Abstract
Autoimmune Regulator (AIRE) regulates central immune tolerance by inducing expression of tissue-restricted antigens in thymic medullary epithelial cells, thereby ensuring elimination of autoreactive T cells. Aire mutations in humans and targeted Aire deletion in mice result in multiorgan autoimmune disease, known in humans as autoimmune polyglandular syndrome type 1 (APS-1). APS-1 is characterized by the presence of adrenal insufficiency, chronic mucosal candidiasis, and/or hypoparathyroidism. Additionally, females often present with gonadal insufficiency and infertility. Aire-deficiency (KO) in mice results in oophoritis and age-dependent depletion of follicular reserves. Here, we found that while the majority of young 6-week-old Aire-KO females had normal follicular reserves, mating behavior, and ovulation rates, 50% of females experienced embryonic loss between gestation day (GD) 5.5 and 7.5 that could not be attributed to insufficient progesterone production or decidualization. The quality of GD0.5 embryos recovered from Aire KO mice was reduced, and when cultured in vitro, embryos displayed limited developmental capacity in comparison to those recovered from wild-type (WT) mice. Further, embryos flushed from Aire KO dams at GD3.5 were developmentally delayed in comparison to WT controls and had reduced trophoblastic outgrowth in vitro. We conclude that AIRE does not play a direct role in uterine decidualization. Rather, reduced fertility of Aire-deficient females is likely due to multiple factors, including oophoritis, delayed preimplantation development, and compromised implantation. These effects may be explained by autoimmune targeting of the ovary, embryo, or both. Alternatively, altered embryonic development could be due to a direct role for AIRE in early embryogenesis.
Keywords: fertility, autoimmune disease, embryo, uterus, ovary, implantation
Although mating and ovulation were normal, oocyte and embryo quality were reduced; peri-implantation embryonic loss was associated with reduced oocyte and embryo developmental potential in vitro.
Introduction
The immune system is dually tasked with the identification and removal of pathogens while remaining tolerant to self-antigens. An appropriate balance between activation and tolerance is essential for reproductive viability in females in several respects. The female immune system must remain tolerant not only to reproductive tract antigens, but also to a host of neoantigens associated with pregnancy, particularly the fetus and the placenta, all the while maintaining its ability to combat infection. Further, immune cells and their secreted products play essential supportive roles in many reproductive processes, including ovulation, development, and regression of the corpus luteum, implantation, placentation, and uterine remodeling [1–4].
Autoimmune disease is broadly defined as an adaptive immune response mounted against self-antigens, and occurs when there is a loss of or failure to develop tolerance to those antigens. Most autoimmune conditions cannot be attributed to a single factor, but instead involve a complex interplay between genetic and environmental factors [5]. Conversely, monogenic autoimmune diseases are rare, but can be fatal at an early age [6]. Autoimmune polyglandular syndrome type 1 (APS-1) is one such disease, and results from functional mutations in the gene-encoding AIRE (autoimmune regulator) [7, 8]. APS-1 is a multiorgan, autosomal recessive disease that is diagnosed based on the presence of at least two of three clinical signs including adrenal insufficiency, chronic mucocutaneous candidiasis, and hypoparathyroidism [9]. Patients also develop a broad range of secondary autoimmune-mediated complications, including autoimmune hepatitis, gastritis, vitiligo, type I diabetes, and primary gonadal insufficiency with infertility [9]. Additionally, some alleles of Aire behave in a dominant negative manner and cause a later-onset, less penetrant disease than that which occurs with complete Aire deficiency [10, 11]. Although many autoimmune diseases preferentially affect women, APS-1 is not sex-specific [12].
The Aire gene encodes a 58-kDa transcriptional regulator expressed predominantly in the thymus, where developing T cells gain immunological competence and are selected for self-tolerance. Specifically, in both mice and humans, AIRE protein localizes to the nuclei of a subset of medullary thymic epithelial cells (mTEC) [13]; a primary function of the protein is to induce ectopic expression of self-antigens in these cells that are otherwise restricted to only one or a few tissues [14]. AIRE acts not as a conventional transcription factor but rather, together with its binding partners, it targets markers of inactive chromatin such as nonmethylated H3K4 to induce transcriptional elongation by releasing stalled RNA polymerase, thus inducing expression of genes that would otherwise be silenced [15–18]. Induced tissue-restricted antigens are then proteolytically processed and presented in the context of major histocompatibility (MHC) molecules to developing thymocytes, either directly by mTEC themselves, or indirectly by local dendritic cells. Recognition of AIRE-regulated self-antigens in the context of MHC results in clonal deletion of developing autoreactive T cells, or alternatively, promotes development of tissue-specific regulatory T cells (TReg) [19–23]. In these respects, AIRE plays an obligatory role in establishment of self-immunological tolerance and prevention of autoimmune disease.
Infertility is estimated to affect 6%–25% of couples worldwide, and has profound social, economic, medical, and psychological repercussions [24, 25]. Female factor infertility accounts for more than half of diagnosed infertility cases, and among the potential causes are a range of autoimmune disorders targeting the ovary directly or secondarily following autoimmune thyroiditis or Addison's disease [26–29]. Additional targets of the immune system important for fertility include sperm, phospholipids, and nuclear antigens [30–32]. A major manifestation of Aire deficiency, in both women with APS-1 and female mice with targeted disruptions in the gene, is infertility [33–35], and in both species mTEC express a number of genes that are restricted to the ovary, placenta, and embryo in an AIRE-dependent manner [14, 36–38]. We previously showed that Aire-deficient (KO) female mice on the Balb/cJ genetic background develop infertility in an age-dependent manner, undergoing ovarian follicular depletion coupled with lymphocytic infiltration and autoantibody formation [35]. By 20 weeks of age, 55% of Aire-KO female mice lack ovarian follicles and oocytes. Even so, young Aire-KO females invariably undergo puberty, albeit slightly delayed, and at 6 weeks of age, exhibit mating behavior and ovulate. However, of these young females only 50% are able to produce a litter, and over time, infertility reaches 100%. The overtly normal reproductive parameters aside from litter production collectively suggest that ovarian senescence alone cannot explain the infertility observed in all of these mice. In this study, we investigate the possibility that uterine and/or embryonic competence is impaired in female Aire-deficient Balb/cJ mice.
Materials and methods
Animals
Mice were housed under pathogen-free conditions at the University of Kansas Medical Center Laboratory Animal Resources or Michigan State University Campus Animal Resources. All experiments complied with NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at KUMC and MSU. Aire-deficient (KO) mice on the Balb/cJ genetic background (>8 generations) were a generous donation by C. Benoist and D. Mathis (Harvard Medical School, Boston, MA) [14, 39] and animals were maintained as heterozygotes. Additional Balb/cJ wild-type (WT) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) as needed. Genotypes were determined by PCR using the following primer set: FWD 5′-GTCCCTGAGGACAAGTTCCA-3′; REV 5′-GGGACTGGTTTAGGTCCACA-3′; using these primers, WT mice generate a 758-bp product, Aire-KO mice yield a 298-bp product, and heterozygous animals produce both. Additional Aire-KO mice containing the same mutation as the Balb/c mice or a different mutation were purchased from the Jackson Laboratory or kindly donated by L. Peltonen, respectively, and genotyped according to published protocols [14, 40].
Fertility assessment
At 6 weeks of age, WT and Aire-KO females were cohabited with a proven Balb/c WT male and checked daily for the presence of a copulatory plug. The duration to initial mating was recorded, and following mating their body weight was measured each morning between 9 and 10 am until sacrifice at gestation day (GD) 5.5, 7.5 or 10.5 (GD0.5 = day of observation of a copulation plug). Animals assessed for pregnancy at GD5.5 received an I.V. injection of Chicago blue dye following anesthesia for easier evaluation of implantation sites, as previously described [41]. Number of implantation and resorption sites was recorded at the time of sacrifice. Animal numbers for each experiment are indicated in the figures and legends.
Determination of serum progesterone levels
Mice were anesthetized with 250 mg/kd avertin IP or isoflurane, and serum was collected via cardiac puncture prior to euthanasia via cervical dislocation. Serum progesterone levels were measured by radioimmunoassay at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA). This assay has a sensitivity of 0.15 ng/mL and a range of 0.15–40 ng/mL, with intra- and interassay coefficients of variation of 3.6% and 9.0%, respectively.
Evaluation of implantation sites
For histological assessment of uterine sites at GD5.5, 6-week-old WT (n = 5) and Aire-KO (n = 7) females were paired with WT males and sacrificed on GD5.5. Whole implantation sites (1–3 per dam, for a total of 10 and 13 sites from WT and KO dams, respectively) were removed, fixed in 4% paraformaldehyde (PFA) overnight, dehydrated through a graded series of ethanol, embedded in paraffin and stored at 4°C. Serial sections (5 μm) were cut, stained with hematoxylin and eosin (Dako, Carpinteria, CA), and viewed by light microscopy. The section with the widest cross-sectional area of the embryo was selected for digital capture and area measurement of the implantation site (excluding myometrium), the primary decidual zone, and the embryo [42]. Area measurements were recorded using ImageJ software (NIH, Bethesda, MD).
Artificial decidualization
To test whether decidualization was impacted by the absence of AIRE in mice, artificial decidualization was performed in WT (n = 5), Aire-heterozygous (n = 3), or Aire-KO (n = 3) mice as previously described [43]. Briefly, WT, heterozygous, or KO mice were ovariectomized and allowed to recover for 2 weeks, at which time the mice received daily subcutaneous injections of 100 ng estradiol for 3 days. The mice were rested for 2 days, and again injected daily with 6.7 ng estradiol and 1 mg progesterone for 7 days (Sigma-Aldrich). On the third day of estradiol and progesterone treatment, 6 h after the estradiol/progesterone treatment, the left uterine horn was exteriorized, and using a burred needle, the endometrium scratched on the anti-mesometrial side to stimulate decidualization, while the right horn served as a nondecidualized control. On the eighth day, the mice were sacrificed, and uterine horns were dissected, weighed and processed for RNA analysis.
Quantitative reverse transcription and polymerase chain reaction
Transcripts for BMP2, CX45, and DPRP in artificial deciduae were quantified by quantitative reverse transcription and polymerase chain reaction (RT-qPCR). RNA was extracted using TRI Reagent (Applied Biosystems, Foster City, CA). Total RNA (1 μg) was reverse transcribed using Superscript III Reverse Transcriptase and random primers (Invitrogen, Carlsbad, CA). TaqMan Gene Expression Assay kits (BMP2, Mm01340178_m1; CX43, Mm00439105_m1; Dprp, Mm01135453_m1; GAPDH, Mm99999915_g1) were purchased from Applied Biosystems (Grand Island, NY). PCR reactions included 20 ng of cDNA combined with TaqMan Universal PCR Master Mix per the manufacturer's instructions in an optical reaction plate before being amplified using an Applied Biosystems 7300 Real Time PCR machine. All RT-qPCR samples were run in triplicate, and the average of the three CT values for each sample and primer set was used in subsequent calculations. Fold changes were calculated using the ddCt method, using average values of WT decidua as a reference.
Transcripts for Aire in reproductive tract tissues and thymus were also quantified. For reproductive tract analysis, 6- to 10-week-old WT females were euthanized at diestrus or estrus, or mated to WT males and euthanized at GD3.5, GD5.5, or GD7.5 to collect the ovaries, oviducts, uteri, and thymus. Estrus cycle stage was evaluated by vaginal cytology, and pregnancy in GD3.5 mice was confirmed by flushing uterine cavities to detect preimplantation blastocysts. Implantation sites at GD5.5 and 7.5 were confirmed macroscopically. RNA was extracted using TRIzol Reagent (ThermoFisher) and reverse transcribed using QuantiTect Reverse Transcriptase (Qiagen). RT-qPCR was performed using the StepOnePlus system together with Taqman primer/probe set for Aire (Mm00477461). Fold changes were calculated using the ddCt method, using the diestrus thymus as a reference.
Immunofluorescence
Expression of AIRE in the female reproductive tract was evaluated by immunofluorescence. Wild-type females were euthanized at diestrus, estrus, or GD3.5, 5.5, or 7.5 as described above. Uterine tissues with visible implantation sites were collected, fixed, and sectioned at 5 μm thickness. The sections were permeabilized with 0.1% Triton-X100 in phosphate-buffered saline (PBS) and blocked with 10% goat serum in PBS to prevent nonspecific antibody binding. Sections were then incubated with rat anti-AIRE primary antibody (clone 5H12, 5 μg/mL; ThermoFisher) at 4°C overnight, washed, and probed with secondary goat anti-rat antibody conjugated to Alexa Fluor 488 (10 μg/mL; catalog number A-11006, Thermo Fisher). Sections were mounted using ProLong Gold Antifade Mountant with DAPI (Thermo Fisher), and images were obtained using a Nikon Eclipse Ti-U inverted microscope system.
Blastocyst culture and trophoblast outgrowth
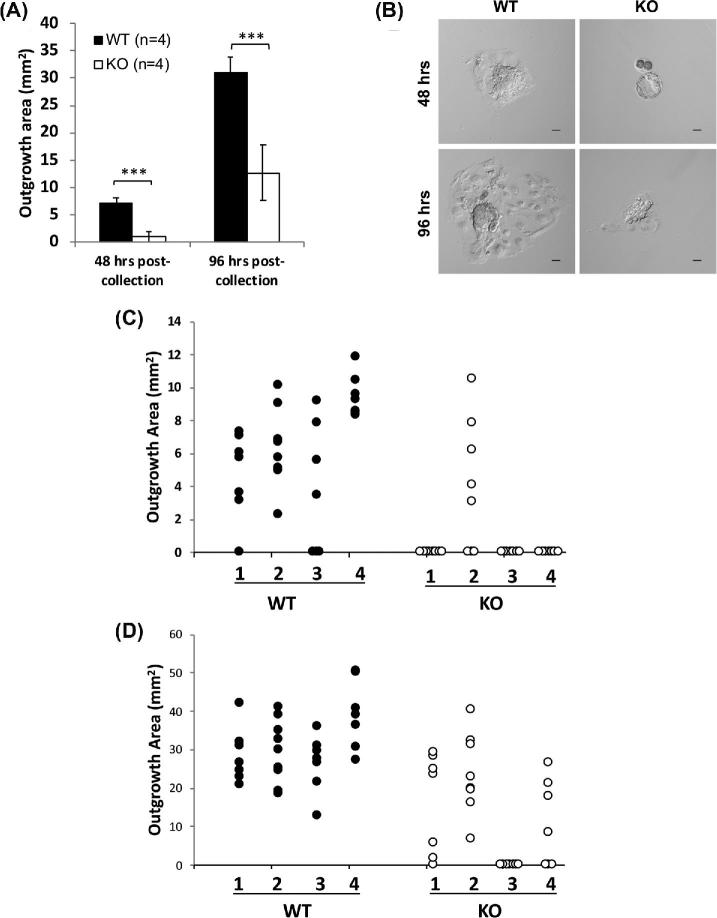
To examine early embryonic development prior to implantation and address the ability for trophoblast cells to proliferate in vitro, 6-week-old WT (n = 4) and Aire-KO (n = 4) females were paired with a WT male and sacrificed on embryonic day 3.5 as described above. The collection, staging, and culture of GD3.5 blastocysts was performed as previously described [44]. Briefly, after removing the reproductive tract, each uterine horn was isolated by cutting the distal and proximal ends of each uterine horn and was just below the fallopian tube and just above the cervix, respectively. Blastocysts were flushed from each uterine horn using a 26-G needle and 400 μl of embryonic stem cell medium (DMEM containing 15% heat-inactivated fetal bovine serum, 0.2% glutamine, 0.2% nonessential amino acids, 0.1 mM 2-mercaptoethanol, 0.2% sodium pyruvate and 50 μg/mL penicillin-streptomycin) washed through fresh microdrops of medium. Each blastocyst was transferred to a single well of a 24-well collagen Type I coated plate (EMD Millipore, Billerica, MA) and covered with fresh media. The number and developmental stage of the GD3.5 embryos was determined at collection [44]. Additionally, images of trophoblast outgrowth were recorded on an inverted light microscope 48- and 96-h postcollection. Total outgrowth area was determined for embryos derived from WT females (n = 30 embryos from 4 mice) and Aire-KO females (n = 30 embryos from 4 mice) using ImageJ software (NIH, Bethesda, MD).
Gestation day 0.5 embryo culture
To assess the quality and developmental potential of GD0.5 embryos, 6-week-old WT (n = 11) and Aire-KO (n = 10) females were sacrificed the afternoon following detection of a copulation plug, and oviducts were flushed with FHM-Hepes (EMD Millipore, Billerica, MA) and incubated in 0.3 mg/mL hyaluronidase (Sigma-Aldrich, St. Louis, MO) for 10 min to remove cumulus cells. Fertilized eggs and embryos were counted and evaluated for quality as described [44]. Briefly, oocytes/embryos with fragmentation or dark granular cytoplasm were considered poor quality. All pronuclear embryos were washed through and cultured in microdrops of KSOM with amino acids and D-Glucose (EMDMillipore, Billerica, MA). At 24 and 96 h of culture, embryos were counted and staged using an inverted microscope.
Statistical analysis
Data on duration to initial mating, gene expression, implantation site and embryo areas, trophoblast outgrowth area and number of ovulations between WT controls and Aire-KO mice were analyzed by two-tailed Student's t-test or, if found to be non-normally distributed, by Mann–Whitney Rank Sum test. Measurements were collected with the operator blinded to genotype. Progesterone values were measured by one-way ANOVA, and weight change over time was analyzed by two-way repeated measures ANOVA. When necessary, data were log10 transformed to normalize. Proportions of mice that mated, ovulated, their pregnancy rates, and developmental stages of embryos were compared by Chi-square analysis. Results were considered significantly different with a P ≤ 0.05.
Results
Peri-implantation embryonic loss in Aire-deficient mice
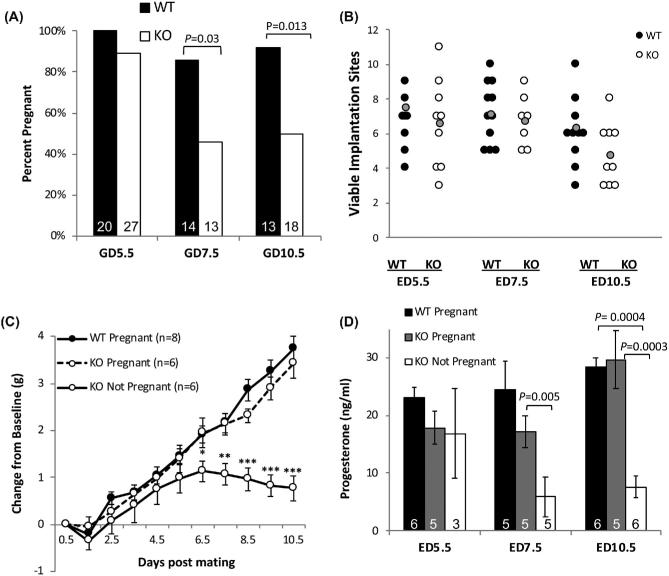
Our previous studies showed that only 50% of 6-week-old female Aire-KO mice on the Balb/cJ genetic background are fertile, with a further reduction in proportion of fertile animals with advancing age. Although ovarian senescence eventually occurs in more than half of Aire-KO mice by 20 weeks, only 25% of mice exhibited abnormal ovarian morphology at 8 weeks, and there were no statistically significant differences in mating behavior or ovulation rate [35]. Thus, the data suggested that the observed fertility rate is lower than could be explained by lack of ovarian function at that age. In agreement with previous results, we found that only 48.5% of 6-week-old Aire-KO female mice cohabitated with WT males became pregnant, despite 93.9% (n = 33) of animals having overtly normal ovaries at this age. Further, the average number of days between pairing and observation of a copulatory plug (WT: 2.06 ± 0.26 days, n = 17; KO: 2.42 ± 0.20, n = 33) and the proportion of total mice to mate (WT: 100%, n = 35; KO: 97.6%, n = 42) were similar between WT and KO mice (P > 0.05), suggesting that ovarian endocrine function is intact at this age. We therefore sought to determine whether low fertility could be the result of embryonic or fetal loss in Aire-KO mice. Aire-KO or WT female mice were mated with intact WT male mice and sacrificed on GD5.5, 7.5, or 10.5, at which time implantation sites were counted. While there was no difference between WT and KO dams in the rate of pregnancy at GD5.5 (P = 0.2), there was a significant reduction in proportion of female mice with viable implantation sites at GD7.5 and 10.5 (Figure 1A; P = 0.03 and 0.01, respectively). Among those with viable implantation sites, the numbers of sites were not different between controls and KO mice on any observed day of gestation (Figure 1B) except for GD10.5, whereby the average number of implantation sites per KO animal was reduced (P = 0.001). Additionally, the timing of pregnancy loss corresponded with a lack of weight gain after GD5.5 (Figure 1C); this was not likely to be caused by impaired food intake by Aire KO females, as weight gain between 4 and 20 weeks in these females was identical to control mice (data not shown).
Figure 1.
Timing of embryonic loss, pregnancy-associated weight gain, and progesterone production in Aire-KO mice. (A) Mean percentage of 6-week-old WT and Aire-KO female mice with implantation sites at GD5.5, 7.5, and 10.5. Data were analyzed by Chi-square analysis. (B) Quantification of implantation sites in 6-week-old WT and Aire-KO mice at GD5.5, 7.5, and 10.5. Each data point represents the number of implantation sites in individual dams; the grey data point represents the mean number of sites per dam. Data were analyzed by Student's t-test (GD5.5 and 7.5) or Mann–Whitney (GD10.5) within gestation day. (C) Weight deviation from baseline in mated 6-week-old WT and Aire-KO females between GD0.5 and GD10.5. Data were analyzed by two-way repeated measures ANOVA on weight; *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D) Mean values for serum progesterone concentrations. Data were analyzed by one-way ANOVA on ranks (GD5.5) or one-way ANOVA (GD7.5, 10.5). A and D, Numbers inside bars represent n for each condition.
To determine whether impaired progesterone production could contribute to embryonic loss, we measured serum progesterone concentrations in mated WT controls and Aire-KO females. At GD5.5, serum progesterone concentrations in KO mice were not significantly different from WT mice, regardless of pregnancy status (P = 0.17; range, 11.37–25.8 ng/mL vs. 17.1–29.5 ng/mL progesterone in KO and WT dams, respectively; Figure 1D), suggesting that progesterone production in KO mice is not impaired and that luteal insufficiency is not the cause of embryonic loss at this stage. By GD7.5 and 10.5, serum progesterone was significantly reduced in KO females found to be nonpregnant (P < 0.05), which is consistent with the timing of loss in serum progesterone in pseudopregnant mice [45, 46].
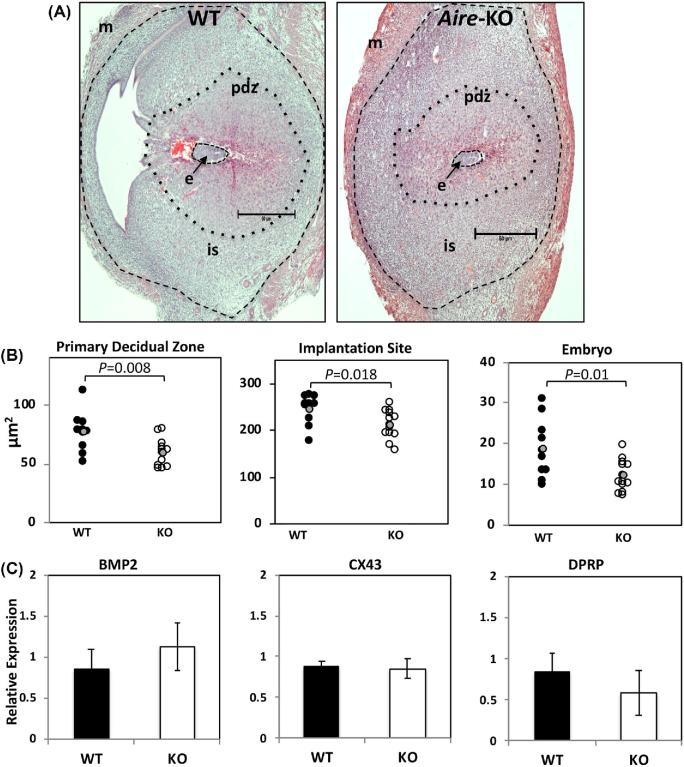
To assess the quality of implantation sites prior to embryonic loss, we next performed histological examination of GD5.5 implantation sites from WT or Aire-KO females mated to WT males. While no overt inflammation of the implantation sites was noted, morphometric analysis revealed that implantation sites, primary decidual zones, and embryo sizes were smaller in KO females than in WT females (Figure 2A and B). This relationship held true whether each site was considered as a discrete experimental unit (Figure 2B), or when sites were averaged within dams and then considered a separate biological replicate (decidual zone, WT: 76.04 ± 6.1, decidual zone, KO: 58.09 ± 4.02 μm; P = 0.020) (implantation site, WT: 247.54 ± 10.12, implantation site, KO: 206.78 ± 9.83 μm; P = 0.017) (embryo, WT: 17.41 ± 1.69, embryo, KO: 11.91 ± 1.04 μm; P = 0.036).
Figure 2.
Histological evaluation of early implantation sites and expression of decidualization markers. (A) Representative images of GD5.5 implantation sites from pregnant WT and Aire-KO female mice, oriented with the mesometrial side to the left. m, myometrium; pdz, primary decidual zone; is, implantation site; e, embryo. Images were taken at using a 4× objective; bar = 50 μm. Dotted lines delimit area measurements used in B. (B) Area measurements of implantation sites, primary decidual zones, and embryos taken from the serial sections. Each data point represents individual deciduae, implantation sites or embryos; 1–3 sites per dam were measured for a total of 10 sites from 5 WT dams, and 13 sites from 7 KO dams. The grey data point represents the mean, and data were analyzed by t-test. (C) mRNA expression for decidualization markers from GD5.5 deciduae. Relative quantity (RQ) indicates fold change over controls. BMP2, Bone morphogenetic protein 2; CX43, Connexin 43; Dprp, Decidual prolactin related protein. Data were analyzed by Student's t-test.
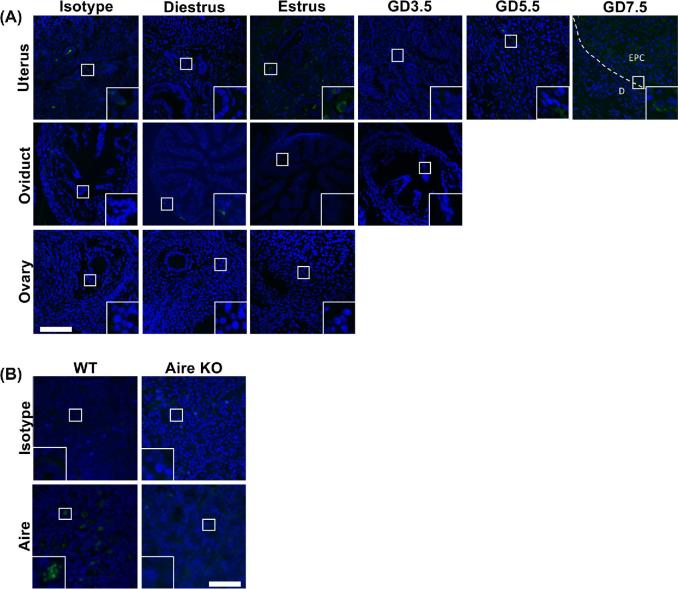
Since decidual areas were found to be smaller in Aire-KO animals, and because a defect in decidualization could have a profound negative impact on embryonic survival, we asked whether a defect in the decidual reaction could be responsible for the smaller implantation sites and reduced embryonic growth. Gene expression of the decidual markers BMP2, Cx43, or DPRP was not altered in Aire-KO females as determined by RT-qPCR (Figure 2C). To examine the possibility that AIRE is expressed in the uterus and directly impacts uterine function during implantation, the temporal expression patterns of Aire mRNA and protein in the female reproductive tract were examined, using WT and Aire KO thymus as positive and negative controls, respectively. Transcripts for Aire were low in all tissues examined compared to the thymus, with the possible exception of the uterus at GD3.5 (Supplementary Figure 1). Immunofluorescence revealed no or only background levels of staining in the ovary, oviduct, or uterus (Figure 3A). This included decidua at GD5.5 or 7.5, although some punctate staining was observed in the ectoplacental cone of GD7.5 in samples incubated with either anti-AIRE antibody or rabbit IgG control antibody. Antibody quality and specificity was verified using thymus of Aire WT mice, in which antibody-reactive nuclear speckles could be observed in medullary epithelial cells; thymuses of Aire KO mice failed to react with the antibody (Figure 3B).
Figure 3.
Immunofluorescence analysis of Aire in the female reproductive tract. WT Balb/c females were euthanized at diestrus (n = 3), estrus (n = 3), GD3.5 (n = 2), GD5.5 (n = 2), and GD7.5 (n = 2). Ovary, oviduct, and uterus were obtained and stained with anti-Aire antibody. (A) Aire immunoreactivity above isotype background levels was not detected in any of the reproductive organs. (B) Aire immunoreactivity in the thymus of WT mice, and absence in Aire KO mice. Images were captured at 200× (A) or 400× (B); insets show digitally magnified regions of interest. Scale bars, 50 μm. EPC, ectoplacental cone; D, decidua.
Because these results differed from those previously reported [47], a possible role for AIRE was investigated using an in vivo artificial decidualization assay. Decidualization was strongly induced in Aire WT, heterozygous, and KO mice (P < 0.001), with no influence of genotype for Aire on responsiveness of uteri to a decidualization stimulus (Figure 4A and B; P = 0.9). Additionally, although biological variability was noted, the levels of induction of BMP2, Cx43, and DPRP were not different (P > 0.05; data not shown). Similar to the lack of expression in GD5.5–7.5 deciduae, Aire mRNA in the artificially decidualized uterus was virtually undetectable (Figure 4C).
Figure 4.
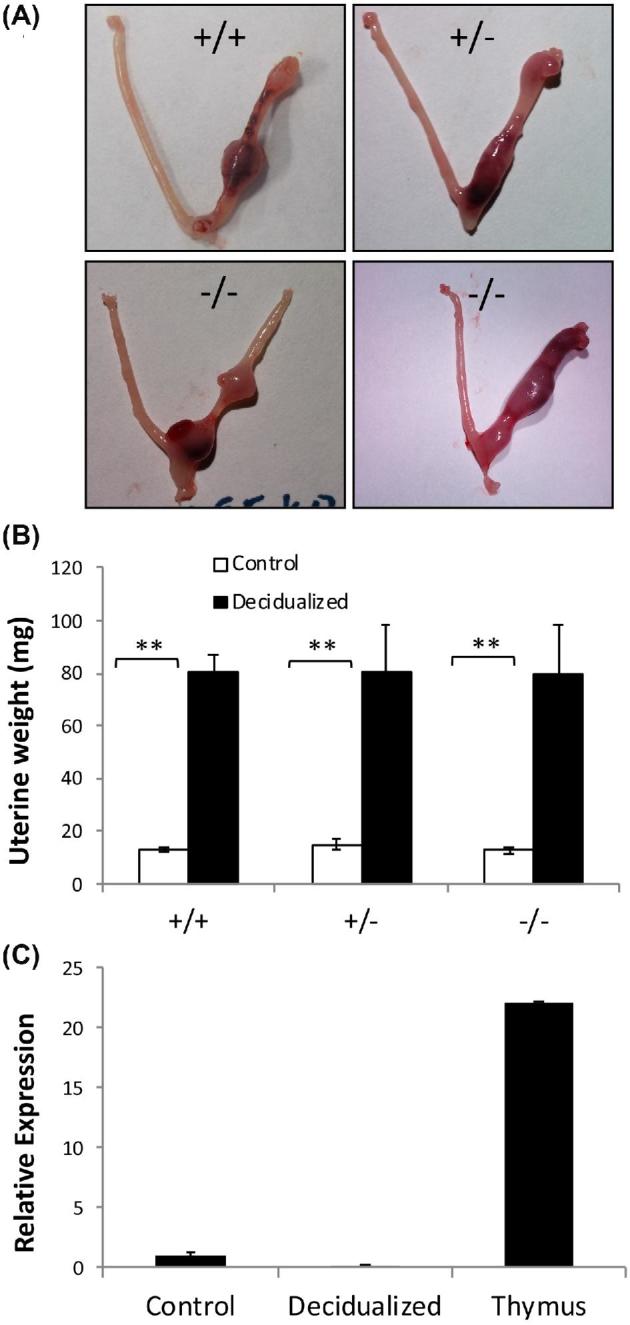
Lack of decidualization defect in Aire-KO mice. (A, B) Ovariectomized mice (+/+, WT, n = 5; +/–, heterozygous, n = 3; –/–, KO, n = 3) were subjected to hormonal stimulation followed by surgical induction of decidualization. In all three groups, there was a highly significant increase in uterine horn weight in response to the decidualization stimulus, with no genotype × treatment interaction. **, P < 0.001, two-way ANOVA on log10-transformed data. (C) mRNA expression for Aire in undecidualized uterus, decidualized uterus, and thymus. Data are expressed as fold difference in quantity relative to the undecidualized uterus samples.
Lastly, we examined the effect of Aire deficiency on the reproductive phenotype of mice on the C57Bl/6 genetic background, reasoning that if Aire plays a critical role in decidualization, this role would be conserved across different strains of the same species. In contrast to the impaired fertility of Aire-deficient mice on the Balb/c background, two different strains of Aire KO mice (n = 3 of each strain) on the C57Bl/6 genetic background gave birth to multiple consecutive litters (2–5) of sizes comparable to controls WT littermate controls (Aire/J: 6.2 ± 0.76/litter, n = 10 litters; Aire/H: 5.9 ± 0.53/litter, n = 13 litters).
Embryos from Aire-deficient dams have delayed embryonic development in vivo and compromised trophoblast growth in vitro
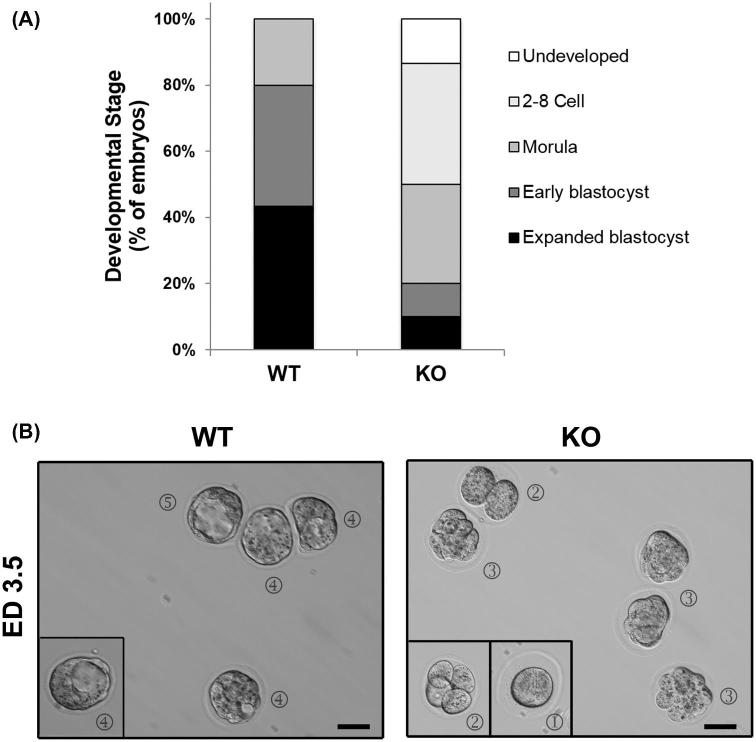
We next examined the kinetics of preimplantation development. Six-week-old WT or Aire-KO females (n = 4 per genotype) were mated to WT males, and the heterozygous embryos were flushed from the uteri of the females on GD3.5. There was no difference in the numbers of embryos collected (WT: 7.5 ± 0.5; KO: 7.5 ± 0.29 embryos per dam; P = 1.0). Differences were evident, however, in the developmental stages of the embryos between strains. In WT dams, embryos of the blastocyst stage were present within all four dams, with the proportion of embryos within dams having reached this stage ranging from 57% to 100%. In KO dams, however, blastocysts were found in only two of the dams, and within these, only one of eight (12.5%) and five of eight (62.5%) embryos had reached the blastocyst stage. In total, 80% and 20% of all of embryos recovered from WT and KO females, respectively, had reached the blastocyst stage (P < 0.05); in the WT, the remaining 20% were morula, while in KOs the remaining were either morula (30%), two to eight cell embryos (37%), or remained undeveloped (13%) (Figure 5A and B).
Figure 5.
Embryonic stage of GD3.5 embryos from WT and Aire-KO mice. (A) Assessment of developmental stage at GD3.5 of embryos collected from WT and KO dams mated to WT males. (B) Representative images of embryos from mated WT and Aire-KO female mice collected at ED3.5. Circled numbers represent stages of development: (1), undeveloped; (2) two–eight cells (2-cell and 4-cell shown); (3) morula; (4) early blastocyst; (5) expanded blastocyst. Images were captured at 200× magnification; scale bar, 50μm.
To assess the developmental competence of the GD3.5 embryos recovered from KO dams, embryos were cultured, and trophoblast outgrowth measured at 48 and 96 h. By 48 h postcollection, 87% of all embryos from WT females had evidence of outgrowth, compared to only 17% of embryos from Aire-KO females (P < 0.05). By 96 h postcollection, all embryos from WT females had trophoblast cell outgrowth, compared to 63% from Aire-KO females (P < 0.001). At both time points, the mean outgrowth area was significantly larger for blastocysts from WT females compared to those from Aire-KO females (P < 0.0001) (Figure 6A and B). Additional differences were noted when data were broken down into embryonic development according to dam. Embryos from all four WT dams exhibited outgrowth at 48 h, whereas in three of the four KO dams, none of the embryos had grown (Figure 6C). From the fourth dam (dam #2 in Figure 6C), five of seven embryos had grown to sizes comparable to those from the WT dams. By 96 h, all embryos from the four WT dams had greatly expanded, whereas all embryos from only KO dam #2 grew comparably (Figure 6C). One KO dam (#3) had no growing embryos; the remaining two had embryos ranging from undeveloped, underdeveloped, and normally developed.
Figure 6.
In vitro growth of GD3.5 embryos from WT and Aire-KO mice. GD3.5 embryos were cultured on collagen-coated plates for 48 and 96 h, and outgrowth area quantified by phase-contrast microscopy. All images were taken using a 10× objective. (A) Average outgrowth area of embryos recovered from four WT and four KO dams. ***, P < 0.0001, Student's t-test. (B) Representative images of embryos recovered from WT and KO dams at 48 and 96 h of culture. (C) Area measurements of embryonic trophoblast outgrowth from WT and Aire-KO dams at 48 h postcollection. (D) Similar to (C), except at 96 h postcollection. Numbers on x-axis in C and D represent individual dams, while each data point represents individual embryos. Images were captured at 200× magnification; scale bar, 50μm.
In vitro culture of GD0.5 embryos from Aire-deficient dams is compromised
Because embryonic delay could already be observed at GD3.5, prior to implantation, we next asked whether early embryonic development was also compromised. GD0.5 embryos from 6-week-old WT controls and Aire-KO females mated with WT males were flushed from oviducts and cultured to the blastocyst stage. The total number of ovulations was comparable between WT (8.36 ± 1.05) and Aire-KO (7.4 ± 1.23) dams (P = 0.5), and all WT (11/11) and all but one KO (9/10) ovulated. However, the proportion of high-quality eggs from Aire-KO females was reduced (Figure 7A; P < 0.0001). Embryos from WT females were able to develop to the blastocyst stage with an overall success rate of 60%, whereas only 27% of embryos from Aire-KO dams reached the blastocyst stage (Figure 7B; P < 0.001). Growth arrest of these embryos became significant at the morula stage (P < 0.05).
Figure 7.
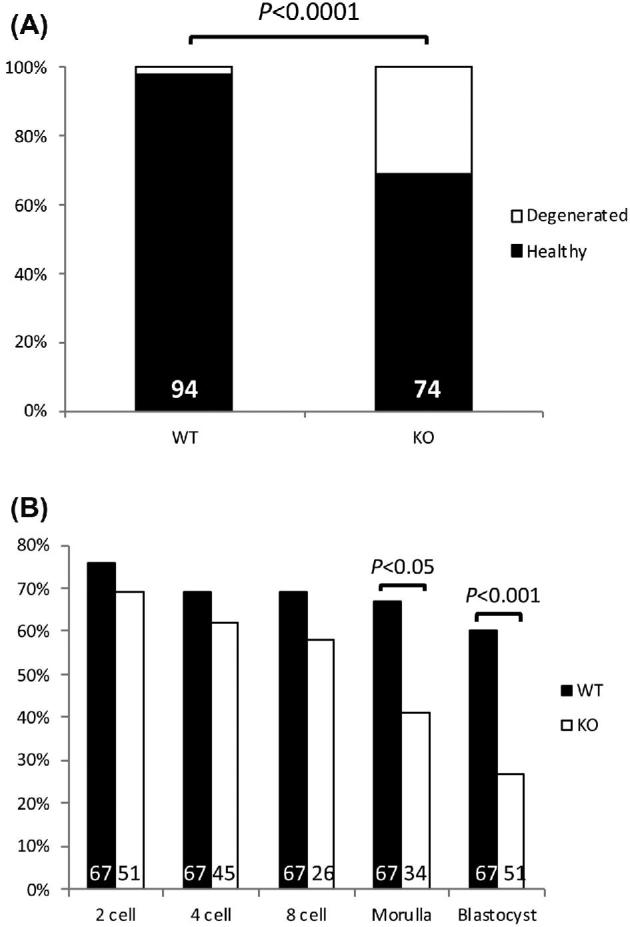
In vitro embryonic development culture. (A) Mean proportions of healthy and degenerated GD0.5 oocytes from mated 6-week-old WT (n = 11) and Aire-KO (n = 9) female mice. Numbers inside bars represent total numbers examined. (B) Mean percentage of flushed oocytes collected at ED0.5 from mated 6-week-old WT (n = 9 dams) and Aire-KO (n = 4–7 dams, depending on stage) female mice to reach 2-cell, 4-cell, 8-cell, morula and blastocyst stages following culture for up to 96 h. Numbers inside each bar represent total number of oocytes or embryos analyzed at each stage. Data were analyzed by Chi-square analysis.
Discussion
The discovery of AIRE and its role in central immune tolerance has fundamentally changed our understanding of how the immune system discriminates between self and nonself. Expression of tissue-restricted proteins in mTEC under the control of AIRE results in the exposure of self-peptide antigens to developing autoreactive thymocytes, which, as a result, are either deleted or directed toward a regulatory T cell phenotype, and thus prevented from causing autoimmune disease. A number of genes otherwise restricted to the female reproductive tract, the embryo, and the placenta are expressed in mTEC and regulated by AIRE, suggesting that AIRE is required for immune tolerance to these organs, and therefore fertility [14, 18, 36, 38]. Consistent with this model, mice with targeted deletion of AIRE exhibit autoimmune targeting of the ovary, and women with APS-1 suffer from high rates of infertility [33, 35].
Several mechanisms could explain the loss of fertility in female Aire-KO mice, including direct roles of Aire in the thymus, ovary/early embryo, or oviduct/uterus or a combination of these. AIRE’s role in the thymus, in which autoimmune disease is prevented, is supported by several lines of evidence, including the gradual increase in proportion of females exhibiting complete and abrupt loss of ovarian follicles, together with the infiltration of T cells into the ovary. However, because ∼50% of peripubertal Aire-KO mice were infertile yet had normal ovulation rates and ovarian reserves [35], we hypothesized that additional postovarian lesions in fertility occur in these mice, and found that indeed, embryonic loss occurs in about half of young Aire-KO females around the time of implantation. Although this timing was suggestive of insufficient progesterone and inappropriate immune responses can impair progesterone production [48, 49], we found that serum progesterone in KO animals at GD5.5 were all within the range of WT females, suggesting that impaired luteal progesterone production was not a causative factor of embryonic loss.
We also asked whether it is likely that AIRE plays a direct role in uterine function, a notion supported by the observation that Aire mRNA can be detected in murine decidual tissue at GD10.5 [50]. Further, Soumya et al. reported that AIRE protein is found in the primary decidual zone of GD5–8 implantation sites, and that local delivery of Aire siRNA impaired implantation [47]. Our present reports, however, are not consistent with these findings. In contrast to Soumya et al., we found only low levels of mRNA in the decidua and failed to observe significant protein expression in this tissue; indeed, both mRNA and protein were low or undetectable throughout the reproductive tract. Lack of availability of the same antibody clone used in previous studies prevented us from making a direct comparison of results; however, our study used stringent positive (thymus) and negative (irrelevant IgG, Aire KO thymus) controls, whereas the Soumya et al. study lacked these controls. Additional evidence countering a direct role of AIRE in decidualization is that global loss of the Aire gene had no significant impact on expression of markers of decidualization in GD5.5 implantation sites. Artificial decidualization stimuli were equally able to cause dramatic increases in uterine wet weight and expression of decidualization genes in KO and WT animals. Finally, Aire deficiency negatively impacts female fertility in mice only on the Balb/c genetic background, whereas fertility of Aire KO mice on the C57Bl/6 background is unimpaired. These differences are most likely explained by differences in MHC haplotype between the two strains (Balb/c: H-2d; C57Bl/6, H-2b), since this feature determines ability to present particular autoantigens to T cells [39]. The lack of conservation of a role for AIRE in fertility across strains of mice argues against a role for this protein in uterine function, since a requisite direct role in fertility would likely be conserved. Taken together, the lack of expression of AIRE in the uterus, the lack of impaired decidualization in Aire-KO mice, and the lack of conserved effect of AIRE deficiency on fertility between background strains of mice, we conclude that uterine AIRE does not play a direct role in decidualization.
On the other hand, our results revealed pronounced effects of maternal Aire deficiency on embryonic growth. Flushing of ova at GD0.5 revealed that although the ovulation rate was similar between WT and KO animals, Aire-KO animals had a higher rate of oocyte degeneration, suggesting that in at least some mice, oocyte quality could have already been impacted by an aberrant immune response prior to ovulation. This idea is supported by multiple lines of evidence. First, ovarian antigens are well-known to be capable of driving an autoimmune response [51–53]. Second, we previously identified abnormal T cell accumulation within the ovaries of Aire-deficient mice as early as 3 weeks of age, and sera from Aire-deficient mice contains autoantibodies to 60–75 kDa ovarian proteins that localize to granulosa cells, luteal cells, and the oocyte [14, 35, 38]. Finally, WT ovaries transplanted into Aire-KO females experienced rapid, near complete follicular depletion in recipients whose ovaries were likewise devoid of oocytes, suggesting a rapid, ovary-specific recall immune response [35]. An incipient anti-ovarian immune response prior to fulminant autoimmune-associated follicular depletion could result in diminished quality of ovulated ova.
Aside from clearly degenerating ova, the developmental potential of zygotes recovered at GD0.5 was also reduced. Indeed, significant arrest occurred at the 8-cell stage with consequent reduction of embryos developing to the morula and blastocyst stages. Consistent with this was the finding that embryos obtained from GD3.5 Aire-KO dams were skewed towards earlier stages of development as compared to those from WT dams. These results might reflect an anti-ovarian autoimmune response as described above, or could indicate a direct role of AIRE in early embryogenesis. Either of these could cause reduced developmental competence or delayed embryonic growth; our finding that implantation rates at GD5.5 were similar in WT and KO dams suggests that, in vivo, embryos did not arrest but rather were developmentally delayed. In some dams, embryos were unable to complete implantation, as evidenced by their smaller size and attrition at GD7.5. Consistent with this, when GD3.5 embryos recovered from Aire-KO dams were allowed to grow in vitro, they did not exhibit as extensive outgrowth of trophoblast as did their WT counterparts. It is possible that this slowed growth resulted in asynchrony between the embryo and maternal environment, which could in turn result in failed completion of implantation. Indeed, implantation and successful pregnancy require precise coordination between embryonic development and uterine receptivity [54, 55]. Embryonic developmental disruption can derail this alignment and result in infertility [56–58].
Some studies have suggested a direct role of AIRE in embryonic growth. As Aire-KO mice are born in Mendelian ratios (our colony: 28% KO, 21% WT, 51% heterozygous), the gene is not mandatory for embryonic development. However, AIRE expression is found in early embryos and stem cells [59, 60], and a role for AIRE in the oocyte and in stem cell self-renewal has been suggested [61]. Consistent with this, we have also detected Aire mRNA in M2-stage oocytes (unpublished data). Intriguingly, a recent study identified AIRE as a mitotic spindle-associated protein with a role in centrosome number and spindle assembly [62]. Further, embryos produced after maternal zygotic deletion of Aire had delayed development at GD3.5, with reduced formation of blastocyst-stage embryos. This phenotype is similar to our observation in global Aire-KO dams, raising the possibility of a critical role for AIRE in early embryo development.
Taken together, it is likely that the observed infertility in 50% of Aire-deficient mice is a result of reduced oocyte quality due to incipient anti-ovarian autoimmune disease, a direct role for AIRE in embryonic growth, and an asynchrony between embryonic growth and the uterine implantation window, altogether resulting in embryonic demise shortly after implantation. Fulminant autoimmune disease later becomes responsible for wholesale follicular depletion. This demonstration of the impact of the absence of AIRE on oocyte and embryonic health extends our understanding of premature ovarian failure, and infertility experienced by APS-1 patients, and further, has important implications on the fundamental mechanisms of embryonic growth and implantation.
Supplementary Material
Acknowledgments
The authors thank Keith Latham for valuable insights and discussion regarding the data from this manuscript, as well as Susmita Jasti, Sarika Kshirsagar, and Sean Nguyen for helpful technical contributions and data insight.
Notes
Edited by Dr. Myriam Hemberger
Footnotes
Grant Support: This work was supported by NIH grants HD062879 and HD045611 (MGP), HD042280 (ATF), and HD082484 (LKC and LKM). Additional support was provided by core facilities of the NIH Kansas IDeA Network of Biomedical Research Excellence (GM103418) and the NIH Center of Biomedical Research Excellence GM104936. Funds for experiments were also provided by Michigan State University and AgBioResearch.
Supplementary data
Supplementary Figure 1. Aire mRNA in thymus and female reproductive organs. Real-type quantitative PCR was conducted to measure the expression of Aire transcripts from Balb/c female reproductive organs at diestrus (n=3), estrus (n=3), GD3.5 (n=2), and GD5.5 (n=2). Ovary at GD3.5 and 5.5, oviduct at GD5.5 were not examined; thymus was examined only at diestrus. GADPH was used as a housekeeping gene to calculate ΔCT values, and the ΔCT value of the diestrus thymus was used to calculate fold change (FC) expression of Aire in each sample. Compared to the Aire transcript levels detected in the thymus at diestrus, amplification was low or undetectable from all organs examined. FC, fold change.
References
- 1. Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Stem Cells Dev 2009; 138(15):2878–2890. [DOI] [PubMed] [Google Scholar]
- 2. Espey LL. Ovulation as an inflammatory reaction—a hypothesis. Biol Reprod 1980; 22:73–106. [DOI] [PubMed] [Google Scholar]
- 3. Pate JL, Toyokawa K, Walusimbi S, Brzezicka E. The interface of the immune and reproductive systems in the ovary: lessons learned from the corpus luteum of domestic animal models. Am J Reprod Immunol 2010; 64:275–286. [DOI] [PubMed] [Google Scholar]
- 4. Zenclussen AC, Hämmerling GJ. Cellular regulation of the uterine microenvironment that enables embryo implantation. Front Immunol 2015; 6:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med 2001; 7:899–905. [DOI] [PubMed] [Google Scholar]
- 6. Su MA, Anderson MS. Monogenic autoimmune diseases: insights into self-tolerance. Pediatr Res 2009; 65:20R–25R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aaltonen J, Bjorses P, Perheentupa J, Horelli-Kuitunen N, Palotie A, Peltonen L, Lee YS, Francis F, Henning S, Thiel C, Leharach H, Yaspo M-L. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 1997; 17:399–403. [DOI] [PubMed] [Google Scholar]
- 8. Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S et al.. Positional cloning of the APECED gene. Nat Genet 1997; 17:393–398. [DOI] [PubMed] [Google Scholar]
- 9. Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med 1990; 322:1829–1836. [DOI] [PubMed] [Google Scholar]
- 10. Oftedal Bergithe E, Hellesen A, Erichsen Martina M, Bratland E, Vardi A, Perheentupa J, Kemp EH, Fiskerstrand T, Viken Marte K, Weetman Anthony P, Fleishman Sarel J, Banka S et al.. Dominant mutations in the Autoimmune Regulator AIRE are associated with common organ-specific autoimmune diseases. Immunity 2015; 42:1185–1196. [DOI] [PubMed] [Google Scholar]
- 11. Proekt I, Miller CN, Jeanne M, Fasano KJ, Moon JJ, Lowell CA, Gould DB, Anderson MS, DeFranco AL. LYN- and AIRE-mediated tolerance checkpoint defects synergize to trigger organ-specific autoimmunity. J Clin Invest 2016; 126:3758–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soderbergh A, Myhre AG, Ekwall O, Gebre-Medhin G, Hedstrand H, Landgren E, Miettinen A, Eskelin P, Halonen M, Tuomi T, Gustafsson J, Husebye ES et al.. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab 2004; 89:557–562. [DOI] [PubMed] [Google Scholar]
- 13. Heino M, Peterson P, Kudoh J, Nagamine K, Lagerstedt A, Ovod V, Ranki A, Rantala I, Nieminen M, Tuukkanen J, Scott HS, Antonarakis SE et al.. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Com 1999; 257:821–825. [DOI] [PubMed] [Google Scholar]
- 14. Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002; 298:1395–1401. [DOI] [PubMed] [Google Scholar]
- 15. Org T, Chignola F, Hetenyi C, Gaetani M, Rebane A, Liiv I, Maran U, Mollica L, Bottomley MJ, Musco G, Peterson P. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep 2008; 9:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koh AS, Kuo AJ, Park SY, Cheung P, Abramson J, Bua D, Carney D, Shoelson SE, Gozani O, Kingston RE, Benoist C, Mathis D. Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc Natl Acad Sci USA 2008; 105:15878–15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abramson J, Giraud M, Benoist C, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell 2010; 140:123–135. [DOI] [PubMed] [Google Scholar]
- 18. Giraud M, Yoshida H, Abramson J, Rahl PB, Young RA, Mathis D, Benoist C. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci USA 2012; 109:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med 2006; 203:2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, Savage PA. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 2013; 339:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 2007; 8:351–358. [DOI] [PubMed] [Google Scholar]
- 22. Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 2015; 348:589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol 2003; 4:350–354. [DOI] [PubMed] [Google Scholar]
- 24. Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 2007; 22:1506–1512. [DOI] [PubMed] [Google Scholar]
- 25. Chandra A, Copen CE. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Nat Health Stat Rep 2013; 67:1–19. [PubMed] [Google Scholar]
- 26. Chen S, Sawicka J, Betterle C, Powell M, Prentice L, Volpato M, Smith BR, Furmaniak J. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison's disease, and premature ovarian failure. J Clin Endocrinol Metab 1996; 81:1871–1876. [DOI] [PubMed] [Google Scholar]
- 27. Abalovich M, Mitelberg L, Allami C, Gutierrez S, Alcaraz G, Otero P, Levalle O. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol Endocrinol 2007; 23:279–283. [DOI] [PubMed] [Google Scholar]
- 28. Falorni A, Laureti S, Candeloro P, Perrino S, Coronella C, Bizzarro A, Bellastella A, Santeusanio F, De Bellis A. Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. Fert Steril 2002; 78:270–279. [DOI] [PubMed] [Google Scholar]
- 29. Winqvist O, Gebre-Medhin G, Gustafsson J, Ritzen EM, Lundkvist O, Karlsson FA, Kampe O. Identification of the main gonadal autoantigens in patients with adrenal insufficiency and associated ovarian failure. J Clin Endocrinol Metab 1995; 80:1717–1723. [DOI] [PubMed] [Google Scholar]
- 30. Mathur S, Baker ER, Williamson HO, Derrick FC, Teague KJ, Fudenberg HH. Clinical significance of sperm antibodies in infertility. Fertil Steril 1981; 36:486–495. [PubMed] [Google Scholar]
- 31. Oshiro BT, Silver RM, Scott JR, Yu H, Ware Branch D. Antiphospholipid antibodies and fetal death. Obstet Gynecol 1996; 87:489–493. [DOI] [PubMed] [Google Scholar]
- 32. Xu L, Chang V, Murphy A, Rock JA, Damewood M, Schlaff W, Zacur HA. Antinuclear antibodies in sera of patients with recurrent pregnancy wastage. Am J Obstet Gynecol 1990; 163:1493–1497. [DOI] [PubMed] [Google Scholar]
- 33. Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab 2006; 91:2843–2850. [DOI] [PubMed] [Google Scholar]
- 34. Perheentupa J. APS-1/APECED: the clinical disease and therapy. Endocrinol Metab Clin North Am 2002; 31:295–320. [DOI] [PubMed] [Google Scholar]
- 35. Jasti S, Warren BD, McGinnis LK, Kinsey WH, Petroff BK, Petroff MG. The autoimmune regulator prevents premature reproductive senescence in female mice. Biol Reprod 2012; 86:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Immunol 2005; 202:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med 2004; 199:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Warren BD, Kinsey WK, McGinnis LK, Christenson LK, Jasti S, Stevens AM, Petroff BK, Petroff MG. Ovarian autoimmune disease: clinical concepts and animal models. Cell Mol Immunol 2014; 11:510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med 2005; 202:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet 2002; 11:397–409. [DOI] [PubMed] [Google Scholar]
- 41. Deb K, Reese J, Paria BC. Methodologies to study implantation in mice. Methods Mol Med 2006; 121:9–34. [DOI] [PubMed] [Google Scholar]
- 42. Edwards AK, Janzen-Pang J, Peng JR, Tayade C. Microscopic anatomy of the pregnant mouse uterus throughout gestation. In: Croy BA, Yamada AT, DeMayo FJ, Adamson SL (eds.), Guide to Investigation of Mouse Pregnancy, ed. Amsterdam: Academic Press; 2014:43–90. [Google Scholar]
- 43. Su RW, Strug MR, Joshi NR, Jeong JW, Miele L, Lessey BA, Young SL, Fazleabas AT. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab 2015; 100:E433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagy A, Gertsenstein M. Manipulating the Mouse Embryo: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 45. Shiotani M, Noda Y, Mori T. Embryo-dependent induction of uterine receptivity assessed by an in vitro model of implantation in mice. Biol Reprod 1993; 49:794–801. [DOI] [PubMed] [Google Scholar]
- 46. Greenwald GS, Rothchild I. Formation and maintenance of corpora lutea in laboratory animals. J Anim Sci 1968; 27 (Suppl 1):129–138. [PubMed] [Google Scholar]
- 47. Soumya V, Padmanabhan RA, Titus S, Laloraya M. Murine uterine decidualization is a novel function of autoimmune regulator beyond immune tolerance. Am J Reprod Immunol 2016; 76:224–234. [DOI] [PubMed] [Google Scholar]
- 48. Aisemberg J, Vercelli CA, Bariani MV, Billi SC, Wolfson ML, Franchi AM. Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PLoS One 2013; 8:e56161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Erlebacher A, Zhang D, Parlow AF, Glimcher LH. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J Clin Invest 114:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang J, Chen Z, Fritz JH, Rochman Y, Leonard WJ, Gommerman JL, Plumb AW, Abraham N, Croy BA. Unusual timing of CD127 expression by mouse uterine natural killer cells. J Leukoc Biol 2012; 91:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tong Z-B, Nelson LM. A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure. Endocrinology 1999; 140:3720–3726. [DOI] [PubMed] [Google Scholar]
- 52. Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev 1997; 18:107–134. [DOI] [PubMed] [Google Scholar]
- 53. Alard P, Thompson C, Agersborg SS, Thatte J, Setiady Y, Samy E, Tung KSK. Endogenous oocyte antigens are equired for rapid induction and progression of autoimmune ovarian disease following day-3 thymectomy. J Immunol 2001; 166:4363–4369. [DOI] [PubMed] [Google Scholar]
- 54. Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science 2002; 296:2185–2188. [DOI] [PubMed] [Google Scholar]
- 55. Ma W-g, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA 2003; 100:2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dickman Z, Noyes RW. The fate of ova transferred into the uterus of the rat. J Reprod Fert 1960; 1:197–212. [Google Scholar]
- 57. Palomino WA, Fuentes A, González RR, Gabler F, Boric MA, Vega M, Devoto L. Differential expression of endometrial integrins and progesterone receptor during the window of implantation in normo-ovulatory women treated with clomiphene citrate. Fert Steril 2005; 83:587–593. [DOI] [PubMed] [Google Scholar]
- 58. Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem 2001; 276:44137–44145. [DOI] [PubMed] [Google Scholar]
- 59. Nishikawa Y, Hirota F, Yano M, Kitajima H, Miyazaki J-i, Kawamoto H, Mouri Y, Matsumoto M. Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J Exp Med 2010; 207:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gu B, Zhang J, Chen Q, Tao B, Wang W, Zhou Y, Chen L, Liu Y, Zhang M. Aire regulates the expression of differentiation-associated genes and self-renewal of embryonic stem cells. Biochem Biophys Res Com 2010; 394:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bin G, Jiarong Z, Shihao W, Xiuli S, Cheng X, Liangbiao C, Ming Z. Aire promotes the self-renewal of embryonic stem cells through Lin28. Stem Cells Dev 2012; 21:2878–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gu B, Lambert J-P, Cockburn K, Gingras A-C, Rossant J. AIRE is a critical spindle-associated protein in embryonic stem cells. eLife 2017; 6:e28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.