Abstract
The purpose of this study was to identify characteristics of family relationships associated with communication of genetic risk and testing behaviors among at-risk relatives in families with an inherited cardiac condition. Data were collected from 53 patients and parents of children with an inherited cardiac condition through interviews, pedigrees, and surveys. Associations were examined among family relationship characteristics and whether at-risk relatives were informed about their risk and tested for disease. Of 1,178 at-risk relatives, 52.5% were informed about their risk and 52.1% of those informed were tested. Emotional closeness, relationship quality, and communication frequency had significant bivariate associations with genetic risk communication. Communication frequency was associated with genetic risk communication and testing in multivariate models. This study provides new insight into the extent of genetic risk communication and testing in families with inherited cardiac conditions. Family relationships, especially communication frequency, are critical factors in family communication of genetic risk.
Keywords: Cardiomyopathy, Hypertrophic [Mesh], Long QT Syndrome [Mesh], cascade screening, genetic risk communication, Disclosure [Mesh]
Awareness of disease risk is critical for prevention or mitigation of disease and its sequelae. When a disease is caused by heritable genetic variations, diagnosis of disease or disease-causing genetic variation in one person identifies increased risk for that person’s biological relatives. Cascade screening is the process of sequentially testing the proband’s (first person in the family diagnosed with disease) relatives for the disease or disease-causing genetic variation. Cascade screening is especially critical for inherited cardiac conditions because these are often undetected until a potentially fatal sudden cardiac event occurs. Cascade screening relies on effective communication of genetic risk to all at-risk relatives in order to initiate preventative interventions. In most countries, the responsibility for communicating genetic risk information to at-risk relatives falls to the proband and not healthcare professionals. In the context of inherited cardiac conditions, it is known that not all at-risk relatives are informed about their risk (Batte et al., 2015; Burns, McGaughran, Davis, Semsarian, & Ingles, 2016; Haukkala et al., 2013; Ormondroyd, Oates, Parker, Blair, & Watkins, 2014), however the extent of communication to specific at-risk relatives is unknown. Factors that impact family communication of genetic risk for inherited cardiac conditions also remain poorly understood. Nurses are well positioned to support family communication of genetic risk given their frequent interactions with patients and their multi-dimensional role as patient advocate, educator, and family care coordinator (Barr et al., 2018).
Inherited Cardiac Conditions & Family Communication
The majority of what is known about family communication of genetic risk has been learned in the context of heritable cancers which tend to manifest in adulthood (Gaff et al., 2007; Wiens, Wilson, Honeywell, & Etchgary, 2013; Wilson et al., 2004; Wiseman, Dancyger, & Michie, 2010). Hypertrophic cardiomyopathy and long QT syndrome are two inherited cardiac conditions that can manifest throughout the lifespan and cause the majority of sudden cardiac death in people under age 30 (Vetter et al., 2008). For both, changes in the electrical system in the heart lead to arrhythmia and sudden death. Historically, hypertrophic cardiomyopathy has been estimated to occur clinically in 1/500 people worldwide (Maron, 2004), although more recent estimates suggest that at least 1/200 people carry a disease-causing genetic variant (Semsarian, Ingles, Maron, & Maron, 2015). The proportion of people with genetic variants who exhibit clinical symptoms (penetrance) of hypertrophic cardiomyopathy is 50%−80% by age 30 and 95% by age 60 (Force et al., 2010), while long QT syndrome has a lifetime penetrance of 60% (Napolitano et al., 2005). Hypertrophic cardiomyopathy is generally characterized by an abnormal thickening of the left ventricular myocardium and myocardial fibrosis, which affect the structure and electrical function of the heart. Long QT syndrome is estimated to occur clinically in as many as 1/2,500 people (Schwartz et al., 2009) and is generally characterized by prolongation of the QT interval on electrocardiogram due to changes in the ion channels affecting the electrical system in the heart. Symptoms of both conditions include shortness of breath, syncope, palpitations, activity intolerance, and chest pain. However, symptoms are often nonexistent, unrecognized, and variable within families, leaving people unaware of their disease until a potentially serious event such as sudden cardiac death occurs. Individuals with hypertrophic cardiomyopathy may also develop serious cardiac complications, including heart failure with preserved or reduced ejection fraction, myocardial ischemia, arrhythmias, and stroke (Gersh et al., 2011; Maron et al., 2016).
Genetic testing can identify disease-causing genetic variants in approximately 70% of probands with hypertrophic cardiomyopathy (Ho, 2012) and in 75% of probands with long QT syndrome (Zumhagen et al., 2012). Identification of a disease-causing variant in the proband allows targeted genetic testing in relatives who may not have clinical evidence of disease. Detection of the disease-causing variant and diagnosis of disease in at-risk relatives facilitate implementation of preventive interventions including routine cardiac screening, lifestyle changes, medications or avoidance of certain medications, placement of an implantable cardioverter defibrillator, and/or surgery, which are effective at significantly reducing risk of sudden death and other complications (Crotti, Celano, Dagradi, & Schwartz, 2008; Maron et al., 2015, 2016). Both diseases are usually inherited in an autosomal dominant pattern giving all first-degree relatives (FDR) including biological parents, siblings, and children of the proband a 50% chance of inheriting the disease-causing genetic variant. When the genetic status of the FDR is unknown, all second-degree relatives (SDR) including half-siblings, grandparents/children, aunts/uncles, and nieces/nephews of the proband have a 25% chance of having the disease-causing variant, and third-degree relatives (TDR) including cousins, great-grandparents/children, great-aunts/uncles, and great-nieces/nephews have a 12.5% chance. In the absence of a confirmatory genetic test result, at-risk relatives should have regular clinical evaluations to monitor for signs and symptoms of disease.
Cascade screening is recommended as the most efficient and cost-effective way to identify and treat individuals in the pre-symptomatic stages of inherited cardiac conditions (Gersh et al., 2011). However, cascade screening for inherited cardiac conditions results in only half of at-risk relatives being tested (Burns et al., 2016; Christiaans, Birnie, Bonsel, Wilde, & van Langen, 2008; Miller, Wang, & Ware, 2013). Although other factors (e.g., accurate understanding of risk, access to healthcare) are important in the cascade screening process, communication of genetic risk within families is a critical, yet poorly understood step in this process (Burns, James, & Ingles, 2018).
Recently, research has started to examine communication of genetic risk in families with inherited cardiac conditions. One study in the United States surveyed members of a national disease support group in which 72% of participants communicated genetic risk for hypertrophic cardiomyopathy with all of their siblings and children, 23% communicated with at least one, but not all of their siblings and children, and 5% communicated with no one (Batte et al., 2015). In Australia, all participants recruited from a national disease registry reported telling at least one FDR, 73% informed at least one SDR, and 60% informed at least one TDR about their risk for LQTS; however, 10% of the participants knew of at least one FDR who had not been informed about their risk (Burns et al., 2016). In a Finnish study of individuals with long QT syndrome diagnosed incidentally through participation in a biobank, 33 of 35 children were informed about their risk for long QT syndrome (Haukkala et al., 2013). While these studies provide evidence that family communication about genetic risk for inherited cardiac conditions is inadequate, our understanding about the extent of communication remains broad and nonspecific and insufficient to develop effective interventions.
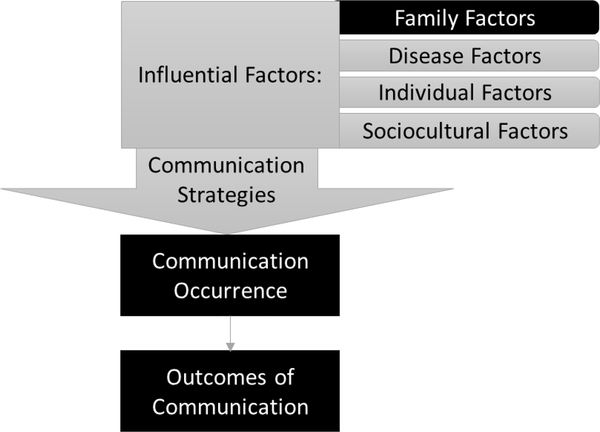
The family communication of genetic risk conceptual framework describes four major elements critical to the process of family communication about genetic risk: influential factors (family, disease, individual, and sociocultural), communication strategies, communication occurrence, and outcomes of communication (Shah & Daack-Hirsch, 2018). These major elements emerged from a review of a body of literature about family communication of genetic risk for non-cardiac conditions (Gaff et al., 2007; Wiens et al., 2013; Wilson et al., 2004; Wiseman et al., 2010). The model describes detailed categories within the major elements identified from a newly emerging body of literature on family communication of genetic risk in the context of inherited cardiac conditions (Shah & Daack-Hirsch, 2018). The study reported here focuses on three elements of the family communication of genetic risk model: influential family factors, communication occurrence, and outcomes of communication of genetic risk (Figure 1). In this study we defined family factors as characteristics of the dyadic relationships between the proband and each of their family members. Family factors that may influence communication of genetic risk for inherited cardiac conditions include the quality of relationships, amount of contact, geographical proximity, and emotional closeness between family members (Shah & Daack-Hirsch, 2018). Our understanding of family factors and communication of genetic risk and testing for inherited cardiac conditions is primarily based on qualitative research (Ormondroyd et al., 2014; Smart, 2010; Whyte, Green, McAllister, & Shipman, 2016). Quantitative evidence has been contradictory on the role of family factors in communication (Batte et al., 2015; Burns et al., 2016), resulting in unclear understanding of the relationship between family factors and communication about genetic risk and testing in families with inherited cardiac conditions.
Figure 1.
Family Communication of Genetic Risk Conceptual Framework (FCGR) developed by Shah & Daack-Hirsch (2018)
Elements in black boxes were the focus of this study. Elements in gray boxes are part of the family communication of genetic risk conceptual framework, but were not specifically measured in this study.
Purpose
The purpose of this study is to identify characteristics of family relationships (geographic proximity, emotional closeness, relationship quality, communication frequency, modes of communication) associated with communication of genetic risk and testing behaviors among at-risk relatives in families with hypertrophic cardiomyopathy or long QT syndrome. This study will also describe the extent of genetic risk communication and testing in these families.
Methods
Design
This study used a cross-sectional design and egocentric social network analysis approach.
Participants
Participants included adult patients diagnosed with hypertrophic cardiomyopathy or long QT syndrome or parents of children under 18 years of age with hypertrophic cardiomyopathy or long QT syndrome and were receiving care at one of two academic medical centers located in the Midwestern United States or attended a patient and family support conference sponsored by these medical centers. To be included in the study, participants had to speak and understand English and have at least one living biological relative. Participants were excluded if they were currently admitted to the hospital for any reason. Participants were recruited in person during scheduled clinic appointments or during the patient and family support conference. Those identified through the electronic health record were recruited via mass mailing. Patients interested in the study were screened in person, online, or over the telephone and informed consent was obtained by the first author. Initial inclusion criteria required participants to identify as the first in their families diagnosed with hypertrophic cardiomyopathy or long QT syndrome. However, early interviews established that many individuals who thought they were the first in the family to be diagnosed had other affected family members or learned of a family history of disease. Thus, true proband status was difficult to ascertain in this population and participants were included if they considered themselves (or their child) to bring the disease to their attention in their family. One family had two individuals take part as probands in the study due to both meeting inclusion criteria. This study was approved by the Author’s Institutional Review Board (IRB ID: 201409704).
Data Collection
Interview.
A semi-structured interview was used to collect participant demographics and disease characteristics, a three-generation family pedigree, and participants’ reports about communication of genetic risk to family members and testing behaviors. The family pedigree had two purposes: 1) to define the participant’s family network, and 2) to determine who in the family was at risk for disease. To define the participant’s family network, participants were instructed to identify all people who were considered family, including those who were not biological relatives (e.g. in-laws, adopted family members), those who were no longer part of the family (e.g. ex-spouses), and those who had died. Identification of those at risk for disease focused on FDR, SDR, and TDR and was based on genetic relationship to the adult participant or affected child if a parent was the participant. Family members who had hypertrophic cardiomyopathy or long QT syndrome ruled out by a parent testing negative for the family mutation were considered not at-risk. The age of FDR was used to determine if children or siblings were minors (less than 18 years old) or if siblings or parents were elderly (65 years or older).
Pedigree.
As part of their interview, participants provided a three-generation pedigree using standardized pedigree construction guidelines (Bennett, French, Resta, & Doyle, 2008). For each at-risk family member, participants indicated whether that person had been informed about his or her risk for disease, who informed him or her about the risk (e.g., proband or another family member), how and when he or she was informed, if he or she was tested for disease, and the results of testing (diagnosed or not). Additional probing questions were used to clarify and confirm participant reports about each family member. Participants’ reports of communication and testing status of relatives were not confirmed by relatives or medical records. Relatives were classified as having been informed about their risk, not having been informed about their risk, or unknown as to whether they had been informed about their risk. For bivariate and multivariate analyses relatives classified as unknown were re-classified as not informed.
Interviews, which took approximately 60 minutes to complete, occurred over the telephone or in person, were audio recorded, and transcribed verbatim. Participants’ pedigrees and responses to interview questions were also recorded on paper during the interview and verified against audio transcripts by the first author. Demographic and disease characteristic data were entered into REDCap data management software (Harris et al., 2009) by a research team member and verified by the first author. Pedigrees were entered into Progeny Clinical (Progeny, 2010) by a research team member and verified by the first author.
Survey.
The survey was personalized for each participant and collected social network data focusing on participants’ perceptions of characteristics of their relationships with each living family network member identified during the interview. The family communication about genetic risk conceptual framework directly informed survey questions which focused on family contact and closeness as a category of influential family factors (Shah & Daack-Hirsch, 2018). Shah and Daack-Hirsch (2018) identified family contact and closeness as geographical proximity, emotional closeness, quality of relationships within the family, and amount of contact between family members. Survey questions directly reflected each of these elements.
Geographical proximity was assessed by the question: Please indicate how far away you currently live from each member of your family by indicating the time it usually takes you to travel to where they live. Participants were advised that travel time should be calculated by which ever mode of transportation you normally use to get to each person (bus, train, car, plane, etc.) and to consider where the family member lives most of the time. Participants selected from the following choices: We live together, less than 30 minutes, less than 1 hour, 1–3 hours, 3–5 hours, 5–8 hours, More than 8 hours but less than 1 day, more than 1 day.
Emotional closeness was assessed by a 10-point Likert scale and the question: Please indicate how close you feel to each member of your family where 1 means not close at all and 10 means extremely close. Participants were reminded that “close” means different things to different people; we want you to answer this question according to what YOU think “close” means in a relationship.
Relationship quality was assessed by a 10-point Likert scale and the question: Please rate your overall relationship with each member of your family where 1 means extremely bad and 10 means extremely good.
Amount of contact between family members was assessed as frequency of communication and modes of communication. Frequency of communication was assessed by the question: Indicate the how often you communicate with each member of your family in general. Response choices were daily, weekly, monthly, yearly, every few years. Participants who did not communicate with a family member had the option to select “No Communication”. Modes of communication were assessed by the question: Indicate the ways you communicate with each member of your family in general. Response choices were face to face (in person), phone, text, video calls (skype, facetime, etc.), social media (Facebook, Instagram, etc.), and letters and participants selected all that applied. Participants who did not communicate with a family member had the option to select “No Communication”.
Construct validity of the survey was ensured through a strong conceptual connection to the family communication of genetic risk conceptual framework (Shah & Daack-Hirsch, 2018). Authors (LS, SDH, AE, AP, JKW) with expertise in family communication about genetic risk and social network analysis confirmed face and content validity of the survey questions. The survey was then piloted and changes were made to question format and wording based on feedback and author observations. Surveys were completed either online using Qualtrics, or on paper. Participants who did not complete surveys received email or telephone reminders to complete the survey. Data from paper surveys were entered into Qualtrics software by a research team member and verified by the first author. Data collection procedures for this study took place between March 2015 and September 2016. Participants received $15 for completing the interview and $15 for completing the survey.
Data Analyses
Descriptive analyses evaluated participant demographic and disease characteristics. Means, medians, standard deviations, and minimum and maximum values for continuous variables and frequencies and proportions for categorical variables were calculated. Characteristics of each proband’s family social network were described using egocentric social network analysis. Egocentric social network analysis facilitates understanding how relationships between people affect health related behaviors (Ersig, Hadley, & Koehly, 2011; Koehly et al., 2003). It assumes that family relationships are interdependent and serve as conduits for resources, including information and influence, and that social network structure influences individual actions (Berkman, Glass, Brisette, & Seeman, 2000; Koehly et al., 2003). In this study, egocentric social networks focused on the relationships of probands with their family members. Although other relationships (e.g. friendships, other social connections) may be important, these are not as critical to communication of genetic risk information in the family, the phenomenon of interest in this study. Family social network characteristics were described in terms of the participant’s perception of relationship characteristics including geographic proximity, emotional closeness, relationship quality, frequency of communication, and modes of communication.
The primary outcome of this study was whether each at-risk relative was informed about his or her risk for hypertrophic cardiomyopathy or long QT syndrome. A secondary outcome was whether at-risk relatives who were informed about their risk were tested for disease, and whether those tested were diagnosed with disease. For all outcomes, frequencies and proportions were calculated overall, then by degree of biological relationship with the proband, and for each type of relative (e.g. parent, sibling, cousin, etc.). Proportions of relatives tested were calculated for all relatives and for those relatives informed about their risk. Proportions of relatives diagnosed with disease were also calculated for all relatives and among only relatives who were tested for disease. Bivariate relationships between relationship characteristics and outcomes were evaluated using logistic regression for FDR and random effects logistic regression for SDR and TDR, controlling for interdependence among relatives from the same family for all relatives where social network data was available. Correlations among relationship characteristics were calculated. Because strong correlation was expected (e.g., those who communicate more frequently likely have more positive relationships), multivariate relationships among non-correlated relationship characteristics and outcomes were evaluated using logistic regression for FDR and random effects logistic regression for SDR and TDR. Stata 14 software was used for analysis (StataCorp, 2015).
Results
Participants
The study included 53 participants. Five (9%) were parents of children diagnosed with disease; these parents, classified as proxy probands, were responsible for communicating risk to family members. Among proxy probands, the average age of affected children was 15 ± 5.3 (range 6–20 years). All 53 participants completed the interview and 48 completed the survey. Most participants completed interviews by telephone (n = 44, 83%), were born in the United States (n = 52, 98%), and were at least 3rd generation Americans (n = 48, 91%). Disease characteristics for all affected individuals (48 adult probands, five affected children) were also obtained (Tables 1, 2). Disease management interventions included implantable cardioverter defibrillators (n = 28, 53%), permanent pacemakers (n = 11, 21%), surgeries including myectomy and denervation (n = 6, 11%), and ablations (n = 7, 13%). All participants had at least one form of health insurance and 29 (55%) currently had life insurance.
Table 1.
Continuous Demographic & Diagnostic Characteristics of Participants & Affected Children of Proxy Probands
| Characteristic | n | Mean ± SD | Median | Range |
|---|---|---|---|---|
| Age of Participant (years) | 53 | 47.6 ± 14.0 | 53 | 18 – 72 |
| Age at Diagnosis (years)* | 53 | 33.5 ± 16.8 | 35 | 11 months – 63 |
| Time with Diagnosis (years)* | 53 | 11.3 ± 10.1 | 8 | 0** – 40 |
| Year of Diagnosis* | 53 | 2004 ± 10.0 | 2007 | 1973–2015 |
indicates the sample included the characteristics of the affected child for the 5 proxy probands.
One participant reported having been diagnosed one hour ago however her healthcare provider reported she had been diagnosed for several years.
Table 2.
Categorical Demographic & Diagnostic Characteristics of Participants & Affected Children of Proxy Probands
| Participant Characteristic (53 adult participants) | n (%) |
|---|---|
| Gender | n = 53 |
| Male | 19 (36) |
| Female | 34 (64) |
| Highest Education Completed | n = 48 |
| Less than high school | 1 (2) |
| High school or GED | 9 (19) |
| Some college | 6 (13) |
| 2-year degree | 14 (29) |
| 4-year degree | 12 (25) |
| Master’s degree | 6 (13) |
| Race/ Ethnicity | n = 53 |
| Hispanic White | 2 (4) |
| Non-Hispanic White | 51 (96) |
| Disease Characteristics (48 adult probands and 5 affected children of the proxy probands) | n (%) |
| Cardiac Diagnosis* | n = 53 |
| Hypertrophic cardiomyopathy | 30 (57) |
| Long QT syndrome | 23 (43) |
| Genetic Test Status* | n = 53 |
| Positive | 30 (57) |
| Negative | 1 (2) |
| Variant of uncertain significance | 1 (2) |
| Currently awaiting genetic test results | 5 (9) |
| No genetic testing done | 14 (27) |
| Did not personally test, but identical twin tested positive | 1 (2) |
| Blood drawn but testing not completed because of billing discrepancy | 1 (2) |
indicates the sample included the characteristics of the affected child for the 5 proxy probands.
Participants’ Families
Among all participants, 1,178 living at-risk FDR, SDR, and TDR were identified, with an average of 22.2 ± 14.7 living, at-risk relatives per participant (range 3–67). FDR represented the smallest group of at-risk relatives (mean 4.3 ± 2.3; range 0–12). Participants averaged 6.8 ± 5.1 living at-risk SDR (range 0–25), and 11.2 ± 11.1 living at-risk TDR (range 0–47). Participants had 10.0 ± 5.6 living non-biological relatives (range 2–24), who were not at risk, but may participate in communication of risk information, or have close relationships with affected or at-risk individuals. Among the 532 non-biological relatives, 46 were spouses or partners of the proband. Others were spouses or partners of other biological relatives, adopted family members, ex-spouses or partners of family members, and step-children.
Family Relationship Characteristics
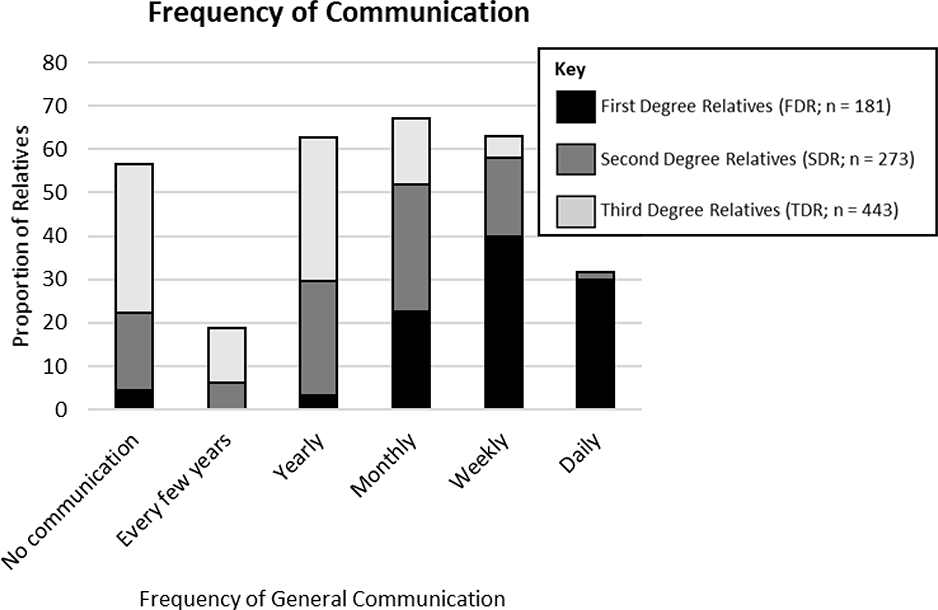
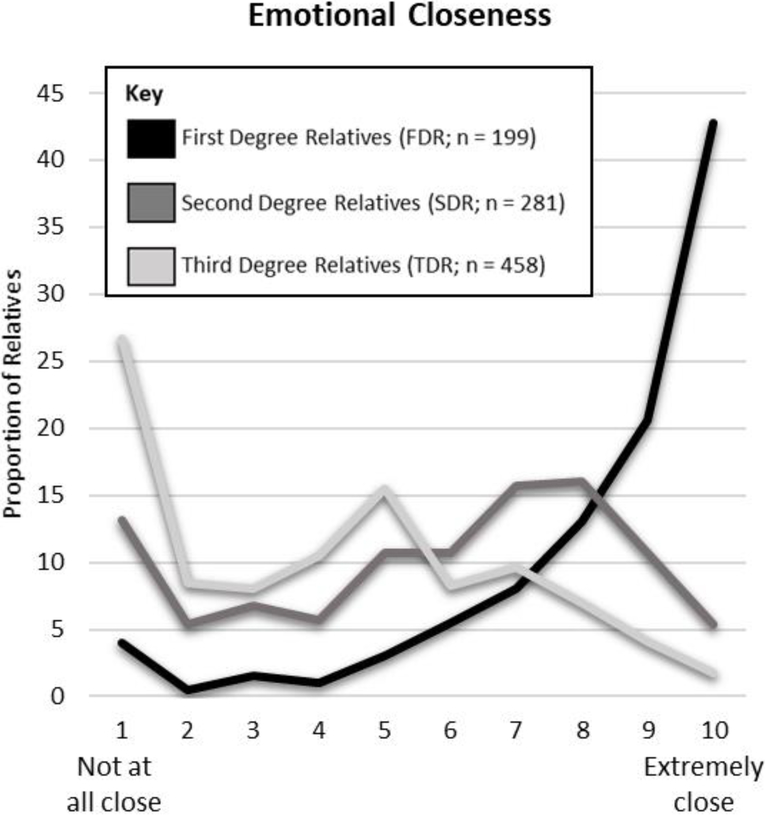
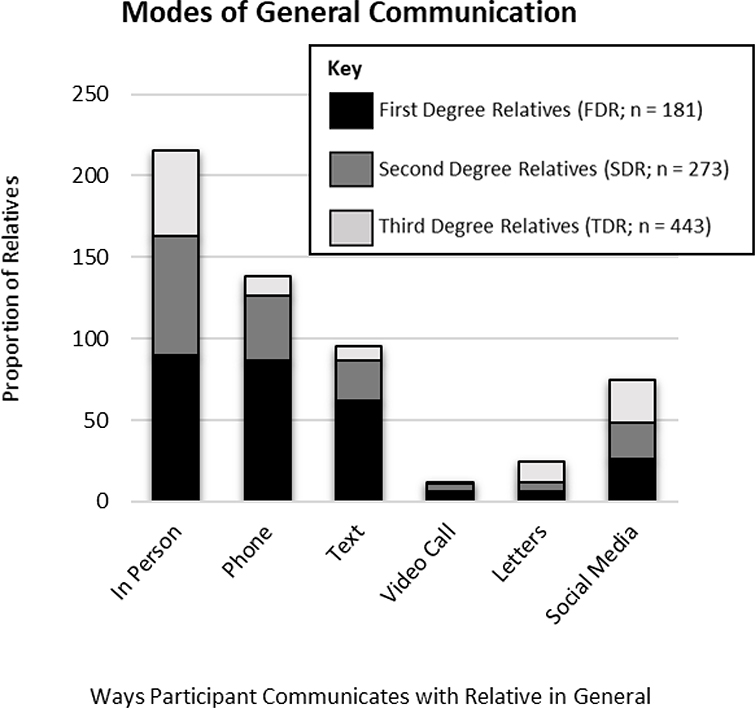
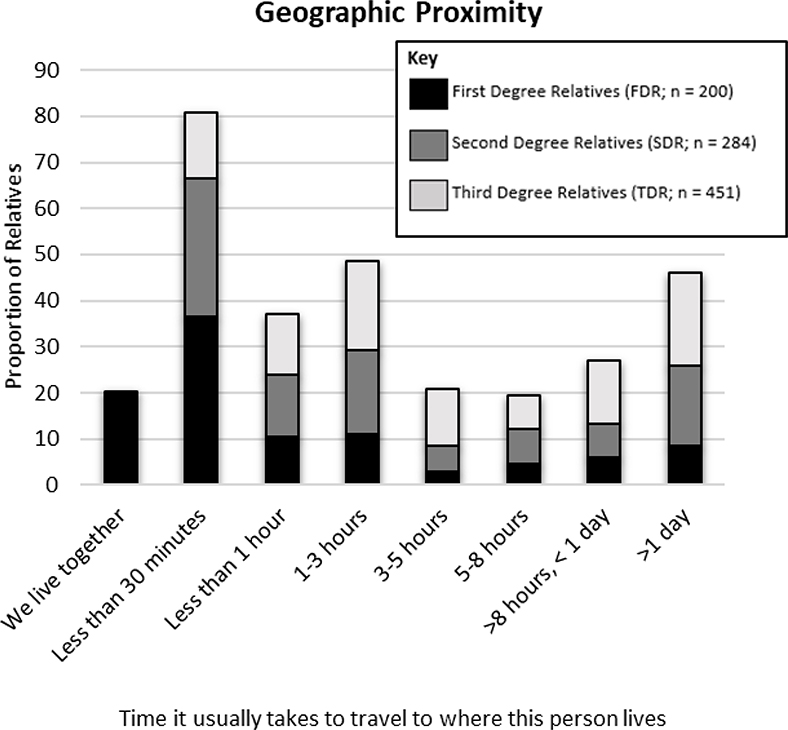
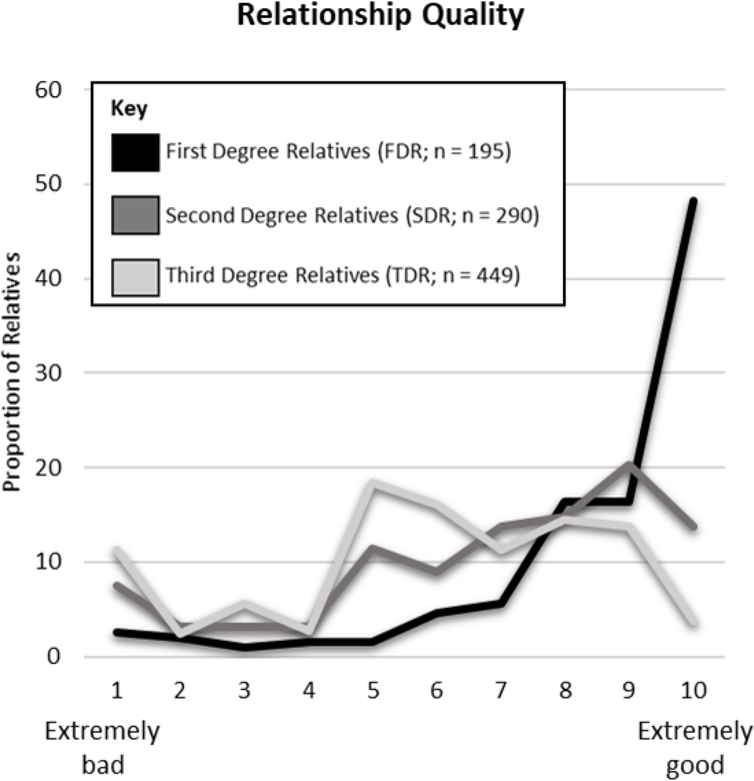
Participants lived closer, were emotionally closer, had more positive relationships, and communicated more frequently with FDR than with SDR and TDR. Characteristics of the relationships between participants and each relative are described in Figures 2–5. The most common mode of communication with relatives in general was in-person communication (Figure 6). Among family relationship characteristics the strongest correlations were between emotional closeness and relationship quality (r = .85), emotional closeness and communication frequency (r = .81), and relationship quality and communication frequency (r = .72). Correlations between geographic proximity and communication frequency (r = −.41), emotional closeness (r = −.31), and relationship quality (r = −.21) were weak.
Figure 2.
Frequency of Communication Between Participants and Family Members
Figure 5.
Emotional Closeness Between Participants and Family Members
Figure 6.
Modes of General Communication Between Participants and Family Members
Communication of Genetic Risk and Testing Behaviors
As shown in Table 3, just over half of the at-risk relatives (n = 618; 52.5%) were informed about their genetic risk. More (90%) FDR were informed, compared to 61% of SDR and 33% of TDR. Within each degree of relationship, communication of risk varied across types of relationships. Among FDR, 96% of children were informed compared to 88% of siblings and 86% of parents. One (50%) of the children who was not informed was an adult (18 years or older); the other child was a minor (less than 18 years of age). All four siblings who were minors were informed about their risk; thirteen siblings were elderly of whom three were not informed of their risk. Half (n = 29) of the at-risk parents were elderly (65 years or older), and 75% (n = 6) of parents not informed of risk were elderly. Among SDR there was greater variation, with grandparents, maternal aunts and uncles, and grandchildren informed about their risk more often than paternal aunts and uncles, half-siblings, and nephews and nieces.
Table 3.
Degree and Type of Relationship and Communication, Testing, & Diagnosis
| Type of Relative | Communication of Risk n (% of row) | Testing & Diagnosis n (% of row) [Conditional % of row]* | |||
|---|---|---|---|---|---|
| Not Informed (n = 289) | Unknown if Informed (n = 271) | Informed (n = 618) | Tested (n = 322) | Diagnosed (n = 116) | |
| FDR (n = 228) | 12 (5) | 11 (5) | 205 (90) | 132 (58) [64] | 56 (25) [42] |
| Identical twin (n = 2) | 0 | 0 | 2 (100) | 1 (50) [50] | 1 (50) [100] |
| Child (n = 63) | 1 (2) | 1 (2) | 61 (96) | 47 (74) [77] | 17 (27) [36] |
| Sibling (n = 105) | 7 (7) | 6 (6) | 92 (88) | 45 (43) [49] | 16 (15) [36] |
| Parent (n = 58) | 4 (7) | 4 (7) | 50 (86) | 39 (67) [78] | 22 (38) [56] |
| SDR (n = 358) | 60 (17) | 79 (22) | 219 (61) | 116 (32) [53] | 40 (11) [34] |
| Half-sibling (n = 15) | 2 (13) | 5 (33) | 8 (53) | 4 (27) [50] | 1 (7) [25] |
| Nephew or niece (n = 170) | 33 (19) | 47 (28) | 90 (53) | 51 (30) [57] | 13 (8) [25] |
| Grandchild (n =28) | 7 (25) | 0 | 21 (75) | 14 (50) [67] | 7 (25) [50] |
| Maternal aunt or uncle (n = 46) | 2 (4) | 8 (17) | 36 (78) | 21 (46) [58] | 10 (22) [48] |
| Paternal aunt or uncle (n = 78) | 22 (28) | 9 (12) | 47 (60) | 15 (19) [32] | 7 (9) [47] |
| Maternal grandparent (n = 11) | 2 (18) | 0 | 9 (81) | 5 (45) [56] | 2 (18) [40] |
| Paternal grandparent (n = 10) | 1 (10) | 1 (10) | 8 (80) | 6 (60) [75] | 0 (0) [0] |
| TDR (n = 592) | 217 (37) | 181 (31) | 194 (33) | 74 (13) [38] | 20 (3) [27] |
| Cousin (n = 467) | 185 (40) | 131 (28) | 151 (32) | 52 (11) [34] | 17 (4) [33] |
| Great-nephew or niece (n =75) | 28 (37) | 19 (25) | 28 (37) | 15 (20) [54] | 2 (3) [13] |
| Nephew or niece from half-sibling (n = 11) | 2 (18) | 7 (64) | 2 (18) | 1 (9) [50] | 0 (0) [0] |
| Parent’s half-sibling (n = 5) | 1 (20) | 2 (40) | 2 (40) | 0 (0) [0] | 0 (0) [0] |
| Great-uncle or aunt (n = 24) | 1 (4) | 14 (58) | 9 (38) | 5 (21) [56] | 0 (0) [0] |
| Great-grandparents (n = 9) | 0 | 8 (89) | 1 (11) | 0 (0) [0] | 0 (0) [0] |
| Parent’s identical twin’s child (n =1) | 0 | 0 | 1 (100) | 1 (100) [100] | 1 (100) [100] |
Conditional percent of row for the Tested column is the number tested out of the number informed about their risk. Conditional percent of row for the Diagnosed column is the number diagnosed out of the number tested.
Six participants (11%) had at least one FDR who was not informed about their risk (range 2–7 FDR not informed). Among these six participants, one informed children and siblings but not parents; one with deceased parents informed children but not siblings; one was adopted, and did not know if the biological parents had been informed of their risk by adoption agency; and three did not communicate with any FDR, SDR, or TDR; These six participants all had hypertrophic cardiomyopathy, five were male, and one had undergone genetic testing (positive result).
The frequency and proportions of testing behaviors and subsequent diagnosis of at-risk family members are reported in Table 3. Approximately half of family members informed about their risk were tested for disease, with the highest proportion among FDR (64%) and lowest among TDR (38%). Among FDR informed about their risk, children and parents tested most often (77–78%) and siblings least often (49%). Of all family members tested, 36% were diagnosed with disease based on participant report.
Family Relationship Characteristics & Communication
Overall, family relationship characteristics were significantly associated with communication among at-risk relatives. Geographic proximity was only significantly associated with communication in FDR (OR = .82, p = .033). Emotional closeness was significantly associated with communication for FDR (OR = 1.40, p < .001), SDR (OR = 1.37, p = .001), and TDR (OR = 1.61, p < .001). Relationship quality was significantly associated with communication for FDR (OR = 1.39, p < .001), SDR (OR = 1.47, p = .001), and TDR (OR = 1.70, p < .001). Communication frequency was significantly associated with communication for FDR (OR = 1.96, p < .001), SDR (OR = 2.60, p < .001), and TDR (OR = 2.43, p < .001).
Family Relationship Characteristics & Testing
All family relationship characteristics except for geographic proximity were significantly associated with testing among at-risk relatives (Table 4). Geographic proximity was not associated with testing for FDR, SDR, or TDR. Emotional closeness was significantly associated with testing for FDR (OR=1.35, p = .005) and TDR (OR = 1.46, p = .009) but not SDR. Relationship quality was associated with testing for only TDR (OR = 1.71, p = .001). Communication frequency was associated with testing for FDR (OR = 1.72, p = .010) and SDR (OR = 1.99, p = .018) but not TDR.
Table 4.
Bivariate Relationships Between Relationship Characteristics and Outcomes
| Informed | Tested | |||||||
|---|---|---|---|---|---|---|---|---|
| Relationship Characteristic | n | OR | 95% CI | p value | n | OR | 95% CI | p value |
| Geographic proximity | ||||||||
| FDR | 195 | .82 | [0.69, 0.98] | .033 | 172 | .86 | [0.71, 1.02] | .095 |
| SDR | 292 | 1.04 | [0.84, 1.30] | .667 | 164 | 1.06 | [0.80, 1.42] | .670 |
| TDR | 455 | .82 | [0.69, 1.01] | .063 | 155 | .93 | [0.68, 1.28] | .662 |
| Emotional closeness | ||||||||
| FDR | 194 | 1.40 | [1.20, 1.65] | <.001 | 171 | 1.35 | [1.09, 1.66] | .005 |
| SDR | 289 | 1.37 | [1.13, 1.65] | .001 | 163 | 1.21 | [0.96, 1.53] | .095 |
| TDR | 463 | 1.61 | [1.30, 2.00] | <.001 | 155 | 1.46 | [1.10, 1.94] | .009 |
| Relationship quality | ||||||||
| FDR | 195 | 1.39 | [1.18, 1.63] | <.001 | 172 | 1.17 | [0.96, 1.44] | .117 |
| SDR | 290 | 1.47 | [1.18, 1.84] | .001 | 164 | 1.12 | [0.89, 1.42] | .326 |
| TDR | 449 | 1.70 | [1.33, 2.16] | <.001 | 152 | 1.71 | [1.25, 2.33] | .001 |
| Communication frequency | ||||||||
| FDR | 181 | 1.96 | [1.40, 2.74] | <.001 | 158 | 1.72 | [1.14, 2.61] | .010 |
| SDR | 273 | 2.60 | [1.65, 4.10] | <.001 | 148 | 1.99 | [1.12, 3.51] | .018 |
| TDR | 443 | 2.43 | [1.59, 3.72] | <.001 | 137 | 1.42 | [0.89, 2.26] | .138 |
Note. OR = odds ratio; CI = confidence interval; FDR = first degree relative; SDR = second degree relative; TDR = third degree relative
Communication frequency was the relationship characteristic most strongly associated with communication and testing for most at-risk relatives. Given the collinearity among emotional closeness, relationship quality, and communication frequency, only the relationship variable with the strongest bivariate relationship with outcomes was selected for inclusion in the multivariate model. Geographic proximity was also included as it was not highly correlated with the other relationship variables. Multivariate models that included communication frequency and geographic proximity were developed for FDR, SDR, and TDR (Table 5). When controlling for geographic distance, communication frequency was significantly associated with communication of genetic risk for SDR (OR = 2.98, p < .001) and TDR (OR = 2.31, p < .001) but not FDR (β = .01, p =.299) and with testing for FDR (OR = 1.81, p = .018), SDR (OR = 1.74, p = .009) but not TDR (OR = 1.39, p = .172). Geographic proximity was not significantly associated with communication or testing for FDR, SDR, or TDR when controlling for communication frequency.
Table 5.
Multivariate Relationships Between Relationship Characteristics and Outcomes
| Model 1: FDR Informed of Disease Risk (n = 180) | Coefficient | 95% CI | p value |
| Communication frequency | .01 | [-0.01, 0.04] | .299 |
| Geographic proximity | -.01 | [-0.02, 0.01] | .341 |
| Intercept | .85 | [.70, 1.01] | <.001 |
| Model 2: SDR Informed of Disease Risk (n = 273) | OR | 95% CI | p value |
| Communication frequency | 2.98 | [1.78, 4.97] | <.001 |
| Geographic proximity | 1.30 | [0.98, 1.73] | .069 |
| Intercept | .05 | [0.01, 0.59] | .016 |
| Model 3: TDR Informed of Disease Risk (n = 433) | OR | 95% CI | p value |
| Communication frequency | 2.31 | [1.49, 3.58] | <.001 |
| Geographic proximity | .93 | [0.75, 1.16] | .535 |
| Intercept | .19 | [0.03, 1.45] | .109 |
| Model 4: FDR Tested for Disease (n =157) | OR | 95% CI | p value |
| Communication frequency | 1.76 | [1.10, 2.84] | .018 |
| Geographic proximity | 1.02 | [0.82, 1.26] | .858 |
| Intercept | .19 | [0.02, 1.99] | .167 |
| Model 5: SDR Tested for Disease (n = 148) | OR | 95% CI | p value |
| Communication frequency | 1.74 | [1.25, 4.77] | .009 |
| Geographic proximity | 1.42 | [0.93, 2.19] | .104 |
| Intercept | .02 | [0.00, 0.65] | .027 |
| Model 6: TDR Tested for Disease (n =136) | OR | 95% CI | p value |
| Communication frequency | 1.39 | [.87, 2.22] | .172 |
| Geographic proximity | .96 | [0.68, 1.34] | .800 |
| Intercept | .13 | [0.01, 1.50] | .103 |
Note. OR = odds ratio; CI = confidence interval; FDR = first degree relative; SDR = second degree relative; TDR = third degree relative
Discussion
The large number of at-risk relatives identified in this study emphasizes the potential impact of cascade screening and the accompanying burden of responsibility placed on probands to effectively communicate with numerous relatives. Similar to Burns et al. (2016), we detected a trend of more communication among FDRs, less to SDR, and even less to TDR, evidence that the traditional cascade screening process breaks down with each successive wave and is not effective in reaching more distant relatives. These findings are important given that current cascade screening recommendations (Gersh et al., 2011) focus on FDR. The large number of high risk SDR and TDR in this study who were not given the opportunity to participate in cascade screening suggests that failure to continue genetic risk communication beyond FDR contributes to lower rates of screening in more distant relatives.
Relatives who are elderly or minors are of special concern for hypertrophic cardiomyopathy and long QT syndrome, which can manifest throughout the lifespan. A desire to protect the elderly has been described as an influential factor in communication of genetic risk for inherited cardiac conditions (Shah & Daack-Hirsch, 2018). Our findings support previous studies describing probands’ decisions to not communicate risk to elderly parents to protect them from guilt or worry, or because they perceived that elderly parents were less likely to be at risk or that information would not be useful given their advanced age (Ormondroyd et al., 2014; Smart, 2010). In contrast to the elderly, a high percentage of children were informed about their risk, a finding consistent with other studies (Batte et al., 2015; Haukkala et al., 2013). However, nearly half of nieces and nephews identified in this study were not informed about their risk, which was surprising given the concern for children that is a distinct characteristic of hypertrophic cardiomyopathy and long QT syndrome (Mangset & Hofmann, 2014; Shah & Daack-Hirsch, 2018; Smart, 2010; Vavolizza et al., 2015). Our data supports previous qualitative research describing communication about genetic risk for inherited cardiac conditions to nieces and nephews being left up to their parents (Haukkala et al., 2013; Vavolizza et al., 2015). Furthermore, a higher proportion of grandchildren were told about their risk (75%), suggesting that probands may have more influence over communication to their children’s children than their siblings’ children.
Previous research about the effects of family contact and closeness on health communication has been somewhat contradictory, potentially due to variations in the measurement and definition of family relationships (Batte et al., 2015; Burns et al., 2016; Shah & Daack-Hirsch, 2018; Smart, 2010). Our findings strongly support an association between family relationships and communication of genetic risk and testing. Among all the relationship characteristics, frequency of communication had the strongest association with genetic risk communication and testing. Practically, assessing general communication frequency may be a useful way to identify relatives likely to not be informed about their risk; alternative means of informing these at-risk relatives (e.g., identifying other family members who could share the information) could then be explored. Conceptually, communication frequency is related to relationship quality and emotional closeness and overemphasis on this single aspect of a relationship may limit our understanding of the entire communication process. The correlations detected between these relationship characteristics further illustrate the complex and multi-dimensional nature of family factors related to communication about genetic risk.
Greater geographic distance between family members has also been suggested to contribute to uncertainty or unwillingness to communicate genetic risk information (Ormondroyd et al., 2014; Smart, 2010); however, when accounting for communication frequency in multivariate models, geographic proximity was no longer significantly associated with communication or testing. This suggests that geographic distance alone may not impede communication, but that the effects of geographic proximity on closeness and contact in family relationships may be more explanatory of communication of genetic risk.
It is also notable that while family letters generated by healthcare providers are suggested for clinical use and may improve family communication about genetic risk (Van Der Roest, Pennings, Bakker, Van Den Berg, & Van Tintelen, 2009), we found that letters are rarely used in routine communication in families and that in-person, telephone, and social media are communication modes used much more frequently in most families. Given that established patterns of communication in families tend to persist when communicating genetic risk information (Shah & Daack-Hirsch, 2018), more commonly used modes of communication may be more acceptable ways of communicating genetic risk to family members than mailed letters. Other strategies such as communication of risk by family members other than the proband may also be effective in increasing communication and testing behaviors (Bowen, Hay, Harris-Wai, Meischke, & Burke, 2017).
Finally, while communication of genetic risk is a necessary step, alone it is not sufficient to ensure that at-risk family members will participate in testing behaviors such as genetic testing or specialized clinical examination. The familial risk perception conceptual framework (Walter & Emery, 2005; Daack-Hirsch et al., 2018) describes awareness of risk for heritable disease as the first step in a process where individuals must develop salience, personalized into vulnerability, in order to reach the step where action is taken. Despite awareness of risk, family members may choose to not have testing for a multitude of reasons.
This study focused only on family relationship factors and their role in genetic risk communication and testing. However, other factors articulated in the family communication of genetic risk conceptual framework including individual factors (gender, age, risk perception), disease factors (e.g. diagnosis, genetic test results), and communication strategies (details of the delivery and content of communications) are suggested to influence genetic risk communication (Shah & Daack-Hirsch, 2018) and testing behaviors. These variables were not included in these analyses because there was too much variability in individual and disease factors between the recruitment sites. Communication strategies were not analyzed because some of our participants had a difficult time remembering specifics about their communication experiences.
Although our sample included a variety of disease experiences, we did not achieve racial or ethnic diversity, an unfortunately persistent problem in studies of family communication about genetic risk. Our sample, which was recruited through two clinical sites, may not be representative of the entire population of individuals with hypertrophic cardiomyopathy and long QT syndrome. Communication and testing behaviors were self-reported by the participant and were not verified with relatives, potentially limiting the validity of the outcome measures. In addition, validity of the survey, beyond content validity was not tested through traditional measures since each survey was personalized for each participant. The cross-sectional design of our study also limits our interpretation of the relationships to associations and we cannot infer causation.
In conclusion, only half of relatives were informed about their risk of hypertrophic cardiomyopathy and long QT syndrome, and only half of those informed were then tested. The relationship between a proband and their relative is an important factor for both communication of genetic risk and testing behaviors. As advocates for patients and their families, nurses caring for individuals and families with inherited cardiac conditions can support family communication about genetic risk by assessing family relationships and helping develop strategies to communicate with at-risk relatives that are likely to be excluded from communication. Interventions designed to improve family communication about genetic risk for inherited cardiac conditions should address family factors as an important component in this complex process.
Figure 3.
Geographic Proximity Between Participants and Family Members
Figure 4.
Relationship Quality Between Participants and Family Members
Acknowledgements
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Grants F31NR014758 and T32NR009759, Midwest Nurses Research Society Dissertation Grant, and the Day Family Scholarship in Genetics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interests
The Authors declare that there are no conflicts of interest.
References
- Barr JA, Tsai LP, Welch A, Faradz SMH, Lane-Krebs K, Howie V, & Hillman W (2018). Current practice for genetic counselling by nurses: An integrative review. International Journal of Nursing Practice, 24(2). e12629. doi: 10.1111/ijn.12629 [DOI] [PubMed] [Google Scholar]
- Batte B, Sheldon JP, Arscott P, Huismann DJ, Salberg L, Day SM, & Yashar BM (2015). Family communication in a population at risk for hypertrophic cardiomyopathy. Journal of Genetic Counseling, 24(2), 336–348. [DOI] [PubMed] [Google Scholar]
- Bennett RL, French KS, Resta RG, & Doyle DL (2008). Standardized human pedigree nomenclature: Update and assessment of the recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling, 17(5), 424–433. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Glass T, Brisette I, & Seeman TE (2000). Social integration, social networks, social support, and health In Berkman IKLF (Ed.), Social Epidemiology (pp. 137–173). New York, NY: Oxford University Press. [Google Scholar]
- Bowen DJ, Hay JL, Harris-Wai JN, Meischke H, & Burke W (2017). All in the family? Communication of cancer survivors with their families. Familial Cancer, 16(4), 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns C, James C, & Ingles J (2018). Communication of genetic information to families with inherited rhythm disorders. Heart Rhythm, 15(5), 780–786. doi: 10.1016/j.hrthm.2017.11.024 [DOI] [PubMed] [Google Scholar]
- Burns C, McGaughran J, Davis A, Semsarian C, & Ingles J (2016). Factors influencing uptake of familial long QT syndrome genetic testing. American Journal of Medical Genetics Part A, 170(2), 418–425. [DOI] [PubMed] [Google Scholar]
- Christiaans I, Birnie E, Bonsel GJ, Wilde AA, & van Langen IM (2008). Uptake of genetic counselling and predictive DNA testing in hypertrophic cardiomyopathy. European Journal of Human Genetics, 16(10), 1201–1207. [DOI] [PubMed] [Google Scholar]
- Crotti L, Celano G, Dagradi F, & Schwartz PJ (2008). Congenital long QT syndrome. Orphanet Journal of Rare Diseases, 3(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daack-Hirsch S, Shah LL, & Cady A (2018). Mental models of cause and inheritance for type 2 diabetes among unaffected individuals who have a positive family history. Qualitative Health Research, 28(4), 534–547. [DOI] [PubMed] [Google Scholar]
- Ersig AL, Hadley DW, & Koehly LM (2011). Understanding patterns of health communication in families at risk for hereditary nonpolyposis colorectal cancer: Examining the effect of conclusive versus indeterminate genetic test results. Health Communication, 26(7), 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force T, Bonow RO, Houser SR, Solaro J, Hershberger RE, Bishow Adhikari ME, . . . Seidman CE (2010). Research priorities in hypertrophic cardiomyopathy: Report of a working group of the National Heart, Lung, and Blood Institute. Circulation, 122, 1130–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, . . . Edwards A (2007). Process and outcome in communication of genetic information within families: A systematic review. European Journal of Human Genetics, 15(10), 999–1011. [DOI] [PubMed] [Google Scholar]
- Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, . . . Yancy CW (2011). 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology, 58(25), e212–260. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukkala A, Kujala E, Alha P, Salomaa V, Koskinen S, Swan H, & Kääriäinen H (2013). The return of unexpected research results in a biobank study and referral to health care for heritable long QT syndrome. Public Health Genomics, 16(5), 241–250. [DOI] [PubMed] [Google Scholar]
- Ho CY (2012). Hypertrophic cardiomyopathy in 2012. Circulation, 125(11), 1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehly LM, Peterson SK, Watts BG, Kempf KK, Vernon SW, & Gritz ER (2003). A social network analysis of communication about hereditary nonpolyposis colorectal cancer genetic testing and family functioning. Cancer Epidemiology, Biomarkers, and Prevention, 12(4), 304–313. [PubMed] [Google Scholar]
- Mangset M, & Hofmann B (2014). LQTS parents’ reflections about genetic risk knowledge and their need to know or not to know their children’s carrier status. Journal of Genetic Counseling, 23(6), 1022–1033. [DOI] [PubMed] [Google Scholar]
- Maron BJ (2004). Hypertrophic cardiomyopathy in childhood. Pediatric Clinics of North America, 51(5), 1305–1346. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Rowin EJ, Casey SA, Lesser JR, Garberich RF, McGriff DM, & Maron MS (2016). Hypertrophic cardiomyopathy in children, adolescents, and young adults associated with low cardiovascular mortality with contemporary management strategies. Circulation, 133(1), 62–73. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Rowin EJ, Casey SA, Link MS, Lesser JR, Chan RH, . . . Maron MS (2015). Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. Journal of the American College of Cardiology, 65(18), 1915–1928. [DOI] [PubMed] [Google Scholar]
- Miller EM, Wang Y, & Ware SM (2013). Uptake of cardiac screening and genetic testing among hypertrophic and dilated cardiomyopathy families. Journal of Genetic Counseling, 22(2), 258–267. [DOI] [PubMed] [Google Scholar]
- Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, Nastoli J, . . . Leonardi S (2005). Genetic testing in the long QT syndrome: Development and validation of an efficient approach to genotyping in clinical practice. Journal of the American Medical Association, 294(23), 2975–2980. [DOI] [PubMed] [Google Scholar]
- Ormondroyd E, Oates S, Parker M, Blair E, & Watkins H (2014). Pre-symptomatic genetic testing for inherited cardiac conditions: A qualitative exploration of psychosocial and ethical implications. European Journal of Human Genetics, 22(1), 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progeny. (2010). Progeny Clinical (Version 8) [computer software]. Delray Beach, FL: Progeny Software LLC. [Google Scholar]
- Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, . . . Spazzolini C (2009). Prevalence of the congenital long-QT syndrome. Circulation, 120(18), 1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsarian C, Ingles J, Maron MS, & Maron BJ (2015). New perspectives on the prevalence of hypertrophic cardiomyopathy. Journal of the American College of Cardiology, 65(12), 1249–1254. [DOI] [PubMed] [Google Scholar]
- Shah LL & Daack-Hirsch S (2018). Family communication about genetic risk of hereditary cardiomyopathies and arrhythmias: An integrative review. Journal of Genetic Counseling, 27(5), 1022–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart A (2010). Impediments to DNA testing and cascade screening for hypertrophic cardiomyopathy and long QT syndrome: A qualitative study of patient experiences. Journal of Genetic Counseling, 19(6), 630–639. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2015). Stata Statistical Software (Release 14) [computer software]. College Station: TX: StataCorp LP. [Google Scholar]
- Van Der Roest WP, Pennings JM, Bakker M, Van Den Berg MP, & Van Tintelen JP (2009). Family letters are an effective way to inform relatives about inherited cardiac disease. American Journal of Medical Genetics, Part A, 149(3), 357–363. [DOI] [PubMed] [Google Scholar]
- Vavolizza R, Kalia I, Aaron K, Silverstein L, Barlevy D, Wasserman D, . . . Dolan S (2015). Disclosing genetic information to family members about inherited cardiac arrhythmias: An obligation or a choice? Journal of Genetic Counseling, 24(4), 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter VL, Elia J, Erickson C, Berger S, Blum N, Uzark K, & Webb CL (2008). Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation, 117(18), 2407–2423. [DOI] [PubMed] [Google Scholar]
- Walter FM, & Emery J (2005). ‘Coming down the line’-patients’ understanding of their family history of common chronic disease. Annals of Family Medicine, 3(5), 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte S, Green A, McAllister M, & Shipman H (2016). Family ommunciation in inherited cardiovascular conditions in Ireland. Journal of Genetic Counseling, 25(6), 1317–1326. [DOI] [PubMed] [Google Scholar]
- Wiens ME, Wilson BJ, Honeywell C, & Etchgary H (2013). A family genetic risk communication framework: Guiding tool development in genetics health services. Journal of Community Genetics, 4, 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BJ, Forrest K, van Teijlingen ER, McKee L, Haites N, Matthews E, & Simpson SA (2004). Family communication about genetic risk: The little that is known. Community Genetics, 7(1), 15–24. [DOI] [PubMed] [Google Scholar]
- Wiseman M, Dancyger C, & Michie S (2010). Communicating genetic risk information within families: A review. Familial Cancer, 9(4), 691–703. [DOI] [PubMed] [Google Scholar]
- Zumhagen S, Stallmeyer B, Friedrich C, Eckardt L, Seebohm G, & Schulze-Bahr E (2012). Inherited long QT syndrome: Clinical manifestation, genetic diagnostics, and therapy. Herzschrittmachertherapie Elektrophysiologie, 23(3), 211–219. [DOI] [PubMed] [Google Scholar]