinal chemistry and drug discovery. This work paves the way to the development of hemoprotein-based catalysts for other types of intramolecular carbene transfer reactions.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the U.S. National Institute of Health grant GM098628. X.R. is supported by Sunivo LLC (US). The authors are grateful to Dr. William Brennessel for assistance with crystallographic analyses. MS and X-ray instrumentation are supported by U.S. National Science Foundation grants CHE-0946653 and CHE-1725028.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Supplementary tables, figures, experimental procedures, characterization data, and crystallographic data are available in the Supporting Information.
The authors declare no competing financial interest.
REFERENCES
- (1).Bisio A, Fontana N, Romussi G, Ciarallo G, De Tommasi N, Pizza C, and Mugnoli A Clerodane diterpenoids from Salvia blepharophylla, Phytochemistry 1999, 52, 1535–1540. [Google Scholar]
- (2).Opatz T, Kolshorn H, and Anke H Sterelactones: New isolactarane type sesquiterpenoids with antifungal activity from Stereum sp IBWF 01060, J. Antibiot 2008, 61, 563–567. [DOI] [PubMed] [Google Scholar]
- (3).Ansiaux C, N’Go I, and Vincent SP Reversible and Efficient Inhibition of UDP-Galactopyranose Mutase by Electrophilic, Constrained and Unsaturated UDP-Galactitol Analogues, Chem. Eur. J 2012, 18, 14860–14866. [DOI] [PubMed] [Google Scholar]
- (4).Min L, Zhang Y, Liang XF, Huang JR, Bao WL, and Lee CS A Biomimetic Synthesis of (+/−)-Basiliolide B, Angew. Chem. Int. Ed 2014, 53, 11294–11297. [DOI] [PubMed] [Google Scholar]
- (5).Kirkland TA, Colucci J, Geraci LS, Marx MA, Schneider M, Kaelin DE, and Martin SF Total synthesis of (+)-ambruticin S, J. Am. Chem. Soc 2001, 123, 12432–12433. [DOI] [PubMed] [Google Scholar]
- (6).Rogers DH, Yi EC, and Poulter CD Enantioselective Synthesis of (+)-Presqualene Diphosphate, J. Org. Chem 1995, 60, 941–945. [Google Scholar]
- (7).Reichelt A, and Martin SF Synthesis and properties of cyclopropane-derived peptidomimetics, Acc. Chem. Res 2006, 39, 433–442. [DOI] [PubMed] [Google Scholar]
- (8).Tang P, and Qin Y Recent Applications of Cyclopropane-Based Strategies to Natural Product Synthesis, Synthesis-Stuttgart 2012, 44, 2969–2984. [Google Scholar]
- (9).Doyle MP, and Forbes DC Recent Advances in Asymmetric Catalytic Metal Carbene Transformations, Chem. Rev 1998, 98, 911–936. [DOI] [PubMed] [Google Scholar]
- (10).Lebel H, Marcoux JF, Molinaro C, and Charette AB Stereoselective cyclopropanation reactions, Chem. Rev 2003, 103, 977–1050. [DOI] [PubMed] [Google Scholar]
- (11).Davies HML, and Denton JR Application of donor/acceptor-carbenoids to the synthesis of natural products, Chem. Soc. Rev 2009, 38, 3061–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zhang ZH, and Wang JB Recent studies on the reactions of alpha-diazocarbonyl compounds, Tetrahedron 2008, 64, 6577–6605. [Google Scholar]
- (13).Lu HJ, and Zhang XP Catalytic C-H functionalization by metalloporphyrins: recent developments and future directions, Chem. Soc. Rev 2011, 40, 1899–1909. [DOI] [PubMed] [Google Scholar]
- (14).Intrieri D, Carminati DM, and Gallo E (2016) Handbook of Porphyrin Science : Recent Advances in Metal Porphyrinoid-Catalyzed Nitrene and Carbene Transfer Reactions (Kadish KM, M. S K, Guilard R Ed.), World Scientific. [Google Scholar]
- (15).Doyle MP, Austin RE, Bailey AS, Dwyer MP, Dyatkin AB, Kalinin AV, Kwan MMY, Liras S, Oalmann CJ, Pieters RJ, Protopopova MN, Raab CE, Roos GHP, Zhou QL, and Martin SF Enantioselective Intramolecular Cyclopropanations of Allylic and Homoallylic Diazoacetates and Diazoacetamides Using Chiral Dirhodium(Ii) Carboxamide Catalysts, J. Am. Chem. Soc 1995, 117, 5763–5775. [Google Scholar]
- (16).Uchida T, Saha B, and Katsuki T Co(II)-salen-catalyzed asymmetric intramolecular cyclopropanation, Tetrahedron Lett 2001, 42, 2521–2524. [Google Scholar]
- (17).Li GY, Zhang J, Chan PWH, Xu ZJ, Zhu NY, and Che CM Enantioselective intramolecular cyclopropanation of cis-alkenes by chiral ruthenium(II) Schiff base catalysts and crystal structures of (Schiff base)ruthenium complexes containing carbene, PPh3, and CO ligands, Organometallics 2006, 25, 1676–1688. [Google Scholar]
- (18).Xu ZJ, Fang R, Zhao C, Huang JS, Li GY, Zhu N, and Che CM cis-beta-Bis(carbonyl) Ruthenium-Salen Complexes: X-ray Crystal Structures and Remarkable Catalytic Properties toward Asymmetric Intramolecular Alkene Cyclopropanation, J. Am. Chem. Soc 2009, 131, 4405–4417. [DOI] [PubMed] [Google Scholar]
- (19).Shen JJ, Zhu SF, Cai Y, Xu H, Xie XL, and Zhou QL Enantioselective Iron-Catalyzed Intramolecular Cyclopropanation Reactions, Angew. Chem. Int. Ed 2014, 53, 13188–13191. [DOI] [PubMed] [Google Scholar]
- (20).Coelho PS, Brustad EM, Kannan A, and Arnold FH Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes, Science 2013, 339, 307–310. [DOI] [PubMed] [Google Scholar]
- (21).Bordeaux M, Tyagi V, and Fasan R Highly Diastereoselective and Enantioselective Olefin Cyclopropanation Using Engineered Myoglobin-Based Catalysts, Angew. Chem. Int. Ed 2015, 54, 1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bajaj P, Sreenilayam G, Tyagi V, and Fasan R Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity, Angew. Chem. Int. Ed 2016, 55, 16110–16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gober JG, Rydeen AE, Gibson-O’Grady EJ, Leuthaeuser JB, Fetrow JS, and Brustad EM Mutating a Highly Conserved Residue in Diverse Cytochrome P450s Facilitates Diastereoselective Olefin Cyclopropanation, Chembiochem 2016, 17, 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tinoco A, Steck V, Tyagi V, and Fasan R Highly Diastereo- and Enantioselective Synthesis of Trifluoromethyl-Substituted Cyclopropanes via Myoglobin-Catalyzed Transfer of Trifluoromethylcarbene, J. Am. Chem. Soc 2017, 139, 5293–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Chandgude AL, and Fasan R Highly Diastereo- and Enantioselective Synthesis of Nitrile-Substituted Cyclopropanes by Myoglobin-Mediated Carbene Transfer Catalysis, Angew. Chem. Int. Ed 2018, 57, 15852–15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Brandenberg OF, Prier CK, Chen K, Knight AM, Wu Z, and Arnold FH Stereoselective Enzymatic Synthesis of Heteroatom-Substituted Cyclopropanes, ACS Catal 2018, 8, 2629–2634. [Google Scholar]
- (27).Knight AM, Kan SBJ, Lewis RD, Brandenberg OF, Chen K, and Arnold FH Diverse Engineered Heme Proteins Enable Stereodivergent Cyclopropanation of Unactivated Alkenes, ACS Central Sci 2018, 4, 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Wang ZJ, Peck NE, Renata H, and Arnold FH Cytochrome P450-catalyzed insertion of carbenoids into N-H bonds, Chem. Sci 2014, 5, 598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sreenilayam G, and Fasan R Myoglobin-catalyzed intermolecular carbene N-H insertion with arylamine substrates, Chem. Commun 2015, 51, 1532–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Tyagi V, Bonn RB, and Fasan R Intermolecular carbene S-H insertion catalysed by engineered myoglobin-based catalysts, Chem. Sci 2015, 6, 2488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kan SBJ, Lewis RD, Chen K, and Arnold FH Directed evolution of cytochrome c for carbon-silicon bond formation: Bringing silicon to life, Science 2016, 354, 1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sreenilayam G, Moore EJ, Steck V, and Fasan R Metal substitution modulates the reactivity and extends the reaction scope of myoglobin carbene transfer catalysts, Adv. Synth. Cat 2017, 359, 2076–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Tyagi V, and Fasan R Myoglobin-Catalyzed Olefination of Aldehydes, Angew. Chem. Int. Ed 2016, 55, 2512–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Tyagi V, Sreenilayam G, Bajaj P, Tinoco A, and Fasan R Biocatalytic Synthesis of Allylic and Allenyl Sulfides through a Myoglobin-Catalyzed Doyle-Kirmse Reaction, Angew. Chem. Int. Ed 2016, 55, 13562–13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Weissenborn MJ, Low SA, Borlinghaus N, Kuhn M, Kummer S, Rami F, Plietker B, and Hauer B Enzyme-Catalyzed Carbonyl Olefination by the E. coli Protein YfeX in the Absence of Phosphines, Chemcatchem 2016, 8, 1636–1640. [Google Scholar]
- (36).Vargas DA, Tinoco A, Tyagi V, and Fasan R Myoglobin-Catalyzed C-H Functionalization of Unprotected Indoles, Angew. Chem. Int. Ed 2018, 57, 9911–9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Chen K, Huang XY, Kan SBJ, Zhang RK, and Arnold FH Enzymatic construction of highly strained carbocycles, Science 2018, 360, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Tinoco A, Wei Y, Bacik J-P, Carminati DM, Moore EJ, Ando N, Zhang Y, and Fasan R Origin of High Stereocontrol in Olefin Cyclopropanation Catalyzed by an Engineered Carbene Transferase, ACS Catal 2019, 9 1514–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Srivastava P, Yang H, Ellis-Guardiola K, and Lewis JC Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation, Nat. Commun 2015, 6, 7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Sreenilayam G, Moore EJ, Steck V, and Fasan R Stereoselective Olefin Cyclopropanation under Aerobic Conditions with an Artificial Enzyme Incorporating an Iron-Chlorin e6 Cofactor, ACS Catal 2017, 7, 7629–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Moore EJ, Steck V, Bajaj P, and Fasan R Chemoselective Cyclopropanation over Carbene Y-H Insertion Catalyzed by an Engineered Carbene Transferase, J. Org. Chem 2018, 83, 7480–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Key HM, Dydio P, Clark DS, and Hartwig JF Abiological catalysis by artificial haem proteins containing noble metals in place of iron, Nature 2016, 534, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wolf MW, Vargas DA, and Lehnert N Engineering of RuMb: Toward a Green Catalyst for Carbene Insertion Reactions, Inorg. Chem 2017, 56, 5623–5635. [DOI] [PubMed] [Google Scholar]
- (44).Ohora K, Meichin H, Zhao LM, Wolf MW, Nakayama A, Hasegawa J, Lehnert N, and Hayashi T Catalytic Cyclopropanation by Myoglobin Reconstituted with Iron Porphycene: Acceleration of Catalysis due to Rapid Formation of the Carbene Species, J. Am. Chem. Soc 2017, 139, 17265–17268. [DOI] [PubMed] [Google Scholar]
- (45).Villarino L, Splan KE, Reddem E, Alonso-Cotchico L, de Souza CG, Lledos A, Marechal JD, Thunnissen AMWH, and Roelfes G An Artificial Heme Enzyme for Cyclopropanation Reactions, Angew. Chem. Int. Ed 2018, 57, 7785–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Hayashi T, Tinzl M, Mori T, Krengel U, Proppe J, Soetbeer J, Klose D, Jeschke G, Reiher M, and Hilvert D Capture and characterization of a reactive haem-carbenoid complex in an artificial metalloenzyme, Nature Catal 2018, 1, 578–584. [Google Scholar]
- (47).Reetz MT Biocatalysis in Organic Chemistry and Biotechnology: Past, Present, and Future, J. Am. Chem. Soc 2013, 135, 12480–12496. [DOI] [PubMed] [Google Scholar]
- (48).Li GY, Wang JB, and Reetz MT Biocatalysts for the pharmaceutical industry created by structure-guided directed evolution of stereoselective enzymes, Bioorg. Med. Chem 2018, 26, 1241–1251. [DOI] [PubMed] [Google Scholar]
- (49).Turner NJ Deracemisation methods, Curr. Opin. Chem. Biol 2010, 14, 115–121. [DOI] [PubMed] [Google Scholar]
- (50).Mugford PF, Wagner UG, Jiang Y, Faber K, and Kazlauskas RJ Enantiocomplementary Enzymes: Classification, Molecular Basis for Their Enantiopreference, and Prospects for Mirror-Image Biotransformations, Angew. Chem. Int. Ed 2008, 47, 8782–8793. [DOI] [PubMed] [Google Scholar]
- (51).Feske BD, Kaluzna IA, and Stewart JD Enantiodivergent, biocatalytic routes to both taxol side chain antipodes, J. Org. Chem 2005, 70, 9654–9657. [DOI] [PubMed] [Google Scholar]
- (52).Wu Q, Soni P, and Reetz MT Laboratory Evolution of Enantiocomplementary Candida antarctica Lipase B Mutants with Broad Substrate Scope, J. Am. Chem. Soc 2013, 135, 1872–1881. [DOI] [PubMed] [Google Scholar]
- (53).Zhang K, Shafer BM, Demars MD 2nd, Stern HA, and Fasan R Controlled oxidation of remote sp3 C-H bonds in artemisinin via P450 catalysts with fine-tuned regio- and stereoselectivity, J. Am. Chem. Soc 2012, 134, 18695–18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Koszelewski D, Grischek B, Glueck SM, Kroutil W, and Faber K Enzymatic Racemization of Amines Catalyzed by Enantiocomplementary omega-Transaminases, Chem. Eur. J 2011, 17, 378–383. [DOI] [PubMed] [Google Scholar]
- (55).France SP, Aleku GA, Sharma M, Mangas-Sanchez J, Howard RM, Steflik J, Kumar R, Adams RW, Slabu I, Crook R, Grogan G, Wallace TW, and Turner NJ Biocatalytic Routes to Enantiomerically Enriched Dibenz[c,e]azepines, Angew. Chem. Int. Ed 2017, 56, 15589–15593. [DOI] [PubMed] [Google Scholar]
- (56).Reetz MT Laboratory Evolution of Stereoselective Enzymes: A Prolific Source of Catalysts for Asymmetric Reactions, Angew. Chem. Int. Ed 2011, 50, 138–174. [DOI] [PubMed] [Google Scholar]
- (57).Arlt D, Jautelat M, and Lantzsch R Syntheses of Pyrethroid Acids, Angew. Chem. Int. Ed 1981, 20, 703–722. [Google Scholar]
- (58).Mandal AK, Borude DP, Armugasamy R, Soni NR, Jawalkar DG, Mahajan SW, Ratnam KR, and Goghare AD New Synthetic Route to (1r)-Cis-(−)-Permethrin, (1r)-Cis-(+)-Cypermethrin and (1r)-Cis-(+)-Deltamethrin (Decis) from (+)-3-Carene, Tetrahedron 1986, 42, 5715–5728. [Google Scholar]
- (59).Talele TT The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules, J. Med. Chem 2016, 59, 8712–8756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


