Abstract
Cardiac involvement in systemic amyloidosis (AL) occurs in ~50% of all AL patients. However once symptomatic heart failure develops, therapeutic options are limited thereby conferring a poor overall prognosis. The median survival is <6 months when AL patients are untreated for the underlying plasma cell dyscrasia. We thus sought to identify risk factors of increased mortality in treatment-naïve, AL cardiac amyloidosis with heart failure. Patients with biopsy-proven AL cardiac amyloid, who presented with heart failure and did not received prior AL treatment, were enrolled between 2004–2014, at the initial visit to the Amyloidosis Center at Boston University Medical Center. Routine laboratory tests, physical examination and echocardiography data were collected. There were 165 predominantly white (76.4%), and male (61%) patients, with a mean age of 61.6 ± 9.5 years. Median survival was 10.9 months (95% CI 6.2–14.7). By multivariate analysis increased relative wall thickness (RWT) [HR 6.70; 95% CI 2.45–18.30), older age (HR 1.04; 95% CI 1.01–1.06), higher New York Heart Association (NYHA) functional class (HR 1.50; 95% CI 1.02–2.2), log brain natriuretic peptide (BNP) levels (HR 1.45; 95% CI 1.15–1.81) and C-reactive protein (CRP) levels (HR 1.02; 95% CI 1.00–1.04) were significant predictors for increased mortality. In conclusion, in treatment-naïve, AL cardiac amyloidosis patients with heart failure symptoms who lack these high-risk features may have a better outcome. These findings might allow for better risk stratification although outcomes are still poor.
Subject terms: Heart failure, Cardiology
Introduction
Systemic amyloidosis (AL) is a uncommon disease characterized by the clonal proliferation of monoclonal immunoglobulin light chains resulting in extracellular fibril formation and amyloid deposits in various organs1. Disruption of the structure and function of normal tissue ensues and symptoms of heart failure inevitably develop. Although targeted treatment in AL amyloidosis aims to induce hematologic remission and prolong survival, only 25–30% of patients with newly diagnosed AL amyloidosis are eligible for high dose melphalan with autologous stem cell transplantation (HDM/SCT), since symptomatic cardiac involvement, more often than not, precludes this high-risk therapeutic option. While amyloid deposition in the heart reportedly occurs in up to half of all AL amyloidosis patients2, once symptoms of heart failure occurs, the prognosis is dismal with a median survival of <6 months if patients remain untreated2,3. Thus, AL cardiac amyloidosis with heart failure is a potentially fatal disease.
Biomarkers of myocardial stress and injury, such as circulating levels of N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) and Troponin T (TnT) are often used to identify patients for optimal therapy selection and risk determination during treatment for systemic amyloidosis4,5; these biomarkers are part of the original Mayo staging for AL amyloidosis for risk stratification4. Subsequently, dFLC (difference in involved versus uninvolved free light chains) was introduced in the revised Mayo staging system6. More recently Lilliness et al., revised and validated the original Mayo staging to include BNP and troponin I (TnI) cutoffs in place of NT-proBNP and TnT offering centers without access to these latter markers, the ability to stage patients with systemic amyloidosis7. Once patients develop AL cardiac amyloidosis, however, the clinical utility of these biomarkers is more uncertain. Nordlinger et al., showed that BNP levels in those with cardiac amyloidosis did not discriminate between patients who had heart failure versus those who were asymptomatic. This suggests that BNP, a measure of wall stress, is unable to differentiate elevated left ventricular (LV) filling pressures from direct cardiac toxicity due to extracellular amyloid deposits8. Importantly, AL cardiac amyloidosis patients with and without heart failure have markedly different survival times2,3. Therefore, the role of these cardiac biomarkers, in those with symptoms of heart failure and AL cardiac amyloidosis, are limited. Thus, there is a need to identify additional clinical markers for prognosis and outcomes in this subgroup of patients with AL cardiac amyloidosis. We sought to identify predictors of mortality in a cohort of treatment naïve patients with light chain cardiac amyloidosis and heart failure.
Methods
Patient data collection
Data was collected over a 10-year period. Two hundred and thirty-six consecutive patients with tissue biopsy proven light chain amyloidosis featuring cardiac involvement and available clinical, laboratory, and echocardiographic data as well as serum samples obtained at initial visit to the Amyloidosis Center were selected for study. Boston University Medical Center (BUMC) Institutional Review Board approved the study (H-29147: “Biomarkers in Cardiac Amyloid”) and all subjects gave written informed consent. All research was performed in accordance with the relevant guidelines/regulations. Patients with transthyretin (TTR)-related forms of amyloidosis were excluded. None of the patients had multiple myeloma. Clinical and laboratory evaluations, including a medical history, physical examination, blood and urine tests including complete cell count, chemistries, serum/urine immunofixation and serum free light chains, chest radiography, electrocardiography and echocardiography, were systematically performed at the first visit to the Amyloidosis Center at BUMC between March 2004 and March 2014.
All subjects had a fat pad tissue biopsy positive for Congo red staining demonstrating the presence of fibrils to confirm the diagnosis of amyloidosis. In all subjects, serum and urine immunofixation electrophoresis, serum free light chain measurements, and bone marrow examination were performed to demonstrate the presence of a plasma cell dyscrasia thus establishing the diagnosis of AL amyloidosis. Amyloid cardiac involvement was determined by the presence of one of the following criteria: low voltage on electrocardiography, elevations in cardiac biomarkers, left ventricular hypertrophy on echocardiogram (in the absence of a history of hypertension or valvular disease), intraventricular septal thickness >12 mm and/or by an endomyocardial biopsy specimen that demonstrated amyloid fibril deposits. Immunohistochemical and biochemical analyses were used to identify the amyloid fibril protein.
All patients were evaluated by a cardiologist at the Amyloidosis Center and had ACC/AHA Stage C/D heart failure. Patients were classified according to New York Heart Association (NYHA) Functional class 1 to 4. Clinical course was monitored with regular follow-up at the Amyloidosis Center or by telephone and/or by contacting referring physicians, if patients were unable to return to the Amyloidosis Center. The endpoint of followup was all cause death obtained from medical records or publicly available databases.
Echocardiography
Two-dimensional echocardiography was performed using the GE VingMed Vivid FiVe Echocardiography System (GE Vingmed, Milwaukee, WI) with a 2.5-MHz phased-array transducer as previously described9. Echocardiograms were performed and analyzed in a blinded manner. Left ventricular ejection fraction (LVEF) was calculated using the modified Simpson’s rule and measurements of systolic and diastolic chamber dimensions (left ventricle end-systolic diameter [LVESD] and left ventricle end-diastolic diameter [LVEDD], respectively) and wall thickness were obtained from 2D imaging according to the recommendations of the American Society of Echocardiography9. The standard cube formula was utilized in order to calculate left ventricular mass10. Relative wall thickness (RWT) was calculated as (2 × posterior wall thickness)/LVEDD.
Biomarker analysis
Blood samples were collected at initial visit to the Amyloidosis Center. BNP, TnI and C-reactive protein (CRP) were measured in addition to routine laboratory testing. However, TnI was only routinely measured beginning in 2008. Testing was performed in the Boston Medical Center clinical laboratory. BNP and TnI was measured by Abbott chemiluminescence. CRP was measured via a rapid Automated High Sensitivity Enzyme Immunoassay (Abbott).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and median with interquartile ranges (IQR). Categorical variables were expressed as number of patients and percentages. Univariate analyses were performed to identify factors associated with increased mortality from demographic, clinical, laboratory, electrocardiography and echocardiographic variables including age, sex, log BNP, troponin I, dFLC, RWT, CRP, LVEF, estimated glomerular filtration rate (eGFR), QRS duration, atrial fibrillation, hemoglobin, systolic blood pressure, interventricular septal thickness (IVS), LV mass index. A P value ≤ 0.05 was considered statistically significant. Covariates were chosen based on statistical significance and entered into a multivariate Cox proportional hazards model. Backward elimination was then conducted with P‐value criteria for retention set at 0.20. In order to ensure that variables were not correlated a priori in the multivariate model, correlation data was determined. This established that variables were not collinear, thus maintaining confidence in the present model.
To define the predictive accuracy of the Cox regression models under considerations we use the time-dependent accuracy measures (sensitivity, specificity, and ROC concepts) proposed by Heagerty et al.11. C-statistic was used to assess the discrimination of the proposed models (revised Mayo criteria which included BNP, troponin I and dFLC). Discrimination characterizes the model’s ability to correctly classify subjects for their actual outcomes.
All analyses were conducted using SAS software version 9.4 (SAS Institute Inc.) or R version 3.5.0 (R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
Results
AL amyloidosis patients presenting between 2004–2014 who had (1) cardiac amyloidosis and (2) symptoms of heart failure were enrolled (n = 236). Among these, 71 patients were excluded because they had received prior therapy directed to the plasma cell dyscrasia prior to their initial evaluation at our center. Thus, 165 non-consecutive treatment-naïve patients with AL cardiac amyloidosis and ACC/AHA Stage C/D heart failure were included in the final analyses. Demographics with clinical characteristics are outlined in Table 1. Mean age was 61.6 ± 9.5 years. The cohort was predominantly male (61%) and white (76.4%). Hypertension and atrial fibrillation occurred in 22.4% and 24.8% of individuals, respectively. All patients had heart failure symptoms and ~50% were NYHA Functional Class 3/4. The kidney was the primary non-cardiac organ affected (36.4%) and most of patients carried the lambda light chain subtype (84.2%). The majority of the patients were on loop diuretics (81.2%). Spironolactone (22.4%) and metolazone (12%) were also prescribed. Not surprisingly, beta-blocker and angiotensin converting enzyme-inhibitor/angiotensin receptor blockers were found only in 38.7% and 21.2%, respectively, as these medications are often poorly tolerated in AL cardiac amyloidosis. The duration of heart failure symptoms was 11.3 ± 8.9 months before the initial evaluation at the Amyloidosis Center. The mean time from diagnosis to the initial visit to the Amyloidosis Center was 2.4 ± 3.4 months (median of 1.0 month, range 0.0–26.0). Cardiac biopsy was performed in 41% of patients.
Table 1.
Clinical Characteristics, Laboratory and Echocardiographic Parameters at Initial Visit to the Amyloidosis Center.
| Value (n = 165) | Median (IQR range) | Normal values | |
|---|---|---|---|
| Age (years) | 61.6 ± 9.5 | 61 (55–69) | |
| Sex: Male (%)/Female (%) | 100 (61%)/65 (49%) | ||
| Race: White (%) | 126 (76.4%) | ||
| Systolic blood pressure (mmHg) | 112.0 ± 18.6 | 108.5 (101–122) | |
| Diastolic blood pressure (mmHg) | 73.0 ± 9.9 | 72 (67–79) | |
| Pulse rate, beats/min | 84.0 ± 15.2 | 83 (73–94) | |
| Body Mass Index (kg/m2) | 25.8 ± 4.6 | 25.5 (23.0–28.0) | |
| Hypertension (%) | 37 (22.4%) | ||
| Diabetes Mellitus (%) | 10 (6.0%) | ||
| Coronary Artery Disease (%) | 29 (17.6%) | ||
| Atrial Fibrillation (%) | 41 (24.8%) | ||
| Lambda Light Chain (%) | 139 (84.2%) | ||
| Kidney Involvement (%) | 60 (36.40%) | ||
| NYHA Class | 2.7 ± 0.6 | 2.5 (2.0–3.0) | |
| NYHA Class 3/4 | 80 (48.50%) | ||
| ACC/AHA Stage C/D (%) | 165 (100%) | ||
| QRS duration (ms) on EKG | 99.0 ± 24.5 | 93 (81–108) | |
| Medication Use: | |||
| Aldactone (%) | 37 (22.4%) | ||
| Loop Diuretic (%) | 135 (81.2%) | ||
| Metolazone (%) | 20 (12%) | ||
| Beta Blocker (%) | 64 (38.8%) | ||
| ACE-I/ARB (%) | 35 (21.2%) | ||
| Anticoagulants (%) | 40 (24.2%) | ||
| Laboratory Data: | |||
| BNP (pg/mL) | 980.4 ± 1035.1 | 654 (301–1208) | 0–176 |
| Troponin I (ng/mL) | 0.4 ± 0.9 | 0.146 (0.067–0.333) | 0–0.033 |
| C-reactive protein (mg/L) | 7.6 ± 12.2 | 4.0 (1.0–9.5) | 0–5.0 |
| Hemoglobin (g/dl) | 13.4 ± 1.7 | 13.4 (12.1–14.5) | 11.8–16.0 |
| Sodium (mmol/L) | 138.5 ± 4.0 | 139 (137–141) | 135–145 |
| MDRD eGFR (ml/min/1.73 m2) | 67.4 ± 25.5 | 66 (53–82) | >60 |
| Uric Acid (mg/dl) | 7.8 ± 2.6 | 7.35 (5.73–9.45) | 2.4–6.0 |
| Alkaline Phosphatase (U/L) | 137.3 ± 86.9 | 110 (82–166) | 25–100 |
| Lactate dehydrogenase (U/L) | 272.2 ± 76.3 | 259 (223.5–315.5) | 171–308 |
| Free kappa light chains (mg/L) | 113.8 ± 372.8 | 11.2 (8.3–21.85) | 3.3–19.4 |
| Free lambda light chains (mg/L) | 390.0 ± 500.4 | 243.0 (69.55–487.9) | 5.7–26.3 |
| Free light chains kappa/lambda ratio | 11.24 ± 42.66 | 0.05 (0.02–0.18) | 0.26–1.65 |
| dFLC (mg/L) | 478.1 ± 558.3 | 291.8 (119–552.6) | |
| Echocardiographic parameters: | |||
| Left ventricular ejection fraction (%) | 49.8 ± 11.6 | 50 (40–60) | ≥50 |
| Left atrium (mm) | 40.2 ± 6.2 | 40 (36–44) | |
| Intraventricular septum (mm) | 14.9 ± 2.7 | 15 (13–16) | 6–10 |
| Posterior wall thickness (mm) | 15.2 ± 3.0 | 15 (13–17) | 6–10 |
| Left ventricular end systolic diameter (mm) | 30.0 ± 6.2 | 30.0 (26.5–34.0) | 21–40 |
| Left ventricular end diastolic diameter (mm) | 40.0 ± 5.8 | 40 (36–44) | <57 |
| Relative wall thickness | 0.8 ± 0.2 | 0.74 (0.63–0.89) | 0.22–0.42 |
| Calculated LV mass (g) | 240.3 ± 75.7 | 223.7 (191.7–271.8) | 67–162 |
| LV mass index (g/m2) | 129.2 ± 35.4 | 124.2 (107.5–145.5) | 50–115 |
Data are expressed as mean ± SD for continuous variables or numbers or percent (%) for categorical variables. IQR, interquartile; AL indicates immunoglobulin light chain amyloidosis; NYHA, New York Heart Association; ECG, electrocardiogram; MDRD eGFR, glomerular filtration rate by Modification of Diet in Renal Disease equation; BNP, brain natriuretic peptide; ACE-I, angiotensin converting enzyme-inhibitor; ARB, angiotensin receptor blocker; ACC/AHA stage, American College of Cardiology/American Heart Association Classification of Heart Failure; dFLC, difference between involved and uninvolved light chains. EKG, electrocardiogram. Anticoagulants include warfarin and non-vitamin K antagonist oral anticoagulants. Normal values for echocardiography are as reported by the American Society of Echocardiography9.
In the original staging, Mayo Stage III disease was defined as TnT ≥0.035 ng/mL and NT-proBNP ≥332 pg/mL4. NT-proBNP levels and TnT were not measured at our institution. It has been proposed and validated that a BNP level of >81 pg/mL and a TnI >0.1 ng/mL provides similar discrimination for cardiac involvement7 as compared to NT-proBNP ≥332 pg/mL and TnT >0.03512,13. Thus, using this association and where both troponin and BNP were available, 64% of patients were characterized as high-risk individuals (Mayo cardiac stage III). Cardiac troponin I and BNP levels were elevated with a mean BNP of 980.4 ± 1056.0 pg/mL (median of 654 pg/mL, range: 15.0–7,161.0) and cardiac troponin I of 0.379 ± 0.980 ng/mL (median of 0.1460 ng/mL, range: 0.0–8.92). Mean LVEF was 49.8 ± 11.6% (median of 50%, range: 15–70) with ~54% of patients presenting with LVEF <50%, i.e., heart failure with reduced ejection fraction (HFrEF).
Predictors of mortality
Univariate analyses, expressed as hazard ratios, are presented in Table 2. The strongest risk factor for all-cause mortality was increased RWT. Others included were age, NYHA functional class, atrial fibrillation, LVEF, IVS, log BNP, dFLC and CRP. Multivariate analysis (Table 2), using a Cox proportional hazards regression model with backward elimination, was used to evaluate interactions among all the risk factors for overall mortality. Age, log BNP, NYHA Class, CRP and RWT persisted as significant risk factors. Remaining univariate risk factors entered into the model were not retained by the final multivariate Cox proportional hazards model.
Table 2.
Univariate and multivariate analysis for risk factors of mortality in AL cardiac amyloidosis with heart failure.
| Univariate Hazard Ratio | P-Value | Multivariate Hazard Ratio | P-Value | |
|---|---|---|---|---|
| RWT | 4.40 (1.87–10.30) | <0.0001 | 6.70 (2.45–18.30) | <0.001 |
| NYHA Class | 2.91 (2.14–3.97) | <0.001 | 1.50 (1.02–2.2) | 0.04 |
| Log BNP | 1.93 (1.58–2.38) | <0.001 | 1.45 (1.15–1.81) | <0.01 |
| Age (years) | 1.04 (1.02–1.06 | <0.0001 | 1.04 (1.01–1.06) | <0.01 |
| CRP | 1.02 (1.00–1.03) | <0.01 | 1.02 (1.00–1.04) | 0.01 |
| Female Sex | 0.87 (0.60–1.26) | 0.47 | — | — |
| Atrial Fibrillation | 1.70 (1.14–2.52) | <0.01 | — | — |
| Systolic Blood Pressure | 0.99 (0.98–1.00) | 0.11 | — | — |
| Troponin I | 1.14 (0.95–1.35) | 0.16 | — | — |
| MDRD eGFR (mL/min/1.73 m2) | 0.99 (0.98–1.00) | 0.051 | — | — |
| LVEF (%) | 0.97 (0.95–0.99) | <0.0001 | — | — |
| IVS (mm) | 1.14 (1.05–1.23) | <0.001 | — | — |
| LV mass index (g/m2) | 0.99 (0.99–1.00) | 0.88 | — | — |
| dFLC | 1.0004 (1.0001–1.0007) | 0.0008 | — | — |
| QRS duration (ms) on EKG | 1.003 (0.99–1.01) | 0.44 | — | — |
NYHA, New York Heart Association; BNP, brain natriuretic peptide; CRP, C-reactive protein; MDRD eGFR, glomerular filtration rate by Modification of Diet in Renal Disease equation; LVEF; left ventricular ejection fraction; RWT, relative wall thickness; IVS, interventricular septum; dFLC, difference between involved and uninvolved light chains; EKG, electrocardiogram.
In order to understand the contribution of these variables in addition to the revised Mayo staging system in predicting mortality, an ROC analysis was performed (Fig. 1). Each significant variable was added to the base model, which includes log BNP, TnI and dFLC. The base model had modest discriminatory ability (AUC 0.67, 95% CI 0.57–0.75). The AUC values were comparable for the addition of each significant variable to the base model: age (AUC 0.67, 95% CI 0.58–0.75), CRP (AUC 0.68, 95% CI 0.58–0.77), RWT (AUC 0.68, 95% CI 0.60–0.76), NYHA class (AUC 0.71, 95% CI (0.62–0.80). An all-inclusive model that utilized all 4 variables in addition to the base model gave the greatest power (AUC 0.73, 95% CI 0.62–0.83), in predicting 1-year mortality in AL cardiac amyloidosis with heart failure.
Figure 1.
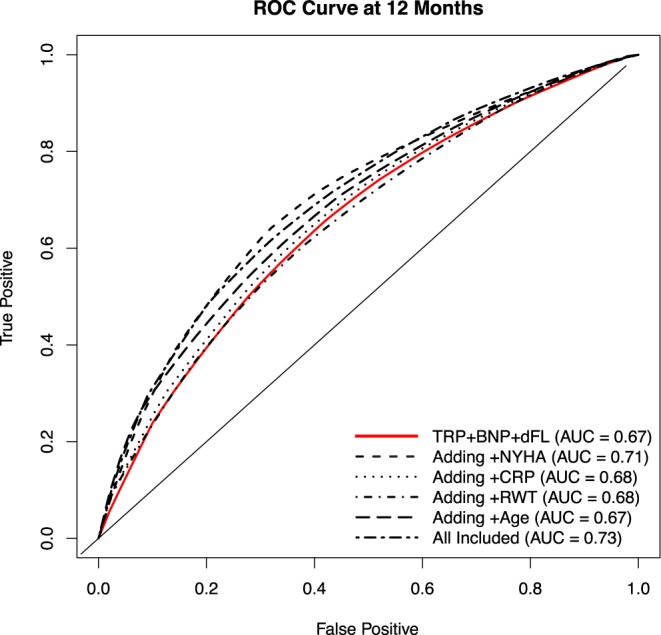
Receiver-operating characteristic (ROC) curves assessing age, NYHA class, RWT and CRP as predictors of 1-year survival in addition to standard variables (BNP, TnI and dFLC) for patients with light chain (AL) cardiac amyloidosis and heart failure.
Relative wall thickness
The cohort was divided into 2 groups based on RWT ≥ and < than the median of 0.74. Baseline demographics and clinical characteristics were compared between these two groups (Table 3). In the group where RWT ≥0.74, patients had worse NYHA functional class, less coronary artery disease and slightly higher hemoglobin levels. Troponin I levels were also insignificantly higher and there was no difference in renal function as measured by MDRD eGFR as compared to RWT <0.74. BNP levels and kidney involvement in AL cardiac amyloidosis did not differ between RWT ≥0.74 and RWT <0.74.
Table 3.
Clinical and Laboratory Characteristics above and below the median for Relative Wall Thickness (RWT).
| RWT ≥0.74 | RWT <0.74 | P values | |
|---|---|---|---|
| Age (years) | 62 ± 10.1 | 61 ± 9.9 | 0.474 |
| Sex: Male (%) Female (%) | 47 (59.5%) 32 (40.5%) | 50 (64%) 28 (35.9%) | 0.552 |
| Race: White (%) | 55 (72.4%) | 64 (83.1%) | 0.081 |
| Systolic blood pressure (mmHg) | 113.4 ± 21.86 | 110.6 ± 14.47 | 0.339 |
| Diastolic blood pressure (mmHg) | 73.7 ± 10.22 | 71.9 ± 9.0 | 0.260 |
| Pulse rate, beats/min | 84.9 ± 16.9 | 81.9 ± 13.8 | 0.238 |
| Body mass index (kg/m2) | 25.4 ± 3.9 | 26.0 ± 5.5 | 0.411 |
| Hypertension (%) | 15 (19%) | 18 (23.1%) | 0.529 |
| Diabetes mellitus (%) | 3 (3.8%) | 5 (6.4%) | 0.697 |
| Coronary artery disease (%) | 9 (11.4%) | 20 (26%) | 0.019* |
| Atrial fibrillation (%) | 20 (25.3%) | 20 (26%) | 0.925 |
| Lambda light chain (%) | 63 (80.8%) | 70 (89.7%) | 0.114 |
| Kidney involvement (%) | 33 (41.8) | 26 (33.8%) | 0.303 |
| NYHA class | 3 (1–4)† | 2.5 (2–3.5)† | 0.059 |
| NYHA class 3/4 | 44 (55.7%) | 32 (41%) | 0.066 |
| QRS duration (ms) on EKG | 97.4 ± 25.86 | 101.9 ± 25.15 | 0.275 |
| Medication Use: | |||
| Aldactone (%) | 19 (24.1%) | 16 (20.5%) | 0.594 |
| Loop diuretic (%) | 67 (84.8%) | 63 (81.8%) | 0.616 |
| Thiazide diuretic (%) | 8 (10.1%) | 11 (14.3%) | 0.427 |
| Beta blocker (%) | 29 (36.7%) | 34 (43.6%) | 0.379 |
| ACE-I/ARB (%) | 15 (19%) | 15 (19.2%) | 0.969 |
| Anticoagulants (%) | 15 (19%) | 25 (32.1%) | 0.060 |
| Laboratory Data: | |||
| BNP (pg/mL) | 739 (372–1368)† | 556 (241–972)† | 0.107 |
| Troponin I (ng/mL) | 0.57 ± 1.39 | 0.22 ± 0.33 | 0.089 |
| Creatinine kinase (U/L) | 135 ± 20.3 | 103 ± 9 | 0.161 |
| C-reactive protein (mg/L) | 6.53 ± 7.43 | 8.68 ± 14.15 | 0.244 |
| Hemoglobin (g/dl) | 13.61 ± 1.72 | 13.04 ± 1.65 | 0.035* |
| Sodium (mmol/L) | 138.5 ± 3.9 | 138.7 ± 3.6 | 0.729 |
| MDRD eGFR (ml/min/1.73 m2) | 67.9 ± 24.8 | 66.9 ± 21.7 | 0.792 |
| Uric acid (mg/dl) | 7.99 ± 2.49 | 7.56 ± 2.52 | 0.297 |
| Alkaline phosphatase (U/L) | 131.5 ± 77.1 | 142.7 ± 95.4 | 0.431 |
| Free kappa light chains (mg/L) | 163.7 ± 478.3 | 63.45 ± 228.7 | 0.096 |
| Free lambda light chains (mg/L) | 401.3 ± 475.7 | 378.2 ± 522.5 | 0.773 |
| Free light chains kappa/lambda ratio | 15.51 ± 52.79 | 7.42 ± 31.72 | 0.247 |
| dFLC (mg/L) | 536.8 ± 581.4 | 418.5 ± 535.0 | 0.188 |
Data are expressed as mean ± SD or median (range) for continuous variables or numbers (%) for categorical variables. †Median (IQR range). AL indicates immunoglobulin light chain amyloidosis; NYHA, New York Heart Association; EKG, electrocardiogram; MDRD eGFR, glomerular filtration rate by Modification of Diet in Renal Disease equation; BNP, brain natriuretic peptide; ACE-I, angiotensin converting enzyme-inhibitor; ARB, angiotensin receptor blocker; dFLC, difference between involved and uninvolved light chains; EKG, electrocardiogram. Anticoagulants include warfarin and non-vitamin K antagonist oral anticoagulants.
As expected IVS (16.2 ± 2.5 vs. 13.6 ± 1.6 mm), posterior wall thickness (17.1 ± 2.8 vs. 13.3 ± 1.9 mm) and LV mass index (136.5 ± 36.8 vs. 121.8 ± 32.6 g/m2) were all significantly increased in RWT ≥0.74 vs. RWT <0.74. Despite the increase in LV mass and wall thickness, LV chamber size was significantly smaller in RWT ≥0.74 than RWT <0.74 (LVESD: 27.9 ± 5.14 vs. 32.1 ± 6.6 mm and LVEDD: 36.5 ± 4.5 vs. 43.5 ± 4.8 mm). LVEF was no different between RWT ≥0.74 and RWT <0.74 (49.2 ± 12.0 vs. 50.7 ± 11.7%).
The Kaplan-Meier estimate depicts a significantly higher mortality in the group with RWT ≥0.74 vs. RWT <0.74 over 10 years (P < 0.01; Fig. 2). The mortality rate at 1-year followup in RWT ≥0.74 was 65%. Median survival time in RWT ≥0.74 was 5.5 months (95% CI 3.1–10.5) with a mean length of followup of 22.1 months. Conversely, the mortality rate at 1-year followup in RWT <0.74 was 39.7%. With RWT <0.74, the median survival time was 16.5 months (95% CI 9.2–39.1; P < 0.01) and the average length of followup was 30.7 months.
Figure 2.
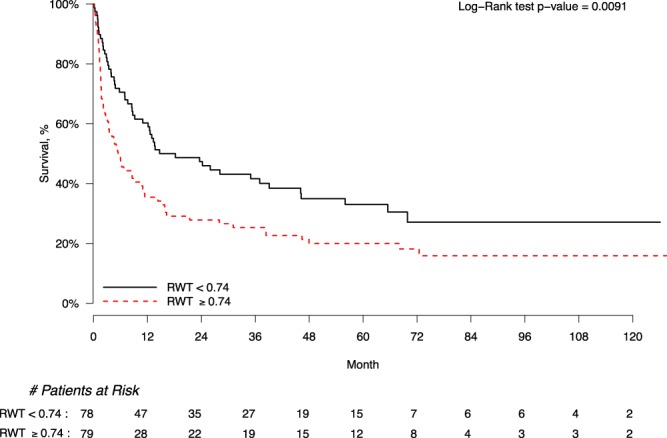
Kaplan Meier Survival curve for RWT above and below the median in AL cardiac amyloidosis with heart failure. Survival was significantly worse in patients with RWT ≥0.74 than those with RWT <0.74 (P < 0.01).
Discussion
Despite some improvement in outcomes14 and advances in novel therapies for systemic AL amyloidosis15, AL patients presenting with cardiac amyloidosis and symptoms of heart failure have the greatest risk of morbidity and mortality. These patients are routinely excluded16 from certain life-saving therapeutic options such as HDM/SCT and also from investigational clinical trials. As seen in this study, AL cardiac amyloidosis patients with symptoms of heart failure presented late and survival was poor. Therefore, it is important to identify clinical variables that could identify patients that were lower risk and thus might be more amenable to higher risk therapies and clinical studies.
RWT, age, BNP, NYHA class, and CRP were markers of mortality in this cohort. RWT is the relationship of LV mass to volume and is the ratio of LV wall thickness to chamber radius. In subjects without cardiovascular disease but with hypertension, increasing RWT is associated with increased mortality17. The change in LV structure has deleterious consequences including deterioration in systolic pump performance and reduction in stroke volumes18. LV mass and wall thickness is greater in ATTRwt cardiac amyloidosis and the cardiac hypertrophy is more often asymmetric compared to AL cardiac amyloidosis19,20. But RWT is much greater in AL cardiac amyloidosis19 because of the smaller LV cavity in this subgroup of cardiac amyloidosis. The cardiac toxicity of amyloid deposits in AL amyloidosis induces cellular oxidative stress, cardiac myocyte dysfunction, impaired cellular autophagy and accelerates cell death21. These may constrain the dilation of the LV that is normally seen with eccentric cardiac remodeling and hypertrophy22 in cardiovascular diseases.
Given a significant majority of our AL amylodosis heart failure patients were Mayo cardiac stage III, the RWT provides an important tool to further aid clinicians in determining prognosis, as patients with RWT above and below the median have significantly different mortality rates. Therefore, increased RWT in AL cardiac amyloidosis with evidence of symptoms of heart failure, in addition to being a marker to discriminate cardiac amyloid hearts from those without amyloid fibril deposition23 ; RWT is useful in identifying “at risk” subjects and provides a simple non-invasive assessment of amyloid fibril burden on LV structure derived from routine echocardiographic measurements, thus conferring its advantage over more novel echocardiographic parameters such as left ventricular strain.
CRP is released after tissue injury/inflammation and is an acute phase reactant. CRP is also an inflammatory marker for cardiovascular events and predicts outcome after myocardial infarction24 and non-amyloid heart failure25. Importantly, AL amyloidosis is not considered a pro-inflammatory disease, unlike AA (amyloid A) which is associated with underlying chronic infections. However, as previously reported, circulating levels of “cardiocentric” matrix metalloproteinases (responsible for extracellular matrix homeostasis)19 are elevated in AL cardiac amyloidosis, suggesting a possible inflammatory milieu in the heart in AL cardiac amyloidosis. Although the present findings suggest that CRP may be important in identifying risk in AL cardiac amyloid with symptomatic heart failure, it is not a known risk marker in AL cardiac amyloidosis and needs to be validated in other cohorts to better define the role of inflammatory responses in AL cardiac amyloidosis.
Non-specific presenting symptoms often delay the diagnosis of amyloidosis. This is likely reflected by the lengthy time lapse (~11 months) between the onset of symptoms to presentation for initial evaluation at the Amyloidosis Center. Rahman et al. showed a similar delay in diagnosis from the onset of symptoms (15.4 months)5. In the present study, this time delay likely contributed to the advanced presentation in symptomatic AL cardiac amyloidosis patients with heart failure, with very elevated BNP and troponin levels at the initial presentation to the Amyloidosis Center. The delay in diagnosis has adverse ramifications on survival and on the ability to provide appropriate and effective treatment, specifically HDM/SCT.
Amyloid fibril deposition in the heart causes a restrictive cardiomyopathy with diastolic impairment19,21 leading to a reduced stroke volume, while LVEF remains relatively preserved26,27. In addition to diastolic function abnormalities, systolic performance is often compromised, even prior to the manifestation of cardiac involvement on standard echocardiography27. Using strain imaging by echocardiography, which was not available for this retrospective analysis, others showed that regional myocardial impairment is evident earlier indicating compromises in systolic pump performance prior to LVEF deterioration and symptomatic heart failure27. Noticeably about half of our cohort had depressed systolic function, i.e., LVEF <50%. As evidenced not only by this data, but also by worse NYHA functional class and elevated cardiac biomarkers, AL cardiac amyloidosis patients presenting with symptoms of heart failure, represents a more advanced stage of presentation.
Limitations of the study
This retrospective, single center cohort study has some limitations. First, the Amyloidosis Center at BUMC is a referral center for the diagnosis and treatment of systemic AL amyloidosis and thus, a referral bias is possible in the selection of patients. Patients also presented at varying stages of disease as noted by the mean duration of heart failure ~11 months and NYHA Class ranged from 2–4. Furthermore, the availability of treatment data after initial referral visit was also limited since many patients were subsequently managed by their primary hematologist/cardiologist. Insights can be gained from the analysis of data of subsequent therapy in patients who received treatment at BUMC. Following the initial visit, 68/76 (89%) of individuals received either chemotherapy alone (63%) or HDM/SCT (37%) after their index visit. Thus, although these patients presented at advanced stages of cardiac disease, they did continue to receive standard treatments. Second, prior studies in AL amyloidosis cohorts showed that cardiac troponin was a risk factor for mortality, a finding not replicated in our cohort. However, cardiac troponin was not part of the initial standard testing at our Amyloid Center until 2008, thus likely influencing its omission from our multi-variate predictive model. Third, the findings are likely center-specific and validation in other cohorts is needed. And finally, not all patients received cardiac biopsy to verify cardiac amyloidosis. However, this is in line with the more contemporary care of these patients, as more non-invasive modalities are used to diagnosis cardiac amyloid involvement28.
In conclusion, AL cardiac amyloidosis patients presenting with symptoms of heart failure have poor outcomes. Other than levels of NT pro-BNP and troponin and perhaps systolic blood pressure16, there have been limited use of clinical data to further risk stratify patients with heart failure due to AL cardiac amyloid. In the present study RWT, older age, worse/higher NYHA functional class, increasing BNP and CRP levels were risk factors for all-cause mortality, with RWT being a simple, non-invasive tool to further risk stratify this already high-risk cohort. Given the limited life expectancy in patients with AL cardiac amyloidosis who develop heart failure, further studies are warranted to determine the effectiveness of these variables in risk stratification and their ability to help guide treatment choices in this high-risk cohort.
Acknowledgements
To the members of the multidisciplinary clinical and research teams of the Amyloidosis Center. This work was supported by a grant from the National Institutes of Health (HL117153) to F.S. and the R. Gordon Darby Research Fund to F.S.
Author Contributions
Conceptualization: Usman Tahir, David Seldin, Flora Sam. Data collection: Usman Tahir, Gheorghe Doros, John Kim, Lawreen Connors, Flora Sam. Project administration: Usman Tahir, Flora Sam. Validation: Usman Tahir, Gheorghe Doros, Flora Sam. Writing (original draft): Usman Tahir, Flora Sam. Writing (review & editing): Usman Tahir, Gheorghe Doros, John Kim, Lawreen Connors, Flora Sam.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
David C. Seldin is deceased.
References
- 1.Falk RH, Skinner M. The systemic amyloidoses: an overview. Adv Intern Med. 2000;45:107–137. [PubMed] [Google Scholar]
- 2.Kyle RA, Greipp PR. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983;58:665–683. [PubMed] [Google Scholar]
- 3.Pellikka PA, et al. Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Arch Intern Med. 1988;148:662–666. doi: 10.1001/archinte.1988.00380030168027. [DOI] [PubMed] [Google Scholar]
- 4.Dispenzieri A, et al. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104:1881–1887. doi: 10.1182/blood-2004-01-0390. [DOI] [PubMed] [Google Scholar]
- 5.Rahman JE, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410–415. doi: 10.1016/j.jacc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilleness B, Ruberg FL, Mussinelli R, Doros G, Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood. 2019;133:215–23. doi: 10.1182/blood-2018-06-858951. [DOI] [PubMed] [Google Scholar]
- 8.Nordlinger M, Magnani B, Skinner M, Falk RH. Is elevated plasma B-natriuretic peptide in amyloidosis simply a function of the presence of heart failure? Am J Cardiol. 2005;96:982–984. doi: 10.1016/j.amjcard.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-X. [DOI] [PubMed] [Google Scholar]
- 11.Heagerty PJ, Zheng Y. Survival Model Predictive Accuracy and ROC Curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 12.Waldo SW, et al. Pro-B-type natriuretic peptide levels in acute decompensated heart failure. J Am Coll Cardiol. 2008;51:1874–1882. doi: 10.1016/j.jacc.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 13.Colucci, W. S. & Chen, H. H. Natriuretic peptide measurement in heart failure, http://www.uptodate.com/contents/natriuretic-peptide-measurement-in-heart-failure? (2017).
- 14.Girnius S, Seldin DC, Cibeira MT, Sanchorawala V. New Hematologic Response Criteria Predict Survival in Patients With Immunoglobulin Light Chain Amyloidosis Treated With High-Dose Melphalan and Autologous Stem-Cell Transplantation. J Clin Oncol. 2013;31:2749–2750. doi: 10.1200/JCO.2013.48.7736. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–615. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- 16.Wechalekar AD, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121:3420–3427. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 17.Tsao CW, et al. Left Ventricular Structure and Risk of Cardiovascular Events: A Framingham Heart Study Cardiac Magnetic Resonance Study. J Am Heart Assoc. 2015;4:e002188. doi: 10.1161/JAHA.115.002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aurigemma GP, et al. Reduced left ventricular systolic pump performance and depressed myocardial contractile function in patients >65 years of age with normal ejection fraction and a high relative wall thickness. Am J Cardiol. 1995;76:702–705. doi: 10.1016/S0002-9149(99)80201-X. [DOI] [PubMed] [Google Scholar]
- 19.Biolo A, et al. Matrix Metalloproteinases and Their Tissue Inhibitors in Cardiac Amyloidosis: Relationship to Structural, Functional Myocardial Changes and to Light Chain Amyloid Deposition. Circ Heart Fail. 2008;1:249–257. doi: 10.1161/CIRCHEARTFAILURE.108.788687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 21.Guan J, Mishra S, Falk RH, Liao R. Current perspectives on cardiac amyloidosis. Am J Physiol Heart Circ Physiol. 2012;302:H544–H552. doi: 10.1152/ajpheart.00815.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–1740. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Pozo E, et al. Revisiting morphological features of cardiac amyloid with cardiac magnetic resonance. J Cardiovasc Magn Reson. 2013;15:154. doi: 10.1186/1532-429X-15-S1-P154. [DOI] [Google Scholar]
- 24.Sabatine MS, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 25.Alonso-Martinez JL, et al. C-reactive protein as a predictor of improvement and readmission in heart failure. Eur J Heart Fail. 2002;4:331–336. doi: 10.1016/S1388-9842(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 26.Apridonidze T, et al. Clinical and echocardiographic correlates of elevated troponin in amyloid light-chain cardiac amyloidosis. Am J Cardiol. 2012;110:1180–1184. doi: 10.1016/j.amjcard.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 27.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 28.Gertz MA. Immunoglobulin light chain amyloidosis: 2018 Update on diagnosis, prognosis, and treatment. Am J Hematol. 2018;93:1169–1180. doi: 10.1002/ajh.25149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


