Abstract
Endometriosis is characterized by extrauterine growth of endometrial tissue accompanied by adverse clinical manifestations including chronic pelvic pain and infertility. Retrograde menstruation, the efflux of endometrium into the peritoneal cavity during menstruation, is believed to contribute to implantation of endometrial tissue and formation of endometriotic lesions at ectopic sites. While it is established through various rodent and nonhuman primate models that endometrial tissue fragments, as well as nondissociated stroma and glands, are capable of seeding endometriosis in a manner mimicking retrograde menstruation, the ability of single endometrial cells to participate in endometriotic processes has not been evaluated due to their failure to establish macroscopic endometriosis. We designed a model by which this capacity can be assessed by examining the integration of individual uterine cells into existing endometriosis lesions in mice. Endometriosis was induced in C57BL/6J female mice followed by intraperitoneal injection of GFP-labeled single uterine cells. We found that freshly introduced uterine cells can successfully integrate and contribute to various cell populations within the lesion. Strikingly, these cells also appeared to contribute to neo-angiogenesis and inflammatory processes within the lesion, which are commonly thought of as host-driven phenomena. Our findings underscore the potential of individual uterine cells to continuously expand lesions and participate in the progression of endometriosis. This model of retrograde menstruation may therefore be used to study processes involved in the pathophysiology of endometriosis.
Keywords: endometriosis, retrograde menstruation, endometrium
Individual endometrial cells contribute to endometriosis lesion expansion, inflammatory burden, and neovasculature in a model of retrograde menstruation.
Introduction
Endometriosis is a debilitating, estrogen-dependent inflammatory disease affecting nearly 10% of reproductive age women. It is characterized by growth of functional endometrial tissue, including glands and stroma outside of the uterine cavity [1]. The chronic pelvic pain and infertility associated with endometriosis underlie the substantial economic and social burdens of the disease, which include increased healthcare costs, loss of productivity at the workplace, and reduced quality of life [2, 3].
Multiple hypotheses have been proposed for the origin of endometriosis [4]. Sampson's theory posits that retrograde menstrual efflux through the fallopian tubes gives rise to ectopic endometrial implants that seed lesion growth [5]. However, while approximately 90% of women aged 15–49 years will exhibit retrograde menstruation, only 5–10% are at risk of developing lesions [6]. Furthermore, in both endometriosis patients and women without the disease, endometrial epithelial and stromal cells are detected in peritoneal fluid during menstruation [7–9]. It was therefore postulated that women at a higher risk of developing endometriosis are those with larger volumes of efflux material. This was supported by clinical observations demonstrating that endometriosis patients have shorter menstrual cycle intervals, heavier menstrual effluent, increased duration of menstrual flow [10, 11], and long durations of uninterrupted menstrual cycles [12, 13], all which contribute to larger and more frequent volumes of retrograde-menstruation material. They were also shown to have more visible tissue fragments in their peritoneal fluid [14]. A study in mice reinforced these observations, demonstrating that more lesions develop with increasing mass of endometrial tissue injected into the peritoneal cavity [15].
Retrograde menstruation is composed of distinct body fluids and cell types: blood, the cells and fluid of the uterine endometrial lining shed in the late secretory phase (specifically, the stratum functionalis and much of the stratum basalis), and immune cells. Blood accumulates beneath the epithelial layer and fills with cell debris, immune cells, and inflammatory exudates. The shed endometrial lining consists of both intact tissue fragments and cells [16]. Identification of stem cells in the uterus and in menstrual blood has led to the hypothesis that endometriotic implants arise from differentiation of a single or a group of progenitor/stem cells originating from neonatal/adult endometrium, bone marrow, or Müllerian duct derivatives in a process of coelemic metaplasia [17–22]. Though stem cell origin implies clonality, clear conclusions regarding a putative clonal process in the development of endometriosis have not been reached [23].
In mice the uterus is remodeled without shedding (menses), such that retrograde menstruation does not occur. Intraperitoneal injection of individual endometrial cells to mimic retrograde menstruation fails to nucleate macroscopic endometriosis, though their mere presence in the peritoneal cavity was shown to enhance macrophage recruitment and increase production of inflammatory cytokines in the peritoneum [24]. Endometriosis induction is therefore commonly performed by directly suturing or injecting organized endometrial fragments or artificially decidualized endometrial tissue into the peritoneal cavity, or alternatively, transplanting single-cell suspensions under the kidney capsule in a manner that supports cell–cell contact, thus allowing for reconstitution of functional endometrial tissue [25–27]. Moreover, intra/retro-peritoneal injections of nondissociated stroma and glands from endometrium of nonhuman primates (i.e. in the form of unmanipulated menstrual endometrium or minced transcervical curettage material) form visible endometriosis in autologous recipients [28]. Finally, injections of nondissociated human endometrial stroma and glands require peritoneal fluid from women with endometriosis to establish macroscopic lesions in athymic mice [29]. In all the above instances, the collective potential rather than the capacity of single endometrial cells to develop at an ectopic site is evaluated. It is also not clear from these models if components in the efflux material other than stromal cells and glandular epithelial cells can functionally integrate into lesions.
Our aim in this study was twofold. First, to develop a model of retrograde menstruation in mice harboring a developing endometriosis-like lesion, which will provide a seeding scaffold for attachment of freshly isolated endometrial cells. Second, to examine the capacity of individual endometrial cells to integrate and contribute to a growing lesion through proliferative, inflammatory, and angiogenic processes that are known to characterize endometriosis.
Methods
Animals
A total of 7- to 8-week-old C57BL/6J (wild type) and syngeneic green fluorescent protein transgenic mice (UBC-GFP; GFP expressed under the direction of the human ubiqutin C promoter) were purchased from Jackson Laboratories. Mice were maintained in environmentally controlled facilities in the Animal Facility at Yale School of Medicine in a room with a 12-h light, 12-h dark cycle (7 am to 7 pm) with ad libitum access to food and water. All animal procedures were performed according to an approved Yale University Institutional Animal Care and Use Committee protocol.
Endometriosis induction and modeling of retrograde menstruation
Endometriosis was induced as previously described [30]. Briefly, diestrous uteri from 7- to 8-week-old cycling females were isolated, horns longitudinally dissected, and the lumen exposed. Four 3 mm2 pieces were sutured onto the peritoneum (two on each side) of syngeneic females, with the luminal side facing the peritoneum. An amount of 1 mg/kg meloxicam (Boehringer Ingelheim) was administered subcutaneously immediately following surgery. The diestrous stage was chosen for tissue harvesting as it is comparable to the human secretory phase preceding menstruation and may therefore more closely represent the endometrial cell population that is shed and deposited in the peritoneal cavity during retrograde menstruation. Females were kept cycling for 4 weeks by changing the cages’ bedding three times per week with fresh male bedding, verified by vaginal cytology.
At 1 week post-surgery, diestrus-stage uterus from syngeneic GFP-expressing donors was isolated, finely minced, and digested in a solution of Hanks balanced salt solution containing 25 mM HEPES (Life Technologies), 1 mg/mL collagenase B (Roche Diagnostics), and 0.1 mg/mL deoxyribonuclease I (Sigma-Aldrich) for 45 min at 37°C with periodic pipetting. Uterine cells were filtered using 70-μm mesh, centrifuged at 2000 rpm at 4°C for 8 min, washed, and resuspended in PBS. A total of 1 × 106 viable cells were injected intraperitoneally into WT recipients in 100 μL volume using a 27-gauge needle. At 4 weeks post endometriosis induction, endometriosis-like mouse lesions were isolated and analyzed by flow cytometry, immunohistochemistry, and immunofluorescence.
Flow cytometry
Mouse endometriosis lesions were digested as described above and cells were incubated with APC-Cy7 conjugated anti-mouse CD45 antibody (1:200; Biolegend 103115) for 30 min, followed by washing with PBS. Flow cytometry was performed to detect GFP+ cells on FACS MoFlo (Beckman Coulter). Gates were applied to forward-scatter/side-scatter dot plots to exclude nonviable cells and cell debris. Data were analyzed using FlowJo V10.
Immunostaining
Mouse endometriosis lesions were fixed in 4% paraformaldehyde, paraffin-embedded and cut into 5-μm sections. Antigen retrieval was accomplished by boiling in sodium citrate (pH 6) for 10 min. For immunohistochemistry, blocking was performed by incubating sections in PBST containing 5% normal rabbit serum (Vector Laboratories) at room temperature for 30 min. Sections were then incubated with goat anti-GFP antibody (1:1000; Abcam: ab6673) overnight at 4°C. For secondary antibody and detection reagents, Vectastain Elite ABC HRP kit (Peroxidase, rabbit anti-goat IgG) and ImmPACT DAB (Vector Laboratories) were used according to the manufacturer's instructions. Tissue sections were counterstained with hematoxylin (Sigma-Aldrich). Images of stained sections were captured using Nikon Eclipse 80i microscope (Nikon).
For immunofluorescence of paraffin-embedded lesions or pellets of dissociated uterine cells, blocking was performed with 10% donkey serum (Vector Laboratories) for 1 h. Sections were then incubated with the following primary antibodies (Abcam) at 4°C overnight: rabbit anti-pan cytokeratin (1:200, ab9377), polyclonal goat anti-GFP antibody (1:400, ab6673), rat anti-CD45 (1:200, ab25386), rabbit anti-CD31 (1:200, ab28634), rabbit anti-vimentin (1:400; ab92547), rabbit anti-PCNA (1:400; ab18197). The secondary antibodies obtained from Thermo Fisher Scientific were diluted 200-fold: Alexa Fluor 568-conjugated donkey anti-goat (A-11057), Alexa Fluor 488-conjugated donkey anti-rabbit (A-21206), or Alexa Fluor 488-conjugated donkey anti-rat (A-21208). Sections were DAPI stained and cover-slipped using Vectashield fluorescent mounting media with DAPI (Vector Laboratories). Sections were imaged using laser scanning confocal microscope (LSM 710; Zeiss) and captured using ZEN software (Carl Zeiss).
Statistical analysis
Mean ± standard error of the mean (SEM) were calculated for the various experiments using GraphPad Prism 6 (GraphPad Software). The percentage of cells expressing CD31, vimentin, and PCNA out of the total integrated (GFP+) cells was counted in immunofluorescent sections and expressed as mean ± SEM. The percentage of cells expressing CD45 out of the total integrated (GFP+) cells was expressed as mean ± SEM.
Results
Individual uterine cells integrate into a developing endometriosis lesion in a model of retrograde menstruation
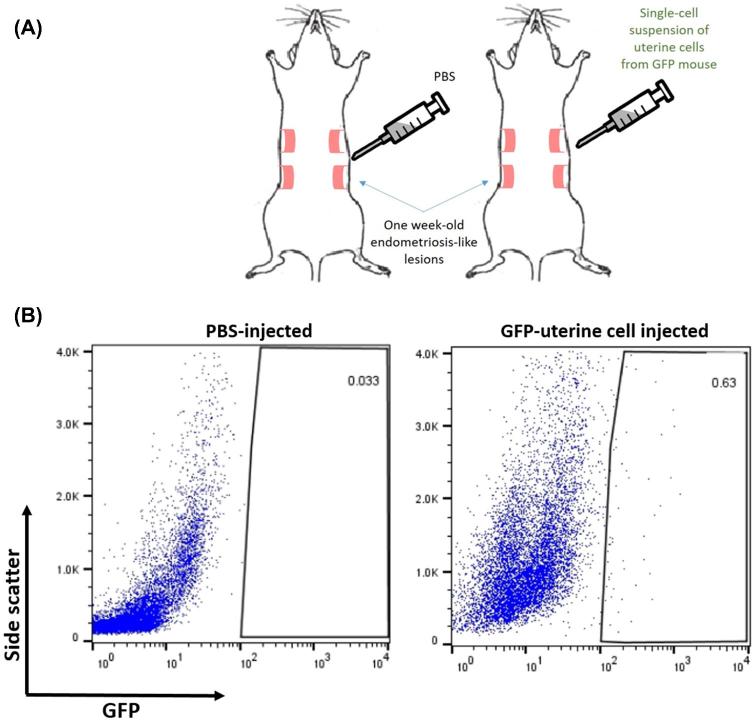
We designed a model to test the capacity of individual uterine cells to become incorporated into a growing endometriosis lesion, seeding endometrial material in a manner reproducing retrograde menstruation. One week following endometriosis induction, donor uteri derived from GFP+ mice were minced, digested filtered, and 1 × 106 cells were injected intraperitoneally into syngenic WT females (Figure 1A). Controls were injected with PBS. Lesions were extracted 3 weeks post injection and analyzed by flow cytometry to evaluate the enrichment of donor uterine cells in the growing lesion. Out of eight mice recipients of GFP-labeled uterine cell injections, all contained at least one lesion that demonstrated integration of GFP+ cells. In total, 13 out of 16 lesions harbored GFP+ cells (81.3%). As shown in Figure 1B, a single administration of uterine cells was sufficient to induce low level of integration within the lesion. The percentage of donor-derived GFP+ cells out of the total cells in the lesion was 0.64% ± 0.24 (n = 14) after subtracting non-specific background in PBS-injected controls (0.03% ± 0.03, n = 5).
Figure 1.
Mouse model of retrograde menstruation. (A) One-week post EMI, female mice were injected with either PBS or single-cell suspension containing 1 × 106 GFP-labeled viable uterine cells. (B) Flow cytometric analysis of GFP+ uterine cells integrated in the endometriosis lesion 4 weeks post EMI. Representative scatter plots from PBS (n = 5) and uterine cell injection (n = 14). Values in boxes denote percentages out of the live cell population.
Integrated cells contribute to lesion makeup, vasculature, and inflammatory burden
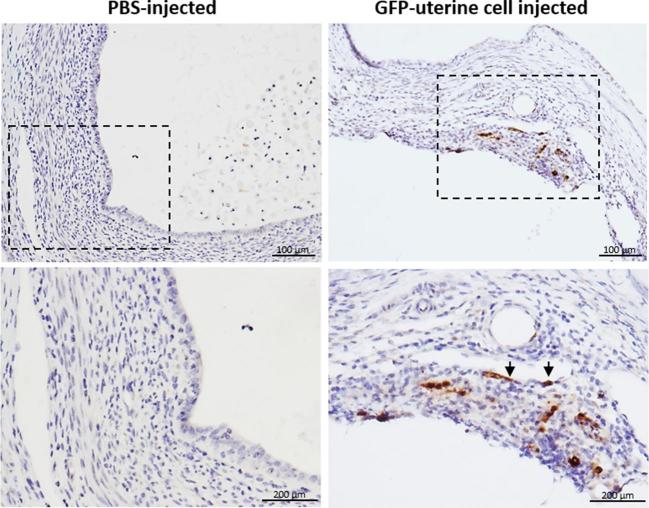
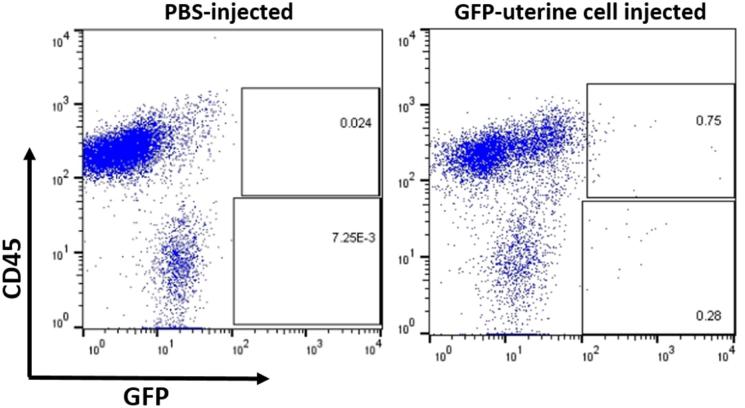
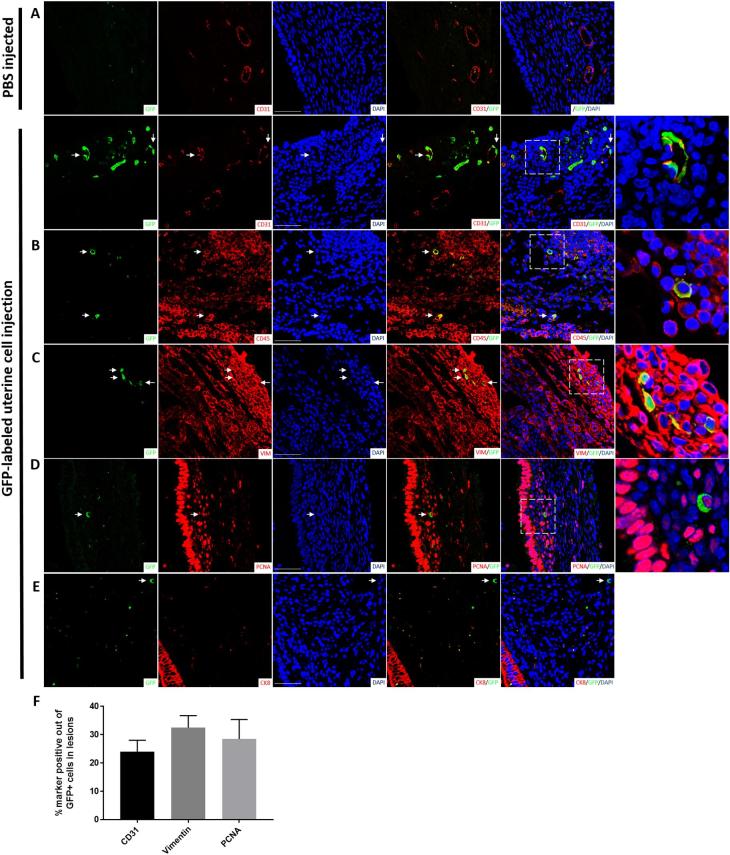
Sections of lesions from mice injected with GFP+ uterine cells were stained with antibody for GFP confirming the presence of donor-derived GFP+ cells within endometriosis lesions by immunohistochemistry (Figure 2). These GFP+ cells were found in the lesion's endometrial stroma with some cells integrated into blood vessels’ wall as endothelial cells (shown with arrows), but no GFP+ cells were found in glandular or luminal epithelium (Figure 2). Flow cytometric analysis using an antibody for the pan-leukocyte surface marker CD45 demonstrated that the majority of GFP+ cells within the endometriotic lesion were hematopoietic cells (69% ± 5%, n = 14) (Figure 3). To further characterize the cellular contribution of injected uterine cells to the endometriotic lesion in our model, we performed immunofluorescence staining with additional markers, including CD31 (endothelial), vimentin (stromal/endothelial), and PCNA (proliferation) markers. As shown in Figure 4A–D, injected GFP+ cells could be found colocalized with each of the above markers. The percentage of CD31, vimentin, and PCNA out of the integrated GFP+ cells was 24.1 ± 4.0, 32.5 ± 4.3, and 28.5 ± 6.9, respectively (Figure 4F). In particular, some lesions exhibited considerable integration of injected cells into lesion vasculature (Figure 4A). GFP+ cells expressing CD45 were also detected in inflammatory clusters within the lesion (Figure 2B). Of note, GFP/cytokeratin 8 co-staining was absent in all lesions containing integrated GFP+ uterine cells (Figure 4E), pointing to failure of epithelial uterine cells in the peritoneal milieu to integrate into growing lesions. To confirm that epithelial cells were indeed present in the starting material, cytokeratin staining was performed on uterine cells that were processed prior to peritoneal injection. Supplementary Figure S1 demonstrates the injected cell suspension does contain numerous epithelial cells. Taken together, these findings suggest that in this model of retrograde menstruation, individual uterine-derived cells (rather than tissue fragments) survive in the peritoneal milieu and functionally integrate into lesions, with the exception of epithelial cells.
Figure 2.
Localization of uterine cells integrated into the endometriotic lesion. Representative immunohistochemical section of an endometriotic lesion demonstrating brown-stained GFP+ uterine cells found in the lesion stroma as well as integrated into blood vessels wall in the lesion (arrowheads) (n = 8, right). Representative section of a lesion from PBS-injected mice (n = 4, left). Lower panel: higher magnification of boxed areas.
Figure 3.
Enrichment of hematopoietic cells derived from cell injection in the endometriosis lesion. Flow cytometric analysis of the percentage of GFP+ cells expressing the pan-hematopoietic marker CD45. Representative plots from PBS (n = 5) and uterine cell injection (n = 14). Values in boxes denote percentages out of the live cell population.
Figure 4.
Profile of uterine cells incorporated into growing mouse endometriosis lesions. Immunofluorescent micrographs of endometriosis lesions 4 weeks post EMI. Representative sections from control (n = 4) and experimental (n = 8) lesions from different mice are shown. Co-staining of GFP-positive uterine cells integrated into a growing endometriosis lesion (green) with (A) CD31, (B) CD45 (section depicting an inflammatory cluster within the lesion), (C) vimentin, (D) PCNA, and (E) CK8 (red). Sections were counterstained with DAPI showing nuclei (blue). Arrowheads point to GFP+ integrated uterine cells. Right column: higher magnification images of dashed areas. Scale bar = 50 μm. (F) Quantification of the percentage of cells double-positive for GFP and CD31 (n = 7 lesions), vimentin (n = 7 lesions), or PCNA (n = 7 lesions).
Discussion
We demonstrate the integration of endometrial cells at the site of endometriosis in the peritoneal cavity and their ability to participate in endometriotic processes. By modeling the fate of single endometrial cells in a model that simulates retrograde menstruation, we were able to dissect their specific contribution to lesion development. Though in this model single cells themselves did not induce or initiate endometriosis processes (as the lesion was already established in the female mice), injected endometrial cells nonetheless integrated into the lesion contributing to various cell subpopulations within it. The relatively low frequency of integration observed in consistent with studies demonstrating the dose dependency of the process of lesion establishment [10, 11, 14, 15].
Our finding that GFP-labeled uterine cells become part of the stromal, hematopoietic, and endothelial makeup of the growing lesion after a single administration of one million cells, as well as the demonstration that injected cells are mitotically active, suggests their functional involvement in developmental, immune, and angiogenic processes within the lesion. The adhesive properties of cells introduced into the peritoneal cavity may be enhanced around the proinflammatory environment of the lesion, supporting integration. Evidence for this comes from a study demonstrating that proinflammatory and profibrotic cytokines in the peritoneum alter the expression of endometrial integrins in endometriotic disease, increasing the probability that refluxed endometrial cells implant in the peritoneum (reviewed by [31]). We did not detect epithelial cells in lesions derived from the injected uterine cells, suggesting that retrograde menstruation may not be a mechanism driving uterine epithelial cells or their progenitors to lesion foci. Interestingly, while evidence suggests the stroma of lesions develops from multiple cells (multicellular origin, like retrograde menstruation), individual glands in lesions were characterized by monoclonal expansion (one-cell origin) [32]. Progenitors/stem cells derived from bone marrow [20] or hematogeneous dissemination [33] may instead contribute to lesion's epithelial structures.
Strikingly, some endometriotic lesions in our study featured an extensive endothelial network derived from the dispersed uterine cells and displayed enrichment of hematopoietic cells (CD45+) among the integrated material. Our results reinforce the validity of our model, as white blood cells were indeed shown to be elevated in peritoneal fluid of women in the menstrual stage of the cycle [34] and immune cells are known to be important mediators of the inflammation associated with endometriosis [35]. That these immune cells are uterus-derived was never before demonstrated.
Previous reports attributed a principal role for retrograde menstrual material in triggering the patient's immunity (i.e. by releasing proinflammatory cytokines that activate and recruit immune cells to the peritoneum [36]). However, it was not clear if endothelial and immune components of the lesion can originate from retrograde menstrual endometrium in addition to being supplied by the ectopic environment (i.e. host-derived). One confounder to revealing whether individual immune cells from menstrual material can become functional building blocks of the ectopic lesion is the tissue-level organization of these cells, which remains intact in most endometriosis models. Using a single cell suspension of endometrial cells, we show for the first time to the best of our knowledge that uterine endothelial and immune cells (or their corresponding progenitors) can independently contribute to blood vessels and inflammation, respectively, within lesions.
One limitation of our study is that we did not isolate progenitor populations from the dissociated uterine sample prior to intraperitoneal injection in order to decipher whether uterine-derived progenitor/stem cells primarily give rise to the various populations we observe in lesions. While the identity of hematopoietic progenitors in the mouse uterus was previously proposed [37], the authors did not attempt to isolate it, noting that these “likely make up a very small percentage” in the uterus. Furthermore, the identity of endothelial progenitors in the mouse uterus is not defined. Finally, the marker combination used to identify mesenchymal stem cells in the endometrial stroma in humans is CD45–/CD146+/CD140b+(PDGFRβ) [38]. In adult female mice, CD146+/CD140b+ cells were colocalized to the perivascular space, though not isolated [39]. We therefore attempted to isolate this population from the mouse uterus by FACS. Only a negligible number of cells in the uterus were able to be sorted using this combination of markers (not shown), and due to the low frequency of integration we observed in this model we did not pursue this approach further.
Our study also raises the question of whether productive integration requires attachment at a random site or juxtaposition with a region in the lesion receptive to the cell type or its corresponding progenitor (i.e. a region providing a suitable niche for the establishment of the specific cell through paracrine/survival signals). Alternatively, cells making transient attachments with lesion structures may migrate within the lesion to areas more suitable for their establishment, as occurs in developmental processes [40]. Such processes were shown to be mediated by guidance cues or through cell mixing [41]. Indeed, recruitment and incorporation of endothelial progenitor cells was shown to require a coordinated sequence of integrin-mediated adhesion and migration, SDF-1/CXCR4-mediated chemoattraction, and ultimately differentiation into endothelial cells (ECs) at the target site [42].
Although some GFP+ CD31+ cells in our study appear to be randomly integrated, the rather proximal EC distribution depicted in Figure 4 supports clonal expansion contributing to neovascularization. While clonal expansion is often attributed to endothelial progenitors, in models of pathophysiological angiogenesis (i.e. injury, ischemia, hypoxia), a specific subset of mature ECs demonstrate high proliferative potential [41]. These ECs are presumed to have progenitor cell characteristic and capable of forming new blood vessels [43]. Indeed, hypoxia and VEGF, both having a well-established proliferative effect on mature ECs [41, 44], are hallmarks of the endometriosis lesion [45, 46]. It is therefore plausible that the lesion environment supports clonal expansion of mature ECs that integrate through retrograde menstruation, as it does in the retina, heart, and skeletal muscle following hypoxia [41].
This study's findings of direct uterine cellular contribution to the endometriotic lesion support the notion that eliminating menstruation should be part of any endometriosis therapy. Interestingly, removing the source of retrograde menstruation (i.e. through hysterectomy) leads to clinical improvement in many patients, demonstrating the importance of a continued supply of new endometrial cells to the persistence of endometriosis. However, the disease still recurs in some even after hysterectomy. This may be due to remaining unresectable or unrecognized lesions, since recurrence of endometriosis symptoms and pelvic pain after hysterectomy appear to correlate with the extent of removal of peritoneal and deeply infiltrating disease [47, 48]. Alternatively, bone marrow derived stem cells may continue to feed endometriosis even after hysterectomy.
In summary, we describe a novel in vivo model of retrograde menstruation allowing for dissecting the specific contribution of individual cells in the retrograde uterine efflux to endometriosis lesion development. Our findings demonstrate that a single injection of dissociated uterine cells stripped of factors known to promote endometriosis establishment (i.e. intact glandular/stromal structures present in fragmented endometrium, peritoneal fluid, artificial environment promoting contact of single cells) is sufficient for cellular integration into an existing lesion. Our study revealed the potential of these singular endometrial cells to contribute to endometriosis processes of angiogenesis, inflammation, and cell proliferation. The study also provides a tool for tracking the fates of individual cells introduced in a manner reproducing retrograde menstruation, allowing for direct comparison of the integration potential of cells in the endometrium of women with and without endometriosis into existing lesions in immunocompromised mice. In the endometriosis milieu created by the endometriosis induction, intrinsic differences in integration and proliferation at the cellular level may be revealed. Moreover, in light of emerging evidence attributing clonal origin to distinct endometriotic foci (reviewed by [23]), the validated model presented herein may have important potential applications for examining the effect of single cell genomic alterations on the pathophysiology of the disease.
Supplementary data
Supplementary Figure S1. Presence of epithelial cells in injected uterine material. Left: H&E staining of digested/dissociated uterus filtered through a 70 μm mesh, followed by immunofluorescent staining (three images on right) of the cellular material using cytokeratin antibody (red) and DAPI (blue). Scale bar = 50 μm.
Notes
Edited by Dr. Haibin Wang
Footnotes
Grant support: This study was supported by NIH R01 HD076422.
Conflict of interest: The authors have no conflict of interests to declare.
References
- 1. Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction 2016; 152:R63–R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 2007; 13(4):395–404. [DOI] [PubMed] [Google Scholar]
- 3. Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, Raine-Fenning N. The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum Reprod Update 2013; 19(6):625–639. [DOI] [PubMed] [Google Scholar]
- 4. Gordts S, Koninckx P, Brosens I. Pathogenesis of deep endometriosis. Fertil Steril 2017; 108(6):872–885.e1. [DOI] [PubMed] [Google Scholar]
- 5. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927; 3:93–110.143. [PMC free article] [PubMed] [Google Scholar]
- 6. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol 1984; 64:151–154. [PubMed] [Google Scholar]
- 7. O DF, Roskams T, Van den Eynde K, Vanhie A, Peterse DP, Meuleman C, Tomassetti C, Peeraer K, D’Hooghe TM, Fassbender A. The presence of endometrial cells in peritoneal fluid of women with and without endometriosis. Reprod Sci 2017; 24(2):242–251. [DOI] [PubMed] [Google Scholar]
- 8. Kruitwagen RF, Poels LG, Willemsen WN, de Ronde IJ, Jap PH, Rolland R. Endometrial epithelial cells in peritoneal fluid during the early follicular phase. Fertil Steril 1991; 55:297–303. [DOI] [PubMed] [Google Scholar]
- 9. van der Linden PJ, Dunselman GA, de Goeij AF, van der Linden EP, Evers JL, Ramaekers FC. Epithelial cells in peritoneal fluid–of endometrial origin? Am J Obstet Gynecol 1995; 173(2):566–570. [DOI] [PubMed] [Google Scholar]
- 10. Matalliotakis IM, Cakmak H, Fragouli YG, Goumenou AG, Mahutte NG, Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet 2008; 277(5):389–393. [DOI] [PubMed] [Google Scholar]
- 11. Vercellini P, De Giorgi O, Aimi G, Panazza S, Uglietti A, Crosignani PG. Menstrual characteristics in women with and without endometriosis. Obstet Gynecol 1997; 90(2):264–268. [DOI] [PubMed] [Google Scholar]
- 12. Sangi-Haghpeykar H, Poindexter AN 3rd. Epidemiology of endometriosis among parous women. Obstet Gynecol 1995; 85(6):983–992. [DOI] [PubMed] [Google Scholar]
- 13. Parazzini F, Cipriani S, Bianchi S, Gotsch F, Zanconato G, Fedele L. Risk factors for deep endometriosis: a comparison with pelvic and ovarian endometriosis. Fertil Steril 2008; 90(1):174–179. [DOI] [PubMed] [Google Scholar]
- 14. Sharpe-Timms KL. Haptoglobin expression by shed endometrial tissue fragments found in peritoneal fluid. Fertil Steril 2005; 84(1):22–30. [DOI] [PubMed] [Google Scholar]
- 15. Dodds KN, Beckett EAH, Evans SF, Hutchinson MR. Lesion development is modulated by the natural estrous cycle and mouse strain in a minimally invasive model of endometriosis. Biol Reprod 2017; 97(6):810–821. [DOI] [PubMed] [Google Scholar]
- 16. Yang H, Zhou B, Prinz M, Siegel D. Proteomic analysis of menstrual blood. Mol Cell Proteomics 2012; 11(10):1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasson IE, Taylor HS.. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci 2008; 1127(1):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant 2008; 17(3):303–311. [DOI] [PubMed] [Google Scholar]
- 19. Maruyama T, Yoshimura Y.. Stem cell theory for the pathogenesis of endometriosis. Front Biosci 2012; 4(8):2754–2763. [DOI] [PubMed] [Google Scholar]
- 20. Du H, Taylor HS.. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007; 25(8):2082–2086. [DOI] [PubMed] [Google Scholar]
- 21. Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med 2015; 33(5):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004; 292(1):81–85. [DOI] [PubMed] [Google Scholar]
- 23. Zhao L, Gu C, Huang K, Han W, Fu M, Meng Y. Endometriosis research using capture microdissection techniques: progress and future applications. Biomed Rep 2016; 5(5):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao X, Yang D, Song M, Murphy A, Parthasarathy S. The presence of endometrial cells in the peritoneal cavity enhances monocyte recruitment and induces inflammatory cytokines in mice: implications for endometriosis. Fertil Steril 2004; 82(Suppl 3):999–1007. [DOI] [PubMed] [Google Scholar]
- 25. Masuda H, Maruyama T, Hiratsu E, Yamane J, Iwanami A, Nagashima T, Ono M, Miyoshi H, Okano HJ, Ito M, Tamaoki N, Nomura T et al.. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc Natl Acad Sci USA 2007; 104:1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pullen N, Birch CL, Douglas GJ, Hussain Q, Pruimboom-Brees I, Walley RJ. The translational challenge in the development of new and effective therapies for endometriosis: a review of confidence from published preclinical efficacy studies. Hum Reprod Update 2011; 17:791–802. [DOI] [PubMed] [Google Scholar]
- 27. Greaves E, Critchley HOD, Horne AW, Saunders PTK. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet Gynecol Scand 2017; 96:644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D’Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Lauweryns JM, Koninckx PR. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am J Obstet Gynecol 1995; 173:125–134. [DOI] [PubMed] [Google Scholar]
- 29. Tabibzadeh S, Miller S, Dodson WC, Satyaswaroop PG. An experimental model for the endometriosis in athymic mice. Front Biosci 1999; 4:c4–9. [DOI] [PubMed] [Google Scholar]
- 30. Tal A, Tal R, Shaikh S, Gidicsin S, Mamillapalli R, Taylor HS. Characterization of cell fusion in an experimental mouse model of endometriosis. Biol Reprod 2019; 100(2):390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rutherford EJ, Hill ADK, Hopkins AM. Adhesion in physiological, benign and malignant proliferative states of the endometrium: microenvironment and the clinical big picture. Cells 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nabeshima H, Murakami T, Yoshinaga K, Sato K, Terada Y, Okamura K. Analysis of the clonality of ectopic glands in peritoneal endometriosis using laser microdissection. Fertil Steril 2003; 80:1144–1150. [DOI] [PubMed] [Google Scholar]
- 33. Li F, Alderman MH, 3rd Tal A, Mamillapalli R, Coolidge A, Hufnagel D, Wang Z, Neisani E, Gidicsin S, Krikun G, Taylor HS. Hematogenous dissemination of mesenchymal stem cells from endometriosis. Stem Cells 2018; 36(6):881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bokor A, Debrock S, Drijkoningen M, Goossens W, Fulop V, D’Hooghe T. Quantity and quality of retrograde menstruation: a case control study. Reprod Biol Endocrinol 2009; 7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang L, Yan Y, Liu Z, Wang Y. Inflammation and endometriosis. Front Biosci 2016; 21:941–948. [DOI] [PubMed] [Google Scholar]
- 36. Laux-Biehlmann A, d’Hooghe T, Zollner TM. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol Sci 2015; 36:270–276. [DOI] [PubMed] [Google Scholar]
- 37. Sun Z, Wu J, Li SH, Zhang Y, Xaymardan M, Wen XY, Weisel RD, Keating A, Li RK. Uterine-derived stem cells reconstitute the bone marrow of irradiated mice. Stem Cells Dev 2015; 24:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update 2016; 22:137–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patterson AL, George JW, Chatterjee A, Carpenter T, Wolfrum E, Pru JK, Teixeira JM. Label-retaining, putative mesenchymal stem cells contribute to murine myometrial repair during uterine involution. Stem Cells Dev 2018; 27(24):1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dorrell MI, Otani A, Aguilar E, Moreno SK, Friedlander M. Adult bone marrow-derived stem cells use R-cadherin to target sites of neovascularization in the developing retina. Blood 2004; 103:3420–3427. [DOI] [PubMed] [Google Scholar]
- 41. Manavski Y, Lucas T, Glaser SF, Dorsheimer L, Gunther S, Braun T, Rieger MA, Zeiher AM, Boon RA, Dimmeler S. Clonal expansion of endothelial cells contributes to ischemia-induced neovascularization. Circ Res 2018; 122:670–677. [DOI] [PubMed] [Google Scholar]
- 42. Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 2004; 95:343–353. [DOI] [PubMed] [Google Scholar]
- 43. Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 2005; 105:2783–2786. [DOI] [PubMed] [Google Scholar]
- 44. Takeshita S, Rossow ST, Kearney M, Zheng LP, Bauters C, Bunting S, Ferrara N, Symes JF, Isner JM. Time course of increased cellular proliferation in collateral arteries after administration of vascular endothelial growth factor in a rabbit model of lower limb vascular insufficiency. Am J Pathol 1995; 147:1649–1660. [PMC free article] [PubMed] [Google Scholar]
- 45. Hsiao KY, Lin SC, Wu MH, Tsai SJ. Pathological functions of hypoxia in endometriosis. Front Biosci (Elite Ed) 2015; 7:309–321. [DOI] [PubMed] [Google Scholar]
- 46. Liu S, Xin X, Hua T, Shi R, Chi S, Jin Z, Wang H. Efficacy of anti-VEGF/VEGFR agents on animal models of endometriosis: a systematic review and meta-analysis. PLoS One 2016; 11:e0166658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rizk B, Fischer AS, Lotfy HA, Turki R, Zahed HA, Malik R, Holliday CP, Glass A, Fishel H, Soliman MY, Herrera D. Recurrence of endometriosis after hysterectomy. Facts Views Vis Obgyn 2014; 6:219–227. [PMC free article] [PubMed] [Google Scholar]
- 48. Schindler AE, Foertig P, Kienle E, Regidor PA. Early treatment of endometriosis with GnRH-agonists: impact on time to recurrence. Eur J Obstet Gynecol Reprod Biol 2000; 93:123–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.